Abstract

Torrefaction is an appealing pretreatment method for improving the fuel properties of kenaf biomass before its utilization in thermochemical processes. This study evaluated and compared the impact of torrefaction on thermal behavior and kinetics during pyrolysis and gasification. Thermogravimetric analysis experiments were conducted at temperatures of 300–1173 K at several heating rates under N2 and CO2 atmospheres. The raw and torrefied kenaf (RK and TK) during CO2 gasification in the low-temperature region (<900 K) was found to exhibit a tendency that was similar to that of N2. However, TK during CO2 gasification resulted in a lower maximum mass loss rate, delayed initiating temperature, and lower devolatilization index due to lower reactivity. In addition, the gasification reaction of CO2 and char was observed to occur in the high-temperature region (> 900 K), thus improving conversion efficiencies. The activation energy for TK in a CO2 atmosphere depending on the conversion was calculated using the distributed activation energy method. The activation of RK during CO2 gasification was higher than that of N2. However, TK during CO2 gasification exhibited a lower activation energy compared to that of N2, indicating its potential as a better feedstock during the CO2 gasification process and the ability to save energy.
1. Introduction
Gasification aims to produce biomass as a gas, which is converted to syngas for liquid biofuel production.1 The produced gas may also be used directly to generate heat and power through combustion.2 Integrated gasification combined cycle systems have higher thermal efficiency compared to direct combustion of biomass. Currently, most gasification plants use coal as a resource, and replacing coal with biomass would be beneficial as biomass is converted into value-added products. However, the direct utilization of biomass as a gasification feedstock is limited by its high moisture content, low grindability, low energy content, and low bulk density.3 Therefore, biomass gasification has been investigated extensively to date, and increasing gasification efficiency through feedstock upgrade using pretreatment methods such as torrefaction is an important research topic.
Torrefaction is also known as mild pyrolysis and in recent years has been preferred for the improvement of the characteristics of biomass as fuel before thermochemical processes. Biomass is heated at 473–573 K in inert gas to produce high-quality solid fuel.4,5 After drying during the torrefaction process, hemicellulose and some cellulose in biomass are thermally decomposed and biochar is produced.6,7 This biochar has a higher calorific value and improved grindability compared to raw biomass and is more effective for application in advanced gasification and combustion technologies.8 Owing to the aforementioned advantages, many studies have focused on evaluating the fuel properties of torrefied biomass, and research is gradually expanding in terms of the gasification kinetics of torrefied biomass.9,10 Gasification kinetics are crucial and have an essential impact on the design, process control, and efficiency of gasification.11 The design and optimization of gasifiers for industrial-scale applications require in-depth understanding of reliable kinetic data and characteristics during gasification.12,13 Although several researchers have studied the effect of torrefied fuel on gasification kinetics and thermal behavior, this subject has not been systematically investigated. Tran et al.5 reported that temperatures of torrefaction had a significant effect on the CO2 gasification of forest residues, and the gasification activation energy of the three samples varied from 260 to 290 kJ/mol. Chew et al.10 evaluated the impact of torrefaction on the gasification behavior and kinetics of three oil palm biomasses: empty fruit bunch, methyl-furans, and palm kernel shells. They reported that torrefied oil palm biomass showed reduced gasification reactivity relative to the nontorrefied analogues due to the removal of volatile matter from biomass after torrefaction. In addition, biomass reactivity during CO2 gasification was affected by the gasification temperature, biomass type, and pretreatment method. Zhang et al.14 investigated the distribution of solid products and the effects of torrefaction conditions on char gasification reactivity, as well as cogasification with coal and torrefied solids. They noted that char produced by torrefied biomass showed faster conversion compared to char produced by raw biomass during CO2 gasification.
Kenaf, which grows in Korea, is an important source of herbaceous biomass. The life cycle of kenaf among herbaceous biomass is relatively short and production costs are relatively low. Owing to the price competitiveness, kenaf is expected to replace woody biomass.15 Therefore, we investigated the characteristics of kenaf in terms of various aspects, such as the effect of torrefaction and how kenaf compares with woody biomass.16,17 Furthermore, we investigated the torrefaction-improved features such as grindability, hydrophobicity, and carbonization. However, to date, studies on the gasification kinetics of torrefied kenaf (TK) biomass, which are essential for large-scale application development, are lacking. In addition, the applicability of N2 and CO2 atmospheres for TK has not been studied and compared comprehensively. Therefore, this study aims to examine the effect of torrefaction on the CO2 gasification behavior and kinetics of kenaf compared to N2 pyrolysis. In addition, we evaluated and compared the CO2 gasification kinetics of raw kenaf (RK) and TK biomass using the distributed activation energy method (DAEM). This study elucidates the kinetics and thermal behavior of the gasification process for TK.
2. Results and Discussion
2.1. Thermal Analysis
Figures 1 and 2 show the mass loss (TG, thermogravimetry) and mass loss rate (DTG) curves for RK and TK under pyrolysis (N2) and gasification (CO2) at a heating rate of 10 K/min. The effects of ambient gas on thermal decomposition of RK and TK were investigated by comparing and evaluating the TG and DTG results in N2 and CO2 atmospheres. Water evaporation, devolatilization, and char reaction are evident in Figure 1. The 300–400 K region was associated with surface moisture and adsorbent water in the sample. In the N2 atmosphere, the devolatilization of RK started at approximately 450 K and reached the maximum mass loss rate at 586 K. The mass loss rate decreased, and there was no change in mass when the temperature was over 900 K, indicating that the thermal decomposition was complete. The decomposition of hemicellulose and cellulose occurred mainly during the initial region of the thermal decomposition, and that of lignin occurred in the latter region. This is similar to previous results showing that there are two thermal decomposition regions for lignocellulose biomass.18 In the CO2 gasification experiments shown in Figure 1, the mass loss tendency of RK and TK in the CO2 atmosphere was similar to that of N2 when the temperature was less than 900 K. However, the maximum mass loss rate of CO2 was less than that of N2 because of the greater heat capacity and lower reactivity of CO2. Nevertheless, an additional mass loss was clearly observed at temperatures exceeding 900 K in the CO2 atmosphere, indicating that the CO2 gasification reaction occurred in the remaining char. These results are consistent with the findings of previous studies.19,20 Char produced by thermal decomposition still contains a large amount of carbon, and incomplete carbonation during the process of thermal decomposition in conjunction with CO2 facilitates the gasification reaction of the remaining char.20,21 Hydrogen and oxygen-related compounds are gradually compressed in the char matrix with increasing temperature, and the fixed carbon content of the remaining char increases, resulting in an inevitable gasification reaction between CO2 and fixed carbon.22
Figure 1.
Mass loss and mass loss rate curves for RK under pyrolysis (N2) and gasification (CO2) at a 10 K/min heating rate.
Figure 2.
Mass loss and mass loss rate curves for TK under pyrolysis (N2) and gasification (CO2) at a 10 K/min heating rate.
Figure 2 shows the thermal decomposition behavior of TK in both N2 and CO2 atmospheres. Evidently, the first strong shoulder on the first half of the devolatilization peak found in RK is not observed for TK, indicating a significant reduction of hemicellulose content.23 In addition, most of the weight loss occurred from 500 to 700 K with the maximum rate and temperature of mass loss at 8.3 mg/min and 599.8 K in N2 and 6.44 mg/min and 599.4 K in CO2, respectively, which was almost identical to the behavior of the cellulose region.24 Furthermore, the maximum mass loss rate of TK was significantly more pronounced than that of RK, and the second shoulder was generally associated with the thermal decomposition of lignin; hence, this observation shows that lignin content increases after the torrefaction process. Yan et al. also reported that the lignin fraction of lignocellulosic biomass was significantly increased as a result of torrefaction.25Figures 1 and 2 show that the maximum mass loss rate and temperature of the DTG peaks in the CO2 gasification region for RK and TK occurred at 3.72 mg/min and 1086.8 K and 6.18 mg/min and 1117.9 K, respectively, indicating that the increased lignin content in char resulted in a greater reaction with CO2.
2.2. Analysis of Crucial Thermal Parameters at Different Heating Rates
The TG and DTG curves for RK and TK under pyrolysis (N2) and gasification (CO2) at heating rates of 5, 10, and 20 K/min are shown in Figures 3 and 4, respectively. The heating rate showed a significant effect on the behavior of the TG and DTG curves and the maximum rate of decomposition. At higher heating rates, a shorter reaction time and thermal progression delay of the sample was observed with respect to heat transfer, indicating that the peak moved into the high-temperature region.18 In addition, the maximum mass loss rate during the thermal decomposition increased, and the conversion rate decreased on increasing the heating rate at the same temperature owing to the thermal hysteresis phenomenon caused by heat resistance.26 This is attributable to the delay in the heat transferred to the internal bulk sample, resulting in the central temperature of the particle being lower than the surface temperature. The increased heating rate increased this temperature difference; furthermore, the sample interior was not supplied with sufficient energy to decompose on time. The higher CO2 gasification reaction of carbon was also observed to occur more significantly at temperatures exceeding 900 K and at a higher heating rate, as mentioned in section 2.1.
Figure 3.
Mass loss and mass loss rate curves for RK under pyrolysis (N2) and gasification (CO2): (a, b) effect of heating rates.
Figure 4.
Mass loss and mass loss rate curves for TK under pyrolysis (N2) and gasification (CO2): (a, b) effect of heating rates.
To compare and evaluate processes quantitatively, the key thermal parameters of pyrolysis (N2) and gasification (CO2) heated at several heating rates are described, which include the initiating temperature (Tin), peak temperature (Tpeak), maximum mass loss rate (Rmax), and devolatilization index (Di), as summarized in Table 1 and Figure 5. In this study, the Di was used to investigate the release behavior of the volatile materials, as shown by He et al.27 and in eq 1 below:
| 1 |
Table 1. Effect of Heating Rates on Key Thermal Parameters for RK (a) and TK (b) under Pyrolysis (N2) and Gasification (CO2)a.
| heating rate (K/min) | Tin (K) | Tpeak (K) | Rmax (mg/min) | ΔT1/2 (K) | Di (10–8 mg/ min-K3) | residual (%) |
|---|---|---|---|---|---|---|
| (a) RK | ||||||
| N2 (pyrolysis) | ||||||
| 5 | 346.86 | 586.07 | 4.08 | 119.60 | 16.77 | 18.95 |
| 10 | 368.83 | 596.66 | 7.81 | 113.92 | 31.17 | 15.70 |
| 20 | 377.98 | 609.63 | 15.24 | 115.82 | 57.08 | 18.83 |
| CO2 (gasification) | ||||||
| 5 | 379.13 | 587.74 | 4.06 | 104.31 | 17.49 | 4.87 |
| 10 | 394.89 | 596.19 | 7.32 | 100.65 | 30.90 | 4.07 |
| 20 | 403.75 | 605.90 | 13.57 | 101.08 | 54.88 | 4.72 |
| (b) TK | ||||||
| N2 (pyrolysis) | ||||||
| 5 | 537.01 | 588.60 | 4.14 | 25.80 | 50.74 | 32.59 |
| 10 | 546.10 | 599.85 | 8.30 | 26.87 | 94.28 | 34.77 |
| 20 | 555.70 | 612.23 | 16.37 | 28.27 | 170.23 | 35.65 |
| CO2 (gasification) | ||||||
| 5 | 543.96 | 589.23 | 3.79 | 22.63 | 52.19 | 5.66 |
| 10 | 547.91 | 599.46 | 6.44 | 25.78 | 76.03 | 3.94 |
| 20 | 560.26 | 609.20 | 12.73 | 24.47 | 152.43 | 6.71 |
Tin, Initial devolatilization temperature at a conversion of 10%; Tpeak, Maximum peak temperature; Rmax, Maximum mass loss rate; ΔT1/2, Temperature interval when R/Rmax is 1/2; Di, Devolatilization index.
Figure 5.
Effect of heating rates on (a) Rmax and (b) Di for raw and torrefied kenaf.
Owing to the thermal hysteresis, the Tpeak, Rmax, and Di increased as the heating rate increased, as observed in both atmospheres, and the Di value for TK was higher than that of RK at all heating rates. For comparison between N2 and CO2 atmospheres, the Di value for CO2 at 5 K/min was slightly higher than that of N2, but the Di value for CO2 at 10 and 20 K/min was 5% less than that of the N2 atmosphere, implying that the CO2 devolatilization performance dropped below that of N2 as the heating rate increased. In addition, Di and Rmax values for TK under CO2 were significantly lower than those of RK under N2, indicating that gasification results in less reactivity with lignin-like compounds produced by torrefaction, which is consistent with the results obtained by other studies.28 In addition, the residuals for CO2 decreased by approximately 74% for RK and 84% for TK because the gasification reaction of the remaining char with CO2 and the reduction ratio for TK was higher than that of RK.
2.3. Kinetic Parameter Analysis
Figure 6 shows conversion as a function of temperature for reaction stages I and II in the N2 and CO2 atmospheres for RK and TK. The model-free activation energy was analyzed using the DAEM. Stages I and II indicate the low-temperature (< 900 K) and high-temperature regions (> 900 K), respectively. In addition, the reaction during stage II only represented CO2 because there was no reaction during stage II for N2. Thus, the kinetic analysis for stage II was only carried out in the high-temperature region, as shown in Table 2. The model-free activation energy using the DAEM at each conversion was obtained from the slope of the linear regression method. The activation energy of RK for N2 was estimated using a conversion of 0.1 and 0.7 because the correlation factor was very low when the conversion exceeded 0.8.29 The estimated squares of the correlation coefficient, R2, corresponding to linear fitting almost exceeded 0.99. The activation energy calculated according to conversion is shown in Figures 7 and 8. The activation energy changed as the reaction progressed during pyrolysis and gasification. This phenomenon occurred because the material was not a pure compound but a mixture of other elements with complex chemical bond structures. Figure 7 shows the variation of activation energies according to conversion for RK and TK under pyrolysis (N2) and gasification (CO2) in stage I. The conversion graph in the reaction during stage I for N2 and CO2 appears similar for both RK and TK. The activation energy according to conversion for RK was higher in the case of CO2 compared to that of N2, yielding average values of 222.42 and 183.29 kJ/mol, respectively. This indicates that the reactivity of CO2 is lower than that of N2. In the case of TK, the activation energies for CO2 were higher as the conversion ratio increased to 0.6, and activation energies for N2 increased significantly when the conversion ratio exceeded 0.6. This shows a higher activation energy of lignin derived from hemicellulose and cellulose by torrefaction during the final stage of the reaction, a tendency that was also observed in a previous study.27 However, the activation energies for CO2 did not change significantly even after the conversion ratio exceeded 0.6, indicating varying patterns in the CO2 atmosphere. Thus, with respect to TK, the average activation energy for CO2 is lower than that of N2, whose average values are 218.29 and 251.87 kJ/mol, respectively. These results show that the energy needed for gasification in the case of TK is lower than that needed for pyrolysis. Figure 8 shows the variation of activation energies according to the conversion ratio for RK and TK under a CO2 environment during stage II. The activation energies for stage II (197.94 and 197.62 kJ/mol for RK and TK, respectively) under a CO2 atmosphere were similar for both the samples. The activation energy gradually decreased as the conversion ratio increased. Furthermore, all samples showed kinetic compensation effects with simultaneous increase or decrease in activation energy and pre-exponential factors for N2 and CO2, as shown in Table 2. This behavior was consistent with the findings of previous studies.30,31 These results suggest that the energy required in the gasification system using TK as feedstock is less than that of RK, which implies that energy can be saved in the system.
Figure 6.

Conversion ratio versus temperature for the pyrolysis and gasification at different heating rates in the reaction during stage I (a, b) and II (c).
Table 2. Activation Energy, Pre-Exponential Factor, and Correlation Coefficient According to the Conversion Ratio in the Reaction during Stage I (a, b) and II (c).
| conversion ratio (α) | activation energy (kJ/mol) | pre-exponential factor (1/s) | R2 |
|---|---|---|---|
| (a) RK (stage I) | |||
| N2 (pyrolysis) | |||
| 0.1 | 180.73 | 9.54E+14 | 0.9971 |
| 0.2 | 182.05 | 6.44E+15 | 0.9987 |
| 0.3 | 186.63 | 1.25E+15 | 0.9965 |
| 0.4 | 188.11 | 7.09E+14 | 0.9981 |
| 0.5 | 183.71 | 1.35E+14 | 0.9990 |
| 0.6 | 180.20 | 3.59E+13 | 0.9996 |
| 0.7 | 181.59 | 2.76E+13 | 1.0000 |
| 0.8 | |||
| 0.9 | |||
| average | 183.29 | 1.36E+15 | 0.9984 |
| CO2 (gasification) | |||
| 0.1 | 206.72 | 2.038E+18 | 0.9891 |
| 0.2 | 215.26 | 2.332E+18 | 0.9903 |
| 0.3 | 232.05 | 2.827E+19 | 0.9900 |
| 0.4 | 243.50 | 1.124E+20 | 0.9915 |
| 0.5 | 235.08 | 7.365E+18 | 0.9940 |
| 0.6 | 218.63 | 1.122E+17 | 0.9953 |
| 0.7 | 205.72 | 4.064E+15 | 0.9961 |
| 0.8 | 192.57 | 1.123E+14 | 0.9827 |
| 0.9 | 122.65 | 6.40E+06 | 0.9412 |
| average | 222.42 | 2.18E+19 | 0.9923 |
| (b) TK (stage I) | |||
| N2 (pyrolysis) | |||
| 0.1 | 175.49 | 3.14E+13 | 1.0000 |
| 0.2 | 172.59 | 5.26E+13 | 1.0000 |
| 0.3 | 176.67 | 2.17E+13 | 1.0000 |
| 0.4 | 178.12 | 1.85E+13 | 0.9999 |
| 0.5 | 184.13 | 4.09E+13 | 0.9998 |
| 0.6 | 216.38 | 1.37E+16 | 0.9981 |
| 0.7 | 320.37 | 1.04E+24 | 0.9976 |
| 0.8 | 385.15 | 2.24E+27 | 0.9985 |
| 0.9 | 457.93 | 1.19E+30 | 0.9948 |
| average | 251.87 | 1.32E+29 | 0.9987 |
| CO2 (gasification) | |||
| 0.1 | 242.74 | 4.058E+20 | 0.8916 |
| 0.2 | 235.62 | 1.461E+19 | 0.9421 |
| 0.3 | 228.24 | 1.216E+18 | 0.9778 |
| 0.4 | 219.78 | 1.082E+17 | 0.9927 |
| 0.5 | 213.80 | 1.742E+16 | 0.9965 |
| 0.6 | 223.37 | 5.263E+16 | 0.9916 |
| 0.7 | 234.20 | 6.463E+16 | 0.9795 |
| 0.8 | 195.55 | 3.159E+12 | 0.9993 |
| 0.9 | 171.30 | 2.623E+09 | 0.9862 |
| average | 218.29 | 4.69E+19 | 0.9730 |
| (c) RK and TK (stage II) – CO2 (gasification) | |||
| RK | |||
| 0.1 | 314.70 | 1.57E+14 | 0.9823 |
| 0.2 | 226.02 | 7.93E+08 | 0.9990 |
| 0.3 | 204.75 | 3.42E+07 | 0.9999 |
| 0.4 | 194.54 | 7.13E+06 | 1.0000 |
| 0.5 | 184.82 | 1.79E+06 | 0.9994 |
| 0.6 | 175.27 | 4.93E+05 | 0.9982 |
| 0.7 | 166.52 | 1.56E+05 | 0.9971 |
| 0.8 | 159.29 | 5.95E+04 | 0.9965 |
| 0.9 | 155.58 | 3.31E+04 | 0.9960 |
| average | 197.94 | 1.75E+13 | 0.9965 |
| TK | |||
| 0.1 | 300.12 | 1.24E+13 | 0.9741 |
| 0.2 | 223.31 | 3.07E+08 | 0.9926 |
| 0.3 | 201.99 | 1.38E+07 | 0.9953 |
| 0.4 | 189.87 | 2.37E+06 | 0.9966 |
| 0.5 | 181.77 | 7.39E+05 | 0.9973 |
| 0.6 | 175.38 | 3.02E+05 | 0.9981 |
| 0.7 | 170.55 | 1.53E+05 | 0.9988 |
| 0.8 | 165.45 | 7.59E+04 | 0.9993 |
| 0.9 | 170.18 | 1.14E+05 | 0.9975 |
| average | 197.62 | 1.38E+12 | 0.9944 |
Figure 7.
Variation of activation energies according to conversion for raw (a) and torrefied kenaf (b) under pyrolysis (N2) and gasification (CO2) during stage I.
Figure 8.
Variation of activation energies according to conversion for raw and torrefied kenaf under gasification (CO2) during stage II.
3. Conclusions
The effect of torrefaction on kenaf under N2 and CO2 atmospheres was examined using thermogravimetric analysis (TGA) data at different heating rates. In the low-temperature region (<900 K), the thermal effect on gasification for TK was similar to that of pyrolysis. However, from the analysis of essential thermal parameters, TK under CO2 gasification resulted in a lower maximum mass loss rate, delayed initiating temperature, and lower Di at the different heating rates, indicating that CO2 gasification has a lower reactivity. However, in the high-temperature region (> 900 K), the gasification reaction of CO2 and char occurred, resulting in high conversion efficiencies in the CO2 atmosphere. In addition, torrefaction led to a larger reaction of CO2 gasification with increased char, which was confirmed by the residual char. The activation energies were analyzed using the DAEM with distinctive stages I and II. TK under a CO2 atmosphere in the entire region remarkably exhibited a lower average activation energy compared to RK, which opposes the tendency observed in the results of RK. The activation energies for the CO2 reaction with char at high temperatures had similar values for both samples. These results suggest that the energy needed for TK under gasification is less than that needed for RK and could thus contribute to saving energy in the system.
4. Materials and Methods
4.1. Materials
Kenaf was used as fuel (Hibiscus cannabinus L.), a sample of which was pretreated by torrefaction. Then, 5 g of RK was added to a sample crucible and N2 was passed through a tube at 1.5 cm3/min to form an inert atmosphere. TK was produced in a fixed-bed furnace under inert N2 gas conditions at 523 K for a residence time of 30 min. The RK and TK samples were prepared at a particle size of < 100 μm using a grinder, and the properties of each sample are listed in Table 3.
Table 3. Fuel Properties of RK and TK.
| sample | RK | TK |
|---|---|---|
| proximate analysis (wt %, as-received) | ||
| moi. | 9.18 | 1.96 |
| VM | 69.42 | 62.12 |
| FC | 17.91 | 30.48 |
| ash | 3.48 | 5.43 |
| FR | 0.26 | 0.49 |
| ultimate analysis (wt %, dry basis) | ||
| C | 43.36 | 52.27 |
| H | 5.69 | 5.27 |
| N | 0.66 | 0.87 |
| Oa | 50.21 | 41.49 |
| S | 0.08 | 0.11 |
| O/C | 1.158 | 0.794 |
| H/C | 0.131 | 0.101 |
| HHV (MJ/kg, AR) | 17.4 | 20.8 |
Calculated by difference.
4.2. Experimental Procedures
The experiments of RK and TK during pyrolysis and gasification were conducted using a thermogravimetric analyzer (SDT Q600, TA Instruments Co.) at three heating rates (5, 10, and 20 K/min). The heating rate was selected as a TGA experiment in which kinetic analysis can be performed using the DAEM.32 In a ceramic crucible, the samples were distributed evenly in a thin layer (17 mg ± 2 mg), and the mass and temperature of the samples were continuously recorded on increasing the temperature at a set heating rate until the final temperature of 1173 K. In the pyrolysis experiments, ultrapure N2 was injected into the thermogravimetric analyzer at a constant flow rate of 100 mL/min, creating an inert gas environment; in the case of gasification experiments, a constant flow rate of ultrapure CO2 was continuously supplied.
4.3. Distributed Activation Energy Model
In this study, kinetic analysis was conducted using the DAEM and a single-step reaction model based on the TGA data on the pyrolysis and gasification of RK and TK to compare and evaluate the characteristics of samples during thermal degradation.33,34 Thus, converting raw materials into products is assumed to be a single-step process.35 The rate constant of the reaction (k) according to the Arrhenius method is expressed as follows:
| 2 |
where k is the reaction rate constant, A is the pre-exponential factor (1/s), E is the activation energy (kJ/mol), R is the gas constant (8.314 J/mol-K), and T is the absolute temperature (K). For biomass conversion from solid to volatile states, the rate equation is expressed as follows:
| 3 |
The conversion ratio (α) is calculated using eq 4
| 4 |
where mi is the initial mass, mt is the mass at time t, and mf is the final mass.
By combining eqs 2 and 3, we get
| 5 |
Rearranging eq 5 into a logarithmic form using the simplified DAEM for the Arrhenius equation yields the following:
| 6 |
From eq 6, the plot
of  versus 1/T yields a straight-line
equation.
versus 1/T yields a straight-line
equation. 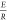 provides the slope of the equation
and
provides the slope of the equation
and 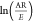 provides the intercept value, while the
value 0.6075 is kept constant for simplicity.
provides the intercept value, while the
value 0.6075 is kept constant for simplicity.
Acknowledgments
This work was supported by the International Energy Joint R&D Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and a financial grant from the Ministry of Trade, Industry and Energy, Republic of Korea (No. 20208520090080).
Author Contributions
B.-H.L., T.V.T., and C.-H.J. conceived and planned the study. T.V.T. conducted the experiments for this research. B.-H.L. contributed to the analysis of the results. B.-H.L. and C.-H.J. contributed to the review of the original and revised manuscripts. B.-H.L. took the lead in writing the manuscript. All authors helped shape the research, discussed the results, and contributed to the final manuscript.
The authors declare no competing financial interest.
References
- Petersen A. M.; Farzad S.; Görgens J. F. Techno-Economic Assessment of Integrating Methanol or Fischer–Tropsch Synthesis in a South African Sugar Mill. Bioresour. Technol. 2015, 183, 141–152. 10.1016/j.biortech.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Soltani S.; Athari H.; Rosen M.; Mahmoudi S.; Morosuk T. Thermodynamic Analyses of Biomass Gasification Integrated Externally Fired, Post-Firing and Dual-Fuel Combined Cycles. Sustainability 2015, 7, 1248–1262. 10.3390/su7021248. [DOI] [Google Scholar]
- Wang L.; Várhegyi G.; Skreiberg O̷. CO2 Gasification of Torrefied Wood: A Kinetic Study. Energy Fuels 2014, 28, 7582–7590. 10.1021/ef502308e. [DOI] [Google Scholar]
- Hu Q.; Yang H.; Xu H.; Wu Z.; Lim C. J.; Bi X. T.; Chen H. Thermal Behavior and Reaction Kinetics Analysis of Pyrolysis and Subsequent In-Situ Gasification of Torrefied Biomass Pellets. Energy Convers. Manage. 2018, 161, 205–214. 10.1016/j.enconman.2018.02.003. [DOI] [Google Scholar]
- Tran K.-Q.; Bui H.-H.; Luengnaruemitchai A.; Wang L.; Skreiberg O̷. Isothermal and Non-Isothermal Kinetic Study on CO2 Gasification of Torrefied Forest Residues. Biomass Bioenergy 2016, 91, 175–185. 10.1016/j.biombioe.2016.05.024. [DOI] [Google Scholar]
- Mamvura T. A.; Danha G. Biomass Torrefaction as an Emerging Technology to Aid in Energy Production. Heliyon 2020, 6, e03531 10.1016/j.heliyon.2020.e03531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-H.; Lin B.-J.; Lin Y.-Y.; Chu Y.-S.; Ubando A. T.; Show P. L.; Ong H. C.; Chang J.-S.; Ho S.-H.; Culaba A. B.; Pétrissans A.; Pétrissans M. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100887 10.1016/j.pecs.2020.100887. [DOI] [Google Scholar]
- van der Stelt M. J. C.; Gerhauser H.; Kiel J. H. A.; Ptasinski K. J. Biomass Upgrading by Torrefaction for the Production of Biofuels: A Review. Biomass Bioenergy 2011, 35, 3748–3762. 10.1016/j.biombioe.2011.06.023. [DOI] [Google Scholar]
- Cahyanti M. N.; Doddapaneni T. R. K. C.; Kikas T. Biomass Torrefaction: An Overview on Process Parameters, Economic and Environmental Aspects and Recent Advancements. Bioresour. Technol. 2020, 301, 122737 10.1016/j.biortech.2020.122737. [DOI] [PubMed] [Google Scholar]
- Chew J. J.; Soh M.; Sunarso J.; Yong S.-T.; Doshi V.; Bhattacharya S. Isothermal Kinetic Study of CO2 Gasification of Torrefied Oil Palm Biomass. Biomass Bioenergy 2020, 134, 105487 10.1016/j.biombioe.2020.105487. [DOI] [Google Scholar]
- Dupont C.; Nocquet T.; Da Costa J. A. Jr.; Verne-Tournon C. Kinetic Modelling of Steam Gasification of Various Woody Biomass Chars: Influence of Inorganic Elements. Bioresour. Technol. 2011, 102, 9743–9748. 10.1016/j.biortech.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Guizani C.; Escudero Sanz F. J.; Salvador S. The Gasification Reactivity of High-Heating-Rate Chars in Single and Mixed Atmospheres of H2O and CO2. Fuel 2013, 108, 812–823. 10.1016/j.fuel.2013.02.027. [DOI] [Google Scholar]
- Al-Farraji A.; Marsh R.; Steer J.; Valera-Medina A. A Kinetics and Performance of Raw and Torrefied Biomass in a Continuous Bubbling Fluidized Bed Gasifier. Waste Biomass Valoriz. 2019, 10, 1365–1381. 10.1007/s12649-017-0167-8. [DOI] [Google Scholar]
- Zhang Y.; Geng P.; Liu R. Synergistic Combination of Biomass Torrefaction and Co-Gasification: Reactivity Studies. Bioresour. Technol. 2017, 245, 225–233. 10.1016/j.biortech.2017.08.197. [DOI] [PubMed] [Google Scholar]
- Amaducci S.; Facciotto G.; Bergante S.; Perego A.; Serra P.; Ferrarini A.; Chimento C. Biomass Production and Energy Balance of Herbaceous and Woody Crops on Marginal Soils in the Po Valley. G.C.B. Bioenergy 2017, 9, 31–45. 10.1111/gcbb.12341. [DOI] [Google Scholar]
- Sh L.; Lee B.-H.; Lee Y.-J.; Jeon C.-H. Comparing the Physicochemical Properties of Upgraded Biomass Fuel by Torrefaction and the Ashless Technique. Appl. Sci. 2019, 9, 5519. 10.3390/app9245519. [DOI] [Google Scholar]
- Sh L.; Lee B.-H.; Jeong T.-Y.; Jeon C.-H. Effects of Different Pretreatment Methods on the Grindability of Pitch Pine Sawdust Biomass and Its Blends With Coal. J. Mech. Sci. Technol. 2020, 34, 2235–2243. 10.1007/s12206-020-0445-4. [DOI] [Google Scholar]
- Slopiecka K.; Bartocci P.; Fantozzi F. Thermogravimetric Analysis and Kinetic Study of Poplar Wood Pyrolysis. Appl. Energy 2012, 97, 491–497. 10.1016/j.apenergy.2011.12.056. [DOI] [Google Scholar]
- Lai Z.; Ma X.; Tang Y.; Lin H. Thermogravimetric Analysis of the Thermal Decomposition of MSW in N2, CO2 and CO2/N2 Atmospheres. Fuel Process. Technol. 2012, 102, 18–23. 10.1016/j.fuproc.2012.04.019. [DOI] [Google Scholar]
- Chen T.; Wu C.; Liu R.; Fei W.; Liu S. Effect of Hot Vapor Filtration on the Characterization of Bio-Oil From Rice Husks With Fast Pyrolysis in a Fluidized-Bed Reactor. Bioresour. Technol. 2011, 102, 6178–6185. 10.1016/j.biortech.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Chen T.; Deng C.; Liu R. Effect of Selective Condensation on the Characterization of Bio-Oil From Pine Sawdust Fast Pyrolysis Using a Fluidized-Bed Reactor. Energy Fuels 2010, 24, 6616–6623. 10.1021/ef1011963. [DOI] [Google Scholar]
- Zhu M. M.; Zhou W. X.; Zhang Z. Z.; Li J. B.; Zhang D. K.. Effect of Temperature on Pyrolysis Products of a Pine Sawdust in an Indirectly Fired Rotary Kiln. Chemeca; 2013, challenging tomorrow2013, 879. [Google Scholar]
- Tran K. Q.; Trinh T. N.; Bach Q. V. Development of a Biomass Torrefaction Process Integrated With Oxy-Fuel Combustion. Bioresour. Technol. 2016, 199, 408–413. 10.1016/j.biortech.2015.08.106. [DOI] [PubMed] [Google Scholar]
- Chen T.; Wu J.; Zhang J.; Wu J.; Sun L. Gasification Kinetic Analysis of the Three Pseudocomponents of Biomass-Cellulose, Semicellulose and Lignin. Bioresour. Technol. 2014, 153, 223–229. 10.1016/j.biortech.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Yan W.; Acharjee T. C.; Coronella C. J.; Vásquez V. R. Thermal Pretreatment of Lignocellulosic Biomass. Environ. Prog. Sustainable Energy 2009, 28, 435–440. 10.1002/ep.10385. [DOI] [Google Scholar]
- Mishra G.; Kumar J.; Bhaskar T. Kinetic Studies on the Pyrolysis of Pinewood. Bioresour. Technol. 2015, 182, 282–288. 10.1016/j.biortech.2015.01.087. [DOI] [PubMed] [Google Scholar]
- He Q.; Ding L.; Gong Y.; Li W.; Wei J.; Yu G. Effect of Torrefaction on Pinewood Pyrolysis Kinetics and Thermal Behavior Using Thermogravimetric Analysis. Bioresour. Technol. 2019, 280, 104–111. 10.1016/j.biortech.2019.01.138. [DOI] [PubMed] [Google Scholar]
- Melkior T.; Jacob S.; Gerbaud G.; Hediger S.; Le Pape L.; Bonnefois L.; Bardet M. NMR Analysis of the Transformation of Wood Constituents by Torrefaction. Fuel 2012, 92, 271–280. 10.1016/j.fuel.2011.06.042. [DOI] [Google Scholar]
- Kaur R.; Gera P.; Jha M. K.; Bhaskar T. Pyrolysis Kinetics and Thermodynamic Parameters of Castor (Ricinus communis) Residue Using Thermogravimetric Analysis. Bioresour. Technol. 2018, 250, 422–428. 10.1016/j.biortech.2017.11.077. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Ma X.; Yao Z.; Yu Q.; Wang Z.; Lin Y. Study of the Pyrolysis of Municipal Sludge in N2/CO2 Atmosphere. Appl. Therm. Eng. 2018, 128, 662–671. 10.1016/j.applthermaleng.2017.09.044. [DOI] [Google Scholar]
- Skodras G.; Nenes G.; Zafeiriou N. Low Rank Coal - CO2 Gasification: Experimental Study, Analysis of the Kinetic Parameters by Weibull Distribution and Compensation Effect. Appl. Therm. Eng. 2015, 74, 111–118. 10.1016/j.applthermaleng.2013.11.015. [DOI] [Google Scholar]
- Chen S.; Meng A.; Long Y.; Zhou H.; Li Q.; Zhang Y. TGA Pyrolysis and Gasification of Combustible Municipal Solid Waste. J. Energy Inst. 2015, 88, 332–343. 10.1016/j.joei.2014.07.007. [DOI] [Google Scholar]
- Mishra R. K.; Mohanty K. Pyrolysis Kinetics and Thermal Behavior of Waste Sawdust Biomass Using Thermogravimetric Analysis. Bioresour. Technol. 2018, 251, 63–74. 10.1016/j.biortech.2017.12.029. [DOI] [PubMed] [Google Scholar]
- Wu W.; Cai J.; Liu R. Isoconversional Kinetic Analysis of Distributed Activation Energy Model Processes for Pyrolysis of Solid Fuels. Ind. Eng. Chem. Res. 2013, 52, 14376–14383. 10.1021/ie4021123. [DOI] [Google Scholar]
- Idris S. S.; Rahman N. A.; Ismail K. Combustion Characteristics of Malaysian Oil Palm Biomass, Sub-Bituminous Coal and Their Respective Blends via Thermogravimetric Analysis (TGA). Bioresour. Technol. 2012, 123, 581–591. 10.1016/j.biortech.2012.07.065. [DOI] [PubMed] [Google Scholar]









