Abstract
The number of bubbles likely to form in a glass of beer is the result of the fine interplay between dissolved CO2, tiny particles or glass imperfections acting as bubble nucleation sites, and ascending bubble dynamics. Experimental and theoretical developments about the thermodynamic equilibrium of dissolved and gas-phase carbon dioxide (CO2) were made relevant to the bottling and service of a commercial lager beer, with 5% alcohol by volume and a concentration of dissolved CO2 close to 5.5 g L–1. The critical radius and the subsequent critical concentration of dissolved CO2 needed to trigger heterogeneous nucleation of CO2 bubbles from microcrevices once the beer was dispensed in a glass were derived. The subsequent total number of CO2 bubbles likely to form in a single glass of beer was theoretically approached as a function of the various key parameters under standard tasting conditions. The present results with the lager beer were compared with previous sets of data measured with a standard commercial Champagne wine (with 12.5% alcohol by volume and a concentration of dissolved CO2 close to 11 g L–1).
1. Introduction
Beer is most probably the oldest alcoholic beverage in the world. Recently, evidence of some 13 000-year-old wheat- and barley-based beer was found inside stone mortars carved into the floor of a cave near Haifa, Israel.1 This discovery challenges previous evidence that beer brewing traced back to the early Neolithic period, about 5000 years ago.2,3 Today, beer is by far the most consumed alcoholic beverage in the world, with a global production of about 1.91 billion hectoliters in 2019.4 The global beer market was valued at USD 606 billion in 2019 and is projected to reach close to USD 700 billion by 2025.5
Beer is generally prepared using four basic ingredients (water, malted cereal grains, yeast, and hops) and undergoes the process of fermentation for a certain time period. Among all types of beers, lager is the most widely consumed and commercially available style of beer.6 Lager beer uses a process of cool fermentation, followed by maturation in cold storage. Bottled or canned lager beers are under a pressure of gas-phase carbon dioxide (CO2), and therefore hold a concentration of dissolved CO2 within the liquid phase, as described in previous articles about corked champagne bottles7,8 and bottled carbonated waters.9 In lager beers, and in sparkling beverages in general, the concentration of dissolved CO2 is an important parameter because it is responsible for the desirable bubbling process.10−12 The presence of dissolved CO2 in beer directly impacts various sensory properties such as the frequency of bubble formation in a glass,13−16 the growth rate of ascending bubbles,17,18 and the perception of dissolved and gas-phase CO2 acting, respectively, on trigeminal receptors19,20 and gustatory receptors.21,22 It was highlighted recently that a minimum concentration of 1.2 g L–1 of dissolved CO2 is required by consumers of sparkling wines to experience a carbonation bite in the mouth.23
The strong interplay between the various parameters at play in a bottle and in a glass of champagne or beer has been the subject of study for about three decades, as presented in several tutorial reviews.12,24−27 A recent publication attempts to determine how many bubbles are likely to form in a glass of champagne using models that combine both ascending bubble dynamics and mass transfer equations.28 As one might expect, the number of bubbles likely to form per glass depends on both the wine and the glass itself. A theoretical relationship was derived, which provides the whole number of bubbles likely to form per glass depending on various parameters such as the concentration of dissolved CO2, wine temperature, glass shape, volume dispensed, and ambient pressure.28 If 100 mL of champagne is poured straight down the middle of a vertically oriented flute, about one million bubbles are likely to nucleate if you resist drinking from your flute. Otherwise, champagne served more gently by pouring down the wall of a tilted flute (a technique that better preserves the dissolved CO229) will yield tens of thousands more bubbles before it goes flat. To the best of our knowledge, the issue of the number of bubbles likely to form in a single glass of beer nevertheless remains unexplored. The highly competitive market of canned or bottled lager beer is therefore still looking for new insights and further developments regarding gas-phase and dissolved CO2 equilibrium and the subsequent CO2 bubble dynamics in glasses.
In this article, experimental and theoretical developments about the thermodynamic equilibrium of dissolved and gas-phase CO2 relevant to the bottling and service of a standard commercial lager beer conditioned in 250 mL glass bottles are conducted. Under standard beer tasting conditions, the critical concentration of dissolved CO2 below which bubble nucleation becomes thermodynamically impossible in a glass was theoretically explored, as well as the issue of the subsequent total number of CO2 bubbles likely to form in the glass along the entire natural degassing process.
2. Results and Discussion
2.1. Temperature-Dependent Solubility of Gas-Phase CO2 in Beer
The solubility of gas-phase CO2 in a liquid phase is governed by Henry’s law, which states that the equilibrium concentration cL of dissolved CO2 in the liquid phase is proportional to the partial pressure of gas-phase CO2, according to the following relationship32
| 1 |
where kH is the temperature-dependent Henry’s constant of gas-phase CO2 in the liquid phase and PCO2 is the partial pressure of gas-phase CO2 in the gas phase (i.e., the gaseous headspace under the cap in case of a beer bottle).
In contrast to the overwhelming collection of data about the solubility of gas-phase CO2 in pure water, data about the CO2 solubility in beers are scarce, as reported in the article by Speers and MacIntosh,33 where several empirically derived CO2 solubility equations and charts from in the past decades are properly listed and discussed. The usual theoretical CO2 solubility models do not account for the complexity and the wide range of styles of present-day beers (in terms of alcohol and carbohydrate concentration, for example). In our beer samples, the Henry’s constant of gas-phase CO2 was approached through the following relationship, established in the early 1960s for hydroalcoholic and sugar solutions, at 20 °C34
| 2 |
with kH being expressed in g L–1 bar–1, a being the alcohol level (displayed in % by volume), and b being the concentration of sugar (displayed in g L–1).
The previous equation proved to be useful in the sparkling wines industry, with champagne and sparkling wines being considered as a first approximation as hydroalcoholic and sugar solutions.11,24,25 Applying the previous equation for the lager beer holding 5% alcohol by volume and no residual sugars (at 20 °C), the Henry’s constant of gas-phase CO2 was found to be kH ≈ 1.6 g L–1 bar. This value, slightly lower than the Henry’s constant of gas-phase CO2 in pure water at 20 °C (≈ 1.7 g L–1 bar–1),9 is indeed in quite good accordance with the main CO2 solubility model reported in the article by Speers and MacIntosh.33
Moreover, the solubility of gas-phase CO2 in a liquid phase is known to be strongly temperature-dependent.11,32−35 The lower the temperature of the liquid phase, the higher the gas solubility and therefore the higher the Henry’s constant, which can be conveniently expressed with a van’t Hoff equation as follows11
| 3 |
where k293 K is the Henry’s constant of gas-phase CO2 in the liquid phase at 20 °C (i.e., ≈3.64 × 10–4 mol m–3 Pa–1 ≈ 1.6 g L–1 in the lager beer), ΔHdiss is the dissolution enthalpy of gas-phase CO2 in the liquid phase (in J mol–1), R is the ideal gas constant (8.31 J K–1 mol–1), and T is the absolute temperature (in K).
Strictly speaking, the presence of ethanol in a water/ethanol mixture modifies the solubility and the subsequent dissolution enthalpy of gas-phase CO2 in the liquid phase compared with pure water, as described in detail in a previous article where gas solubility data and Henry’s constants for carbon dioxide in water/ethanol mixtures are reported.36 Nevertheless, for a beer with less than 10% alcohol by volume, the Henry’s law constant of CO2 and the enthalpy of solution do not differ significantly from those in pure water,36 with ΔHdiss ≈ −20 kJ mol–1.37Equation 3 with the appropriate parameters gives the Henry’s constant of CO2 in our lager beer stored at a realistic tasting temperature of 6 °C as kH6 °C ≈ 2.4 g L–1 bar–1. This Henry’s constant will be used in the following.
2.2. Thermodynamic Equilibrium in the Sealed Bottles
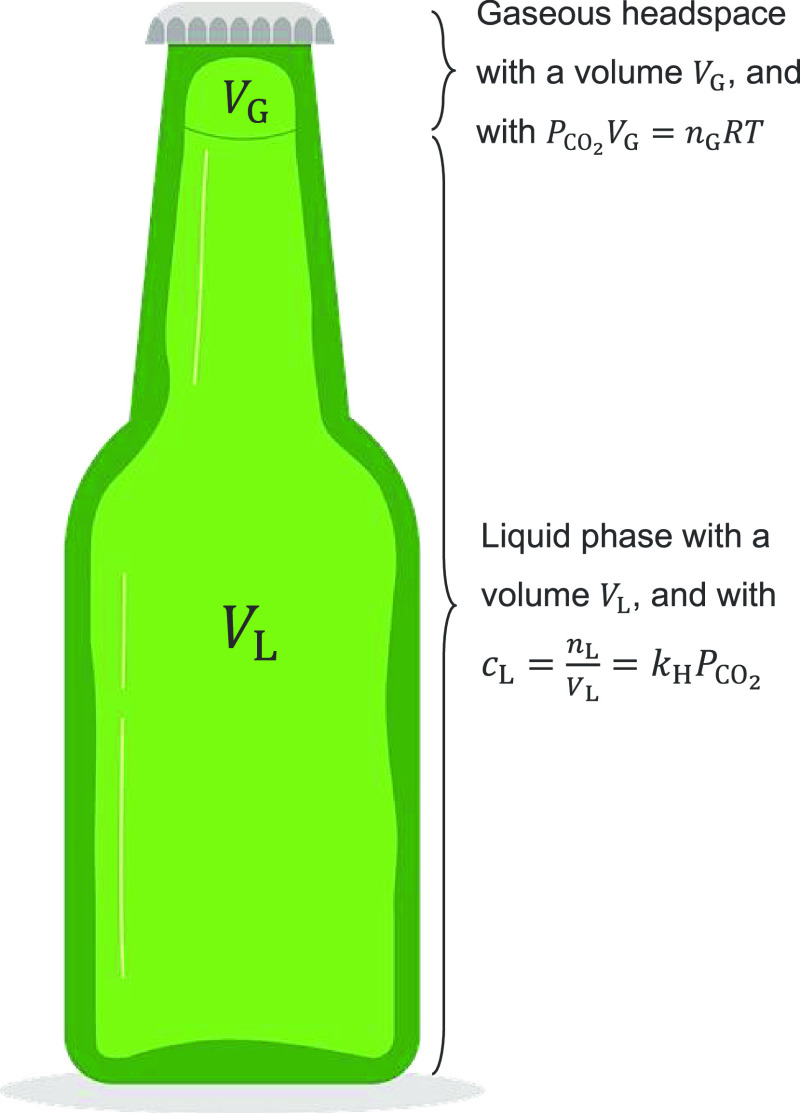
In the beer bottles hermetically sealed with a crown cap, a volume VG of gas phase in the headspace under the cap cohabits with a volume VL of beer (i.e., the liquid phase). In the sealed bottles, the total number of moles of CO2, denoted nT, is a conserved quantity that decomposes into nG moles in the gas phase and nL moles in the liquid phase. Therefore nT = nG + nL. In the realistic pressure range found in a beer bottle (a few bar), it will be safely assumed that the gas-phase volume under the cap is ruled by the ideal gas law (i.e., with PCO2VG = nGRT). Finally, dissolved and gas-phase CO2 follow the system of equations described hereafter, as exemplified in Figure 1
 |
4 |
By combining the three equations of the previous system, the theoretical dependence on temperature of the pressure of gas-phase CO2 found in the sealed lager beer bottles can be determined according to the following equation, with every parameter displayed in the International System of Units (SI).
| 5 |
The previous equation is valid for any sealed bottle or can of sparkling beverage, as discussed in more details in a recent article.9 It is nevertheless noteworthy to mention that the headspace volume is very small in the lager beer bottles (with VG ≈ 5 mL) compared to the liquid-phase volume (with VL ≈ 250 mL). Therefore, by considering the high solubility of CO2 in the liquid phase, the total amount of CO2 found in the sealed bottle is finally very close to the amount of dissolved CO2 found in the liquid phase. Thus, nT ≈ nL.
Figure 1.
Scheme of a capped beer bottle exemplifying the thermodynamic equilibrium experienced by dissolved and gas-phase CO2 between the liquid phase and the gaseous headspace under the crown cap (courtesy of K dapple-designer/Pixabay).
Finally, by replacing nT in eq 5 with nL = cbottleVL, and kH with the van’t Hoff eq 3, the pressure of gas-phase CO2 in sealed lager beer bottles can be determined in the temperature range between 0 and 20 °C (Figure 2). As a comparison, the temperature-dependent pressure of gas-phase CO2 in the headspace of a standard 750 mL bottle of commercial Champagne wine is also displayed in Figure 2.7,25 The pressure of gas-phase CO2 in a bottle of champagne is much higher than that in a bottle of lager beer because the concentration of yeast-fermented dissolved CO2 is about twice as high in a standard corked bottle of champagne than in the sealed bottles of the present commercial lager beer.
Figure 2.
Pressure of gas-phase CO2 which prevails within the 250 mL sealed lager beer bottles in the range of temperature between 0 and 20 °C. For comparison purposes, the temperature-dependent pressure of gas-phase CO2 found in a standard 750 mL corked bottle of champagne appears in red.7 Reproduced from ref (7) with permission from Springer Nature.
2.3. Critical Radius Required for CO2 Bubble Nucleation
In a beer bottle hermetically sealed, the capacity of CO2 to remain dissolved in the liquid phase is achieved by the pressure of gas-phase CO2 of several bar found in the headspace under the crown cap, as shown in Figure 2. But, as soon as the cap is removed from a bottle and the beer is dispensed in a glass, the thermodynamic equilibrium of dissolved and gas-phase CO2 is broken. The partial pressure PCO2atm of gas-phase CO2 in ambient air is near 0.4 mbar. Following Henry’s law at 6 °C, the new stable concentration of dissolved CO2 should be only ceq = kHPCO2 ≈ 1 mg L–1. Therefore, almost all of the dissolved CO2 retained in beer must desorb from the liquid phase (through bubbling and by diffusion through the free air/liquid interface, as already described in detail in champagne glasses).25
Foam and persistent bubbling being essential characteristics of lager beers, many consumers attach premium importance to both the number and size of bubbles likely to form in a glass.38−40 Nevertheless, in liquids weakly supersaturated with dissolved CO2, such as sparkling beverages in general, bubble formation is limited by an energy barrier.41 To overcome the nucleation energy barrier and grow freely, CO2 bubbles need pre-existing gas cavities immersed in the liquid phase, with radii of curvature larger than a critical radius. This process is referred to as nonclassical heterogeneous bubble nucleation.41 In a previous work, the critical radius of curvature r* required for bubble nucleation has been determined according to the following relationship, with every parameter displayed in the SI units42
| 6 |
with γ being the surface tension of the liquid/gas interface, kH being the strongly temperature-dependent Henry’s law constant of CO2 in water (expressed in mol m–3 Pa–1), P0 being the ambient pressure (≈105 Pa), and cL being the dissolved CO2 concentration in the liquid phase (expressed in mol m–3).
Strictly speaking, the surface tension γ of aqueous solutions is also temperature-dependent, but in the range of temperatures between 0 and 20 °C, the surface tension of pure water varies less than 3%.43 The surface tension of lager beers can thus be taken as 43 mN m–1, as determined in a previous work.44 By replacing all parameters in eq 6 by their numerical values displayed in correct units, the critical radius r* required to enable nonclassical heterogeneous bubble nucleation (at 6 °C) was found to be ≈ 0.7 μm for the lager beer dispensed in the glass (with cL = c0 ≈ 5.2 g L–1). By contrast, for a typical champagne dispensed at a tasting temperature close to 10 °C, holding about 8 g L–1 of dissolved CO2 after pouring in a flute,29 and with a surface tension close to 47 mN m–1,44r* is near 0.3 μm. The temperature dependence of the critical radius of curvature r* required for bubble nucleation in a glass is plotted in Figure 3 for both the lager beer and champagne, in the range of temperatures between 0 and 20 °C. The critical radius r* is systematically about twice as high in beer than in champagne mainly because the concentration of dissolved CO2cL is much higher in champagne than in beer after the pouring process.
Figure 3.
Temperature dependence of the critical radius of curvature r* required for bubble nucleation in a glass, immediately after serving, for both the lager beer and champagne, in the range of temperatures between 0 and 20 °C.
Careful observation through combined microscopy and high-speed video imaging revealed that most of the bubble nucleation sites found in glasses poured with sparkling beverages were located on pre-existing gas cavities trapped inside microcrevices in the glass wall (done by the glassmaker to trigger effervescence)17 or inside tiny hollow cellulose fibers,11,16,42 as seen in the two micrographs displayed in Figure 4. In most cases, the radii of curvature r of the pre-existing gas cavities trapped inside fibers or microcrevices were much higher than the critical radius r* required for nonclassical heterogeneous bubble nucleation.
Figure 4.

Two micrographs showing the network of microcrevices responsible for nonclassical heterogeneous bubble nucleation in laser-etched glasses (bar = 100 μm) (a) and a particle with a micrometric gas cavity trapped inside, acting as a bubble nucleation site in a glass poured with champagne (bar = 20 μm) (b).
2.4. Critical Concentration of Dissolved CO2 Required for Bubbling
Under the usual conditions of consumption of a sparkling beverage (i.e., in a glass), the concentration of dissolved CO2cL was found to continuously decrease with time.45−47 Following eq 6, r* is thus found to increase with time. Eventually, as the concentration of dissolved CO2 found in the liquid bulk reaches the critical value cL* expressed hereafter, the bubbling process becomes thermodynamically impossible because of lack of dissolved CO2.8
| 7 |
where r is the radius of curvature of the gas cavity acting as a bubble nucleation site.
The critical concentration of dissolved CO2cL* required for bubble nucleation in the glass is plotted in Figure 5 as a function of the radius of curvature r of pre-existing gas cavities acting as bubble nucleation sites (in the range between 1 and 10 μm), for both the lager at 6 °C and champagne at 10 °C. cL decreases with r, which means in practice that the bubbling process will progressively stop from every bubble nucleation sites in a glass of beer or champagne, with the smallest nucleation sites becoming inactive first. Moreover, because kH is highly temperature-dependent, it can also be concluded that the colder the beer is in the glass, the higher will be the critical concentration of dissolved CO2 needed to produce bubbles.
Figure 5.
Critical concentration of dissolved CO2cL* required for bubble nucleation in a glass as a function of the radius of curvature r of pre-existing gas cavities acting as bubble nucleation sites, for both the lager beer and champagne dispensed at 6 and 10 °C, respectively.
2.5. Ascending Beer Bubble Dynamics
The pioneering observations about bubbles rising in-line in a glass of beer were conducted in the early 1990s, by Shafer and Zare.45 They reported that the diameter of bubbles linearly increases with time as they rise toward the liquid surface. About a decade later, high-speed photography and video imaging were applied to progressively decipher the physicochemical processes behind the dynamics of bubbles ascending in champagne and beer glasses.13−18,44 By combining fundamental developments in bubble dynamics rising at small and intermediate Reynolds numbers with mass transfer equations, the following relationship was derived which links the diameter d of a buoyant CO2 bubble ascending in a liquid phase supersaturated with dissolved CO2 with several parameters (in the SI units).25
| 8 |
where g is the acceleration due to gravity (≈9.8 m s–2) and h is the distance traveled by a bubble from its nucleation site.
Following eq 8, the volume of a CO2 bubble that reaches the liquid surface and is finally withdrawn from the liquid phase, can therefore be expressed as follows
| 9 |
Under standard tasting conditions, for a similar distance traveled by ascending bubbles, the volume ratio between a beer bubble and a champagne bubble can be expressed as follows
| 10 |
with the subscripts and superscripts B and C referring to beer and champagne, respectively.
From the previous relationship, by considering the tasting temperatures of beer and champagne as being 6 and 10 °C, respectively, and by considering the initial concentration of dissolved CO2 in beer and champagne equivalent to ≈5.2 and ≈8 g L–1, respectively, the volume ratio between a beer bubble and a champagne bubble is close to 0.4. At the beginning of tasting, champagne bubbles should therefore be about 2.5 times larger in volume than beer bubbles, as illustrated in Figure 6.
Figure 6.
High-speed photographs showing ascending and growing bubbles in a glass of beer (a), as compared with bubbles ascending and growing in a flute poured with champagne (b) (bar = 1 mm).
Finally, because the growth rate of a CO2 bubble along its journey toward the liquid/air interface is strongly dependent on several parameters of both the liquid phase and the glass, the total number of bubbles likely to form in a single glass of beer from the initial reservoir c0 of dissolved CO2 should therefore also depend on all of these parameters.
2.6. How Many Bubbles in Your Glass of Beer?
The issue of the number of bubbles likely to form in a glass of bubbly or sparkling water was discussed recently.9,28 This number is the result of the interplay between the initial concentration of dissolved CO2 found in the glass after pouring, the critical concentration of dissolved CO2 below which bubble formation becomes thermodynamically impossible, and the volume of bubbles as they reach the liquid/air interface. The total number N of ascending bubbles likely to form in a glass was thus found to obey the following relationship9,28
| 11 |
where V is the volume of beverage dispensed in the glass and h is the distance between bubble nucleation sites and the liquid/air interface (considered as being the liquid level in the glass if most of bubble nucleation sites are located at the bottom of the glass).
By replacing cL* in eq 11 by its theoretical relationship given in eq 7, the total number of bubbles likely to form in a glass of beer can be rewritten as follows (with parameters displayed in the SI units)
| 12 |
By replacing parameters of the previous equation by their numerical value displayed in the correct unit, the total number of bubbles likely to form in 250 mL of the 6 °C lager beer, whose concentration of dissolved CO2c0 ≈ 5.2 g L–1 after having been dispensed in the glass holding a liquid level of 8.9 cm, is plotted in Figure 7 (versus the radius of curvature r of the pre-existing gas cavities acting as bubble nucleation sites in the realistic range between 1 and 10 μm). The total number of bubbles likely to nucleate in the glass of beer increases with r because the critical concentration of dissolved CO2, below which bubbling becomes thermodynamically impossible, decreases with increasing r, thus making heterogeneous nucleation of CO2 bubbles still thermodynamically possible at decreasing concentrations of dissolved CO2.
Figure 7.
Theoretical total number of CO2 bubbles likely to nucleate in a glass poured with 250 mL of beer at 6 °C (with a beer level of 8.9 cm) plotted versus the radius of curvature of gas cavities acting as bubble nucleation sites at the bottom of the glass (see inset). For comparison, the theoretical total number of CO2 bubbles likely to form in a flute poured with 100 mL of a standard champagne dispensed at 10 °C (with a level of champagne of 7.4 cm) appears in red.28 Reprinted (Adapted) with permission from ref (28). Copyright 2014 American Chemical Society.
The total number of bubbles likely to nucleate in the glass of lager beer is compared with the total number of bubbles likely to nucleate in a flute poured with 100 mL of a standard champagne classically dispensed at 10 °C.28 The same global trend can be observed for the total number of bubbles likely to form in the flute of champagne versus r, but the interplay between the various parameters at play in a glass of beer and in a glass of champagne brings out a surprising result. Above a limiting radius rL ≈ 1.5 μm, the total number of CO2 bubbles likely to form is higher in the glass of beer than in the champagne flute, whereas the trend reverses below this limiting radius.
The bubble-counting model provided by eq 12 considers a simplified situation where all of the bubble nucleation sites are located at the same level in the glass, with pre-existing gas pockets showing identical radii of curvature r. In real tasting conditions, there is a collection of various microcrevices and tiny particles, varying in sizes and forms at the bottom of a glass and on its walls. Therefore, there must be a collection of various pre-existing gas pockets (showing various r) acting as CO2 bubble nucleation sites, thus complicating the situation. The simplified bubble-counting model discussed here should therefore be aimed at estimating the order of magnitude of number of bubbles likely to form in beer glasses.
3. Conclusions
Beer making has been practiced for millenaries, undergoing technical improvements and constant refining, but the pursuit of this art can still benefit from the latest advances in science. Experimental and theoretical developments about the thermodynamic equilibrium of dissolved and gas-phase CO2 were made relevant to the conditioning of a standard commercial lager beer (with 5% alcohol by volume and a concentration of dissolved CO2 close to 5.5 g L–1) in 250 mL glass bottles. Under tasting conditions, the critical radius and the subsequent critical concentration of dissolved CO2 needed to trigger heterogeneous nucleation of CO2 bubbles from microcrevices were derived once the beer was dispensed in a glass. Accordingly, the subsequent total number of CO2 bubbles likely to form in a single glass of beer, along the entire natural degassing process, was theoretically approached as a function of the various key parameters at play.
4. Materials and Methods
4.1. Lager Beer
A standard commercial lager beer (Heineken, France) with 5% alcohol by volume (conditioned in standard 250 mL glass bottles sealed with a cap) was used for this set of experiments. At least 48 h before each set of experiments, beer bottles were stored in a refrigerator at 6 °C.
4.2. Glass and the Pouring Process
A batch of four identical 500 mL machine-blown glasses (ARC International, France) was used for this set of experiments. Glasses were thoroughly washed with a dilute aqueous acetic acid solution, rinsed with distilled water, and then dried in a drying oven at 60 °C. After uncapping a bottle stored at 6 °C, the whole 250 mL of beer was gently poured in a tilted glass to prevent too much turbulence and subsequent over-foaming (as it would usually be done by servers or beer tasters).29
4.3. Physicochemical Parameters and Data Analysis
Concentrations of dissolved CO2 in the lager beer were determined in two distinct steps: (1) in the bottle, immediately after uncapping but before pouring beer (denoted cbottle) and (2) in the tasting glass, immediately after pouring the 250 mL of beer (denoted c0). Dissolved CO2 concentrations were determined according to the official method recommended by the International Office of Vine and Wine (OIV), based on the article by Caputi et al.30 This method requires the use of carbonic anhydrase (labeled C2522 Carbonic Anhydrase Isozyme II from bovine erythrocytes and provided from Sigma-Aldrich). This titrimetric determination of dissolved CO2 has been routinely used since the past decade in the science of champagne and sparkling wines and is reported in minute details by Liger-Belair et al.31
The density of the beer was measured, at 6 °C, with a digital density meter (Mettler Toledo 30PX) based on the oscillating U-tube technique. The dynamic viscosity of beer was also measured, at 6 °C, with an Ubbelohde capillary viscometer (Schott Gerate). Beer densities and viscosities were measured with beer samples first degassed under vacuum.
To enable a statistical treatment, measurements of dissolved CO2, density, and viscosity were done on four distinct bottles. Table 1 compiles the concentrations of dissolved CO2 found in the commercial lager beer used in this study (at 6 °C), the Henry’s constant of CO2 in beer, as well as the lager beer density and viscosity. For comparison, the same physicochemical parameters for a standard commercial Champagne wine (dispensed at 10 °C in a standard flute) are also reported in Table 1.25
Table 1. Concentrations of Dissolved CO2, Henry's Constant of CO2, Viscosity and Density of the Commercial Lager Beer Stored at 6 °Ca.
| parameter | beer (at 6 °C) | champagne (at 10 °C) |
|---|---|---|
| [CO2] cbottle (g L–1) | 5.49 ± 0.08 | ≈11 |
| [CO2] c0 (g L–1) | 5.19 ± 0.05 | ≈8 |
| Henry’s constant kH (g L–1 bar–1) | ≈2.4 | ≈2.1 |
| viscosity η (mPa·s) | 2.42 ± 0.03 | ≈2.2 |
| density ρ (kg m–3) | 1010 ± 1 | ≈103 |
For comparison purposes, we also have reported orders of magnitude of dissolved CO2 concentrations found in a standard commercial Champagne wine stored at 10 °C (in a 750 mL bottle before pouring and after having dispensed 100 mL of champagne in a vertically oriented flute), Henry’s constant of CO2 in champagne, as well as the champagne dynamic viscosity and density. Standard deviations correspond to the root-mean-square deviations of the data provided by four distinct bottles and subsequent pouring.
Acknowledgments
G.L.-B. and C.C. are indebted to the CNRS for supporting their team and research.
Glossary
Nomenclature
- c0
initial concentration of dissolved CO2 in the liquid phase immediately after pouring the beer in the glass (g L–1)
- cbottle
concentration of dissolved CO2 in the bottled beer after removing the cap but before pouring the beer in the glass (g L–1)
- cL
concentration of dissolved CO2 in the liquid phase (g L–1)
- cL*
critical concentration of dissolved CO2 below which bubbling becomes thermodynamically impossible (g L–1)
- g
acceleration due to gravity (=9.81 m s–2)
- h
level of liquid in the glass (m)
- kH
Henry’s law constant of gas-phase CO2 in the liquid phase (mol m–3 Pa–1)
- N
total number of CO2 bubbles likely to nucleate in a glass
- nG
amount of gas-phase CO2 in the headspace of a sealed bottle (mol)
- nL
amount of dissolved CO2 in a sealed bottle (mol)
- nT
amount of CO2 found in a sealed bottle (mol)
- P0
ambient pressure (≈105 Pa)
- PCO2
partial pressure of gas-phase CO2 found in the sealed bottle (Pa)
- PCO2atm
partial pressure of gas-phase CO2 found in ambient air (≈ 40 Pa ≈ 0.4 mbar)
- r
radius of curvature of the pre-existing gas cavity immersed in the liquid phase and acting as a CO2 bubble nucleation site (m)
- r*
critical radius of curvature required to enable bubble nucleation from a pre-existing gas cavity (m)
- rL
limiting radius of curvature for which the theoretical total numbers of bubbles nucleated in a glass of beer and in a champagne flute are identical (m)
- R
ideal gas constant (=8.31 J K–1 mol–1)
- T
temperature (K)
- V
volume of beer poured in the glass (m3)
- VG
volume of gas phase in the headspace of the sealed bottle (m3)
- VL
volume of liquid found in the sealed bottle (m3)
- ΔHdiss
dissolution enthalpy of gas-phase CO2 in the liquid phase (J mol–1)
- γ
surface tension (N m–1)
- η
dynamic viscosity (Pa·s)
- ρ
density (kg m–3)
Author Contributions
G.L.-B. and C.C. conceived and designed the research. C.C. performed the experiments. G.L.-B. and C.C. analyzed the data. G.L.-B. wrote the paper. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
References
- Liu L.; Wang J.; Rosenberg D.; Zhao H.; Lengyel G.; Nadel D. Fermented beverage and food storage in 13 000 y-old stone mortars at Raqefet Cave, Israel: Investigating Natufian ritual feasting. J. Archaeol. Sci. 2018, 21, 783–793. 10.1016/j.jasrep.2018.08.008. [DOI] [Google Scholar]
- McGovern P.Uncorking the Past: The Quest for Wine, Beer, and Other Alcoholic Beverages; University of California Press: Berkeley, 2011. [Google Scholar]
- Wang J.; Liu L.; Ball T.; Yu L.; Li Y.; Xing F. Revealing a 5000-y-old beer recipe in China. Proc. Nat. Acad. Sci. U.S.A. 2016, 113, 6444–6448. 10.1073/pnas.1601465113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer production worldwide from 1998 to 2019. https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed Dec 25, 2020).
- Beer Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2021-2026. https://www.imarcgroup.com/beer-market (accessed Dec 25, 2020).
- Denny M.Froth! The Science of Beer; The Johns Hopkins University Press: Baltimore, 2009. [Google Scholar]
- Liger-Belair G.; Cordier D.; Honvault J.; Cilindre C. Unveiling CO2 heterogeneous freezing plumes during champagne cork popping. Sci. Rep. 2017, 7, 10938 10.1038/s41598-017-10702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liger-Belair G.; Carvajal-Pérez D.; Cilindre C.; Facque J.; Brevot M.; Litoux-Desrues F.; Chaperon V.; Geoffroy R. Evidence for moderate losses of dissolved CO2 during aging on lees of a champagne prestige cuvee. J. Food Eng. 2018, 233, 40–48. 10.1016/j.jfoodeng.2018.03.026. [DOI] [Google Scholar]
- Liger-Belair G. Carbon dioxide in bottled carbonated waters and subsequent bubble nucleation under standard tasting condition. J. Agric. Food Chem. 2019, 67, 4560–4567. 10.1021/acs.jafc.9b00155. [DOI] [PubMed] [Google Scholar]
- Barker G. S.; Jefferson B.; Judd S. J. The control of bubble size in carbonated beverages. Chem. Eng. Sci. 2002, 57, 565–573. 10.1016/S0009-2509(01)00391-8. [DOI] [Google Scholar]
- Liger-Belair G. The physics and chemistry behind the bubbling properties of champagne and sparkling wines: A state-of-the-art review. J. Agric. Food Chem. 2005, 53, 2788–2802. 10.1021/jf048259e. [DOI] [PubMed] [Google Scholar]
- Vega-Martinez P.; Enriquez O.; Rodríguez-Rodríguez J. Some Topics on the physics of bubble dynamics in beer. Beverages 2017, 3, 38 10.3390/beverages3030038. [DOI] [Google Scholar]
- Liger-Belair G.; Parmentier M.; Jeandet P. Modeling the kinetics of bubble nucleation in champagne and carbonated beverages. J. Phys. Chem. B 2006, 110, 21145–21151. 10.1021/jp0640427. [DOI] [PubMed] [Google Scholar]
- Uzel S.; Chappell M. A.; Payne S. J. Modeling the cycles of growth and detachment of bubbles in carbonated beverages. J. Phys. Chem. B 2006, 110, 7579–7586. 10.1021/jp056531x. [DOI] [PubMed] [Google Scholar]
- Lee W. T.; Devereux M. G. Foaming in stout beers. Am. J. Phys. 2011, 79, 991–998. 10.1119/1.3620416. [DOI] [PubMed] [Google Scholar]
- Lee W. T.; McKechnie J. S.; Devereux M. G. Bubble nucleation in stout beers. Phys. Rev. E 2011, 83, 051609 10.1103/PhysRevE.83.051609. [DOI] [PubMed] [Google Scholar]
- Liger-Belair G. Modeling the losses of dissolved carbon dioxide from laser-etched champagne glasses. J. Phys. Chem. B 2016, 120, 3724–3734. 10.1021/acs.jpcb.6b01421. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Xu Z. “Fizzics” of bubble growth in beer and champagne. Elements 2008, 4, 47–49. 10.2113/GSELEMENTS.4.1.47. [DOI] [Google Scholar]
- Kleemann A.; Albrecht J.; Schöpf V.; Haegler K.; Kopietz R.; Hempel J. M.; Linn J.; Flanagin V.; Fesl G.; Wiesmann M. Trigeminal perception is necessary to localize odors. Physiol. Behav. 2009, 97, 401–405. 10.1016/j.physbeh.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Meusel T.; Negoias S.; Scheibe M.; Hummel T. Topographical differences in distribution and responsiveness of trigeminal sensitivity within the human nasal mucosa. Pain 2010, 151, 516–521. 10.1016/j.pain.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J.; Yarmolinsky D.; von Buchholtz L.; Oka Y.; Sly W.; Ryba N. J.; Zucker C. S. The taste of carbonation. Science 2009, 326, 443–445. 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel A.; Hofmann T. Carbonic anhydrase IV mediates the fizz of carbonated beverages. Angew. Chem., Int. Ed. 2010, 49, 2975–2977. 10.1002/anie.200906978. [DOI] [PubMed] [Google Scholar]
- McMahon K. M.; Culver C.; Ross C. F. The production and consumer perception of sparkling wines of different carbonation levels. J. Wine Res. 2017, 28, 123–134. 10.1080/09571264.2017.1288092. [DOI] [Google Scholar]
- Liger-Belair G.; Polidori G.; Jeandet P. Recent advances in the science of champagne bubbles. Chem. Soc. Rev. 2008, 37, 2490–2511. 10.1039/b717798b. [DOI] [PubMed] [Google Scholar]
- Liger-Belair G. Effervescence in Champagne and sparkling wines: From grape harvest to bubble rise. Eur. Phys. J.: Spec. Top. 2017, 226, 3–116. 10.1140/epjst/e2017-02678-7. [DOI] [Google Scholar]
- Zenit R.; Rodriguez-Rodriguez J. The fluid mechanics of bubbly drinks. Phys. Today 2018, 71, 44–50. 10.1063/PT.3.4069. [DOI] [Google Scholar]
- Gonzalez Viejo C.; Torrico D. D.; Dunshea F. R.; Fuentes S. Bubbles, foam formation, stability and consumer perception of carbonated drinks: A review of current, new and emerging technologies for rapid assessment and control. Foods 2019, 8, 596 10.3390/foods8120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liger-Belair G. How many bubbles in your glass of bubbly?. J. Phys. Chem. B 2014, 118, 3156–3163. 10.1021/jp500295e. [DOI] [PubMed] [Google Scholar]
- Liger-Belair G.; Bourget M.; Villaume S.; Jeandet P.; Pron H.; Polidori G. On the losses of dissolved CO2 during champagne serving. J. Agric. Food Chem. 2010, 58, 8768–8775. 10.1021/jf101239w. [DOI] [PubMed] [Google Scholar]
- Caputi A.; Ueda M.; Walter P.; Brown T. Titrimetric determination of carbon dioxide in wine. Am. J. Enol. Vitic. 1970, 21, 140–144. [Google Scholar]
- Liger-Belair G.; Villaume S.; Cilindre C.; Jeandet P. Kinetics of CO2 fluxes outgassing from champagne glasses in tasting conditions: The role of temperature. J. Agric. Food Chem. 2009, 57, 1997–2003. 10.1021/jf803278b. [DOI] [PubMed] [Google Scholar]
- Smith F. L.; Harvey A. H. Avoid common pitfalls when using Henry’s law. Chem. Eng. Prog. 2007, 103, 33–39. 10.1016/j.cej.2006.10.001. [DOI] [Google Scholar]
- Speers R. A.; MacIntosh A. J. Carbon dioxide solubility in beer. J. Am. Soc. Brew. Chem. 2013, 71, 242–247. 10.1094/ASBCJ-2013-1008-01. [DOI] [Google Scholar]
- Agabaliantz G. G. Bases scientifiques de la technologie des vins mousseux. Bull. OIV 1963, 36, 703–714. [Google Scholar]
- Kuntzleman T. S.; Sturgis A. Effect of temperature in experiments involving carbonated beverages. J. Chem. Educ. 2020, 97, 4033–4038. 10.1021/acs.jchemed.0c00844. [DOI] [Google Scholar]
- Dalmolin I.; Skovroinski E.; Biasi A.; Corazza M. L.; Dariva C.; Vladimir Oliveira J. Solubility of carbon dioxide in binary and ternary mixtures with ethanol and water. Fluid Phase Equilib. 2006, 245, 193–200. 10.1016/j.fluid.2006.04.017. [DOI] [Google Scholar]
- Lide D. R.; Frederikse H. P.. Handbook of Chemistry and Physics, 76th ed.; CRC Press: Boston, 1995. [Google Scholar]
- Hepworth N. J.; Varley J.; Hind A. Characterizing gas bubble dispersions in beer. Food Bioprod. Process. 2001, 79, 13–20. 10.1205/09603080151123317. [DOI] [Google Scholar]
- Bamforth C. W. The relative significance of physics and chemistry for beer foam excellence: Theory and practice. J. Inst. Brew. 2004, 110, 259–266. 10.1002/j.2050-0416.2004.tb00620.x. [DOI] [Google Scholar]
- Bamforth C.; Russell I.; Stewart G.. Beer: A Quality Perspective; Academic Press: Oxford, 2011. [Google Scholar]
- Jones S. F.; Evans G. M.; Galvin K. P. Bubble nucleation from gas cavities: A review. Adv. Colloid Interface Sci. 1999, 80, 27–50. 10.1016/S0001-8686(98)00074-8. [DOI] [Google Scholar]
- Liger-Belair G.; Vignes-Adler M.; Voisin C.; Robillard B.; Jeandet P. Kinetics of gas discharging in a glass of champagne: The role of nucleation sites. Langmuir 2002, 18, 1294–1301. 10.1021/la0115987. [DOI] [Google Scholar]
- Vargaftik N. B.; Volkov B. N.; Voljak L. D. International tables of the surface tension of water. J. Phys. Chem. Ref. Data 1983, 12, 817–820. 10.1063/1.555688. [DOI] [Google Scholar]
- Liger-Belair G.; Marchal R.; Robillard B.; Dambrouck T.; Vignes-Adler M.; Maujean A.; Jeandet P. On the velocity of expanding spherical bubbles rising in-line in supersaturated hydroalcoholic solutions: Application to bubble trains in carbonated beverages. Langmuir 2000, 16, 1889–1895. 10.1021/la990653x. [DOI] [Google Scholar]
- Shafer N. E.; Zare R. N. Through a beer glass darkly. Phys. Today 1991, 44, 48–52. 10.1063/1.881294. [DOI] [Google Scholar]
- Liger-Belair G.; Conreux A.; Villaume S.; Cilindre C. Monitoring the losses of dissolved carbon dioxide from laser-etched champagne glasses. Food Res. Int. 2013, 54, 516–522. 10.1016/j.foodres.2013.07.048. [DOI] [Google Scholar]
- Liger-Belair G.; Sternenberg F.; Brunner S.; Robillard B.; Cilindre C. Bubble dynamics in various commercial sparkling bottled waters. J. Food Eng. 2015, 163, 60–70. 10.1016/j.jfoodeng.2015.04.016. [DOI] [Google Scholar]