Abstract

Clean water is one of the sustainable development goals set by the United Nations for 2030. The development of effective but worldwide affordable strategies is essential to guarantee this achievement. Photocatalysis technology fulfills these criteria whenever the photocatalyst is sustainable and nontoxic. In this article, a cellulose-paper modified with a polyamide-titanium dioxide (TiO2) nanocomposite by dip-coating is evaluated to degrade estrogens efficiently under solar light. The study deepens on the synergic combination of the sorptive capacity of the polyamide and the activity of TiO2. The photocatalytic performance was studied under artificial and sunlight in a miniaturized experimental setup (batch of six reactors). Also, the effects of the dispersed/immobilized catalyst, irradiation time, and adsorption evaluation were studied under kinetic conditions. The photocatalyst composition, considering the polyamide (nylon-6) and TiO2 amounts and the dipping cycles, was studied by a response surface methodology, and the reusability of the photocatalytic cellulose-paper was investigated. The LED source provided removal efficiencies of 65, 62, and 52% (for estrone, 17β-estradiol, and estriol, respectively) after 420 min of light exposure. Under sunlight, the efficiency increased up to 99.5% for estrone and 17β-estradiol and 98.5% for estriol after 180 min of irradiation. The sustainable character of the cellulosic substrate, the low toxicity of the nanocomposite ingredients, and its performance under sunlight make the material attractive for in-field application.
Introduction
In 2010, the United Nations (UN) clearly stated the human right to clean water and sanitation.1 Although this issue is mostly solved in many nations, it is a recurrent health and economic concern in low-income countries. According to a joint World Health Organization (WHO) and United Nations Children’s Fund (UNICEF) report, 785 million people did not have access to a basic drinking water service in 2017.2 The UN has challenged the scientific community to design strategies that make possible the worldwide access to clean water by the definition of a specific sustainable goal for 2030.3 These strategies should be affordable to be implemented easily and at low cost. The removal of pollutants from water is one of the action areas, and in this context, photocatalysis is a very attractive strategy due to its performance and simplicity.4
Although different materials have been evaluated as photocatalysts,5−8 TiO2 nanoparticles (NPs) are the most used due to their high efficiency, low price, and nontoxic character. They can be directly dispersed in the contaminated water as a (nano) powder, although this is not too practical in large-scale processes as the NPs are hard to be recovered after being deployed. Also, isolated TiO2 NPs have a wide band gap (≈3.2 eV), which is only active in the UV region.9,10 This limitation can be overcome by the photosensitization of the inorganic semiconductors by tuning their bandgap response to the visible region. This process can be undertaken by doping it with nonmetals, incorporating or depositing noble metals (ions), and synthesizing nanocomposites.11 Polymer-TiO2 nanocomposites have received growing attention for their applications in photocatalysis, sensing, and photoelectric applications. The immobilization of the nanocatalyst on a polymeric substrate/support can play several complementary roles, namely, mechanically stabilize the photocatalyst, eliminate the need of recovering the NPs suspended in the medium after the water treatment, and increase the interaction with organic compounds via selective adsorption.12,13
Several polymer-based TiO2 photocatalysts have been developed for the visible-light degradation of pollutants, including polypropylene,14 aromatic polyamide dendrimer/polystyrene,15 polyamides such as nylon-6 (N6),16,17 polyurethane,18 polyethylene,19 poly(vinyl alcohol),20 polythiophene,12 and even recycled polymers.21 N6 is widely used in many fields because of its low price, favorable mechanical strength, and high thermal and chemical stability. Therefore, the hybrid material obtained from N6 and TiO2 is a potential candidate for many applications.16,22,23
Cellulose paper is an excellent substrate for the fabrication of functional materials as it is sustainable, porous, and presents a large superficial area.24−27 Also, it can be easily modified by covalent bonding or simple deposition of functional groups/phases on its surface. The latter approach is simpler and reduces the solvents/reagents required. The synthesis of functional papers by dip coating, which has been recently reviewed,28 has allowed the preparation of papers covered with polymers,29−31 nanoparticles,32 and nanocomposites.33 In a previous communication,34 we proposed a photocatalytic paper, and the present article deepens the understanding of the synergic combination of sorption and photocatalysis using a relevant environmental problem, the removal of hormones from water, as a model.
Endocrine disrupting compounds (EDCs) are dangerous pollutants that can be found at low concentrations in environmental waters.35 EDCs are pseudo-persistent substances and, therefore, a long-term environmental issue.36 EDCs interfere with the endocrine system at the molecular level, blocking natural hormones’ function or mimicking them, thus producing adverse effects on both humans and wildlife. EDCs are a broad term that includes hormones (natural or artificially administered) and chemicals of different nature.37 The presence of hormones in the environment has increased due to their extensive medical and veterinary uses.38,39 In this sense, estrone (E1), 17β-estradiol (E2), and estriol (E3) are potent biogenic estrogens that are usually detected in sewage40 and they have been reported as the significant reason for estrogenicity in the effluents of sewage treatment plants.41,42
Estrogens are resistant to biodegradation in conventional wastewater treatment processes making these substances a significant concern regarding the water quality43,44 and an excellent challenge to evaluate the performance of photocatalysis. This work describes a N6-TiO2-coated cellulose-paper photocatalyst for the efficient sorption/photodegradation of E1, E2, and E3. The ingredients of the photocatalytic cellulose-paper present low toxicity. In fact, the reported oral LD50 in rats for cellulose and TiO2 nanoparticles is 5000 mg/kg.45,46 Nylon 6 can be considered nontoxic,47 while its monomer, ε-caprolactam, presents an oral LD50 in rats of 1200–1600 mg/kg.48 This aspect and the good performance of the photocatalytic cellulose-paper under sunlight make the material attractive for in-field application. Photocatalysis can be applied in two different scenarios: the treatment of a large volume of water for community sanitization or smaller volumes for individual or small groups. In the latter context, the use of the photocatalysts in water bottles is really interesting.49 Although plastic bottles are cheaper and their solar irradiation does not induce excessive leaching of hazardous compounds,50 the use of glass bottles can be an answer to the worldwide plastic environmental crisis.51 This study works in this scenario as the assays are performed in closed glass vials.
Results and Discussion
The UV−vis absorption spectrum for the photocatalytic paper (henceforth PCP for simplicity) presents a wider maximum absorption band with a sharper peak in the UV region (Figure S1). In the same Figure S1, the normalized emission spectrum of a visible LED source and sunlight is also presented for comparative purposes. Although, according to the overlapping curves, sunlight is clearly the best option to achieve a rapid degradation of the studied compounds, the photocatalytic performance of the material was initially evaluated under LED irradiation to understand more clearly the effect of pollutant sorption in the overall process.
The polymer plays a double role in this application. On the one hand, it stabilizes the NPs mechanically, avoiding their dispersion into the water. On the other hand, the polymer retains the hormones by a double mechanism,52 hydrophobic interaction with the polymeric backbone and H-bonding with the amide group, close to the photocatalytic hotspots. This aspect can be inferred from the results presented in Figure 1 where the activities of dispersed TiO2 NPs and PCP are compared. Under the preliminary experimental conditions indicated in the figure caption, PCP always provided a higher removal of the estrogens at the two times studied. Another preliminary study was developed to evaluate the contribution of the sorption to this removal. Figure S2 shows the comparison between the removal efficiency of the paper containing only N6 and the photocatalytic paper containing a mixture of nylon and TiO2. The results demonstrated that both effects (sorption and photodegradation) were behind the removal of the hormones. However, a major contribution has been achieved using the PCP, which indicates its efficiency for the photodegradation of the compounds.
Figure 1.

Comparison between dispersed and immobilized TiO2 in the degradation of estrogens. Membrane composed of 3% N6 and 4% TiO2 dipped two times; 5 mL of a mixture of the analytes at 1 mg L–1 in ultrapure water; after irradiating the solutions for 4 and 7 h, the solutions were kept in contact with the PC in the dark for 30 min; constant agitation at 500 rpm.
Photocatalytic Cellulose-Paper Composition and Surface Characterization
In this study, a Box–Behnken design was used as a chemometric tool to achieve the ideal proportion of N6/TiO2 and the number of dips to provide the maximum removal of estrogens from the solution. The effects of N6 and TiO2 percentages (0.5, 2.0, 3.5% w/v and 0, 2.5, 5.0% w/v, respectively) and dips (1, 2, and 3) on the withdrawal of estrogens were studied by a three-factor Box–Behnken design. The geometric means of the chromatographic peak areas for all the analytes were used as the signals. The response surfaces obtained with this optimization are shown in Figure 2.
Figure 2.
Effect of PCP for the estrogen’s removal. Conditions: 5 mL of a mixture of the analytes at 1 mg L–1 in ultrapure water, 30 min of previous adsorption without the light source, 180 min of photoreaction under constant agitation at 500 rpm using a visible-light LED lamp.
Analyzing the results in Figure 2 obtained through a special cubic model with an R2 of 0.9224, the best removal results were obtained using 3.5% N6, 5% TiO2, and dipping three times the paper. This result corroborates the hypothesis that both N6 and TiO2 contribute to the estrogen removal in a synergic effect since poor results were obtained when values below 1% of each compound were used. Minimum quantities of both elements are required to guarantee an effective intermolecular interaction between the polymer and the hormones that bring them close to the photocatalytic hotspots. Also, three dips were considered sufficient to create a PCP with a satisfactory amount of nanocomposite material.
After the selection of the best PCP composition, surface characterization of the synthesized material was performed. Figure S3 shows the SEM image of the PCP where it is possible to observe the efficient nanocomposite deposition over the cellulose fibers.
The modified papers were subsequently studied by Raman spectroscopy and imaging (Figure S4). The Raman spectrum of cellulose paper (Figure S4d1) is dominated by the CCC and CCO vibrations of glucose rings at about 380 and 435 cm–1 and 435 and 458 cm–1, respectively.53 CH2 vibrations appear as a broad signal at about 2980 cm–1, and skeletal vibrations produce a band at ca. 1380 cm–1. Moreover, the band at about 520 cm–1 corresponds to the CCC vibration of the ring and glycoside bond, while the symmetric and symmetric vibrations of glycoside bonds can be observed at about 1096 and 1120 cm–1.34 The intensity of such bands is used to create the Raman image of Figure S4a, depicting the intensity of the cellulose band in the range 1085.6–1161.3 cm–1, showing the different fibers within the cellulose network of paper. As can be seen in the spectrum of Figure S4d2, the modification of paper with the polyamide decreases the intensity of the Raman features of paper along with the appearance of a Raman peak at about 1648 cm–1 (Amide I). Figure S4b shows a Raman image at a higher magnification of the modified substrate, representing the amide I band intensity (region 1627.6–1670 cm–1).
On the other hand, upon modification of the paper with the nanocomposite 3, the spectrum is dominated by the signatures of TiO2 (Figure S4d4). Nevertheless, in regions with low coverage of TiO2, contribution to the Raman spectra of cellulose paper, nylon, and TiO2 can be observed (Figure S4d3). The main bands observed in the spectrum of TiO2 can be assigned, according to the literature,54,55 to the six active modes of anatase. Figure S4c depicts a Raman image of the cellulose paper modified with the nanocomposite obtained by monitoring the region 110.9–174.7 cm–1 corresponding to the Eg band of TiO2. As can be seen, TiO2 NPs are distributed along the cellulose substrate covering the surface of paper.
Adsorption Kinetics Models
To fully understand the E1, E2, and E3 adsorption mechanisms onto the PCP, the adsorption process was investigated at different times (0–420 min, C0 = 1 mg L–1) under dark conditions, and the kinetic data were fitted to four models, namely, pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion models. The parameters are reported in Table 1. The adsorption kinetics of the estrogens by the PCP fitted the pseudo-second-order kinetic model (R2 > 0.98892), indicating that the adsorption process was controlled by both estrogen concentration and adsorption sites on the PCP (Figure S5A).56 Similar results have been reported for the retention of E1, E2, and E3 onto N-propyl-modified mesoporous silica,57 few-layered BN nanosheets,58 and raw lotus seedpod biochar.59
Table 1. Different Types of Kinetic Models for the Adsorption of E3, E2, and E1 at C0 = 1 mg L–1a.
| pseudo-first
order |
pseudo-second
order |
Elovich |
intraparticle
diffusion |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| analyte | k1 (min–1) | qe (mg g–1) | R2 | k2 (g mg–1 min–1) | qe (mg g–1) | R2 | α (mg g–1 min–1) | β (g mg–1) | R2 | k1 (mg g–1 min–1/2) | I (mg g–1) | R2 |
| E3 | 0.00784 | 0.37751 | 0.87942 | 0.07200 | 0.42463 | 0.99266 | 0.04964 | 13.8677 | 0.95732 | 0.01751 | 0.09556 | 0.85561 |
| E2 | 0.00832 | 0.83038 | 0.91481 | 0.01443 | 1.2462 | 0.98892 | 0.05561 | 3.96936 | 0.94395 | 0.05528 | 0.13262 | 0.90485 |
| E1 | 0.01225 | 0.25776 | 0.91009 | 0.06298 | 0.67577 | 0.99881 | 0.09078 | 8.40054 | 0.92105 | 0.02824 | 0.17643 | 0.77644 |
qe, amounts of E1, E2, and E3 adsorbed at equilibrium. qt, amounts of E1, E2, and E3 adsorbed at a given time t. k1, first-order rate constant. k2, pseudo-second-order rate constant. α, initial hormone sorption. β, desorption constant. kI, intraparticle diffusion rate constant. I, parameter related to the thickness of the boundary layer.
The model indicates that 60 min was the equilibrium time, as can be seen in the plot of qe vs t demonstrated in Figure S5B. The adsorption amounts of E1, E2, and E3 at equilibrium were 0.637, 1.118, and 0.409 mg g–1, respectively. These values are in accordance with the logarithm of the octanol/water partition coefficients (log Kow) for the compounds. In fact, the log Kow values for E1, E2, and E3 are 3.13, 4.01, and 2.45, respectively. The results suggested a rapid adsorption rate at the beginning, which can be ascribed to the presence of a large number of available sites.56
Photocatalytic Studies
The photocatalytic degradation of E1, E2, and E3 using the PCP has been carried out under visible LED light and sunlight irradiation. The experiments were also performed in dark conditions to study the sorption contribution. The removal efficiency (η) of these processes was calculated using following equation
| 1 |
where C0 and Ct are the initial and residual concentrations of the estrogens at various intervals of time, respectively.
Under dark conditions, the adsorption process corresponds (at 420 min) to 54, 60, and 36% of the E1, E2, and E3 removal, respectively. In addition to the removal of the pollutants by adsorption, their photocatalytic degradation was observed for both visible LED light and sunlight treatments. The degradation efficiency was 99.5% of E1 and E2 and 98.5% of E3 after 180 min of sunlight exposure. For the visible LED lamp, removal efficiencies of 65, 62, and 52% for E1, E2, and E3 were reached after 420 min of visible LED exposure.
Also, a competitive effect for the active sites was observed for both the adsorption and photodegradation processes. Figure S6 describes the competition study evaluating each compound individually and into a mixture under dark and LED light conditions. Differences in the removal efficiencies for E3, E2, and E1 were 7, 13, and 10% greater under dark conditions and 17, 25 and 15% greater under visible LED conditions when studying each compound individually and the mixture, respectively.
Figure 3 depicts the chromatograms obtained by analyzing an aqueous mixture of E1, E2, and E3 being irradiated by sunlight and visible LED at different times. E1, E2, and E3 peaks decreased apparently with increasing irradiation time, implying the degradation/removal of the compounds from the solution with consequent formation/decay of by-products and intermediates. Different by-product peaks were found in the chromatograms. These differences can be ascribed to different pathways or different snapshots of the degradation process.60 Further investigations will be carried out, using high-resolution mass spectrometry, to elucidate the chemical identity of the unknown peaks.
Figure 3.
Chromatograms obtained for an aqueous mixture of E1, E2, and E3 irradiated at different times under (A) sunlight and (B) visible LED light conditions.
Kinetics of E1, E2, and E3 Degradation
To further understand the kinetics of E1, E2, and E3 degradation under visible LED light and sunlight irradiation, the data were adjusted to a pseudo-first-order equation
| 2 |
where C is the concentration of the estrogens, t is the reaction time (min), and k is the pseudo-first-order rate constant (min–1). If the initial concentration of estrogens is C0, eq 2 can be simplified as
| 3 |
The arrangement of eq 3 leads to eq 4
| 4 |
Figure 4 presents the kinetics of E1, E2, and E3 degradation fitted to this model, and the kinetic parameters for E1, E2, and E3 photodegradation under different light sources were determined and can be seen in Table 2. These results indicate a good fitting, with R2 higher than 0.98260 and 0.99066 for the visible LED light and sunlight, respectively. The good fitting suggests that the reaction depends only on the estrogen concentration, as the hydroxyl radical concentration at the PCP surface is high, and it can be considered almost constant.61 The proposed model is in agreement with others from the literature for the photodegradation of E1, E2, and E3 promoted by immobilized and dispersed TiO2 under several radiation sources.4,41,43,62
Figure 4.
Photodegradation kinetics under (A) visible LED light and (B) sunlight irradiation.
Table 2. Pseudo-First-Order Kinetic Parameters of E3, E2, and E1 Photodegradation under Visible LED Light and Sunlight at C0 = 1 mg L–1.
| LED
visible lamp |
sunlight |
|||||
|---|---|---|---|---|---|---|
| analyte | k1 (min–1) | R2 | t1/2 (min–1) | k1 (min–1) | R2 | t1/2 (min–1) |
| E3 | 0.00832 | 0.99581 | 83.34 | 0.01059 | 0.99658 | 65.46 |
| E2 | 0.01724 | 0.98260 | 40.21 | 0.02521 | 0.99262 | 27.50 |
| E1 | 0.01992 | 0.99474 | 34.80 | 0.02126 | 0.99066 | 32.61 |
The k values for the estrogen degradation under sunlight were slightly higher than those obtained for the visible LED light with ksunlight = 0.02126, 0.02521, and 0.01059 min–1 vs kLED = 0.01992, 0.01724, and 0.00832 min–1 for E1, E2, and E3, respectively. Interestingly, despite similar k values under LED and sunlight conditions, the mixture of E1, E2, and E3 was completely removed after only 180 min under sunlight whereas only 60% of the estrogens was removed after 420 min of LED light irradiation treatment. This aspect is ascribed to the different intensity of the sources.
The k values for the estrogen degradation followed the tendency kE1 > kE2 > kE3 for LED light and kE2 > kE1 > kE3 for sunlight, which was consistent with the previous E1, E2, and E3 photocatalytic behaviors.40,60
Reusability of the Photocatalytic Cellulose-Paper
Finally, to investigate the photochemical stability and reusability of the synthesized PCP, repeated experiments were carried out for the photodegradation of E1, E2, and E3 under sunlight using the same PCP. The cleaning procedure between cycles was adopted as being 1 mL of a mixture of acetonitrile/methanol (50:50) for 15 min. After the cleaning step, the supernatant was injected, and no signal of the analytes was detected, indicating that all the adsorbed compounds were photodegraded by the material. As can be seen in Figure S7, after three cycles, the photocatalytic activity was maintained higher than 95% for all the compounds, indicating that the developed PCP is considered fairly stable under the studied experimental conditions and the same PCP can be used at least three times maintaining the efficiency.
Photocatalytic Cellulose-Paper in Context
The performance of the PCP must be done externally (compared with other counterparts) and internally (evaluating the pros and cons). Table 3 compares the main features of different methods described in the literature4,39,41,63−65 for the photocatalytic degradation of E1, E2, and E3 mostly through TiO2-based nanomaterials with the PCP described in this work. The data suggest very interesting photoactivity, particularly regarding the high removal efficiency achieved using the sunlight as the radiation source. The synergic combination of the paper substrate and the polymer containing TiO2 NPs allowed a photocatalytic activity in the visible region comparable to studies performed under UV conditions.41,64 In addition, this study evaluated an equimolar mixture of the estrogenic hormones, which resembles the real conditions usually found in the environment. Besides being the only study combining a polymer and TiO2 NPs for the removal of estrogens, the k values obtained by the kinetics studies are very similar to the others. These formidable features make the proposed photocatalyst paper a very promising tool to be explored for photodegradation purposes.
Table 3. Comparison of the Performance of Some Reported Methods for the Removal of E1, E2, and E3.
| analyte | C0 (mg L–1) | kinetics (min–1) | removal (%) | light source | catalyst | ref |
|---|---|---|---|---|---|---|
| E1, E2, E3 | 1.0 | pseudo-first order, k = 0.02126, 0.02521, and 0.01059 min–1 | 99.5, 99.5, and 98.5% in 180 min | sunlight 14.5 mW cm–2 | paper-based nylon-TiO2, 0.0222 g | this work |
| E1, E2 | 0.05 | k = 0.068 and 0.012 min–1 | 97.0 and 49.2% in 50 min | UV lamp, λ = 253.7 nm, 350 μW cm–2 | UV/H2O2, 10 mg L–1 | (63) |
| E1 | 1.0 | pseudo-first order, k = 0.01 min–1 | 100% in 18 min to UVA and 93% in 60 min to white LED | UVA (λ = 365 nm) and cool white (λ > 420 nm) | 4% Au-TiO2 nanocomposite, 50 mg L–1 | (41) |
| E3 | 2.88 | pseudo 1st order, k = 0.021 min–1 | 100% in 180 min | two black light lamps, λ = 365 nm, 15 W, 1500 μW cm–2 | TiO2 P25, 20 mg L–1 | (64) |
| E2 | 1.0 | k = 0.043 min–1 | 99% in 240 min | solar simulator, λ = 280–400 nm, 450 W | nanocrystalline TiO2, 20 mg L–1 | (65) |
| E2 | 2.0 μM | pseudo-first order, k = 0.0025 min–1 | 50.86% in 300 min | sunlight, 1910 μW cm–2 | nanotubular TiO2 | (4) |
| E2 | 3.0 | pseudo-first order, k = 0.0983 min–1 | 99.5% in 60 min | mercury lamp, 20 W | 5% Fe/Bi2SiO5, 500 mg L–1 | (39) |
An honest internal evaluation of the PCP, indicating the pros and cons, is essential to present a realistic view of the material and a route for future improvements.
The PCP is simple to synthesize, and the components are relatively cheap and nontoxic, but the dip-coating technique requires a solvent. A procedure to recover (by evaporation/condensation) this solvent would be interesting to reduce the price of the material and the environmental impact of the synthesis in potential large-scale production.
The PCP is mechanically stable at the macroscopic level, and its components are nontoxic. A deep study of the potential leaching of the polymer and the NPs and the evaluation of the effect of the photocatalysis on the N6 degradation would be also interesting for investigation.
Conclusions
In this study, a PCP was successfully synthesized and characterized and its photoactivity effectiveness was properly evaluated toward E1, E2, and E3 visible-driven degradation. The PCP combines the sorption capacity of a polyamide with the photocatalytic activity of TiO2 NPs. Under the optimized experimental conditions, the PCP exhibited a high degradation performance to the sunlight, having a range of 98.5–99.5% of the estrogenic hormones E1, E2, and E3 removed from the solution after 180 min of irradiation. Also, the PCP was stable, and it can be reused. The facile synthesis via simple dipping of the cellulose paper in a nanocomposite medium paves the way for novel solar-driven photocatalytic applications using several polymer/semiconductor ensembles. The analysis of the degradation product by high-resolution mass spectrometry (HRMS) will be considered in the next projects. Due to the higher polarity of the compounds, which presents retention times shorter than the parent compounds, LC-HRMS or direct infusion HRMS can be a better option for these studies.
The developed PCP is cheap and has low toxicity. Further work will be focused on its in-field application for the removal of contaminants as its small design is fully compatible with individual water bottles.
Experimental Section
Chemicals and Materials
With a few exceptions, the reagents were purchased from Sigma-Aldrich (Madrid, Spain). Stock (1 g L–1) and working standards (1 mg L–1) of estrone (E1), 17β-estradiol (E2), and estriol (E3) were built in methanol and Milli-Q water (Millipore Corp., Madrid, Spain). Formic acid (98%), N6 pellets, pure cellulose filter paper (0.170 mm in thickness, Filtros Anoia), and TiO2 nanoparticles (dp: 25 nm) were used for the preparation of the photocatalytic cellulose-paper. Acetonitrile (ACN, 99.9%) and methanol (MeOH, 99.9%), both from PanReac Applichem ITW reagents (Madrid, Spain), were also used.
Analytical Measurements
The quantification of the target pollutants in water samples was performed by liquid chromatography coupled to a UV detector (HP1100 series liquid chromatograph (Agilent, Palo Alto, CA), which provides good sensitivity levels as it is indicated in Table S1. The chromatographic separation took place on an Agilent Eclipse XDB-C18 column (150 mm × 4.6 mm i.d. × 5.0 μm) (Waltham, MA, USA) using a flow rate of 1 mL min–1 for the mobile phase. A gradient elution mode was used. The initial mobile phase’s composition consisted of 70% component A (water) and 30% component B (ACN). The percentage of component B was linearly increased from 30 to 70% (from 4 to 7 min) and then decreased again to 30% (from 7 to 8 min) for column re-equilibration. 20 μL of solutions was automatically injected by an autosampler, and the pollutants were measured at 280 nm.
Synthesis and Characterization of the Photocatalytic Paper
The PCPs were prepared by immersing three times a paper ring (o.d. 1.7 cm × i.d. 0.6 cm) into a dispersion containing 3.5% w/v N6 and 5% w/v TiO2 NPs prepared in formic acid. After each dip, the paper was dried entirely. The dipping position was changed after each material deposition to achieve a more homogeneous coating. The physical/chemical characterization of the nanocomposite paper-based films was carried out by microscopy and Raman spectroscopy as indicated in the Supporting Information.
Adsorption/Photocatalytic Studies
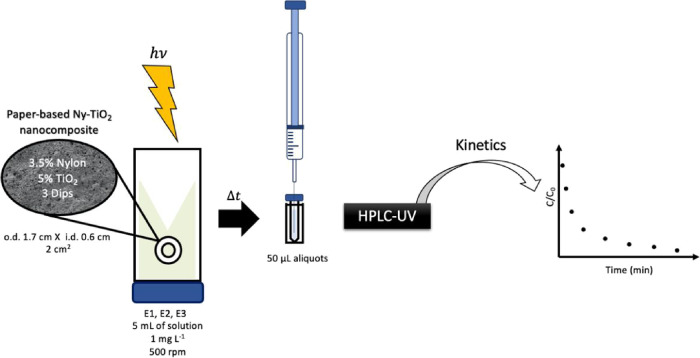
The activity of PCP was studied by the photodegradation of E1, E2, and E3 under visible LED light and sunlight irradiation. A diagram describing the overall procedure is shown in Figure 5. A visible LED light (10 W Aigostar LED lamp, 3000 K, 90 mA, 230 V/50 Hz, 800 lm), positioned above an orbital agitator containing six glass vessels (10 mL headspace vials) used as photoreactors, was used as the light source. The experimental apparatus was placed inside a dark chamber to ensure any interference from other light sources. The as-prepared PCP in a ring format with an area of 2 cm2 of each side was placed in a vial with 5 mL of an aqueous solution of each analyte (starting concentration of 1 mg L–1). During the photoreaction, the vial was stirred at 500 rpm while irradiated by the radiation source. The vials were positioned face down for better irradiation. Supernatant aliquots of 50 μL were taken at different times and analyzed by LC. For studies under sunlight, the same procedure was adopted, but the experimental apparatus was placed directly exposed to the sunlight. The procedure was also performed at room temperature under dark conditions to evaluate the adsorption kinetics.
Figure 5.
Schematic description of the experimental photodegradation procedure.
The PCP composition was evaluated through a Box–Behnken design consisting of 15 experiments, according to Table S2. The studied variables were N6 amount (0.5, 2, and 3.5% w/v), TiO2 percentage (0, 2.5, and 5% w/v), and total dips (1, 2, and 3). StatSoft Statistic 8.0 and Microsoft Excel were used for the statistical study.
For the adsorption process, four different kinetics models66 were evaluated as it is indicated in the Supporting Information.
Acknowledgments
Financial support from the “Fondo Europeo de Desarrollo Regional (FEDER)” and Junta de Andalucía (Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Project 1262884) is gratefully acknowledged. G.M. expresses her gratitude to the Brazilian governmental agency Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for the scholarship supported by the Programa Institucional de Internacionalização of the Universidade Federal de Santa Catarina (CAPES/PRINT-UFSC, Project number 88887.310569/2018-00). The authors would like to acknowledge the Central Service for Research Support (SCAI) of the University of Córdoba for assistance on the acquisition of SEM micrographs.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00128.
UV–vis absorption spectrum of the photocatalytic paper and emission spectra of the light sources; removal efficiency of the photocatalytic paper compared with an N6-coated paper without TiO2; scanning electron microscopy of the photocatalytic paper; Raman spectroscopy studies; adsorption kinetics of the estrogens by the photocatalytic paper; study of competition for the active sites of the photocatalytic paper; photocatalytic paper reusability under sunlight irradiation; calibration of the direct injection LC-UV method; experimental design of the optimization of the photocatalytic paper; kinetics studies (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Assembly U. N. G.Resolution 64/292. The Human Right to Water and Sanitation; UN-Water: 2010. [Google Scholar]
- United Nations Children’s Fund; World Health Organization. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017: Special Focus on Inequalities; World Health Organization: 2019. [Google Scholar]
- United Nations . A/RES/70/1. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: 2015. [Google Scholar]
- Kim S.; Cho H.; Joo H.; Her N.; Han J.; Yi K.; Kim J.-O.; Yoon J. Evaluation of Performance with Small and Scale-up Rotating and Flat Reactors; Photocatalytic Degradation of Bisphenol A, 17β–Estradiol, and 17α–Ethynyl Estradiol under Solar Irradiation. J. Hazard. Mater. 2017, 336, 21–32. 10.1016/j.jhazmat.2017.04.047. [DOI] [PubMed] [Google Scholar]
- Valenzuela M. A.; Bosch P.; Jiménez-Becerrill J.; Quiroz O.; Páez A. I. Preparation, Characterization and Photocatalytic Activity of ZnO, Fe2O3 and ZnFe2O4. J. Photochem. Photobiol., A 2002, 148, 177–182. 10.1016/S1010-6030(02)00040-0. [DOI] [Google Scholar]
- Sharma M.; Jain T.; Singh S.; Pandey O. P. Photocatalytic Degradation of Organic Dyes under UV–Visible Light Using Capped ZnS Nanoparticles. Sol. Energy 2012, 86, 626–633. 10.1016/j.solener.2011.11.006. [DOI] [Google Scholar]
- Rajabi H. R.; Khani O.; Shamsipur M.; Vatanpour V. High-Performance Pure and Fe3+-Ion Doped ZnS Quantum Dots as Green Nanophotocatalysts for the Removal of Malachite Green under UV-Light Irradiation. J. Hazard. Mater. 2013, 250-251, 370–378. 10.1016/j.jhazmat.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Rajabi H. R.; Arjmand H.; Kazemdehdashti H.; Farsi M. A Comparison Investigation on Photocatalytic Activity Performance and Adsorption Efficiency for the Removal of Cationic Dye: Quantum Dots vs. Magnetic Nanoparticles. J. Environ. Chem. Eng. 2016, 4, 2830–2840. 10.1016/j.jece.2016.05.029. [DOI] [Google Scholar]
- Pant H. R.; Pandeya D. R.; Nam K. T.; Baek W.; Hong S. T.; Kim H. Y. Photocatalytic and Antibacterial Properties of a TiO2/Nylon-6 Electrospun Nanocomposite Mat Containing Silver Nanoparticles. J. Hazard. Mater. 2011, 189, 465–471. 10.1016/j.jhazmat.2011.02.062. [DOI] [PubMed] [Google Scholar]
- Zangeneh H.; Farhadian M.; Zinatizadeh A. A. N (Urea) and C N (L-Asparagine) Doped TiO2-CuO Nanocomposites: Fabrication, Characterization and Photodegradation of Direct Red 16. J. Environ. Chem. Eng. 2020, 8, 103639. 10.1016/j.jece.2019.103639. [DOI] [Google Scholar]
- Singh S.; Mahalingam H.; Singh P. K. Polymer-Supported Titanium Dioxide Photocatalysts for Environmental Remediation: A Review. Appl. Catal., A 2013, 462-463, 178–195. 10.1016/j.apcata.2013.04.039. [DOI] [Google Scholar]
- Liang H.; Li X. Visible-Induced Photocatalytic Reactivity of Polymer–Sensitized Titania Nanotube Films. Appl. Catal., B 2009, 86, 8–17. 10.1016/j.apcatb.2008.07.015. [DOI] [Google Scholar]
- Jbeli A.; Hamden Z.; Bouattour S.; Ferraria A. M.; Conceição D. S.; Ferreira L. F. V.; Chehimi M. M.; do Rego A. M. B.; Rei Vilar M.; Boufi S. Chitosan-Ag-TiO2 Films: An Effective Photocatalyst under Visible Light. Carbohydr. Polym. 2018, 199, 31–40. 10.1016/j.carbpol.2018.06.122. [DOI] [PubMed] [Google Scholar]
- Giovannetti R.; Amato C. A. D.’.; Zannotti M.; Rommozzi E.; Gunnella R.; Minicucci M.; di Cicco A. Visible Light Photoactivity of Polypropylene Coated Nano-TiO2 for Dyes Degradation in Water. Sci. Rep. 2016, 5, 17801. 10.1038/srep17801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y.; Lei H.; Huo J. Innovative Controllable Photocatalytic Degradation of Polystyrene with Hindered Amine Modified Aromatic Polyamide Dendrimer/ Polystyrene-Grafted-TiO2 Photocatalyst under Solar Light Irradiation. Polym. Degrad. Stab. 2015, 118, 1–9. 10.1016/j.polymdegradstab.2015.04.005. [DOI] [Google Scholar]
- Mejía M. I.; Marín J. M.; Restrepo G.; Pulgarín C.; Kiwi J. Photocatalytic Evaluation of TiO2/Nylon Systems Prepared at Different Impregnation Times. Catal. Today 2011, 161, 15–22. 10.1016/j.cattod.2010.09.005. [DOI] [Google Scholar]
- Zhang H.; Zhu H. Preparation of Fe-Doped TiO2 Nanoparticles Immobilized on Polyamide Fabric. Appl. Surf. Sci. 2012, 258, 10034–10041. 10.1016/j.apsusc.2012.06.069. [DOI] [Google Scholar]
- Lin L.; Wu Q.; Gong X.; Zhang Y. Preparation of TiO2 Nanotubes Loaded on Polyurethane Membrane and Research on Their Photocatalytic Properties. J. Anal. Methods Chem. 2017, 2017, 1–6. 10.1155/2017/9629532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W.; Luo Y.; Song S.; Dong X.; Yu X. High Photocatalytic Degradation Activity of Polyethylene Containing Polyacrylamide Grafted TiO2. Polym. Degrad. Stab. 2013, 98, 1754–1761. 10.1016/j.polymdegradstab.2013.05.027. [DOI] [Google Scholar]
- Liu X.; Chen Q.; Lv L.; Feng X.; Meng X. Preparation of Transparent PVA/TiO2 Nanocomposite Films with Enhanced Visible-Light Photocatalytic Activity. Catal. Commun. 2015, 58, 30–33. 10.1016/j.catcom.2014.08.032. [DOI] [Google Scholar]
- EL-Mekkawi D. M.; Abdelwahab N. A.; Mohamed W. A. A.; Taha N. A.; Abdel-Mottaleb M. S. A. Solar Photocatalytic Treatment of Industrial Wastewater Utilizing Recycled Polymeric Disposals as TiO2 Supports. J. Cleaner Prod. 2020, 249, 119430. 10.1016/j.jclepro.2019.119430. [DOI] [Google Scholar]
- Pant H. R.; Bajgai M. P.; Nam K. T.; Seo Y. A.; Pandeya D. R.; Hong S. T.; Kim H. Y. Electrospun Nylon-6 Spider-Net like Nanofiber Mat Containing TiO2 Nanoparticles: A Multifunctional Nanocomposite Textile Material. J. Hazard. Mater. 2011, 185, 124–130. 10.1016/j.jhazmat.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Abdal-hay A.; Mousa H. M.; Khan A.; Vanegas P.; Lim J. H. TiO2 Nanorods Coated onto Nylon 6 Nanofibers Using Hydrothermal Treatment with Improved Mechanical Properties. Colloids Surf., A 2014, 457, 275–281. 10.1016/j.colsurfa.2014.05.058. [DOI] [Google Scholar]
- Pasupuleti R. R.; Tsai P.-C.; Ponnusamy V. K. Low-Cost Disposable Poly(Ethyleneimine)-Functionalized Carbon Nanofibers Coated Cellulose Paper as Efficient Solid Phase Extraction Sorbent Material for the Extraction of Parahydroxybenzoates from Environmental Waters. Chemosphere 2021, 267, 129274. 10.1016/j.chemosphere.2020.129274. [DOI] [PubMed] [Google Scholar]
- Kaneko K.; Hara M.; Nishino T.; Maruyama T. One-Step Biotinylation of Cellulose Paper by Polymer Coating to Prepare a Paper-Based Analytical Device. Anal. Chem. 2020, 92, 1978–1987. 10.1021/acs.analchem.9b04373. [DOI] [PubMed] [Google Scholar]
- Das D.; Senapati S.; Nanda K. K. “Rinse, Repeat”: An Efficient and Reusable SERS and Catalytic Platform Fabricated by Controlled Deposition of Silver Nanoparticles on Cellulose Paper. ACS Sustainable Chem. Eng. 2019, 7, 14089–14101. 10.1021/acssuschemeng.9b02651. [DOI] [Google Scholar]
- d’Halluin M.; Rull-Barrull J.; Bretel G.; Labrugère C.; Le Grognec E.; Felpin F.-X. Chemically Modified Cellulose Filter Paper for Heavy Metal Remediation in Water. ACS Sustainable Chem. Eng. 2017, 5, 1965–1973. 10.1021/acssuschemeng.6b02768. [DOI] [Google Scholar]
- Ríos-Gómez J.; Lucena R.; Cárdenas S. Paper-Based Sorptive Phases Prepared by Dip Coating. LC·GC Eur. 2020, 33, 60–66. [Google Scholar]
- Ríos-Gómez J.; Lucena R.; Cárdenas S. Paper Supported Polystyrene Membranes for Thin Film Microextraction. Microchem. J. 2017, 133, 90–95. 10.1016/j.microc.2017.03.026. [DOI] [Google Scholar]
- Ríos-Gómez J.; García-Valverde M. T.; López-Lorente Á. I.; Toledo-Neira C.; Lucena R.; Cárdenas S. Polymeric Ionic Liquid Immobilized onto Paper as Sorptive Phase in Microextraction. Anal. Chim. Acta 2020, 1094, 47–56. 10.1016/j.aca.2019.10.021. [DOI] [PubMed] [Google Scholar]
- Díaz-Liñán M. C.; García-Valverde M. T.; Lucena R.; Cárdenas S.; López-Lorente A. I. Dual-Template Molecularly Imprinted Paper for the Determination of Drugs of Abuse in Saliva Samples by Direct Infusion Mass Spectrometry. Microchem. J. 2021, 160, 105686. 10.1016/j.microc.2020.105686. [DOI] [Google Scholar]
- Ríos-Gómez J.; Fresco-Cala B.; García-Valverde M.; Lucena R.; Cárdenas S. Carbon Nanohorn Suprastructures on a Paper Support as a Sorptive Phase. Molecules 2018, 23, 1252. 10.3390/molecules23061252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Liñán M. C.; García-Valverde M. T.; López-Lorente A. I.; Cárdenas S.; Lucena R. Silver Nanoflower-Coated Paper as Dual Substrate for Surface-Enhanced Raman Spectroscopy and Ambient Pressure Mass Spectrometry Analysis. Anal. Bioanal. Chem. 2020, 412, 3547–3557. 10.1007/s00216-020-02603-x. [DOI] [PubMed] [Google Scholar]
- Ríos-Gómez J.; Ferrer-Monteagudo B.; López-Lorente Á. I.; Lucena R.; Luque R.; Cárdenas S. Efficient Combined Sorption/Photobleaching of Dyes Promoted by Cellulose/Titania-Based Nanocomposite Films. J. Cleaner Prod. 2018, 194, 167–173. 10.1016/j.jclepro.2018.05.100. [DOI] [Google Scholar]
- Caliman F. A.; Gavrilescu M. Pharmaceuticals, Personal Care Products and Endocrine Disrupting Agents in the Environment - A Review. CLEAN - Soil, Air, Water 2009, 37, 277–303. 10.1002/clen.200900038. [DOI] [Google Scholar]
- Gmurek M.; Olak-Kucharczyk M.; Ledakowicz S. Photochemical Decomposition of Endocrine Disrupting Compounds – A Review. Chem. Eng. J. 2017, 310, 437–456. 10.1016/j.cej.2016.05.014. [DOI] [Google Scholar]
- Sornalingam K.; McDonagh A.; Zhou J. L. Photodegradation of Estrogenic Endocrine Disrupting Steroidal Hormones in Aqueous Systems: Progress and Future Challenges. Sci. Total Environ. 2016, 550, 209–224. 10.1016/j.scitotenv.2016.01.086. [DOI] [PubMed] [Google Scholar]
- Pereira R. O.; Postigo C.; de Alda M. L.; Daniel L. A.; Barceló D. Removal of Estrogens through Water Disinfection Processes and Formation of By-Products. Chemosphere 2011, 82, 789–799. 10.1016/j.chemosphere.2010.10.082. [DOI] [PubMed] [Google Scholar]
- Li L.; Long Y.; Chen Y.; Wang S.; Wang L.; Zhang S.; Jiang F. Facile Synthesis of Fe/Bi2SiO5 Nanocomposite with Enhanced Photocatalytic Activity for Degradation of 17β-Estradiol(E2). Solid State Sci. 2018, 83, 143–151. 10.1016/j.solidstatesciences.2018.07.008. [DOI] [Google Scholar]
- Li Puma G.; Puddu V.; Tsang H. K.; Gora A.; Toepfer B. Photocatalytic oxidation of multicomponent mixtures of estrogens (estrone (E1), 17β-estradiol (E2), 17α-ethynylestradiol (EE2) and estriol (E3)) under UVA and UVC radiation: Photon absorption, quantum yields and rate constants independent of photon absorption. Appl. Catal., B 2010, 99, 388–397. 10.1016/j.apcatb.2010.05.015. [DOI] [Google Scholar]
- Sornalingam K.; McDonagh A.; Zhou J. L.; Johir M. A. H.; Ahmed M. B. Photocatalysis of Estrone in Water and Wastewater: Comparison between Au-TiO2 Nanocomposite and TiO2, and Degradation by-Products. Sci. Total Environ. 2018, 610-611, 521–530. 10.1016/j.scitotenv.2017.08.097. [DOI] [PubMed] [Google Scholar]
- Kovacic M.; Kopcic N.; Kusic H.; Bozic A. L. Solar Driven Degradation of 17β-Estradiol Using Composite Photocatalytic Materials and Artificial Irradiation Source: Influence of Process and Water Matrix Parameters. J. Photochem. Photobiol., A 2018, 361, 48–61. 10.1016/j.jphotochem.2018.05.015. [DOI] [Google Scholar]
- Zhang W.; Li Y.; Su Y.; Mao K.; Wang Q. Effect of Water Composition on TiO2 Photocatalytic Removal of Endocrine Disrupting Compounds (EDCs) and Estrogenic Activity from Secondary Effluent. J. Hazard. Mater. 2012, 215-216, 252–258. 10.1016/j.jhazmat.2012.02.060. [DOI] [PubMed] [Google Scholar]
- Sornalingam K.; McDonagh A.; Canning J.; Cook K.; Johir M. A. H.; Zhou J. L.; Ahmed M. B. Photocatalysis of 17α-Ethynylestradiol and Estriol in Water Using Engineered Immersible Optical Fibres and Light Emitting Diodes. J. Water Process Eng. 2020, 33, 101075. 10.1016/j.jwpe.2019.101075. [DOI] [Google Scholar]
- Cavender F. L.Synthetic Polymers-Cellulosics, Other Polysaccharides, Polyamides, and Polyimides. In Patty’s Toxicology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 935–964. 10.1002/0471435139.tox091.pub2. [DOI] [Google Scholar]
- Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016, 14, e04545 10.2903/j.efsa.2016.4545. [DOI] [Google Scholar]
- Hu X.-C.; Yang H. H.. Polyamide and Polyester Fibers. In Comprehensive Composite Materials; Elsevier: 2000; pp. 327–344. 10.1016/B0-08-042993-9/00060-7. [DOI] [Google Scholar]
- Wu Y.-H.; Wu M.-L.; Lin C.-C.; Chu W.-L.; Yang C.-C.; Lin R. T.; Deng J.-F. Determination of Caprolactam and 6-Aminocaproic Acid in Human Urine Using Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr., B 2012, 885-886, 61–65. 10.1016/j.jchromb.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Cowie B. E.; Porley V.; Robertson N. Solar Disinfection (SODIS) Provides a Much Underexploited Opportunity for Researchers in Photocatalytic Water Treatment (PWT). ACS Catal. 2020, 10, 11779–11782. 10.1021/acscatal.0c03325. [DOI] [Google Scholar]
- Schmid P.; Kohler M.; Meierhofer R.; Luzi S.; Wegelin M. Does the Reuse of PET Bottles during Solar Water Disinfection Pose a Health Risk Due to the Migration of Plasticisers and Other Chemicals into the Water?. Water Res. 2008, 42, 5054–5060. 10.1016/j.watres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Derraik J. G. B. The Pollution of the Marine Environment by Plastic Debris: A Review. Mar. Pollut. Bull. 2002, 44, 842–852. 10.1016/S0025-326X(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Reyes-Gallardo E. M.; Lucena R.; Cárdenas S. Silica Nanoparticles–Nylon 6 Composites: Synthesis, Characterization and Potential Use as Sorbent. RSC Adv. 2017, 7, 2308–2314. 10.1039/C6RA24739C. [DOI] [Google Scholar]
- Kavkler K.; Demšar A. Application of FTIR and Raman Spectroscopy to Qualitative Analysis of Structural Changes in Cellulosic Fibres. Tekstilec 2012, 55, 19–44. [Google Scholar]
- Ohsaka T.; Izumi F.; Fujiki Y. Raman Spectrum of Anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. 10.1002/jrs.1250070606. [DOI] [Google Scholar]
- Choi H. C.; Jung Y. M.; Kim S. B. Size Effects in the Raman Spectra of TiO2 Nanoparticles. Vib. Spectrosc. 2005, 37, 33–38. 10.1016/j.vibspec.2004.05.006. [DOI] [Google Scholar]
- Gao P.; Liang Z.; Zhao Z.; Wang W.; Yang C.; Hu B.; Cui F. Enhanced Adsorption of Steroid Estrogens by One-Pot Synthesized Phenyl-Modified Mesoporous Silica: Dependence on Phenyl-Organosilane Precursors and PH Condition. Chemosphere 2019, 234, 438–449. 10.1016/j.chemosphere.2019.06.089. [DOI] [PubMed] [Google Scholar]
- Gao P.; Yang C.; Liang Z.; Wang W.; Zhao Z.; Hu B.; Cui F. N-Propyl Functionalized Spherical Mesoporous Silica as a Rapid and Efficient Adsorbent for Steroid Estrogen Removal: Adsorption Behaviour and Effects of Water Chemistry. Chemosphere 2019, 214, 361–370. 10.1016/j.chemosphere.2018.09.115. [DOI] [PubMed] [Google Scholar]
- Liu G.; Zhang Z.; Yan C.; Wang Y.; Ma X.; Gao P.; Feng Y. Adsorption of Estrone with Few-Layered Boron Nitride Nanosheets: Kinetics, Thermodynamics and Mechanism. Chemosphere 2018, 207, 534–542. 10.1016/j.chemosphere.2018.05.129. [DOI] [PubMed] [Google Scholar]
- Liu N.; Liu Y.; Zeng G.; Gong J.; Tan X.; JunWen; Liu S.; Jiang L.; Li M.; Yin Z. Adsorption of 17β-Estradiol from Aqueous Solution by Raw and Direct/Pre/Post-KOH Treated Lotus Seedpod Biochar. J. Environ. Sci. 2020, 87, 10–23. 10.1016/j.jes.2019.05.026. [DOI] [PubMed] [Google Scholar]
- Mayer B. K.; Johnson C.; Yang Y.; Wellenstein N.; Maher E.; McNamara P. J. From Micro to Macro-Contaminants: The Impact of Low-Energy Titanium Dioxide Photocatalysis Followed by Filtration on the Mitigation of Drinking Water Organics. Chemosphere 2019, 217, 111–121. 10.1016/j.chemosphere.2018.10.213. [DOI] [PubMed] [Google Scholar]
- Oliveira H. G.; Ferreira L. H.; Bertazzoli R.; Longo C. Remediation of 17-α-Ethinylestradiol Aqueous Solution by Photocatalysis and Electrochemically-Assisted Photocatalysis Using TiO2 and TiO2/WO3 Electrodes Irradiated by a Solar Simulator. Water Res. 2015, 72, 305–314. 10.1016/j.watres.2014.08.042. [DOI] [PubMed] [Google Scholar]
- Arlos M. J.; Liang R.; Hatat-Fraile M. M.; Bragg L. M.; Zhou N. Y.; Servos M. R.; Andrews S. A. Photocatalytic Decomposition of Selected Estrogens and Their Estrogenic Activity by UV-LED Irradiated TiO2 Immobilized on Porous Titanium Sheets via Thermal-Chemical Oxidation. J. Hazard. Mater. 2016, 318, 541–550. 10.1016/j.jhazmat.2016.07.048. [DOI] [PubMed] [Google Scholar]
- Ma X.; Zhang C.; Deng J.; Song Y.; Li Q.; Guo Y.; Li C. Simultaneous Degradation of Estrone, 17β-Estradiol and 17α-Ethinyl Estradiol in an Aqueous UV/H2O2 System. Int. J. Environ. Res. Public Health 2015, 12, 12016–12029. 10.3390/ijerph121012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Sánchez I. M.; Tuberty S.; Hambourger M.; Bandala E. R. Resource Efficiency Analysis for Photocatalytic Degradation and Mineralization of Estriol Using TiO2 Nanoparticles. Chemosphere 2017, 184, 1270–1285. 10.1016/j.chemosphere.2017.06.046. [DOI] [PubMed] [Google Scholar]
- Mboula V. M.; Héquet V.; Andrès Y.; Gru Y.; Colin R.; Doña-Rodríguez J. M.; Pastrana-Martínez L. M.; Silva A. M. T.; Leleu M.; Tindall A. J.; Mateos S.; Falaras P. Photocatalytic Degradation of Estradiol under Simulated Solar Light and Assessment of Estrogenic Activity. Appl. Catal., B 2015, 162, 437–444. 10.1016/j.apcatb.2014.05.026. [DOI] [Google Scholar]
- Konggidinata M. I.; Chao B.; Lian Q.; Subramaniam R.; Zappi M.; Gang D. D. Equilibrium, Kinetic and Thermodynamic Studies for Adsorption of BTEX onto Ordered Mesoporous Carbon (OMC). J. Hazard. Mater. 2017, 336, 249–259. 10.1016/j.jhazmat.2017.04.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.