Abstract
As a common inorganic silicon material, nano-SiO2 is extremely hydrophilic due to the presence of a large number of hydroxyl groups on its surface, which limits its application in some fields. In this research, hexadecyltrimethoxysilane (HDTMS) was used to modify nano-SiO2, and the results of water contact angle (WCA), Fourier transform infrared (FTIR), two-dimensional correlation spectroscopy (2D-COS), thermogravimetric (TG) analysis, and scanning electron microscopy (SEM) indicated that the hydrophobic long-chain alkyl of HDTMS was successfully grafted onto the surface of nano-SiO2. When the ratio of nano-SiO2 and HDTMS was 0.25:1, the WCA of nano-SiO2 modified with HDTMS (HDTMS-nano-SiO2) reached 170.9°, which was about 5.62 times higher than that before modification, and the superhydrophobic property was obtained. The novelty of this work lies in the modified nano-SiO2 with a WCA of over 170° and the analysis of the modification mechanism with the help of 2D-COS. This study can provide a reference for the hydrophobic modification of nano-SiO2 and its application field expansion.
1. Introduction
Nano-SiO2 is nontoxic and odorless with large specific surface area and light weight. Because of the excellent barrier, mechanical, optical, and flame retardant properties, it has attracted great attention in the fields of ceramics, paper making, plastics, metals, and photovoltaics.1−6 For instance, it can be added to polymer cells as a filler to slow down the battery’s capacity decay.7 Yu et al. prepared polylactic acid/thermoplastic polyurethane/ hydrophobic nano-SiO2 composites and improved the hydrophobic and mechanical properties simultaneously.8 Kumar et al. modified nano-SiO2 with polymethylmethacrylate to obtain both hydrophobic and corrosion-resistant coatings.9 Elkalla et al. synthesized polystyrene/hydrophobic nano-SiO2 composite particles by oil-in-water Pickering emulsion polymerization.10
It can be seen that the hydrophobic property is one of the important properties of nano-SiO2 being studied and applied. Nano-SiO2 is hydrophilic due to its large number of hydroxyl groups on the surface, so it is necessary to conduct hydrophobic modification research on it so as to expand its application field and deepen the research on the material surface.11 The key to preparing a superhydrophobic coating on the substrate surface is to construct the rough structure of binary micro-nano or modify it with low-surface-energy substances to reduce its surface energy.12−15 As a typical low-surface-energy substance, fluorocarbons have been commonly investigated in hydrophobic modification of such materials.16−18 Dou et al. modified nano-SiO2 with 1H,1H,2H,2H-perfluorodecyltrichoxysilane (PFDS), and the WCA reached 130.6°.19 Lin et al. successfully prepared a hydrophobic silica gel film with porosity over 90% by the sol–gel method using the hydrophobic fluorocarbon functional group–CF3 as a modified group, which can be used to absorb CO2.20 Nevertheless, fluorinated compounds are expensive and may accumulate and become toxic in organisms and the environment.21,22 Therefore, some hydrophobically modified substances without fluorine have caused extensive concern, most of which work by the reaction between the hydroxyl groups on the surface of nanoparticles and the hydrophobic groups of the modified substances.23−27 The silane coupling agent is one of the most commonly used modified substances with special properties, such as hydrolyzable chemical groups and organic functional groups. Its silanoxy groups are consequently reactive to inorganic substances, and its organic functional groups are reactive or compatible with organic substances.28−30 Kapridaki et al. prepared a titanium–SiO2–polydimethylsiloxane nanocomposite hydrophobic coating, which had excellent self-cleaning properties and could be used for cultural relic protection.31 Li et al. obtained a hydrophobic paper with a static contact angle of 163° and a rolling angle of 3° by spraying nano-SiO2 with octadecyltrichlorosilane (OTS).32 Tsuru et al. modified nano-SiO2 with methyltriethoxysilane (MTES) to obtain hydrophobic nano-SiO2 with a WCA of over 150°.33
Hexadecyltrimethoxysilane (HDTMS) is also a silane coupling agent, which has been frequently used in the hydrophobic modification of nano-SiO2. Xu et al. prepared HDTMS-modified nano-SiO2 via the sol–gel reaction under alkaline conditions, which was coated onto the cotton fabric sample. Then, the sample was endowed with hydrophobicity, the WCA of which was 152.1°.34 Sohrabi et al. modified nano-SiO2 with HDTMS and deposited it on glass. As a result, the glass had a WCA of 139.5° with 0.1% of the modified nano-SiO2.35 Xiong et al. synthesized a kind of functional nano-SiO2 with 2,4-dihydroxybenzophenone and HDTMS and then introduced it onto the surface of cotton fabric. The results indicated that the modified cotton fabric not only showed strong UV-resistant ability but also obtained the hydrophobic property with a WCA of 153°. Moreover, the modified cotton fabric could keep these properties after being abraded 300 times or laundered 20 times.36 Therefore, it can be seen that HDTMS has been successfully applied as a kind of hydrophobic modifier, which can make nano-SiO2 more hydrophobic. Besides, because of being widely used for cotton fabric, HDTMS-modified hydrophobic nano-SiO2 probably has potential in other materials and fields.
To study this subject, in this paper, we propose to utilize HDTMS as the modifier to modify the surface of nano-SiO2 and to explore the influence of different proportions of reactants on the hydrophobic property of nano-SiO2. WCA, FTIR, 2D-COS, TG, and SEM analysis were used to analyze the results. As a result, superhydrophobic HDTMS-nano-SiO2 was prepared successfully, the WCA of which reached 170.9°. In addition, the chemical mechanism of modification in this study was revealed. To our knowledge, hydrophobic nano-SiO2 modified by the silane coupling agent with a WCA of over 170° has not been reported. Moreover, the temperature sensitivity of chemical functional groups and the sequence of chemical changes were investigated with the help of 2D-COS, which has not been widely used in this field. The results of this study can provide a reference for the hydrophobic modification of nano-SiO2.
2. Results and Discussion
2.1. Hydrophobicity Analysis
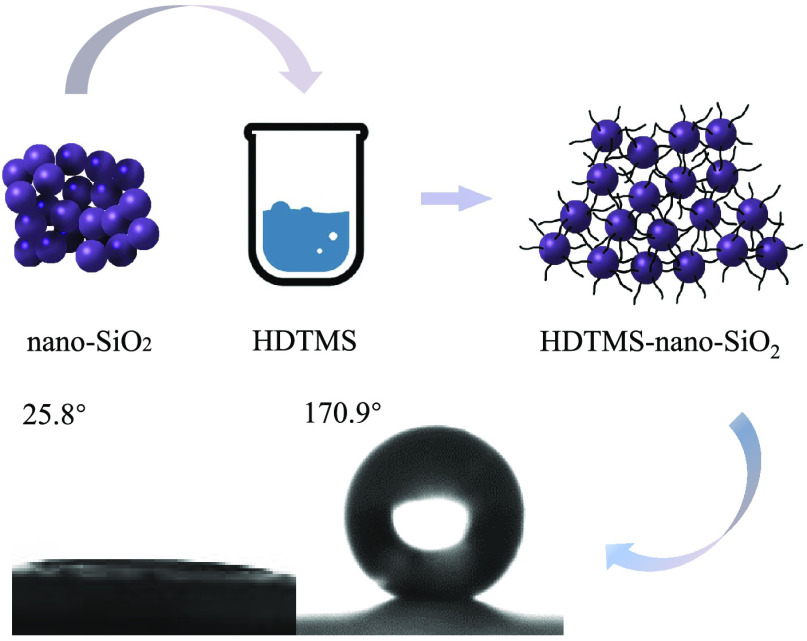
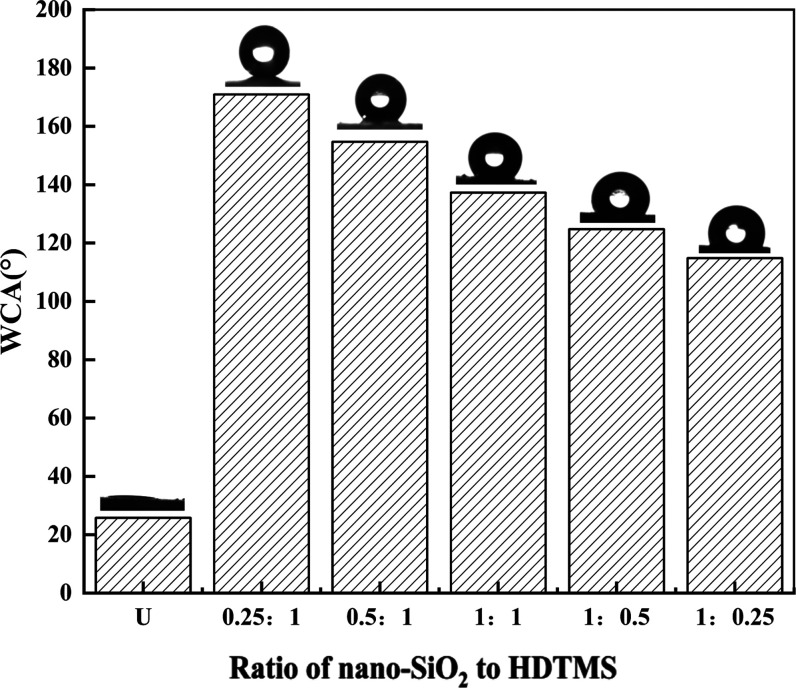
The test results of the WCA value of HDTMS-nano-SiO2 obtained by the reaction of different proportions of nano-SiO2 and HDTMS are shown in Figure 1. Unmodified nano-SiO2 was so hydrophilic that water drops spread out quickly when hitting its surface, with a WCA of only 25.8°. When the ratios of nano-SiO2 to HDTMS were 0.25:1, 0.5:1, 1:1, 1:0.5, and 1:0.25, the WCA values of the modified nano-SiO2 were 170.9, 154.7, 137.3, 124.8, and 114.8°, respectively. This indicates that the hydrophobicity of nano-SiO2 modified by HDTMS was improved, and the hydrophobicity achieved the best effect when the ratio of them was 0.25:1. Compared with nano-SiO2 before modification, the WCA increased by about 5.62 times.
Figure 1.
WCA values of unmodified nano-SiO2 (U) and HDTMS-nano-SiO2 when the ratios of nano-SiO2 to HDTMS were 0.25:1, 0.5:1, 1:1, 1:0.5, and 1:0.25.
2.2. FTIR Analysis
The change of functional groups of nano-SiO2 before and after modification was analyzed by FTIR, and the results are shown in Figure 2. HDTMS, nano-SiO2, and HDTMS-nano-SiO2 all had absorption peaks at 1100 and 800 cm–1, which were the antisymmetric and symmetric contraction vibration peaks of the Si–O–Si bond, respectively.37,38 At about 3440 and 950 cm–1, both HDTMS-nano-SiO2 and nano-SiO2 had absorption peaks. These peaks were assigned to the stretching vibration peaks of silicon hydroxyl groups on the surface of nano-SiO2 particles,39 and the absorption peaks of HDTMS-nano-SiO2 in these two places were slightly weaker than that of nano-SiO2, which proved that the surface of HDTMS-nano-SiO2 had been successfully modified and the hydroxyl groups were relatively reduced. Compared with the infrared spectra of the unmodified nano-SiO2, three new absorption peaks representing the stretching vibrations of −CH3, −CH2, and the C–O bond, respectively, appeared at about 2925, 2854, and 1467 cm–1, which indicated that the long-chain hydrophobic alkyl groups of HDTMS had been successfully grafted onto the surface of nano-SiO2.11,40
Figure 2.
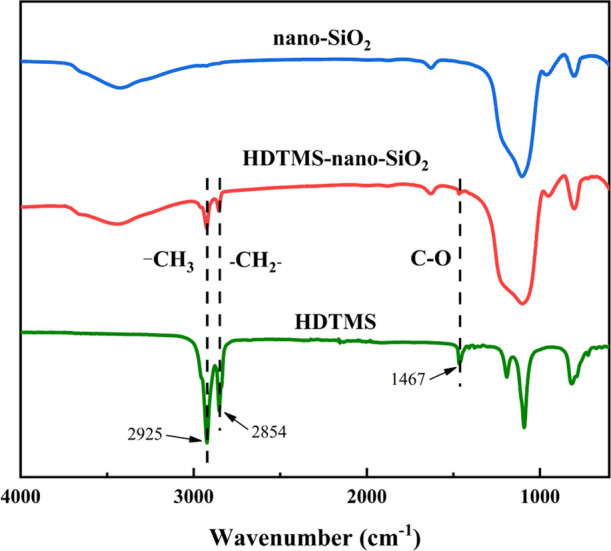
FTIR spectra of HDTMS, nano-SiO2, and HDTMS-nano-SiO2.
2.3. 2D-COS Analysis
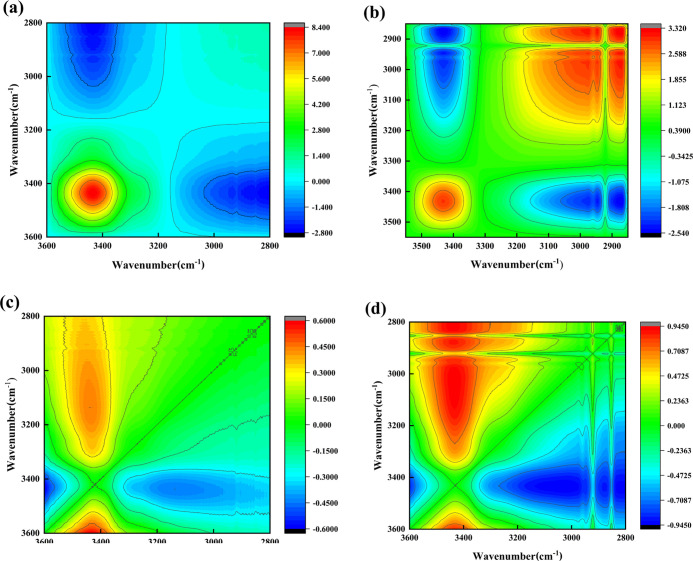
2D-COS results are depicted in Figure 3. The synchronous graph of 2D-COS was used to indicate the degree of coordination between the two spectral intensity changes. The value of the automatic peak located on the diagonal is always positive.41−46 In the synchronous figure of HDTMS-nano-SiO2, three automatic peaks appeared at about 2925, 2854, and 3440 cm–1, which represented −CH3, −CH2–, and hydroxyl groups, respectively, on the surface of nano-SiO2. In the synchronous graph of this band, there was only an automatic peak at about 3440 cm–1, proving that the surface hydroxyl groups of nano-SiO2 were sensitive to temperature perturbation to a certain extent before and after modification. Besides, the existence of diagonal cross peaks on both sides shows that functional groups may exist in intramolecular or intermolecular interactions. The parsing rules are that two variables under perturbation of changes in the spectral peaks are positively related if the value is positive. On the contrary, these two variables under perturbation of changes in the spectral peaks are negatively related with a negative value.41−46
Figure 3.
Synchronous graphs of nano-SiO2 (a) and HDTMS-nano-SiO2 (b) and the asynchronous graphs of nano-SiO2 (c) and HDTMS-nano-SiO2 (d).
The asynchronous graph represents the degree of difference in the intensity changes of the two spectra. There are no automatic peaks and only cross peaks with positive or negative values in an asynchronous graph, which is antisymmetric about the diagonal. According to the rules of Noda, when synchronous correlation peak intensity Φ(ν1,ν2) and asynchronous correlation peak intensity Ψ(ν1,ν2) have the same sign, the change of the correlation peak of ν1 is earlier than that of ν2. Inversely, when synchronous correlation peak intensity Φ(ν1,ν2) and asynchronous correlation peak intensity Ψ(ν1,ν2) have different signs, the change of the correlation peak of ν1 is posterior to that of ν2. Therefore, the order of change of these groups with temperature perturbation can be acquired by combining their synchronous and asynchronous graphs.41−46 It could be found that the cross peaks (2925, 3854) cm–1 of HDTMS-nano-SiO2 had the same sign in synchronous and asynchronous graphs, hence the change of −CH3 at 2925 cm–1 was prior to that of −CH2– at 2854 cm–1. Because of the different signs in the synchronous and asynchronous graphs of the cross peaks (3440, 2854) cm–1, so the change of the hydroxyl groups at 3440 cm–1 is later than the change of −CH2– at 2854 cm–1. Overall, the sequence of changes of these three groups was −CH3, −CH2–, and hydroxyl groups. Combined with the results of FTIR analysis, it could be deduced that the reaction mechanism of hydrophobic modification was that HDTMS directly reacted with the hydroxyl groups of nano-SiO2, thus grafting its hydrophobic long-chain alkyl group onto the surface of nano-SiO2.47 The reaction process and the interaction of HDTMS and nano-SiO2 are presented in Figure 4.
Figure 4.
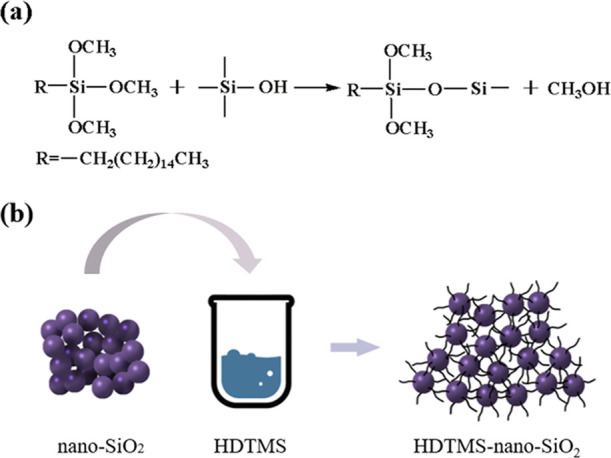
Reaction process (a) and the interaction of HDTMS and nano-SiO2 (b).
2.4. TG Analysis
As exhibited in Figure 5, in the range of 20–150 °C, both nano-SiO2 and HDTMS-nano-SiO2 had a small amount of mass loss, and the mass loss of nano-SiO2 was more than that of HDTMS-nano-SiO2. Combined with FTIR and 2D-COS analysis, it could be inferred that the main component of mass loss was the hydroxyl groups on the surface of nano-SiO2. With the increase of temperature, the mass loss of nano-SiO2 was very slow, and the final mass remained about 95%. However, HDTMS-nano-SiO2 was decomposed for the second time in the range of 150–500 °C, resulting in a large mass loss of the sample with a final remaining mass of about 63%, which was mainly caused by the decomposition of long-chain alkyl on its surface. This also indicated that −CH2(CH2)14CH3 was efficaciously grafted onto the surface of HDTMS-nano-SiO2.
Figure 5.
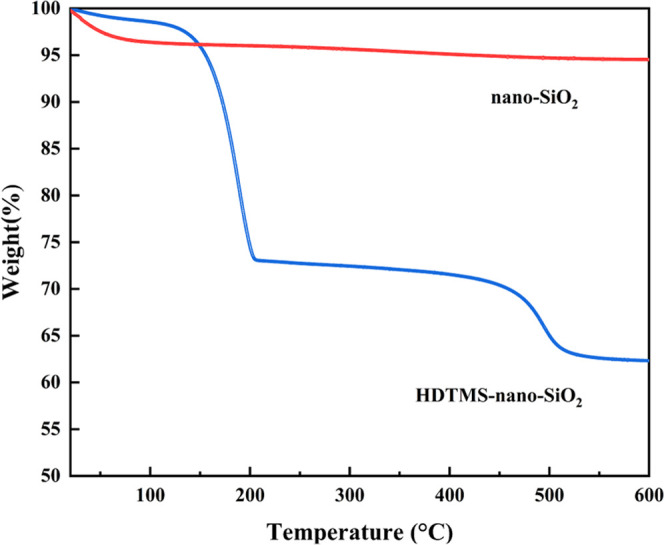
TG analysis of nano-SiO2 and HDTMS-nano-SiO2.
2.5. SEM Analysis
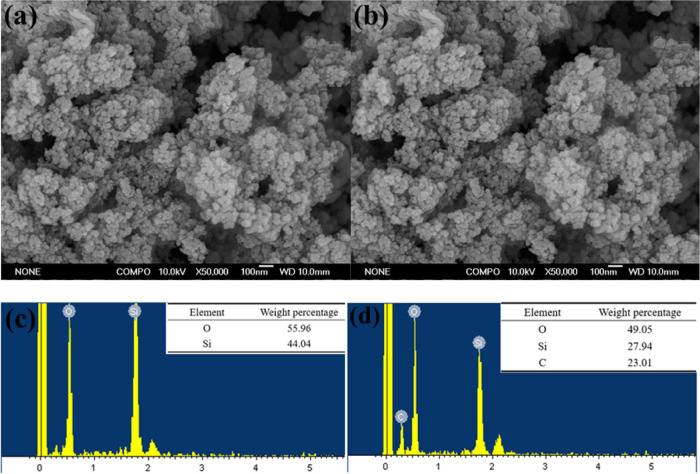
The SEM images are shown in Figure 6a,b. It could be found that the particle size of nano-SiO2 before and after modification was relatively uniform, which was about tens of nanometers. The agglomeration phenomenon appeared, but the agglomeration degree of HDTMS-nano-SiO2 was slightly lower than that of nano-SiO2 because of the reduction of hydroxyl groups on the surface after modification. In the process of practical application, it can be dispersed in the corresponding solvent by means of mechanical stirring and ultrasonic wave. In addition, the chemical element composition of nano-SiO2 and HDTMS-nano-SiO2 were measured, which is demonstrated in Figure 6c,d. According to the comparative analysis of the data in the figures, it is apparent that the elemental composition of nano-SiO2 was only Si and O. After modification, a certain proportion of the C element was added to the elemental composition of HDTMS-nano-SiO2, which confirmed the modification of nano-SiO2. The presence of −CH2– and −CH3 led to the appearance of the C element.
Figure 6.
SEM images of nano-SiO2 (a) and HDTMS-nano-SiO2 (b). EDS images of nano-SiO2 (c) and HDTMS-nano-SiO2 (d).
3. Conclusions
In this study, nano-SiO2 with a ratio of 0.25:1 to HDTMS was utilized to modify nano-SiO2. Consequently, hydrophobic HDTMS-nano-SiO2 was obtained, the WCA of which reached 170.9°. Through FTIR and 2D-COS analyses, it was verified that nano-SiO2 had been successfully modified and that the sequence of the reaction of functional groups in the modification reaction was −CH3, −CH2–, and hydroxyl groups. The results of TG analysis showed that HDTMS-nano-SiO2 was weightless in a temperature range of 150–500 °C due to the presence of long-chain alkyl. By observing the SEM images, it could be found that HDTMS-nano-SiO2 agglomerated less because of the reduction of the hydroxyl groups. EDS elemental analysis also further confirmed the existence of the C element in HDTMS-nano-SiO2. This research can provide a reference for the research for the hydrophobic modification of nano-SiO2.
4. Experimental Section
4.1. Reagents
Nano-SiO2 with an average particle size of tens of nanometers and HDTMS (≥85%) were purchased from McLean Biochemical Technology Co., Ltd., (Shanghai, China). Anhydrous ethanol (≥99.7%) was purchased from Modern Oriental Science and Technology Development Co., Ltd., (Beijing, China). All of the above reagents were analytically pure and had not been further purified before use.
4.2. Preparation of HDTMS-Nano-SiO2
When the ratio of nano-SiO2 and HDTMS was 1.4, HDTMS-nano-SiO2 could be superhydrophobic. After being blended with organic silane, it could be made into composite coatings. These coatings showed hydrophobicity on paper, glass, metals, and other materials, with all of the WCA values reached 150°.11 According to the previous research, nano-SiO2 and HDTMS with different mass ratios of 0.25:1, 0.5:1, 1:1, 1:0.5, and 1:0.25 were prepared. They were dispersed in 300 mL of anhydrous ethanol and stirred for 1 h in a thermostatic water bath at 90 °C, separately. Then, the obtained products were cleaned with deionized water and filtered, and the solid part was dried in a vacuum drying oven at 60 °C for 24 h. Finally, HDTMS-nano-SiO2 was obtained.
4.3. Characterization
WCA: Static water contact angles before and after modification of nano-SiO2 were measured by a contact angle analyzer (OCA-20, Germany). Deionized water (2 μL) was used as the test fluid. Five parts of each sample were measured and the mean value was calculated to characterize and analyze the change in its hydrophobic performance.
FTIR: FTIR (Spectrum GX) was carried out using the potassium bromide tablet method. The spectral range was 400–4000 cm–1, and each sample was scanned 32 times with a resolution of 4 cm–1. The sample powder was mixed with the potassium bromide powder in a ratio of 1:100, then the sampler–powder mixture was ground with a mortar and pestle to ensure uniformity, and the tablet was pressed at a pressure of 10 Mpa.
2D-COS: The samples were loaded into the variable temperature accessories and heated at a heating rate of 2 °C/min. The infrared spectra were collected every 10 °C with a temperature range of 50–120 °C. The 2D analysis software 2DShige developed by Shigeaki Morita was used for data analysis and the 2D infrared spectra were obtained.45−47
TG: TG analysis was conducted by a thermogravimetric analyzer (TA Q50) in the atmosphere of nitrogen, which was meant to test and investigate the thermal stability of nano-SiO2 before and after modification. The temperature range was 20–600 °C, and the heating rate was 10 °C/min in the process.
SEM: SEM (JSM-6700F, Japan) was utilized to characterize and analyze the microscopic morphology of nano-SiO2 before and after modification, and the samples were pasted with a conductive adhesive and sprayed with gold for observation. In addition, the elements of the sample were tested quantitatively by instrument-prepared energy-dispersive X-ray spectroscopy (EDS).
Acknowledgments
The authors gratefully acknowledge MOE Key Laboratory of Wooden Material Science and Application, Beijing Forestry University, China.
The authors declare no competing financial interest.
References
- Hsu C. C.; Lan W. L.; Chen N. P.; Wu C. C. The Hydrophobic and Omnidirectional Antireflection Coating of SiO2 Nanospheres with C18-TEOS. Opt. Laser Technol. 2014, 58, 202–206. 10.1016/j.optlastec.2013.11.019. [DOI] [Google Scholar]
- Singh P.; Saroj A. L. Effect of SiO2 Nano-particles on Plasticized Polymer Blend Electrolytes: Vibrational, Thermal, and Ionic Conductivity Study. Polym.-Plast. Technol. Mater. 2021, 60, 298–305. 10.1080/25740881.2020.1793202. [DOI] [Google Scholar]
- Valluri S. K.; Schoenitz M.; Dreizin E. Preparation and Characterization of Silicon-Metal Fluoride Reactive Composites. Nanomaterials 2020, 10, 2367 10.3390/nano10122367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukmanikrishnan B.; Jo C. H.; Choi S. J.; Ramalingam S.; Lee J. Flexible Ternary Combination of Gellan Gum, Sodium Carboxymethyl Cellulose, and Silicon Dioxide Nanocomposites Fabricated by Quaternary Ammonium Silane: Rheological, Thermal, and Antimicrobial Properties. ACS Omega 2020, 5, 28767–28775. 10.1021/acsomega.0c04087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H. Z.; Zheng L. W. Nanocomposite Films Based on Cellulose Reinforced with Nano-SiO2: Microstructure, Hydrophilicity, Thermal Stability, and Mechanical Properties. Cellulose 2013, 20, 1737–1746. 10.1007/s10570-013-9941-3. [DOI] [Google Scholar]
- Huang Q. B.; Xu M. M.; Sun R. C.; Wang X. H. Large Scale Preparation of Graphene Oxide/Cellulose Paper with Improved Mechanical Performance and Gas Barrier Properties by Conventional Papermaking Method. Ind. Crops Prod. 2016, 85, 198–203. 10.1016/j.indcrop.2016.03.006. [DOI] [Google Scholar]
- Jiang G. X.; Maeda S.; Yang H. B.; Saito Y.; Tanase S.; Sakai T. All Solid-state Lithium-polymer Battery Using Poly (Urethane Acrylate)/Nano-SiO2 Composite Electrolytes. J. Power Sources 2005, 141, 143–148. 10.1016/j.jpowsour.2004.09.004. [DOI] [Google Scholar]
- Yu F.; Huang H. X. Simultaneously Toughening and Reinforcing Poly (Lactic Acid)/Thermoplastic Polyurethane Blend via Enhancing Interfacial Adhesion by Hydrophobic Silica Nanoparticles. Polym. Test. 2015, 45, 107–113. 10.1016/j.polymertesting.2015.06.001. [DOI] [Google Scholar]
- Madhan Kumar A.; Latthe S. S.; Sudhagar P.; Obot I. B.; Gasem Z. M. In-situ Synthesis of Hydrophobic SiO2-PMMA Composite for Surface Protective Coatings: Experimental and Quantum Chemical Analysis. Polymer 2015, 77, 79–86. 10.1016/j.polymer.2015.09.030. [DOI] [Google Scholar]
- Elkalla E.; Kaewsaneha C.; Elaissari A. Synthesis of Polystyrene/Hydrophobic SiO2 Composite Particles via Oil-in-Water Pickering Emulsion Polymerization. Polym. Eng. Sci. 2019, 59, E195–E199. 10.1002/pen.24906. [DOI] [Google Scholar]
- Xu P. P.; Huang Y. W.; Wang B. Preparation and Performance of Fluorine-free Superhydrophobic Coatings Based on Modified Silica and Polysiloxane. Fine Chem. 2019, 36, 2009–2015. [Google Scholar]
- Miyauchi Y.; Ding B.; Shiratori S. Fabrication of a Silver-ragwort-leaf-like Super-hydrophobic Micro/nanoporous Fibrous Mat Surface by Electrospinning. Nanotechnology 2006, 17, 5151–5156. 10.1088/0957-4484/17/20/019. [DOI] [Google Scholar]
- Sun T.; Feng L.; Gao X.; Jiang L. Bioinspired Surfaces with Special Wettability. Acc. Chem. Res. 2005, 38, 644–652. 10.1021/ar040224c. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Zhang X.; Tian C.; Gao Z. Analysis of Wetting Characteristics on Microstructured Hydrophobic Surfaces for the Passive Containment Cooling System. Sci. Technol. Nucl. Install. 2015, 2015, 365–370. 10.1155/2015/652731. [DOI] [Google Scholar]
- Aljallis E.; Sarshar M. A.; Datla R.; Sikka V.; Jones A.; Choi C. Experimental Study of Skin Friction Drag Reduction on Superhydrophobic Flat Plates in High Reynolds Number Boundary Layer Flow. Phys. Fluids 2013, 25, 025103 10.1063/1.4791602. [DOI] [Google Scholar]
- Chen K.; Zhou S.; Yang S.; Wu L. Fabrication of All-water-based Self-repairing Superhydrophobic Coatings Based on UV-responsive Microcapsules. Adv. Funct. Mater. 2015, 25, 1035–1041. 10.1002/adfm.201403496. [DOI] [Google Scholar]
- Zhao J.; Li Z. J.; Zhang M.; Meng A. Super-hydrophobic Surfaces of SiO2-coated SiC Nanowires: Fabrication, Mechanism and Ultraviolet-durable Super-hydrophobicity. J. Colloid Interface Sci. 2015, 444, 33–37. 10.1016/j.jcis.2014.12.057. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Wang H.; Niu H.; Gestos A.; Lin T. Robust. Self-healing Superamphiphobic Fabrics Prepared by Two-step Coating of Fluoro-Containing Polymer, Fluoroalkyl Silane, and Modified Silica Nanoparticles. Adv. Funct. Mater. 2013, 23, 1664–1670. 10.1002/adfm.201202030. [DOI] [Google Scholar]
- Dou W. W.; Wang P.; Zhang D.; Yu J. Q. An Efficient Way to Prepare Hydrophobic Antireflective SiO2 Film by Sol-gel Method. Mater. Lett. 2016, 167, 69–72. 10.1016/j.matlet.2015.12.146. [DOI] [Google Scholar]
- Lin Y. F.; Chen C. H.; Tung K. L.; Wei T. Y.; Lu S. Y.; Chang K. S. Mesoporous Fluorocarbon-modified Silica Aerogel Membranes Enabling Long-term Continuous CO2 Capture with Large Absorption Flux Enhancements. ChemSusChem 2013, 6, 437–442. 10.1002/cssc.201200837. [DOI] [PubMed] [Google Scholar]
- Darmanin T.; Guittard F. Superoleophobic Surfaces with Short Fluorinated Chains?. Soft Matter 2013, 9, 5982–5990. 10.1039/c3sm50643f. [DOI] [Google Scholar]
- Prevedouros K.; Cousins I. T.; Buck R. C.; Korzeniowski S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Zhang X. X.; Cai S.; You D.; Yan L. H.; Lv H. B.; Yuan X. D.; Jiang B. Template-Free Sol-gel Preparation of Superhydrophobic Ormosil Films for Double-wavelength Broadband Antireflective Coatings. Adv. Funct. Mater. 2013, 23, 4361–4365. 10.1002/adfm.201203059. [DOI] [Google Scholar]
- Park E. J.; Sim J. K.; Jeong M. G.; Seo H. O.; Kim Y.D. Transparent and Superhydrophobic Films Prepared with Polydimethylsiloxane-coated Silica Nanoparticles. RSC Adv. 2013, 3, 12571–12576. 10.1039/c3ra42402b. [DOI] [Google Scholar]
- Ogihara H.; Xie J.; Saji T. Factors Determining Wettability of Superhydrophobic Paper Prepared by Spraying Nanoparticle Suspensions. Colloids Surf., A 2013, 434, 35–41. 10.1016/j.colsurfa.2013.05.034. [DOI] [Google Scholar]
- Kulkarni S. A.; Ogale S. B.; Vijayamohanan K. P. Tuning the Hydrophobic Properties of Silica Particles by Surface Silanization Using Mixed Self-assembled Monolayers. J. Colloid Interface Sci. 2008, 318, 372–379. 10.1016/j.jcis.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Li L.; Li B.; Dong J.; Zhang J. Roles of Silanes and Silicones in Forming Superhydrophobic and Superoleophobic Materials. J. Mater. Chem. A 2016, 4, 13677–13725. 10.1039/C6TA05441B. [DOI] [Google Scholar]
- Zhang Y.; Fang F.; Wang C.; Wang L. D.; Wang X. J.; Chu X. Y.; Li J. H.; Fang X.; Wei Z. P.; Wang X. H. Hydrophobic Modification of ZnO Nanostructures Surface Using Silane Coupling Agent. Polym. Compos. 2014, 35, 1204–1211. 10.1002/pc.22769. [DOI] [Google Scholar]
- Kim N.; Shin D. H.; Lee Y. T. Effect of Silane Coupling Agents on the Performance of RO Membranes. J. Membr. Sci. 2007, 300, 224–231. 10.1016/j.memsci.2007.05.039. [DOI] [Google Scholar]
- Huang H. C.; Hsieh T. E. Highly Stable Precursor Solution Containing ZnO Nanoparticles for the Preparation of ZnO Thin Film Transistors. Nanotechnology 2010, 21, 295707 10.1088/0957-4484/21/29/295707. [DOI] [PubMed] [Google Scholar]
- Kapridaki C.; Maravelaki-Kalaitzaki P. TiO2-SiO2-PDMS Nano-composite Hydrophobic Coating with Self-cleaning Properties for Marble Protection. Prog. Org. Coat. 2013, 76, 400–410. 10.1016/j.porgcoat.2012.10.006. [DOI] [Google Scholar]
- Li J.; Wan H. Q.; Ye Y. P.; Zhou H. D.; Chen J. M. One-step Process to Fabrication of Transparent Superhydrophobic SiO2 Paper. Appl. Surf. Sci. 2012, 261, 470–472. 10.1016/j.apsusc.2012.08.034. [DOI] [Google Scholar]
- Tsuru T.; Nakasuji T.; Oka M.; Kanezashi M.; Yoshioka T. Preparation of Hydrophobic Nanoporous Methylated SiO2 Membranes and Application to Nanofiltration of Hexane Solutions. J. Membr. Sci. 2011, 384, 149–156. 10.1016/j.memsci.2011.09.018. [DOI] [Google Scholar]
- Xu L. H.; Wang L. M.; Shen Y.; Ding Y.; Cai Z. S. Preparation of Hexadecyltrimethoxysilane-modified Silica Nanocomposite Hydrosol and Superhydrophobic Cotton Coating. Fibers Polym. 2015, 16, 1082–1091. 10.1007/s12221-015-1082-x. [DOI] [Google Scholar]
- Sohrabi B.; Mansouri F.; Khalifan S. Z. The Study of Glass Superhydrophobicity by Modified SiO2-Hexadecyltrimethoxysilane (SiO2-m-HDTMS) Nanoparticles and Mixture of Surfactants-Science Direct. Prog. Org. Coat. 2019, 131, 73–81. 10.1016/j.porgcoat.2019.02.010. [DOI] [Google Scholar]
- Xiong M. M.; Ren Z. H.; Liu W. J. Fabrication of Superhydrophobic and UV-resistant Surface on Cotton Fabric via Layer-by-layer Assembly of Silica-based UV Absorber. J. Dispersion Sci. Technol. 2020, 41, 1703–1710. 10.1080/01932691.2019.1634589. [DOI] [Google Scholar]
- Wu G. M.; Liu D.; Chen J.; Liu G. F.; Kong Z. W. Preparation and Properties of Super Hydrophobic Films from Siloxane-modified Two-component Waterborne Polyurethane and Hydrophobic Nano SiO2. Prog. Org. Coat. 2019, 127, 80–87. 10.1016/j.porgcoat.2018.06.016. [DOI] [Google Scholar]
- Zhang H. Y.; Li B. B.; Sun D.; Miao X. L.; Gu Y. L. SiO2-PDMS-PVDF Hollow Fiber Membrane with High Flux for Vacuum Membrane Distillation. Desalination 2018, 429, 33–43. 10.1016/j.desal.2017.12.004. [DOI] [Google Scholar]
- Cheng D.; Wen Y. B.; An X. Y.; Zhu X. H.; Ni Y. H. Tempo-oxidized Cellulose Nanofibers (TOCNs) as a Green Reinforcement for Waterborne Polyurethane Coating (WPU) on Wood. Carbohydr. Polym. 2016, 151, 326–334. 10.1016/j.carbpol.2016.05.083. [DOI] [PubMed] [Google Scholar]
- Petcu C.; Purcar V.; Spataru C. I.; Alexandrescu E.; Somoghi R.; Trica B.; Nitu S. G.; Panaitescu D. M.; Donescu D.; Jecu M. L. The Influence of New Hydrophobic Silica Nanoparticles on the Surface Properties of the Films Obtained from Bilayer Hybrids. Nanomaterials 2017, 7, 47 10.3390/nano7020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T. T.; Fu C. L.; Mu Z. G.; Zhang Z. L.; Bao M. Two-Dimensional Infrared Correlation Spectroscopy Analysis. J. Univ. Jinan 2007, 21, 124–129. [Google Scholar]
- Gui X. Y.; Liu C.; Xu J. H.; Duan F. L.; Fang S. W.; Li F. Y. Two-Dimensional Perturbation Correlation Infrared Spectroscopy Analysis of Animal Manure Biochar. Spectrosc. Spectral Anal. 2020, 40, 3606–3612. [Google Scholar]
- Liu J.; Zhang Q. H.; Ma F.; Zhang S. F.; Zhou Q.; Huang A. M. Three-step Identification of Infrared Spectra of Similar Tree Species to Pterocarpus Santalinus Covered with Beeswax. J. Mol. Struct. 2020, 1218, 128484 10.1016/j.molstruc.2020.128484. [DOI] [Google Scholar]
- Dou X. M.; Yuan B.; Zhao H. Y.; Yin G. Z.; Yukihiro O. Generalized Two-dimensional Correlation Spectroscopy-theory and Applications in Analytical Field. Sci. China, Ser. B: Chem. 2004, 47, 257–266. 10.1360/03yb0085. [DOI] [Google Scholar]
- Morita S.; Ozaki Y.; Noda I. Global Phase Angle Description of Generalized Two-dimensional Correlation Spectroscopy: 1. Theory and its Simulation for Practical Use. Appl. Spectrosc. 2001, 55, 1618–1621. 10.1366/0003702011954189. [DOI] [Google Scholar]
- Noda I. Generalized Two-dimensional Correlation Method Applicable to Infrared, Raman and Other Types of Spectroscopy. Appl. Spectrosc. 1993, 47, 1329–1336. 10.1366/0003702934067694. [DOI] [Google Scholar]
- Cao H. X.; Feng X. J.; Huo J. C. Synthesis and Surface Chemical Modification of the Monodisperse Silica Microsphere. J. Synth. Cryst. 2016, 45, 2050–2060. [Google Scholar]