Abstract
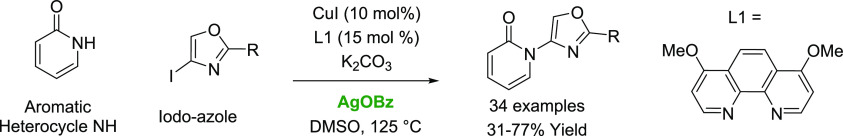
In the literature, C–N coupling methods for the reaction of iodo-oxazole with 2-pyridinone were found to be low yielding. C–N coupling using silver benzoate additives with CuI catalysts and 4,7-dimethoxy-1,10-phenanthroline ligands has been developed to afford synthetically useful yields of the desired heterobicycle product. The reaction conditions are applied to the coupling of a range of iodo-heterocycles with 2-pyridinone. The coupling of a variety of NH-containing heterocycles with 4-iodo-oxazole is also demonstrated. The use of 2-, 4-, or 5-iodo-oxazole allows for the coupling of pyridinone to each oxazole position.
1. Introduction
Functionalized oxazoles are frequently featured in drug designs.1 The scope of the original Gabriel-Robinson oxazole synthesis (Scheme 1a)2,3 has been improved through the use of milder dehydrating reagents and improved access to cyclization precursors.4,5 These strategies remain, however, inherently less divergent, and single-step methods to diversify substituent groups on an oxazole core are highly desirable for use in medicinal chemistry. While C–C coupling with oxazoles has been well-developed using metalation,6 direct Pd cross-coupling,7−11 Suzuki,12−14 Negishi,15 or nickel-catalyzed decarbonylation16 examples of C–N coupling are less prevalent in the literature. This can be attributed to slow C–N bond forming reductive elimination reactions using electron-rich oxazole electrophiles.17 Amination of halo-oxazoles at the electron-rich C(4)-position,18 in particular, is limited to a singular report of Buchwald-Hartwig coupling of piperidines (Scheme 1b).19 In this work, we report optimization of conditions and scope of the copper-catalyzed coupling of halo-azoles with aromatic nitrogen heterocycles.
Scheme 1. Synthesis of Functionalized Oxazoles.
In the course of our drug discovery efforts, we required a divergent synthesis of N-(oxazol-4-yl)pyridones (Scheme 1d). A C–N coupling was envisioned to enable their rapid and modular synthesis. The Buchwald-Hartwig amination reaction20 has been developed into one of the most useful methods to forge C–N bonds.21 This includes the coupling of aryl-halides with common nitrogen heterocycles22 or amides using Pd catalysts.23 Buchwald24,25 and Taillefer26 also pioneered the use of auxiliary ligands to expand the scope of C–N coupling via Ullman chemistry. Specifically, Buchwald’s use of CuI and phenanthroline to catalyze the heteroarylation of 2-hydroxypyridines bears directly on the present case (Scheme 1c).27
2. Results and Discussion
Initial attempts to couple 2-pyridinone with 4-iodo-2-phenyloxazole proved unsatisfactory. Two sets of conditions employing Pd catalysts previously applied to couplings of 4-halo-oxazole failed to furnish the desired bicyclic product (Table 1, entries 1–2). The Buchwald group has also shown that use of the CuI catalyst with the 4,7-dimethoxy-1,10-phenanthroline ligand (L1) is an optimal ligand for heterocycle coupling,28 and in our case, the yield improved to 14% (entry 3). Switching the ligand to phenanthroline provided <5% product (entry 4). Amide coupling conditions using TMEDA or trans-1,2-methylamino-cyclohexane (L3) also did not provide any of the desired product (entry 5, 6).
Table 1. Optimization of Coupling Conditions.
Average HPLC assay yield for two reactions using an internal standard.
THF, 40 °C.
0.05 M.
0.025 M.
Isolated yield.
Using 4-bromo-2-phenyloxazole in place of 4-iodo-2-phenyloxazole 1a.
The use of silver additives to increase the reaction yield for the palladium-catalyzed direct arylation of aryl iodides has been reported. By sequestering iodide with Ag2CO3 rather than K2CO3 the yield is increased from 64 to 99%.29 For copper catalysis, direct arylation of benzodithiophene-S,S-tetraoxide with aryl iodides was found to be most efficient with Ag2CO3.30 In a subsequent computation study, it was determined that the Ag additive reduces the rate-limiting Ph–I insertion through a Ag–I interaction and oxidative addition of Ph–I on Ag.31 For Cu-catalyzed amide arylations using excess bidentate ligands, Ar–I insertion is rate-limiting.32 We therefore attempted to increase the yield of the oxazole amide coupling by the addition of Ag2CO3, and a modest increase in the yield to 23% was observed (entry 7). We then screened other bases (entries 8–11) and found K2CO3 to be best (entry 11, 53%). We then recognized that the solubility of Ag2CO3 could be limiting the reaction and switched to AgOBz; the yield increased to 63% (entry 12). By lowering the reaction concentration, we saw further improvement in the reaction yield, 72% at 0.05 M (entry 13) and 81% yield at 0.025 M (entry 14). We then isolated the product in 77% yield. Use of 4-bromo-2-phenyloxazole in place of 4-iodo-2-phenyloxazole is lower yielding (entry 15, 20%).
The scope of N-heterocycles beyond 2-pyridinone was then investigated (Figure 1). 3-Substituted 2-pyridinones afforded modest yields (3, Br, 55%; 4: MeO, 54%; 5: EtO, 42%; 6: Me, 48%; and 7: F, 47%).
Figure 1.
Scope of the N-heterocycle coupling partner.
Pyridazin-3(2H)-one (8, 54%) or 6-methylpyridazin-3(2H)-one (9, 58%) coupled in a similar yield. Pyrazole (10, 45%) or 4-iodopyrazole (11, 31%) proved more challenging. Imidazole 12 coupled well (77%); however, 4-bromoimidazole 13 was less efficiently coupled (53%). 1,2,4 Triazole (14, 46%) or 5-methylpyrimidine-2,4-dione (15, 51%) both coupled at the N1-position. The pyridinone regioisomer 3-bromopyridin-4-one coupled in 44% yield (16). Next, we tested the scope of fused bicyclic pyridinones. While imidazo[1,2-a]pyrazin-8-one (18, 36%) proved to be one of the most challenging substrates, thieno[2,3-c]pyridin-7-one (19, 76%) or furo[2,3-c]pyridin-7(6H)-one (20, 58%) worked well. Indazole (21, 31%) was lower yielding than 6-chloro-indole (22, 56%). Benzimidazole (23, 65%) or 6-aza-indole (24, 62%) coupled in a moderate yield.
The reaction of 2-pyridinone was then explored with different iodo-heterocycles (Figure 2). 2-Iodo-benzoxazole (25, 53%), 2-iodo-benzthiazole (26, 77%), 2-iodo-benzthiophene (27, 48%), 2-iodo-5-phenyl-1,3,4-oxadiazole (28, 60%), 3-iodo-1-phenyl-pyrazole (29, 65%), 5-iodo-2-phenylthiazole (30, 41%), and 5-bromo-2-iodo-1-methyl-1H-benzo[d]imidazole (31, 47%) were all coupled successfully.
Figure 2.
Scope of iodo-heterocycle coupling with 2-pyridone to provide N-pyridin-2-one heterocycles.
The coupling of iodo-oxazole regioisomers with 2-pyridinone is presented in Figure 3. Use of 2-iodo-5-phenyl-oxazole provides the 2-substituted product 32 in 63% yield. Use of 5-iodo-2-phenyloxazole as the substrate provides the 5-substituted product 33 in 49% yield. Using this method, we can generate all three oxazole-pyridinone regioisomers. Sterically encumbered 4-iodo-5-methyl-2-phenyloxazole is also competent in the coupling reaction to provide 34 in 41% yield.
Figure 3.
Synthesis of oxazole positional isomers.
3. Conclusions
In summary, by using AgOBz as an additive, we have been able to extend the scope of the Buchwald’s copper-catalyzed aryl amine coupling reaction to include the coupling of iodo-azoles with NH-containing heterocycles. We have demonstrated that these coupling conditions can be used to couple oxazole to a diverse range of heterocycles of pharmaceutical interest. High selectivity for coupling of aromatic iodides allows incorporation of additional aromatic halides, I (11), Br (3, 13, 16), or Cl (22), that can subsequently be used for further derivatization.
4. Experimental Section
4.1. General Information
All reactions were conducted under an atmosphere of air unless otherwise indicated, using a Teflon-coated magnetic stir bar at the temperature given. Commercial reagents and anhydrous solvents were used without further purification. Organic solvents, silver benzoate, 4,7-dimethoxyphenanthroline, potassium carbonate, copper(I) iodide, pyridone, and 3-iodo-N-phenyl-pyrazole (1f) were purchased from Sigma-Aldrich. 2-Iodobenzothiophene (1d) was purchased from Frontier Scientific. Reactions were monitored by liquid chromatography-mass spectroscopy (LC–MS) (Agilent Technologies G6100 Series LC/MSD Single Quad). Flash chromatography was carried out on a CombiFlash Rf+ purification system using RediSep Rf Gold silica gel (20–40 μm), purchased from Teledyne Isco, Inc. Preparative LC was performed on a Teledyne Isco CombiFlash EZ Prep equipped with a Luna 5 μm 100 Å 100 × 30 mm LC column. Organic solutions were concentrated under reduced pressure on a Heidolph rotary evaporator. 1H, 13C{1H}, 19F, and 31P NMR spectra were recorded on a Bruker Advance (400 MHz) spectrometer. 1H and 13C{1H} spectra are internally referenced to residual proton solvent signals (DMSO referenced at δ 2.50 ppm for 1H and δ 39.52 ppm for 13C; chloroform referenced at δ 7.26 ppm for 1H and δ 77.16 ppm for 13C). Chemical shifts (δ) are reported in parts per million (ppm). The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad. High resolution mass spectral data were determined on a Synapt G2 QTOF mass spectrometer.
4.2. Synthesis of 4-Iodo-2-phenyoxazole
1,3-Oxazole (1.00 mL, 14.9 mmol, 1 equiv) was dissolved into a mixture of anhydrous THF (6.4 mL) and anhydrous DMPU (5.2 mL) and cooled to −78 °C. LHMDS (32 mL, 32 mmol, 2.1 equiv) was then added dropwise and stirred for 1 h. After this time, solid iodine (7.7 g, 30 mmol, 2 equiv) was added to the reaction mixture and stirred for an additional 30 min at −78 °C. The cooling bath was then removed, and the reaction mixture was left to warm to room temperature and stirred for 48 h under a low positive pressure of N2. The reaction mixture was then poured into a mixture of aqueous Na2S2O3 (100 mL) and diethyl ether (100 mL). The organic layer was washed with brine (100 mL) and dried over MgSO4. After filtration, the solvent was removed in vacuo. The residue was purified by flash chromatography (silica, hexanes/EtOAc, 9:1) to afford 2,4-diiodooxazole (3.701 g, 77% yield). Characterization matches with literature values.33
Under nitrogen, Pd(OAc)2 (6.12 mg, 0.027 mmol, 0.030 equiv) and 1,3,5-triaza-7-phosphaadamantane (9.44 mg, 0.054 mmol, 0.050 equiv) were added to degassed acetonitrile (0.45 M). After stirring for 5 min, this solution was transferred to a separate vessel under nitrogen containing 2,4-diiodooxazole (350 mg, 1.00 mmol, 1.0 equiv), phenylboronic acid (146 mg, 1.20 mmol, 1.2 equiv), and potassium hydrogen phosphate (695 mg, 3.27 mmol, 3.3 equiv). The sealed vessel was heated at 60 °C for 18 h. Upon cooling to room temperature, LC–MS showed 100% conversion of the starting material, 19.5:1 mono:bis product ratio, and > 20:1 C2:C4 product isomer ratio. The reaction mixture was filtered and the solid was washed with DCM. The solvents were removed by rotary evaporation and the title compound was purified by column chromatography (silica gel, EtOAc/hexane) to give a white solid (502 mg, 42% yield). Spectra are consistent with reported literature.14
4.3. General Procedures for the Synthesis of Heteroaryl Halides
4.3.1. Heteroaryl Halide Synthesis Procedure A
Modified from a literature procedure.34 To a flame-dried vial was added 1,3-azole (2.52 mmol, 1.0 equiv), 1,10-phenanthroline (2.52 mmol, 1.0 equiv), LiOtBu (5.04 mmol, 2.0 equiv), CuBr2 (0.126 mmol, 0.05 equiv), and iodine (3.78 mmol, 1.5 equiv). Dry 1,4-dioxane (2 mL) was then added to the mixture and heated to 80 °C. The mixture was cooled to room temperature and filtered through a short pad of silica gel. The silica gel was washed with EtOAc (20 mL) and the combined filtrate was concentrated under reduced pressure then purified by silica gel column chromatography to afford the title compound(s).
4.3.2. Heteroaryl Halide Synthesis Procedure B
To a flame-dried vial was added 1,3-azole (6.20 mmol, 1.0 equiv), N-iodosuccinimide (13.64 mmol, 2.2 equiv), and chloroform (4 mL). To the reaction mixture was added three drops of trifluoroacetic acid and then heated to 65 °C. Once the starting material was consumed, the reaction was cooled then diluted with dichloromethane and washed with aqueous sodium bicarbonate and brine. The organic phase was then dried over sodium sulfate and filtered. The filtrate was concentrated and the product purified by silica gel column chromatography to yield the title compound(s).
4.3.3. 2-Iodobenzo[d]oxazole (1b)
General Procedure A was used to obtain 1b as a white solid, yield 89% (549 mg). Analytical data are consistent with the values reported in the literature.35
4.3.4. 2-Iodobenzo[d]thiazole (1c)
General Procedure A was used to obtain 1c as a white solid, yield 55% (362 mg). Analytical data are consistent with the values reported in the literature.36
4.3.5. 2-Iodo-5-phenyl-1,3,4-oxadiazole (1e)
General Procedure A was used to obtain 1e as a white solid, yield 76% (521 mg). Analytical data are consistent with the values reported in the literature.37
4.3.6. 5-Iodo-2-phenylthiazole (1g)
General Procedure B was used to obtain 1g as a white solid, 63% yield (1.12 g). 1H NMR (400 MHz, CDCl3): δ 7.43–7.46 (m, 3H), 7.87 (s, 1H), 7.87–7.90 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3): δ 70.05, 126.44, 129.10, 130.50, 133.00, 151.46, 173.62 HRMS (ESI), C9H6INSH [M + H] + calculated m/z 287.9344; found m/z 287.9336.
4.3.7. 2-Iodo-5-bromo-1-methyl-1H-benzo[d]imidazole (1h)
General Procedure A was used to obtain 1h as a white solid, 48% yield (408 mg). 1H NMR (400 MHz, DMSO-d6): δ 3.76 (s, 3H), 7.38 (dd, J = 8.56, 1.71 Hz, 1H), 7.59 (d, J = 8.56 Hz, 1H), 7.79 (d, J = 1.71 Hz, 1H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 34.17, 110.29, 112.69, 114.57, 120.91, 125.63, 135.70, 146.38. HRMS (ESI), C8H6BrIN2H [M + H]+ calculated m/z 336.8837; found 336.8835.
4.3.8. 2-Iodo-5-phenyloxazole (1i)
General Procedure B was used to obtain 1i as a white solid. Yield 52% (873 mg). Analytical data are consistent with the values reported in the literature.38
4.3.9. 5-Iodo-2-phenyloxazole (1j)
General Procedure B was used to obtain 1j as a white solid. Yield 64% (1.08 g). 1H NMR (400 MHz, CDCl3): δ 7.25 (s, 1H), 7.43–7.47 (m, 3H), 7.99–8.03 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3): δ 86.65, 126.21, 126.70, 128.84, 130.79, 136.98, 166.42 HRMS (ESI) C9H6INOH [M + H]+ calculated m/z 271.9572; found m/z 271.9576.
4.3.10. 4-Iodo-5-methyl-2-phenyl-1,3-oxazole (1k)
5-Methyl-2-phenyl-oxazole was synthesized using a literature procedure.39 5-Methyl-2-phenyl-oxazole (150 mg, 0.94 mmol) was then dissolved in THF (0.2 M) and cooled to −78 °C. To the solution was added a solution of n-butyllithium (2.5 M, 1.1 equiv) and the reaction mixture stirred for 1 h. Solid I2 (1 equiv) was then added to the solution and the reaction was allowed to stir for another hour. The reaction mixture was then diluted with water, extracted with CH2Cl2 (3×), washed once with aqueous brine, and dried over Na2SO4 to afford a white powder (41% yield, 110 mg). 1H NMR (400 MHz, CDCl3): δ 2.42 (s, 3H), 7.42–7.46 (m, 3H), 7.97–8.02 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3): δ 11.22, 81.73, 126.03, 126.73, 128.78, 130.49, 150.82, 161.88. HRMS (ESI) C10H8INOH [M + H]+ calculated m/z 285.9729; found m/z 285.9724.
4.3.11. General Procedure for C–N Coupling
To a 40 mL vial charged with a Teflon stir bar was added heteroaryl iodide 1a–1k (0.1 mmol, 1.0 equiv), copper(I) iodide (0.01 mmol, 0.10 equiv), 4,7-dimethoxy-1,10-phenanthroline (0.015 mmol, 0.15 equiv), silver(I) benzoate (0.1 mmol, 1 equiv), potassium carbonate (0.20 mmol, 2.0 equiv), aromatic heterocycle NH (1.1 equiv), and DMSO (3 mL, 0.033 M). The reaction mixture was stirred at 125 °C for 18 h, unless stated otherwise. The reaction was filtered through a pad of celite and rinsed with EtOAc, the filtrate was diluted with H2O (3 mL), extracted with EtOAc (3 × 10 mL), washed again with saturated aqueous LiCl (5 mL), and dried over Na2SO4 then filtered off. The crude mixture was then concentrated, and the title compound purified via column chromatography using a heptane/ethyl acetate solvent system unless stated otherwise.
4.3.12. 4-Pyridonyl-2-phenyloxazole (2)
The title compound was synthesized according to the general procedure for C–N coupling using pyridin-2(1H)-one (10.5 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 2 as a white solid in 77% yield (18.4 mg). 1H NMR (400 MHz, CDCl3): δ 6.42 (t, J = 6.72 Hz, 1H), 6.73 (d, J = 9.05 Hz, 1H), 7.42 (ddd, J = 9.05, 6.60, 1.96 Hz, 1H), 7.46–7.55 (m, 3H), 8.04–8.14 (m, 2H), 8.61 (dd, J = 7.21, 1.83 Hz, 1H), 8.74 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 106.53, 121.06, 126.51, 126.79, 128.89, 130.44, 130.90, 132.89, 137.74, 138.84, 158.99, 160.73. HRMS (ESI) C14H10N2O2Na [M + Na]+ calculated m/z 261.0640; found m/z 261.0628.
4.3.13. 3-Bromo-1-(2-phenyl-1,3-oxazol-4-yl)pyridin-2(1H)-one (3)
The title compound was synthesized according to the general procedure for C–N coupling using 3-bromopyridine-2(1H)-one (19.0 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 3 as a white solid in 55 yield% (17.3 mg). 1H NMR (400 MHz, CDCl3): δ 6.34 (t, J = 7.21 Hz, 1H), 7.49–7.53 (m, 3H), 7.83 (dd, J = 7.09, 1.71 Hz, 1H), 8.08–8.12 (m, 2H), 8.66 (dd, J = 7.09, 1.71 Hz, 1H), 8.77 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 106.26, 116.08, 126.13, 126.17, 128.58, 130.39, 130.68, 132.05, 137.33, 140.29, 156.40, 158.77. HRMS (ESI) C14H9N2O2Na [M + Na]+ calculated m/z 338.9745; found m/z 338.9748.
4.3.14. 3-Methoxy-1-(2-phenyl-1,3-oxazol-4-yl)pyridin-2(1H)-one (4)
The title compound was synthesized according to the general procedure for C–N coupling using 3-methoxy-2(1H)-pyridinone (14.0 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 4 as a white solid in 54% yield (14.5 mg). 1H NMR (400 MHz, CDCl3): δ 3.85–3.90 (m, 3H), 6.34 (t, J = 7.34 Hz, 1H), 6.67 (dd, J = 7.34, 1.47 Hz, 1H), 7.46–7.53 (m, 3H), 8.06–8.13 (m, 2H), 8.23 (dd, J = 7.21, 1.59 Hz, 1H), 8.77 (s, 1H). 13C{1H} NMR (400 MHz, CDCl3): δ 56.05, 105.51, 111.21, 123.81, 126.46, 126.83, 128.86, 130.50, 130.82, 137.82, 149.83, 156.31, 158.85. HRMS (ESI) C15H12N2O3Na [M + Na]+ calculated m/z 291.0746; found m/z 291.0742.
4.3.15. 3-Ethoxy-1-(2-phenyl-1,3-oxazol-4-yl)pyridin-2(1H)-one (5)
The title compound was synthesized according to the general procedure for C–N coupling using 3-ethoxy-2(1H)-pyridinone (15.2 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 5 as a white solid in 42% yield (15.3 mg). 1H NMR (400 MHz, CDCl3): δ 1.53 (t, J = 6.97 Hz, 3H), 4.06 (q, J = 7.09 Hz, 2H), 6.32 (t, J = 7.34 Hz, 1H), 6.66 (dd, J = 7.34, 1.22 Hz, 1H), 7.45–7.54 (m, 3H), 8.07–8.14 (m, 2H), 8.23 (dd, J = 7.34, 1.47 Hz, 1H), 8.77 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ14.52, 64.53, 105.61, 112.09, 123.64, 126.46, 126.87, 128.86, 130.47, 130.81, 137.91, 149.09, 156.42, 158.83 HRMS (ESI) C16H14N2O3Na [M + Na]+ calculated m/z 305.0902; found m/z 305.0898.
4.3.16. 3-Methyl-1-(2-phenyl-1,3-oxazol-4-yl)pyridin-2(1H)-one (6)
The title compound was synthesized according to the general procedure for C–N coupling using 3-methylpyridine-2(1H)-one (12.0 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 6 as a white solid in 48% yield (12.0 mg). 1H NMR (400 MHz, CDCl3): δ 2.25 (s, 3H), 6.34 (t, J = 6.97 Hz, 1H), 7.29 (s, 1H), 7.46–7.54 (m, 3H), 8.07–8.14 (m, 2H), 8.50 (dd, J = 7.21, 1.10 Hz, 1H), 8.75 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 17.22, 106.45, 126.47, 126.85, 128.86, 129.80, 130.24, 130.43, 130.83, 136.08, 138.04, 158.88, 161.25. HRMS (ESI) C15H12N2O2Na [M + Na]+ calculated m/z 275.0797; m/z found 275.0788.
4.3.17. 3-Fluoro-1-(2-phenyl-1,3-oxazol-4-yl)pyridin-2(1H)-one (7)
The title compound was synthesized according to the general procedure for C–N coupling using 3-fluoropyridin-2(1H)-one (12.4 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 7 as a white solid in 47% yield (12.2 mg). 1H NMR (400 MHz, CDCl3): δ 6.34 (td, J = 7.34, 4.65 Hz, 1H), 7.17 (ddd, J = 9.05, 7.34, 1.71 Hz, 1H), 7.48–7.53 (m, 3H), 8.07–8.12 (m, 2H), 8.42 (dt, J = 7.34, 1.47 Hz, 1H), 8.76 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 104.42, 104.48, 119.09, 119.26, 126.54, 126.57, 128.18, 128.23, 128.93, 130.67, 131.08, 159.17. HRMS (ESI) C14H9FN2O2Na [M + Na]+ calculated m/z 279.0546; found m/z 279.0541.
4.3.18. 2-(2-Phenyl-1,3-oxazol-4-yl)pyridazin-3(2H)-one (8)
The title compound was synthesized according to the general procedure for C–N coupling using pyridazin-3(2H)-one (11.0 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 8 as a white solid in 54% yield (12.9 mg). 1H NMR (400 MHz, CDCl3): δ 7.12 (dd, J = 9.54, 1.47 Hz, 1H), 7.31 (dd, J = 9.54, 3.67 Hz, 1H), 7.46–7.52 (m, 3H), 8.13–8.20 (m, 3H), 8.69 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 126.62, 126.84, 128.78, 130.13, 130.51, 130.77, 131.01, 137.54, 139.69, 158.53, 160.12. HRMS (ESI) C13H9N3O2Na [M + Na]+ calculated m/z 262.0592; found m/z 262.0591.
4.3.19. 6-Methyl-2-(2-phenyl-1,3-oxazol-4-yl)pyridazin-3(2H)-one (9)
The title compound was synthesized according to the general procedure for C–N coupling using 6-methylpyridazin-3(2H)-one (12 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 9 as colorless oil in 58% yield (14.6 mg). 1H NMR (400 MHz, CDCl3): δ 2.55 (s, 3H), 7.05 (d, J = 9.54 Hz, 1H), 7.21 (d, J = 9.29 Hz, 1H), 7.46–7.52 (m, 3H), 8.15 (dd, J = 6.60, 2.93 Hz, 2H), 8.63 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 21.37, 126.57, 126.84, 128.75, 129.97, 130.85, 131.00, 133.00, 139.49, 146.31, 158.32, 160.15. HRMS (ESI) C14H11N3O2Na [M + Na]+ calculated m/z 276.0749; found m/z 276.0738.
4.3.20. 2-Phenyl-4-(1H-pyrazol-1-yl)-1,3-oxazole (10)
The title compound was synthesized according to the general procedure for C–N coupling using 1H-pyrazole (7.5 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 10 as a white solid in 45% yield (9.4 mg). 1H NMR (400 MHz, DMSO-d6): δ 6.56 (t, J = 1.96 Hz, 1H), 7.57–7.60 (m, 3H), 7.78–7.80 (m, 1H), 8.01–8.06 (m, 2H), 8.30 (d, J = 2.45 Hz, 1H), 8.53 (s, 1H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 107.87, 126.54, 126.58, 127.16, 128.90, 129.78, 131.77, 141.38, 142.27, 160.31. HRMS (ESI) C12H9N3ONa [M + Na]+m/z 234.0643; found m/z 234.0634.
4.3.21. 4-(4-Iodo-1H-pyrazol-1-yl)-2-phenyl-1,3-oxazole (11)
The title compound was synthesized according to the general procedure for C–N coupling using 4-iodo-1H-pyrazole (21.0 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 11 as a white solid in 31% yield (10.4 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.56–7.61 (m, 3H), 7.90 (s, 1H), 8.04 (dd, J = 6.60, 2.93 Hz, 2H), 8.48 (s, 1H), 8.60 (s, 1H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 60.85, 126.43, 126.61, 127.73, 129.80, 131.87, 133.29, 140.72, 146.96, 160.36. HRMS (GC CI-MS) C12H8IN3O [M]+• calculated m/z 336.9712; found m/z 336.9699.
4.3.22. 4-(1H-Imidazol-1-yl)-2-phenyl-1,3-oxazole (12)
The title compound was synthesized according to the general procedure for C–N coupling using 1H-imidazole (7.5 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 12 as a white solid in 77% yield (16.3 mg). 1H NMR (400 MHz, DMSO-d6): δ ppm 7.15 (br s, 1H), 7.57–7.61 (m, 3H), 7.70 (br s, 1H), 8.02–8.07 (m, 2H), 8.22 (s, 1H), 8.65 (s, 1H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 118.16, 126.43, 126.62, 127.60, 129.77, 130.00, 131.84, 135.89, 138.21, 160.51. HRMS (ESI) C12H9N3OH [M + H]+ calculated m/z 212.0824; found m/z 212.0827.
4.3.23. 4-(4-Bromo-1H-imidazol-1-yl)-2-phenyl-1,3-oxazole (13)
The title compound was synthesized according to the general procedure for C–N coupling using 4-bromo-1H-imidazole (16.0 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 13 as a white solid in 53% yield (15.2 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.58–7.61 (m, 3H), 7.90 (s, 1H), 8.01–8.05 (m, 2H), 8.24 (s, 1H), 8.65 (s, 1H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 116.21, 117.60, 126.27, 126.67, 128.30, 129.80, 131.98, 136.24, 137.44, 160.59. HRMS (ESI) C12H8BrN3OH [M + H]+ calculated m/z 289.9929; found m/z 289.9933. The assignment is based on analogy to arylations of 4-bromo-1H-imidazole as the heterocycle.40
4.3.24. 1-(2-Phenyl-1,3-oxazol-4-yl)-1H-1,2,4-triazole (14)
The title compound was synthesized according to the general procedure for C–N coupling using 1,2,4-triazole (7.6 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 14 as a white solid in 46% yield (9.8 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.57–7.62 (m, 3H), 8.02–8.07 (m, 2H), 8.31 (s, 1H), 8.70 (s, 1H), 9.13 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 126.27, 126.70, 129.22, 129.84, 132.04, 138.35, 143.58, 153.42, 160.77. HRMS (ESI) C11H8N4OH [M + H]+ calculated m/z 213.0776; found m/z 213.0776.
4.3.25. 5-Methyl-1-(2-phenyl-1,3-oxazol-4-yl)pyrimidine-2,4(1H,3H)-dione (15)
3-Benzoyl-5-methyl-2,4(1H,3H)-pyrimidinedione was synthesized via a literature procedure41 and was used as the heterocycle (25.0 mg) coupling partner. The title compound was synthesized according to the general procedure for C–N coupling. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 15 as a white solid in 51% yield (13.6 mg). 1H NMR (400 MHz, DMSO-d6): δ 1.91 (d, J = 0.98 Hz, 3H), 7.56–7.60 (m, 3H), 8.02–8.07 (m, 2H), 8.25 (d, J = 1.22 Hz, 1H), 8.44 (s, 1H), 11.76 (s, 1H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 12.61, 111.05, 126.46, 126.57, 129.22, 129.75, 131.75, 136.06, 137.09, 149.13, 158.91, 163.91. HRMS (ESI) C14H11N3O3Na [M + Na]+ calculated m/z 292.0698; found m/z 292.0684.
4.3.26. 3-Bromo-1-(2-phenyl-1,3-oxazol-4-yl)pyridin-4(1H)-one (16)
The title compound was synthesized according to the general procedure for C–N coupling using 3-bromopyridine-4(1H)-one (19.0 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 16 as a white solid in 44% yield (13.8 mg). 1H NMR (400 MHz, CDCl3): δ 6.61 (d, J = 7.58 Hz, 1H), 7.49–7.57 (m, 3H), 7.87 (s, 1H), 7.92 (dd, J = 7.83, 2.20 Hz, 1H), 8.07 (dd, J = 7.58, 1.71 Hz, 2H), 8.41 (d, J = 2.20 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 116.55, 117.10, 124.59, 125.95, 126.70, 129.10, 131.74, 135.57, 136.59, 141.82, 161.65, 173.62. HRMS (ESI) C14H9BrN2O2H [M + H]+ calculated m/z 316.9926; found m/z 316.9928.
4.3.27. 8-Methyl-6-(2-phenyl-1,3-oxazol-4-yl)imidazo[1,2-c]pyrimidin-5(6H)-one (17)
The title compound was synthesized according to the general procedure for C–N coupling using 8-methyl-6-imidazo[1,2-c]pyrimidin-5(6H)-one (16.3 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 17 as a white solid in 67% yield (18.2 mg). 1H NMR (400 MHz, CDCl3): δ 2.48 (d, J = 1.22 Hz, 3H), 7.48 (d, J = 1.47 Hz, 1H), 7.52 (quin, J = 3.18 Hz, 3H), 7.85 (d, J = 1.47 Hz, 1H), 8.09–8.14 (m, 2H), 8.19 (d, J = 1.22 Hz, 1H), 8.46 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 13.28, 109.03, 113.47, 124.37, 126.44, 126.53, 128.53, 128.89, 131.03, 132.82, 137.33, 144.13, 145.56, 159.37 HRMS (ESI) calculated for C16H12N4O2H [M + H]+ 293.1039 found 293.1035.
4.3.28. 7-(2-Phenyl-1,3-oxazol-4-yl)imidazo[1,2-a]pyrazin-8(7H)-one (18)
The title compound was synthesized according to the general procedure for C–N coupling using imidazo[1,2-a]pyrazin-8(7H)-one (17.0 mg) as the heterocycle. The product was purified by silica gel column chromatography DCM/methanol 90:10 to provide 18 as a white solid in 36% yield (9.2 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.57 (s, 1H), 7.59–7.62 (m, 3H), 7.83 (d, J = 6.11 Hz, 1H), 7.95 (s, 1H), 7.98 (d, J = 6.11 Hz, 1H), 8.08 (dd, J = 6.60, 2.93 Hz, 2H), 8.77 (s, 1H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 109.20, 115.92, 118.71, 126.46, 126.62, 129.81, 130.15, 131.82, 133.70, 136.82, 137.33, 151.37, 158.79. HRMS (ESI) C15H10N4O2Na [M + Na]+ calculated m/z 301.0702; found m/z 301.0699.
4.3.29. 6-(2-Phenyl-1,3-oxazol-4-yl)thieno[2,3-c]pyridin-7(6H)-one (19)
The title compound was synthesized according to the general procedure for C–N coupling using thieno[2,3-c]pyridin-7(6H)-one (16.6 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 19 as a white solid in 76% yield (22.3 mg). 1H NMR (400 MHz, CDCl3): δ 6.88 (d, J = 7.34 Hz, 1H), 7.26–7.30 (m, 2H), 7.51 (m, J = 3.30 Hz, 3H), 7.77 (d, J = 5.14 Hz, 1H), 8.10–8.15 (m, 1H), 8.54 (d, J = 7.34 Hz, 1H), 8.73 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 103.84, 124.42, 126.44, 126.82, 128.67, 128.85, 129.74, 130.08, 130.80, 134.16, 138.00, 144.50, 156.76, 158.87. HRMS (ESI) C16H10N2O2SNa [M + Na]+m/z 317.0361; found m/z 317.0356.
4.3.30. 6-(2-Phenyl-1,3-oxazol-4-yl)furo[2,3-c]pyridin-7(6H)-one (20)
The title compound was synthesized according to the general procedure for C–N coupling using furo[2,3-c]pyridin-7(6H)-one (14.8 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 20 as a white solid in 58% yield (16.1 mg). 1H NMR (400 MHz, CDCl3): δ 6.69–6.75 (m, 2H), 7.49–7.53 (m, 3H), 7.82 (d, J = 1.96 Hz, 1H), 8.09–8.14 (m, 2H), 8.47 (d, J = 7.34 Hz, 1H), 8.76 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 101.19, 107.47, 126.49, 126.84, 128.24, 128.89, 129.96, 130.86, 132.30, 137.88, 143.38, 148.97, 151.85, 158.89. HRMS (ESI) C16H10N2O3Na [M + Na]+ calculated m/z 301.0589; found m/z 301.0588.
4.3.31. 1-(2-Phenyl-1,3-oxazol-4-yl)-1H-indazole (21)
The title compound was synthesized according to the general procedure for C–N coupling using 1H-indazole (13.0 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 21 as a white solid in 31% yield (8.1 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.30–7.35 (m, 1H), 7.57–7.60 (m, 1H), 7.61 (d, J = 2.20 Hz, 3H), 7.89–7.93 (m, 1H), 8.12–8.15 (m, 2H), 8.37–8.40 (m, 1H), 8.44 (s, 1H), 8.64 (s, 1H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 112.53, 121.89, 122.75, 124.92, 126.60, 126.74, 127.54, 128.51, 129.81, 131.71, 137.44, 138.84, 141.85, 160.28. HRMS (ESI) C16H11N3ONa [M + Na]+ calculated m/z 284.0800; found m/z 284.0807. The assignment based on analogy to literature arylation of indazoles.42,43
4.3.32. 6-Chloro-1-(2-phenyl-1,3-oxazol-4-yl)-1H-indole (22)
The title compound was synthesized according to the general procedure for C–N coupling using 6-chloro-1H-indole (17.0 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 22 as a white solid in 54% yield (15.8 mg). 1H NMR (400 MHz, CDCl3): δ 6.69 (d, J = 3.42 Hz, 1H), 7.19 (dd, J = 8.44, 1.83 Hz, 1H), 7.51–7.54 (m, 3H), 7.58 (d, J = 8.31 Hz, 1H), 7.61 (d, J = 3.42 Hz, 1H), 7.81 (d, J = 1.47 Hz, 1H), 7.90 (s, 1H), 8.13–8.16 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3): δ 104.71, 111.72, 121.68, 121.96, 125.12, 125.53, 126.57, 126.87, 126.95, 128.08, 128.92, 130.98, 135.45, 139.97, 160.63. HRMS (ESI) C17H11ClN2OH [M + H]+ calculated m/z 295.0638; found m/z 295.0650.
4.3.33. 1-(2-Phenyl-1,3-oxazol-4-yl)-1H-benzimidazole (23)
The title compound was synthesized according to the general procedure for C–N coupling using 1H-benzimidazole (13.0 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 2:1 to provide 23 as a white solid in 65% yield (16.8 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.34–7.45 (m, 2H), 7.58–7.61 (m, 3H), 7.78–7.81 (m, 1H), 7.93–7.97 (m, 1H), 8.07–8.11 (m, 2H), 8.72 (s, 1H), 8.86 (s, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 117.77, 120.83, 123.33, 124.23, 125.53, 126.55, 126.66, 129.0, 131.28, 132.20, 137.52, 141.28, 144.05, 161.18. HRMS (ESI) C16H11N3OH [M + H]+ calculated m/z 262.0980; found m/z 262.0981.
4.3.34. 1-(2-Phenyl-1,3-oxazol-4-yl)-1H-pyrrolo[2,3-c]pyridine (24)
The title compound was synthesized according to the general procedure for C–N coupling using 6-azaindole (13.0 mg) as the heterocycle. The product was purified by silica gel column chromatography toluene:EtOAc 1:1 to provide 24 as a white solid in 62% yield (16.8 mg). 1H NMR (400 MHz, DMSO-d6): δ 6.85 (d, J = 3.18 Hz, 1H), 7.60–7.63 (m, 3H), 7.69 (d, J = 5.38 Hz, 1H), 8.09 (d, J = 3.18 Hz, 1H), 8.10–8.14 (m, 2H), 8.31 (d, J = 5.38 Hz, 1H), 8.87 (s, 1H), 9.34 (s, 1H). 13C{1H} NMR (400 MHz, DMSO-d6) δ 104.43, 115.96, 126.62, 126.67, 127.40, 129.80, 130.36, 131.76, 131.99, 134.31, 135.24, 139.51, 140.34, 160.18 HRMS (ESI) C16H11N3OH [M + H]+ calculated m/z 262.0980; found m/z 262.0990.
4.3.35. 1-(1,3-Benzoxazol-2-yl)pyridin-2(1H)-one (25)
The title compound was synthesized according to the general procedure for C–N coupling using 1b and pyridone (11.0 mg) as the heterocycle and stirred for 1 h. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 25 as a white solid in 53% yield (11.5 mg). 1H NMR (400 MHz, DMSO-d6): δ 6.47 (br d, J = 0.98 Hz, 1H), 6.61 (m, 1H), 7.52 (s, 2H), 7.62–7.69 (m, 1H), 7.83–7.89 (m, 2H), 7.93–7.97 (m, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 106.98, 111.24, 120.33, 122.84, 125.18, 125.88, 135.32, 139.99, 140.75, 150.22, 155.37, 160.90. HRMS (ESI) C12H8N2O2Na [M + Na]+ calculated m/z 235.0483; found m/z 235.0483.
4.3.36. 1-(1,3-Benzothiazol-2-yl)pyridin-2(1H)-one (26)
The title compound was synthesized according to the general procedure for C–N coupling using 1c and pyridone (10.5 mg) as the heterocycle and stirred for 3 h. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 26 as a white solid in 77% yield (16.8 mg). 1H NMR (400 MHz, CDCl3): δ 6.33 (d, J = 6.79, 1H), 6.72 (d, J = 7.09, 1H), 7.39–7.55 (m, 4H), 7.66 (dd, J = 7.09, 1H), 7.81 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3): δ ppm 6.44–6.51 (m, 1H), 6.80 (d, J = 9.29 Hz, 1H), 7.39–7.55 (m, 2H), 7.95 (dd, J = 16.02, 8.19 Hz, 1H), 8.98 (dd, J = 7.34, 1.71 Hz, 1H). HRMS (ESI) C12H8N2OSNa [M + Na]+ calculated m/z 251.0255; found m/z 251.0264.
4.3.37. 1-(1-Benzothiophen-2-yl)pyridin-2(1H)-one (27)
The title compound was synthesized according to the general procedure for C–N coupling using 1d and pyridinone (10.4 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 27 as a white solid in 48% yield (10.5 mg). 1H NMR (400 MHz, CDCl3): δ 6.33 (td, J = 6.79, 1.34 Hz, 1H), 6.72 (d, J = 9.29 Hz, 1H), 7.36–7.45 (m, 4H), 7.66 (dd, J = 7.09, 1.96 Hz, 1H), 7.77–7.86 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3): δ 106.96, 117.72, 122.09, 122.15, 123.83, 124.75, 125.12, 136.75, 136.79, 138.29, 139.69, 141.18, 161.59. HRMS (ESI) C13H9NOSNa [M + Na]+ calculated m/z 250.0303; found m/z 250.0303.
4.3.38. 1-(5-Phenyl-1,3,4-oxadiazol-2-yl)pyridin-2(1H)-one (28)
The title compound was synthesized according to the general procedure for C–N coupling using 1e and pyridinone (10.5 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 28 as a white solid in 60% yield (13.2 mg). 1H NMR (400 MHz, CDCl3): δ 6.33 (t, J = 6.79, 1.34 Hz, 1H), 6.72 (m, 1H), 7.36–7.45 (m, 5H), 7.77–7.86 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3): δ 107.55, 122.78, 123.44, 127.37, 128.81, 129.44, 130.40, 132.63, 135.69, 141.69, 160.73. HRMS (ESI) C13H9N3O2Na [M + Na]+ calculated m/z 262.0592; found m/z 262.0597.
4.3.39. 1-(1-Phenyl-1H-pyrazol-3-yl)pyridin-2(1H)-one (29)
The title compound was synthesized according to the general procedure for C–N coupling using 1f and pyridinone (10.5 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 29 as a white solid in 65% yield (14.6 mg). 1H NMR (400 MHz, CDCl3): δ 6.29–6.34 (m, 1H), 6.71 (dd, J = 9.19, 0.49 Hz, 1H), 7.18 (d, J = 2.45 Hz, 1H), 7.31–7.36 (m, 1H), 7.40 (ddd, J = 9.11, 6.66, 2.08 Hz, 1H), 7.46–7.52 (m, 2H), 7.71 (dd, J = 7.82 Hz, 2H), 7.98 (d, J = 2.69 Hz, 1H), 8.14 (dd, J = 7.09, 1.96 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3): δ 104.12, 106.39, 118.98, 122.05, 126.85, 127.95, 129.56, 135.42, 139.64, 139.78, 149.30, 161.75. HRMS (ESI) C14H11N3ONa [M + Na]+ calculated m/z 260.0800; found m/z 260.0795.
4.3.40. 1-(2-Phenyl-1,3-thiazol-5-yl)pyridin-2(1H)-one (30)
The title compound was synthesized according to the general procedure for C–N coupling using 1g and pyridinone (10.5 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 30 as a white solid in 41% yield (10.5 mg). 1H NMR (400 MHz, CDCl3): δ 6.38 (td, J = 6.66, 1.10 Hz, 1H), 6.75 (d, J = 9.29 Hz, 1H), 7.41–7.44 (m, 1H), 7.44–7.49 (m, 3H), 7.70 (dd, J = 7.09, 1.96 Hz, 1H), 7.91 (s, 1H), 7.94–7.99 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3): δ 107.52, 121.69, 126.35, 129.07, 130.33, 133.37, 134.94, 135.10, 136.13, 139.73, 160.44, 166.69. HRMS (ESI) C14H10N2OSH [M + H]+ calculated m/z 255.0592; found m/z 255.0592.
4.3.41. 1-(5-Bromo-1-methyl-1H-benzimidazol-2-yl)pyridin-2(1H)-one (31)
The title compound was synthesized according to the general procedure for C–N coupling using 1h and pyridone (11.0 mg) as the heterocycle and stirred for 4 h. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 31 as a white solid in 47% yield (15.0 mg). 1H NMR (400 MHz, DMSO-d6): δ 3.68 (s, 3H), 7.38 (m, 2H), 7.42 ( J = 8.31, 1H), 7.53 (d, J = 8.31, 1H), 7.68 (d, J = 1.71, 1H), 8.02 (m, 1H), 8.29 (dd, J = 1.71, 1H).13C{1H} NMR (101 MHz, CDCl3): δ 29.06, 99.99, 110.13, 113.31, 115.24, 121.59, 121.77, 125.19, 140.17, 140.40, 148.27, 154.33, 160.27. HRMS (ESI) C13H10BrN3ONa [M + Na]+ calculated m/z 327.9886; found m/z 327.9897.
4.3.42. 1-(5-Phenyl-1,3-oxazol-2-yl)pyridin-2(1H)-one (32)
The title compound was synthesized according to the general procedure for C–N coupling using 1i and pyridinone (10.5 mg) as the heterocycle and stirred for 6 h. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 32 as a white solid in 63% yield (15.1 mg). 1H NMR (400 MHz, CDCl3): δ 6.29 (td, J = 6.72, 0.73 Hz, 1H), 6.67 (d, J = 9.29 Hz, 1H), 7.35 (d, J = 9.29, 1H), 7.37–7.46 (m, 4H), 7.58 (dd, J = 6.72, 1.96 Hz, 1H), 7.65–7.70 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3): δ 106.58, 122.09, 122.55, 124.42, 127.08, 128.97, 129.07, 135.58, 140.70, 151.85, 152.64, 161.13 HRMS (ESI) C14H10N2O2Na [M + Na]+ calculated m/z 261.0640; found m/z 261.0647.
4.3.43. 1-(2-Phenyl-1,3-oxazol-5-yl)pyridin-2(1H)-one (33)
The title compound was synthesized according to the general procedure for C–N coupling using 1j and pyridinone (10.5 mg) as the heterocycle. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 33 as a white solid in 49% yield (11.7 mg). 1H NMR (400 MHz, CDCl3): δ 6.35–6.40 (m, 1H), 6.70 (d, J = 9.54 Hz, 1H), 7.39 (ddd, J = 9.23, 6.79, 2.08 Hz, 1H), 7.47–7.50 (m, 3H), 7.73–7.76 (s 1H), 7.83 (dd, J = 7.21, 1.83 Hz, 1H), 8.02–8.06 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3): δ 107.01, 119.37, 121.90, 126.23, 126.63, 128.93, 130.77, 132.52, 139.15, 143.86, 157.10, 159.70. HRMS (ESI) C14H10N2O2Na [M + Na]+ calculated m/z 261.0640; found m/z 261.0642.
4.3.44. 1-(5-Methyl-2-phenyl-1,3-oxazol-4-yl)pyridin-2(1H)-one (34)
The title compound was synthesized according to the general procedure for C–N coupling using 1k. The product was purified by silica gel column chromatography Hep:EtOAc 1:1 to provide 34 as opaque oil in 41% yield (9.0 mg). 1H NMR (400 MHz, CDCl3): δ 2.44 (s, 3H), 6.29–6.34 (m, 1H), 6.69 (d, J = 9.54 Hz, 1H), 7.40–7.50 (m, 4H), 7.57 (dd, J = 6.97, 2.08 Hz, 1H), 7.97–8.03 (m, 2H). 13C{1H} NMR (101 MHz, DMSO-d6): δ 11.16, 106.45, 120.94, 126.06, 126.81, 129.74, 131.30, 134.37, 138.92, 141.61, 143.60, 158.15, 160.79. HRMS (ESI) C15H12N2O2Na [M + Na]+ calculated m/z 275.0797; found m/z 275.0794.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (AG002132) (S.B.P.), as well as by support from the Brockman Foundation (S.B.P.) and the Sherman Fairchild Foundation (S.B.P.).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00458.
Experimental details and compound characterization data (PDF)
Stanley B. Prusiner is a member of the Scientific Advisory Boards of ViewPoint Therapeutics and New Ventures Inc. and a member of the Supervisory Board of Priavoid, none of which have contributed financial or any other support to these studies.
The authors declare no competing financial interest.
Supplementary Material
References
- Zhang H.-Z.; Zhao Z.-L.; Zhou C.-H. Recent Advance in Oxazole-Based Medicinal Chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. 10.1016/j.ejmech.2017.12.044. [DOI] [PubMed] [Google Scholar]
- Wasserman H. H.; Vinick F. J. Mechanism of the Robinson-Gabriel Synthesis of Oxazoles. J. Org. Chem. 1973, 38, 2407–2408. 10.1021/jo00953a028. [DOI] [Google Scholar]
- Robinson R. CCXXXII.—A New Synthesis of Oxazole Derivatives. J. Chem. Soc. Dalton 1909, 95, 2167–2174. 10.1039/CT9099502167. [DOI] [Google Scholar]
- Wipf P.; Miller C. P. A new synthesis of highly functionalized oxazoles. J. Org. Chem. 1993, 58, 3604–3606. 10.1021/jo00066a004. [DOI] [Google Scholar]
- Zhou R.-R.; Cai Q.; Li D.-K.; Zhuang S.-Y.; Wu Y.-D.; Wu A.-X. Acid-Promoted Multicomponent Tandem Cyclization to Synthesize Fully Substituted Oxazoles via Robinson–Gabriel-Type Reaction. J. Org. Chem. 2017, 82, 6450–6456. 10.1021/acs.joc.7b00763. [DOI] [PubMed] [Google Scholar]
- Haas D.; Mosrin M.; Knochel P. Regioselective Functionalization of the Oxazole Scaffold Using TMP-Bases of Mg and Zn. Org. Lett. 2013, 15, 6162–6165. 10.1021/ol403019c. [DOI] [PubMed] [Google Scholar]
- Shibahara F.; Yamauchi T.; Yamaguchi E.; Murai T. One-pot Sequential Direct C–H Bond Arylation of Azoles Catalyzed by [Pd(phen)2](PF6)2: Synthetic Methods for Triarylated Azoles. J. Org. Chem. 2012, 77, 8815–8820. 10.1021/jo301621t. [DOI] [PubMed] [Google Scholar]
- Liégault B.; Petrov I.; Gorelsky S. I.; Fagnou K. Modulating Reactivity and Diverting Selectivity in Palladium-Catalyzed Heteroaromatic Direct Arylation Through the Use of a Chloride Activating/Blocking Group. J. Org. Chem. 2010, 75, 1047–1060. 10.1021/jo902515z. [DOI] [PubMed] [Google Scholar]
- Liégault B.; Lapointe D.; Caron L.; Vlassova A.; Fagnou K. Establishment of Broadly Applicable Reaction Conditions for the Palladium-Catalyzed Direct Arylation of Heteroatom-Containing Aromatic Compounds. J. Org. Chem. 2009, 74, 1826–1834. 10.1021/jo8026565. [DOI] [PubMed] [Google Scholar]
- Strotman N. A.; Chobanian H. R.; Guo Y.; He J.; Wilson J. E. Highly Regioselective Palladium-Catalyzed Direct Arylation of Oxazole at C-2 or C-5 with Aryl Bromides, Chlorides, and Triflates. Org. Lett. 2010, 12, 3578–3581. 10.1021/ol1011778. [DOI] [PubMed] [Google Scholar]
- Besselièvre F.; Lebrequier S.; Mahuteau-Betzer F.; Piguel S. C-H Bond Activation: A Versatile Protocol for the Direct Arylation and Alkenylation of Oxazoles. Synthesis 2009, 2009, 3511–3518. 10.1055/s-0029-1216987. [DOI] [Google Scholar]
- Ferrer Flegeau E.; Popkin M. E.; Greaney M. F. Suzuki Coupling of Oxazoles. Org. Lett. 2006, 8, 2495–2498. 10.1021/ol060591j. [DOI] [PubMed] [Google Scholar]
- Solomin V. V.; Radchenko D. S.; Slobodyanyuk E. Y.; Geraschenko O. V.; Vashchenko B. V.; Grygorenko O. O. Widely Exploited, Yet Unreported: Regiocontrolled Synthesis and the Suzuki–Miyaura Reactions of Bromooxazole Building Blocks. Eur. J. Org. Chem. 2019, 2019, 2884–2898. 10.1002/ejoc.201900032. [DOI] [Google Scholar]
- Strotman N. A.; Chobanian H. R.; He J.; Guo Y.; Dormer P. G.; Jones C. M.; Steves J. E. Catalyst-Controlled Regioselective Suzuki Couplings at Both Positions of Dihaloimidazoles, Dihalooxazoles, and Dihalothiazoles. J. Org. Chem. 2010, 75, 1733–1739. 10.1021/jo100148x. [DOI] [PubMed] [Google Scholar]
- Geier M. J.; Wang X.; Humphreys L. D.; Calimsiz S.; Scott M. E. Warming Up to Oxazole: Noncryogenic Oxazole Metalation and Negishi Coupling Development. Synlett 2019, 30, 1776–1781. 10.1055/s-0037-1611909. [DOI] [Google Scholar]
- Muto K.; Yamaguchi J.; Musaev D. G.; Itami K. Decarbonylative Organoboron Cross-Coupling of Esters by Nickel Catalysis. Nat. Commun. 2015, 6, 7508. 10.1038/ncomms8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. F.; Richards S.; Barañano D.; Paul F. Influences on the Relative Rates for C–N Bond-Forming Reductive Elimination and β-Hydrogen Elimination of Amides. A Case Study on the Origins of Competing Reduction in the Palladium-Catalyzed Amination of Aryl Halides. J. Am. Chem. Soc. 1996, 118, 3626–3633. 10.1021/ja954121o. [DOI] [Google Scholar]
- Zeinali N.; Oluwoye I.; Altarawneh M.; Dlugogorski B. Z. Kinetics of Photo-Oxidation of Oxazole and its Substituents by Singlet Oxygen. Sci. Rep. 2020, 10, 3668. 10.1038/s41598-020-59889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather A. C.; Martinot T. A. Data-Rich Experimentation Enables Palladium-Catalyzed Couplings of Piperidines and Five-Membered (Hetero)aromatic Electrophiles. Org. Process Res. Dev. 2019, 23, 1725–1739. 10.1021/acs.oprd.9b00233. [DOI] [Google Scholar]
- Forero-Cortés P. A.; Haydl A. M. The 25th Anniversary of the Buchwald–Hartwig Amination: Development, Applications, and Outlook. Org. Process Res. Dev. 2019, 23, 1478–1483. 10.1021/acs.oprd.9b00161. [DOI] [Google Scholar]
- Ruiz-Castillo P.; Buchwald S. L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antilla J. C.; Baskin J. M.; Barder T. E.; Buchwald S. L. Copper–Diamine-Catalyzed N-Arylation of Pyrroles, Pyrazoles, Indazoles, Imidazoles, and Triazoles. J. Org. Chem. 2004, 69, 5578–5587. 10.1021/jo049658b. [DOI] [PubMed] [Google Scholar]
- Yin J.; Buchwald S. L. Palladium-Catalyzed Intermolecular Coupling of Aryl Halides and Amides. Org. Lett. 2000, 2, 1101–1104. 10.1021/ol005654r. [DOI] [PubMed] [Google Scholar]
- Surry D. S.; Buchwald S. L. Diamine Ligands in Copper-Catalyzed Reactions. Chem. Sci. 2010, 1, 13–31. 10.1039/c0sc00107d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapars A.; Antilla J. C.; Huang X.; Buchwald S. L. A General and Efficient Copper Catalyst for the Amidation of Aryl Halides and the N-Arylation of Nitrogen Heterocycles. J. Am. Chem. Soc. 2001, 123, 7727–7729. 10.1021/ja016226z. [DOI] [PubMed] [Google Scholar]
- Monnier F.; Taillefer M. Catalytic C-C, C-N, and C-O Ullmann-Type Coupling Reactions. Angew. Chem., Int. Ed. 2009, 48, 6954–6971. 10.1002/anie.200804497. [DOI] [PubMed] [Google Scholar]
- Altman R. A.; Buchwald S. L. Cu-Catalyzed N- and O-Arylation of 2-, 3-, and 4-Hydroxypyridines and Hydroxyquinolines. Org. Lett. 2007, 9, 643–646. 10.1021/ol062904g. [DOI] [PubMed] [Google Scholar]
- Altman R. A.; Buchwald S. L. 4,7-Dimethoxy-1,10-phenanthroline: An Excellent Ligand for the Cu-Catalyzed N-Arylation of Imidazoles. Org. Lett. 2006, 8, 2779–2782. 10.1021/ol0608505. [DOI] [PubMed] [Google Scholar]
- Campeau L.-C.; Parisien M.; Jean A.; Fagnou K. Catalytic Direct Arylation with Aryl Chlorides, Bromides, and Iodides: Intramolecular Studies Leading to New Intermolecular Reactions. J. Am. Chem. Soc. 2006, 128, 581–590. 10.1021/ja055819x. [DOI] [PubMed] [Google Scholar]
- Khambhati D. P.; Sachinthani K. A. N.; Rheingold A. L.; Nelson T. L. Regioselective Copper-Catalyzed Direct Arylation of Benzodithiophene-S, S-Tetraoxide. Chem. Commun. 2017, 53, 5107–5109. 10.1039/C7CC01781B. [DOI] [PubMed] [Google Scholar]
- Ajitha M. J.; Pary F.; Nelson T. L.; Musaev D. G. Unveiling the Role of Base and Additive in the Ullmann-Type of Arene-Aryl C–C Coupling Reaction. ACS Catal. 2018, 8, 4829–4837. 10.1021/acscatal.8b00837. [DOI] [Google Scholar]
- Strieter E. R.; Blackmond D. G.; Buchwald S. L. The Role of Chelating Diamine Ligands in the Goldberg Reaction: A Kinetic Study on the Copper-Catalyzed Amidation of Aryl Iodides. J. Am. Chem. Soc. 2005, 127, 4120–4121. 10.1021/ja050120c. [DOI] [PubMed] [Google Scholar]
- Flegeau E. F.; Popkin M. E.; Greaney M. F. Direct Arylation of Oxazoles at C2. A Concise Approach to Consecutively Linked Oxazoles. Org. Lett. 2008, 10, 2717–2720. 10.1021/ol800869g. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Ding F.; Li J.; Lu K.; Lu X.; Wang B.; Yu P. Direct C–H Iodination of 1,3-Azoles Catalysed by CuBr2. Tetrahedron Lett. 2015, 56, 511–513. 10.1016/j.tetlet.2014.12.029. [DOI] [Google Scholar]
- Zhang C.-P.; Wang Z.-L.; Chen Q.-Y.; Zhang C.-T.; Gu Y.-C.; Xiao J.-C. Copper-Mediated Trifluoromethylation of Heteroaromatic Compounds by Trifluoromethyl Sulfonium Salts. Angew. Chem., Int. Ed. 2011, 50, 1896–1900. 10.1002/anie.201006823. [DOI] [PubMed] [Google Scholar]
- Do H.-Q.; Daugulis O. A General Method for Copper-Catalyzed Arene Cross-Dimerization. J. Am. Chem. Soc. 2011, 133, 13577–13586. 10.1021/ja2047717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich S. H.; Knochel P. (tmp)2Zn·2 MgCl2·2 LiCl: A Chemoselective Base for the Directed Zincation of Sensitive Arenes and Heteroarenes. Angew. Chem., Int. Ed. 2007, 46, 7685–7688. 10.1002/anie.200701984. [DOI] [PubMed] [Google Scholar]
- Ferrer Flegeau E.; Popkin M. E.; Greaney M. F. Regioselective Palladium Cross-Coupling of 2,4-Dihalooxazoles: Convergent Synthesis of Trisoxazoles. J. Org. Chem. 2008, 73, 3303–3306. 10.1021/jo800121y. [DOI] [PubMed] [Google Scholar]
- Senadi G. C.; Hu W.-P.; Hsiao J.-S.; Vandavasi J. K.; Chen C.-Y.; Wang J.-J. Facile, Selective, and Regiocontrolled Synthesis of Oxazolines and Oxazoles Mediated by ZnI2 and FeCl3. Org. Lett. 2012, 14, 4478–4481. 10.1021/ol301980g. [DOI] [PubMed] [Google Scholar]
- Kirsch P.; Jakob V.; Oberhausen K.; Stein S. C.; Cucarro I.; Schulz T. F.; Empting M. Fragment-Based Discovery of a Qualified Hit Targeting the Latency-Associated Nuclear Antigen of the Oncogenic Kaposi’s Sarcoma-Associated Herpesvirus/Human Herpesvirus 8. J. Med. Chem. 2019, 62, 3924–3939. 10.1021/acs.jmedchem.8b01827. [DOI] [PubMed] [Google Scholar]
- Shatila R. S.; Bouhadir K. H. Two Simple Protocols for the Preparation of Diallylaminoethyl-Substituted Nucleic Bases: A Comparison. Tetrahedron Lett. 2006, 47, 1767–1770. 10.1016/j.tetlet.2006.01.035. [DOI] [Google Scholar]
- Nagaradja E.; Chevallier F.; Roisnel T.; Dorcet V.; Halauko Y. S.; Ivashkevich O. A.; Matulis V. E.; Mongin F. Deproto-Metallation Using a Mixed Lithium–Zinc Base and Computed CH Acidity of 1-Aryl 1H-Benzotriazoles and 1-Aryl 1H-Indazoles. Org. Biomol. Chem. 2014, 12, 1475–1487. 10.1039/c3ob42380h. [DOI] [PubMed] [Google Scholar]
- Damkaci F.; Alawaed A.; Vik E. N-Picolinamides as Ligands for Ullmann-Type C–N Coupling Reactions. Tetrahedron Lett. 2016, 57, 2197–2200. 10.1016/j.tetlet.2016.04.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.