Abstract
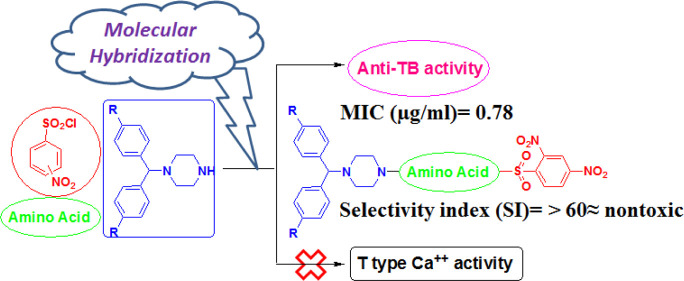
A series of novel benzhydryl piperazine-coupled nitrobenzenesulfonamide hybrids were synthesized with good to excellent yields. They were tested for in vitro inhibition of mycobacterial activity against the Mycobacterium tuberculosis H37Rv strain, in vitro cytotoxicity MTT (RAW 264.7cells) assay, nutrient starvation (H37Rv strain), and ability to block Cav3.2 T-type calcium channels. Novel hybrids did not inhibit T-type calcium channels, whereas they showed excellent antituberculosis (TB) activity and low cytotoxicity with a selectivity index of >30. A direct impact of the amino acid linker was not observed. Studied hybrids exhibited good inhibition activities, and the 2,4-dinitrobenzenesulfonamide group emerged as a promising scaffold for further drug design by hybridization approaches for anti-TB therapy.
Introduction
Mycobacterium tuberculosis (Mtb) is the bacterium that causes tuberculosis (TB) and most frequently affects the lungs. TB is highly prevalent, with the World Health Organization estimates indicating that as many as 1.8 billion people are infected with Mtb. Last year, 10 million contracted TB, leading to over 1 million deaths. TB is considered to be among the top 10 causes of death worldwide and the predominant cause of infection by a single pathogen.1 The United Nations General Assembly has urged efforts to end TB. Mtb has the propensity to adapt to treatments, thus leading to multidrug-resistant (MDR) and extremely drug-resistant (XDR) strains and thus an increasing dearth of available antibiotic treatments. One of the challenges is the fact that Mtb has an unusual composition and structure of the mycobacterial cell wall that prevents efficient entry of antibiotic drugs, thus contributing to resistance. Individuals infected with MDR-TB require treatment with a cocktail of 6–8 drugs over an extended period of up to 24 months.2 Hence, the requirement for effective chemotherapeutic agents to reduce the treatment period and to combat this disease is much higher than ever before.
There has been a major effort by the research community to devise a new tool kit of anti-TB drugs by either modifying existing drugs or developing entirely new agents.3 Efforts have been steered toward the hybridization of chemical entities with biological activities for the effective development of new drugs against TB. Such a rational approach has the potential to overcome MDR and XDR TB strains that may occur due to interruption in the first-line drug therapy, which is normally for 6 months. Molecular hybridization (MH) is one among the emerging drug design efforts which are based on the incorporation of different pharmacophores to obtain a novel hybrid. Typically, the resultant hybrid may exhibit enhanced activity compared to the parent scaffold due to the resultant dual action mode. This has so far led to the development of a number of novel anti-TB scaffolds.4
Piperazine as a precursor and/or linker is a prime scaffold for enhancing the druggability of compounds. Piperazine is useful for targeting multiple pharmacological activities,5 and it is present in more than 300 clinical drugs presently in use. Disubstituted nitrogen in the piperazine ring plays an important role in exerting selectivity and potency against various biological targets.6,7
Benzhydrylpiperazine groups are found in bioactive molecules that have antihistaminic activities, dopaminergic, antiviral, and anticancer properties.8,9 Antimicrobial inhibition has been reported10−12 and is expected to increase anti-TB activity probably by enhancing the lipophilicity of molecules. Moreover, a smaller substituent such as hydrogen/fluorine at the fourth position on the aromatic diphenyl ring may decrease molecular refractivity. However, benzhydrylpiperazines such as cyclizine, chlorcyclizine, flunarizine, and cetirizine can act as calcium channel blockers and are presently used clinical drugs. In our earlier report, substituted benzhydrylpiperazines gave rise to a new T-type calcium channel blocker with efficacy as an analgesic.13
The wide applicability of a sulfonamide framework in drug design is attributed to the use of inexpensive starting materials and the adoption of a simple synthetic strategy to wangle diverse molecules. This includes compounds targeting a range of diseases14 and drugs with antimycobacterial activity.15 Lipophilicity plays a crucial role in biological activities. A sulfonamide group may contribute to enhanced anti-TB activity by increasing lipophilicity.15,16 Sulfonyl piperazine is an attractive scaffold because of its cost-effectiveness, low toxicity, and the fact that it was reported as a possible anti-TB candidate.17−20 However, electron-deficient aromaticity induced by dinitrosubstituted benzene sulfonamide drastically enhanced the in vitro anti-TB activity.21
Amino acids are an important class for the design and development of potential pharmaceutical drugs and are well utilized in molecular modification tools such as hybridization and prodrug design.22 Strategically linking amino acids to an active pharmacophore has been reported to yield novel hybrids that make drug candidates less toxic, have reasonable bioavailability/permeability, have better metabolic and pharmacokinetic properties,23 and have been successfully incorporated into peptidomimetics and peptide analogues.24
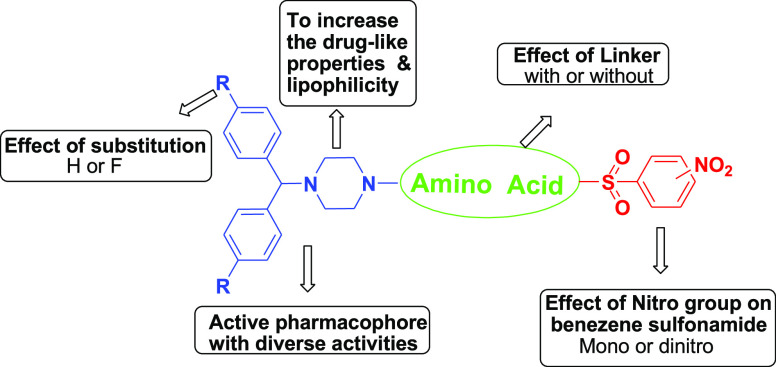
The hybridization of biologically active molecules with chemical alterations is a powerful tool for drug discovery as it enhances pharmaceutical, pharmacokinetic, or pharmacodynamic parameters. We designed and synthesized hybrid derivatives integrated into three parts: benzhydrylpiperazine as the key core moiety, amino acids as a linker for enhancing desired pharmacophoric behavior, and nitrobenzene sulfonamide for lipophilicity control based on the molecular hybridization approach. The variations in the proposed scaffold were accomplished in all three parts for a better structure–activity relationship, as shown in (Figure 1)
Figure 1.
Hybridization approach.
Results and Discussion
Syntheses
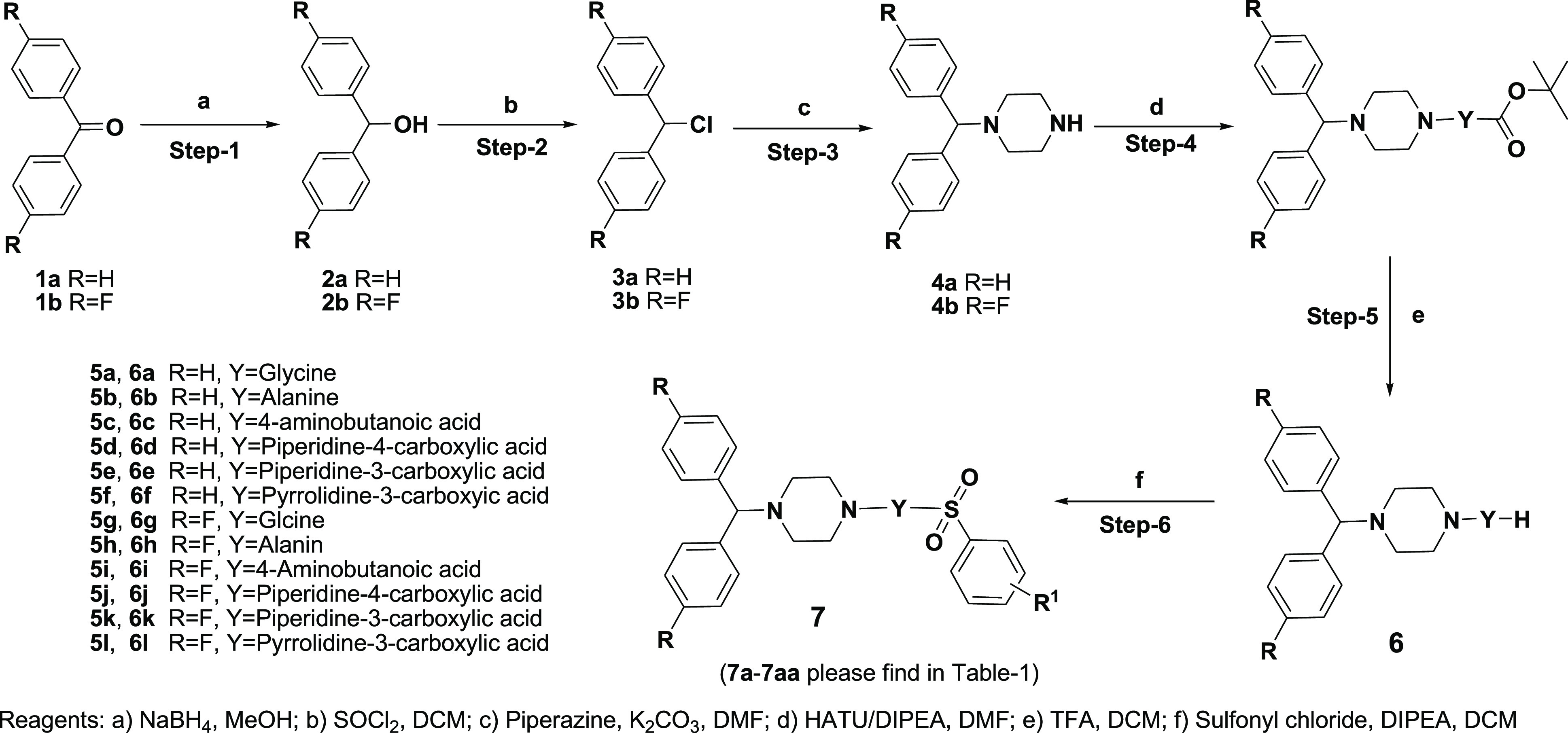
Scheme 1 describes the synthesis of designed hybrids 7a–aa. We adopted a reported procedure13,25,26 with slight modification in the reduction of appropriate benzophenones 1a–b into compounds 2a–b by sodium borohydride. Dehydroxy chlorination of 2a–b was achieved by thionyl chloride, followed by treatment with piperazine to yield 4a–b {1-benzhydryl piperazine (4a) and 1-(bis(4-fluorophenyl)methyl) piperazine (4b)}. Treatment of substituted benzhydryl piperazine with the appropriate Boc-protected amino acids catalyzed by HATU and DIPEA yielded the amide derivatives 5. Boc deprotection was achieved by trifluoroacetyl (TFA) to yield corresponding amine derivatives 6a–l. Commercially available nitro-substituted benzene sulfonyl chloride was treated with amines 6a–l using DIPEA as a base for compounds 7a–aa. All of the synthesized products were purified by column chromatography utilizing silica gel (100–200 mesh) with 2–6% methanol in dichloromethane as the eluent, followed by triturating with a mixture of n-pentane/diethyl ether (1:1). The synthesized compounds were confirmed by analytical and spectral data (1H NMR, 13C NMR, liquid chromatography–mass spectrometry, and elemental analysis). The general procedure to prepare the target compounds 7a–aa and the spectral characterization of compounds 7a–aa is included in the Supporting Information.
Scheme 1. Synthesis of 1-Benzhydryl-4-(arylsulfonyl)piperazine.
In Vitro Antimycobacterial Activity
All hybrids 7a–aa were screened for in vitro anti-TB against M. tuberculosis H37Rv (ATCC27294) using the agar dilution method for the determination of the minimum inhibitory concentration (MIC) in triplicate. The minimum concentration required to completely inhibit bacterial growth is denoted as MIC. The preliminary MIC values (1 g/mL) of 7a–aa and standard drugs for correlation are presented in Table 1.
Table 1. Antimycobacterial Activities of Compound 7a–aa against the Mtb H37Rv Strain.
| s. no | R | R(1) | Y | MIC (μg/mL) | s. no | R | R(1) | Y | MIC (μg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| 7a | -H | 2,4-dinitro | 1.56 | 7p | -H | 2-nitro | piperidine-4-carboxylic acid | >25 | |
| 7b | -H | 2-nitro | >25 | 7q | -H | 4-nitro | piperidine-4-carboxylic acid | >25 | |
| 7c | -H | 4-nitro | >25 | 7r | -F | 2,4-dinitro | piperidine-4-carboxylic acid | 1.56 | |
| 7d | -F | 2,4-dinitro | >25 | 7s | -H | 2,4-dinitro | piperidine-3-carboxylic acid | 0.78 | |
| 7e | -F | 2-nitro | >25 | 7t | -H | 2-nitro | piperidine-3-carboxylic acid | >25 | |
| 7f | -H | 4-nitro | >25 | 7u | -H | 4-nitro | piperidine-3-carboxylic acid | >25 | |
| 7g | -H | 2,4-dinitro | glycine | 12.50 | 7v | -F | 2,4-dinitro | piperidine-3-carboxylic acid | 0.78 |
| 7h | -F | 2,4-dinitro | glycine | 25 | 7w | -F | 2-nitro | piperidine-3-carboxylic acid | >25 |
| 7i | -F | 2-nitro | glycine | >25 | 7x | -F | 4-nitro | piperidine-3-carboxylic acid | >25 |
| 7j | -F | 4-nitro | glycine | 25 | 7y | -H | 2,4-dinitro | pyrrolidine-3-carboxylic acid | 0.78 |
| 7k | -H | 2,4-dinitro | alanine | 0.78 | 7z | -F | 2,4-dinitro | pyrrolidine-3-carboxylic acid | 0.78 |
| 7l | -F | 2,4-dinitro | alanine | 0.78 | 7aa | -F | 2,4-dinitro | 4-amino butanoic acid | 0.78 |
| 7m | -F | 2-nitro | alanine | >25 | Isoniazid | 0.05 | |||
| 7n | -F | 4-nitro | alanine | 25 | Rifampicin | 0.10 | |||
| 7o | -H | 2,4-dinitro | piperidine-4-carboxylic acid | 0.78 | Ethambutol | 1.56 | |||
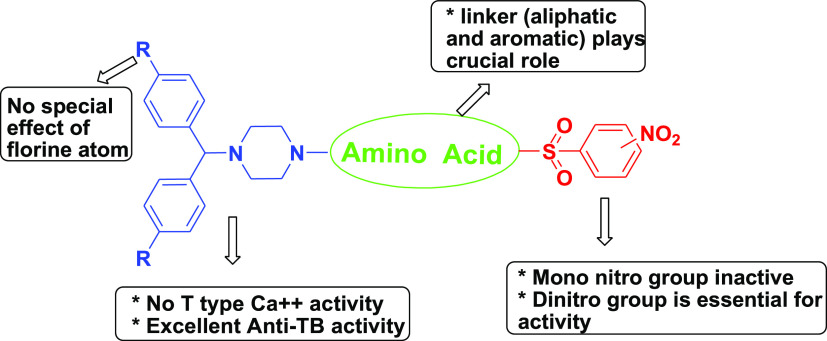
These hybrids were screened and compared to isoniazid (0.05 μg/mL), rifampicin (0.1 μg/mL), and ethambutol (1.56 μg/mL), the well-known anti-TB drugs in clinical use. All compounds exhibited in vitro activity against Mtb with MIC ranging from 0.78 to >25 μg/mL. The preliminary MIC data confirmed that all 2,4-dinitrobenzenesulfonamide derivatives were more potent than the corresponding 2- or 4-nitro derivatives. Among all these hybrids, 7a and 7r exhibited MIC at 1.56 μg/mL and were equally active as ethambutol, while 7y, 7s, 7o, 7z, 7v, 7k, 7aa, and 7l displayed MIC at 0.78 μg/mL and were superior to Ethambutol, comparable to rifampicin and inferior with respect to isoniazid. 2- or 4-Nitrobenzenesulfonamide derivatives were found to be less potent than standard drugs. The obtained results are in accordance with findings reported in the literature,21 namely, electron-withdrawing groups such as 2,4-dinitro on the phenyl ring of the sulfonamide group were found to be the most potent analogues. Introducing fluorine can lead to a tremendous increase in biological activities.27,28 Therefore, the hydrogen of benzhydryl piperazine was replaced with a fluorine atom. Unfortunately, no significant improvement in activity was observed by fluorine-containing hybrids. Amino acids such as alanine, pyrrolidine-3-carboxylic acid, piperidine-3-carboxylic acid, and 4-aminobutanoic acid emerged as better linkers without improving activity.
Cytotoxicity Determination
The active compounds in the in vitro antimycobacterial activity test were further tested for in vitro cytotoxicity using the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay against mouse macrophage RAW 264.7 cells at 50 μg/mL. The MTT assay is a colorimetric assay in which the yellow tetrazolium salt MTT is reduced to purple formazan crystals by metabolically active cells. The darker the solution, the greater the number of viable, metabolically active cells. The percentage inhibition of cells was investigated in triplicate; the approximate IC50, selectivity index (SI), and nutrient starvation (H37Rv strain) are shown in Table 2.
Table 2. In Vitro Cytotoxicity and Nutrient Starvation Studies of Active Derivatives.
| compound | MIC (μg/mL) | % cell inhibition at 50 μg/mL | IC50 approximation (μg/mL) | SI index (IC50/MIC) | nutrient starvation |
|---|---|---|---|---|---|
| 7y | 0.78 | 59.6 | >50 | >60 | 1.5 folds |
| 7s | 0.78 | 59.2 | >50 | >60 | 2.5 folds |
| 7o | 0.78 | 57.2 | >50 | >60 | 1.5 folds |
| 7z | 0.78 | 60.4 | >50 | >60 | 1.5 folds |
| 7v | 0.78 | 55.7 | >50 | >60 | 1.2 folds |
| 7k | 0.78 | 61.4 | >50 | >60 | 2.2 folds |
| 7aa | 0.78 | 59.2 | >50 | >60 | 1.0 folds |
| 7l | 0.78 | 60.2 | >50 | >60 | 1.8 folds |
| 7a | 1.56 | 57.6 | >50 | >30 | 0.8 fold |
| 7r | 1.56 | 57.2 | >50 | >30 | 2.2 folds |
| Isoniazid | 0.05 | 97 | >25 | >500 | 1.5 folds |
| Rifampicin | 0.1 | 90 | >25 | >250 | 1.8 folds |
2,4-Dinitrobenzenesulfonamide hybrids exhibited cell percentage inhibition in the range of 55.7–61.4%. Compounds that showed <50% inhibition were considered inactive. The ratio between in vitro cytotoxicity (IC50 in μg/mL) and anti-TB activity (MIC in μg/mL) determines the SI. Compounds that exhibited SI values greater than 10 in H37Rv cells were considered nontoxic. The SI values for all are >30, indicating low toxicity with high selectivity and further suitability in an endeavor to attain lead molecules for further drug development.
Nutrient Starvation Model
M. tuberculosis cultures were starved of nutrients in PBS for 6 weeks.
After 6 weeks, the culture was treated with the synthesized hybrids along with standard drugs at a concentration of 10 μg/mL. Isoniazid and rifampicin were selected for standard comparison with inhibition of 1.5-fold and 1.8-fold reduction, respectively. All tested hybrids displayed a log reduction in the range from 0.8-fold to 2.5-fold. This indicates that all compounds exhibited better activities than standard drugs on dormant cultures except 7s, 7k, and 7r. Three hybrids offer compelling inhibition of growth of M. tuberculosis (above 2-fold) in the current model as compared to the control, as shown in Figure 2.
Figure 2.
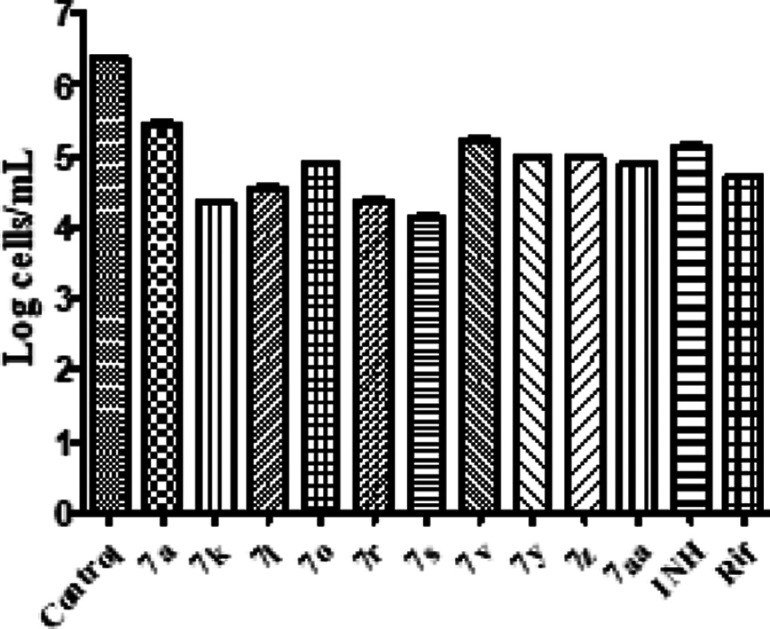
Biological activities of the active compounds against M. tuberculosis in the nutrient starvation model. Bacterial count estimation (mean ± S.D., n = 3) for control and treated groups were conducted by using the MPN (most probable number) assay (p < 0.0001, two-way ANOVA using GraphPad Prism software.)
T-Type Ca2+ Channel Blocking Activity
Given that certain piperazines can be potent T-type channel blockers, the blocking effects of all hybrids 7a–aa were tested on transiently expressed Cav3.2 T-type calcium channels at concentrations of 10 and 30 μM. This was done by using whole-cell patch-clamp recordings identical to what we described recently.13 None of the compounds mediated an inhibition greater than 20% at 30 μM concentrations, and this corresponds to an IC50 of 120 μM or greater. Hence, we conclude that the derivatives examined here are unlikely to mediate and significant T-type channel blocking activity at doses at which they are effective as antimicrobial agents.
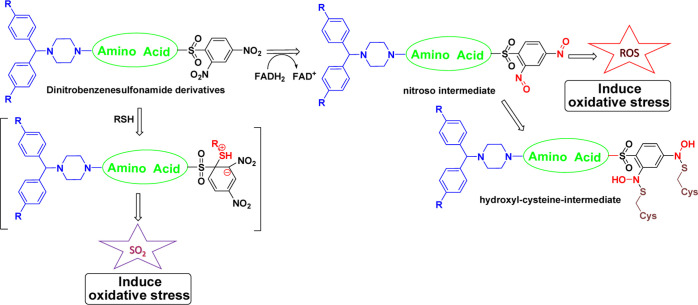
Multiple possible mechanisms of action for these sulfonamide derivatives have been postulated. It was hypothesized that the 2,4-dinitrobenzenesulfonamides may produce oxidative stress29 via formation of reactive oxygen species (ROS) and sulfur dioxide (SO2). The nitro group can be reduced by cofactor FADH2 to a corresponding nitroso intermediate. Cine addition to target-active hydroxyl intermediates thereby causes the subsequent suicide inhibition of the enzyme.21 An electron-deficient benzene ring with a thiol produces a Meisenheimer complex that dissociates to generate SO2, as shown in Figure 3.30 Apart from oxidative stress, carbonic anhydrase inhibitors,31 dihydropteroate synthase and dihydrofolate reductase inhibition,32 resistance to the aminoglycoside antibiotic kanamycin A,33 and an undefined mechanism associated with Wag3133,34 may be responsible for desired inhibition.
Figure 3.
Proposed mechanism of oxidative stress by 2,4-dinitrobenzenesulfonamides.
There was no influence when using the fluorine atom instead of hydrogen on activity. Amino acids were able to retain anti-TB activity, as shown in Figure 4.
Figure 4.
Brief SAR of novel hybrids.
Experimental Section
General
All the reagents and anhydrous solvents were purchased from Sigma-Aldrich, Alfa Aesar, and TCI. All reactions were carried out under a nitrogen atmosphere, and solvents were transferred using a syringe. Nuclear magnetic resonance spectra (1H NMR and 13C NMR) were recorded in DMSO-d6. The chemical shifts are reported in parts per million, splitting patterns are designated as follows: s, singlet; d, doublet; t, triplet; q, quadruplet; m, multiplet; brs, broad singlet; dd, doublet of doublet, and the coupling constant (J) is expressed in Hertz (Hz). Melting points (mps) were analyzed in open capillary tubes; thin-layer chromatography was carried out on silica gel F-254 plates purchased from Merck. Compounds 5a–5K were synthesized as per the literature.25,26
Syntheses
General Procedure for the Synthesis of Compounds 7a–7aa
To a stirred solution of compounds 5a–5K (1.0 equiv) in DCM (10.0 mL), TFA (3.0 equiv) was added and stirred at r.t. for 2h. The reaction mass was evaporated to dryness and codistilled twice with DCM to yield amines 6a–6k as TFA salts (not isolated), dissolved in DCM (10.0 mL) and DIPEA (5.0 equiv), followed by appropriate addition of sulfonyl chloride (1.2 equiv), and stirred at r.t. for 1 h. The reaction mixture was evaporated, and the crude extract was purified by silica gel column chromatography using ethyl acetate in n-hexane (20–40% v/v) as the eluent to yield compounds 7a–7aa.
1-Benzhydryl-4-((2,4-dinitrophenyl)sulfonyl)piperazine (7a)
Using 4a and 2,4-dinitro benzenesulfonyl chloride as starting materials, compound 7a was obtained with yield: 65%, off-yellow solid. mp 196–200 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.37 (4H, brs, 2 × piperazine–CH2), 3.25 (4H, brs, 2 × piperazine–CH2), 4.36 (1H, s, CH), 7.16 (2H, t, J = 7.2, 2 × Ar–H), 7.26 (4H, t, J = 7.5, 4 × Ar–H), 7.37 (4H, d, J = 7.4, 4 × Ar–H), 8.22 (1H, d, J = 8.7, Ar–H), 8.57 (1H, dd, J = 8.7 & 2.1, Ar–H), 9.00 (1H, d, J = 2.0, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 45.94, 50.42, 73.89, 119.93, 126.81, 126.97, 127.46, 128.54, 132.22, 133.74, 142.07, 147.83, 150.15; MS (ESI positive) m/z: 483.02 [M + H]+. Elemental analysis calculated for C23H22N4O6S: C, 57.25; H, 4.60; N, 11.61. Found: C, 57.28; H, 4.63; N, 11.65.
1-Benzhydryl-4-((2-nitrophenyl)sulfonyl)piperazine (7b)
Using 4a and 2-nitrobenzene sulfonyl chloride as starting materials, compound 7b was obtained with yield: 72%, off-white solid. mp 132–136 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.36 (4h, brs, 2 × piperazine–CH2), 3.19 (4H, brs, 2 × piperazine–CH2), 4.33 (1H, s, CH), 7.16 (2H, t, J = 7.2, 2 × Ar–H), 7.26 (4H, t, J = 7.4, 4 × Ar–H), 7.37 (4H, d, J = 7.3, 4 × Ar–H), 7.84–7.99 (4H, m, 4 × Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 45.89, 50.48, 74.06, 124.09, 126.92, 127.46, 128.52, 130.42, 132.18, 134.84, 142.16, 147.97; MS (ESI positive) m/z: 437.9 [M + H]+. Elemental analysis calculated for C23H23N3O4S: C, 63.14; H, 5.30; N, 9.60. Found: C, 63.18; H, 5.35; N, 9.58.
1-Benzhydryl-4-((4-nitrophenyl)sulfonyl)piperazine (7c)
Using 4a and 4-nitrobenzene sulfonyl chloride as starting materials, compound 7c was obtained with yield 46%, off-white solid; mp 216–218 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.35 (4H, brs, 2 × piperazine–CH2), 2.99 (4H, brs, 2 × piperazine–CH2), 4.31 (1H, s, CH), 7.14 (2H, t, J = 7.2, 2 × Ar–H), 7.23 (4H, t, J = 7.4, 4 × Ar–H), 7.33 (4H, d, J = 7.3, 4 × Ar–H), 7.98 (2H, d, J = 8.8, 2 × Ar–H), 8.45 (2H, d, J = 7.2, 2 × Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 46.00, 50.21, 74.01, 124.67, 126.91, 127.41, 128.49, 129.09, 140.45, 142.14, 150.07; MS (ESI positive) m/z: 437.9 [M + H]+. Elemental analysis calculated for C23H23N3O4S: C, 63.14; H, 5.30; N, 9.60. Found: C, 63.17; H, 5.36; N, 9.59.
1-(Bis(4-fluorophenyl)methyl)-4-((2,4-dinitrophenyl)sulfonyl)piperazine (7d)
Using 4b and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7d was obtained with yield: 65%, off-brown solid; mp 192–196 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.35 (4H, brs, 2 × piperazine–CH2), 3.24 (4H, brs, 2 × piperazine–CH2), 4.45 (1H, s, CH), 7.12 (4H, t, J = 7.5, 4 × Ar–H), 7.38 (4H, t, J = 8.2, 4 × Ar–H), 8.22 (1H, d, J = 8.7, Ar–H), 8.58 (1H, dd, J = 8.7 & 2.0, Ar–H), 8.99 (1H, d, J = 2.0, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 45.89, 50.23, 71.86, 115.22, 115.43, 119.92, 126.80, 129.27, 129.35, 132.23, 133.78, 137.94, 147.80, 150.11, 159.86, 162.28; MS (ESI positive) m/z: 518.92 [M + H]+. Elemental analysis calculated for C23H20F2N4O6S: C, 53.28; H, 3.89; N, 10.81. Found: C, 53.26; H, 3.90; N, 10.85.
1-(Bis(4-fluorophenyl)methyl)-4-((2-nitrophenyl)sulfonyl)piperazine (7e)
Using 4b and 2-nitrobenzenesulfonyl chloride as starting materials, compound 7e was obtained with yield: 77%, off-white solid; mp 120–124 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.34 (4H, brs, 2 × piperazine–CH2), 3.18 (4H, brs, 2 × piperazine–CH2), 4.43 (1H, s, CH), 7.09 (4H, t, J = 8.7, 4 × Ar–H), 7.39 (4H, t, J = 8.2, 4 × Ar–H), 7.84–7.99 (4H, m, 4 × Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 45.88, 50.30, 72.00, 115.23, 115.44, 124.11, 128.44, 129.29, 129.37, 130.44, 132.21, 134.89, 138.07, 147.97, 159.86, 162.28; MS (ESI positive) m/z: 474.04 [M + H]+. Elemental analysis calculated for C23H21F2N3O4S: C, 58.34; H, 4.47; N, 8.87. Found: C, 58.37; H, 4.51; N, 8.91.
1-(Bis(4-fluorophenyl)methyl)-4-((4-nitrophenyl)sulfonyl)piperazine (7f)
Using 4b and 4-nitrobenzenesulfonyl chloride as starting materials, compound 7f was obtained with yield: 73%, yellow solid; mp 222–226 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.34 (4H, brs, 2 × piperazine–CH2), 2.98 (4H, brs, 2 × piperazine–CH2), 4.40 (1H, s, CH), 7.07 (4H, t, J = 8.8, 4 × Ar–H), 7.34 (4H, m, J = 5.7, 4 × Ar–H), 7.98 (2H, d, J = 8.7, 2 × Ar–H), 8.45 (2H, d, J = 8.7, 2 × Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 45.97, 50.03, 71.96, 115.18, 115.39, 124.66, 129.09, 129.22, 129.30, 138.05, 140.47, 150.08, 159.82, 162.24; MS (ESI positive) m/z: 473.9 [M + H]+. Elemental analysis calculated for C23H21F2N3O4S: C, 58.34; H, 4.47; N, 8.87. Found: C, 58.35; H, 4.49; N, 8.90.
N-(2-(4-Benzhydrylpiperazin-1-yl)-2-oxoethyl)-2,4-dinitrobenzenesulfonamide (7g)
Using 5a and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7g was obtained with yield 32%, off-white solid; mp 206–210 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.18 (2H, brs, piperazine–CH2), 2.25 (2H, brs, piperidine-CH2), 3.34 (4H, brs, 2 × piperazine–CH2), 3.96 (2H,s, J = 4.8, CH2), 4.28 (1H, s, CH), 7.17 (2H, t, J = 7.3, 2 × Ar–H), 7.28 (4H, t, J = 7.4, 4 × Ar–H), 7.40 (4H, d, J = 7.3, 4 × Ar–H), 8.25 (1H, d, J = 8.6, Ar–H), 8.43 (1H, brs, N H), 8.59 (1H, dd, J = 8.7 & 1.9, Ar–H), 8.83 (1H, d, J = 1.8, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 41.52, 44.10, 50.92, 51.34, 74.57, 119.82, 126.90, 127.52, 128.50, 131.70, 138.50, 142.30, 147.20, 149.33, 165.42; MS (ESI positive) m/z: 540.16 [M + H]+. Elemental analysis calculated for C25H25N5O7S: C, 55.65; H, 4.67; N, 12.98. Found: C, 55.64; H, 4.68; N, 13.01.
N-(2-(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl)-2-oxoethyl)2,4-dinitrobenzene Sulfonamide (7h)
Using 5f and 2,4-nitrobenzenesulfonyl chloride as starting materials, compound 7h was obtained with yield: 40%, off-yellow solid; mp 246–250 °C; 1H NMR (DMSO-d6,400 MHz): δ 2.16 (2H, brs, piperazine–CH2) 2.23 (2H, brs, piperazine–CH2), 3.34 (4H, brs, 2 × piperazine–CH2), 3.98 (2H, d, J = 5.2, CH2), 4.38 (1H, s, CH), 7.12 (4H, t, J = 8.7, 4 × Ar–H), 7.41 (4H, t, J = 8.3, 4 × Ar–H), 8.25 (1H, d, J = 8.7, Ar–H), 8.44 (1H, t, J = 5.2, NH), 8.60 (1H, dd, J = 8.7 & 2.2, Ar–H), 8.82 (1H, d, J = 2.1, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 41.48, 43.98, 44.07, 50.68, 51.11, 72.45, 115.16, 115.37, 119.79, 126.91, 129.30, 129.38, 131.68, 138.15, 138.48, 147.19, 149.33, 159.83, 162.24, 165.41; MS (ESI positive) m/z: 576.06 [M + H]+. Elemental analysis calculated for C25H23F2N5O7S: C, 52.17; H, 4.03; N, 12.17. Found: C, 52.19; H, 4.05; N, 12.19.
N-(2-(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl)-2-oxoethyl)2-nitrobenzene Sulfonamide (7i)
Using 5f and 2-nitrobenzenesulfonyl chloride as starting materials, compound 7i was obtained with yield: 69%, off-white solid; mp 136–140 °C; 1H NMR (DMSO-d6, 400 MHZ): δ 2.16 (2H, br d, 2 × piperazine–CH2), 2.22 (2H, br d, 2 × piperazine–CH2), 3.34 (4H, brs, 2 × piperazine–CH2), 3.90 (2H, d, J = 5.38, CH2), 4.37 (1H, s, CH), 7.11 (4H, t, J = 8.7, 4 × Ar–H), 7.41 (4H, q, J = 8.3, 4 × Ar–H), 7.81 (2H, m, 2 × Ar–H), 7.93 (2H, m, 2 × Ar–H), 8.02 (1H, m, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 44.73, 51.30, 73.55, 115.97, 116.17, 125.19, 130.11, 130.19, 130.57, 133.49, 133.54, 134.81, 138.81, 147.94, 160.60, 163.62, 166.40; MS (ESI positive) m/z: 531.12 [M + H]+. Elemental analysis calculated for C25H24F2N4O5S: C, 56.60; H, 4.56; N, 10.56. Found: C, 56.59; H, 4.55; N, 10.59.
N-(2-(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl)-2-oxoethyl)4-nitrobenzene Sulfonamide (7j)
Using 5f and 4-nitrobenzenesulfonyl chloride as starting materials, compound 7j was obtained with yield: 47%, off-white solid; mp 176–180 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.15 (2H, br d, piperazine–CH2), 2.20 (2H, br d, piperazine–CH2), 3.33 (4H, brs, 2 × piperazine–CH2), 3.81 (2H, d, J = 5.5, CH2), 4.37 (1H, s, CH), 7.11 (4H, t, J = 8.6, 4 × Ar–H), 7.40 (4H, t, J = 8.1, 4 × Ar–H), 8.03 (2H, d, J = 8.6, 2 × Ar–H), 8.14 (1H, t, J = 5.4, NH), 8.36 (2H, d, J = 8.6, 2 × Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 41.40, 43.68, 44.08, 50.70, 51.18, 72.48, 115.17, 115.38, 115.50, 115.72, 124.17, 129.31, 129.39, 132.37, 132.47, 138.15, 146.29, 149.37, 159.83, 162.25, 165.30; MS (ESI positive) m/z: 531.04 [M + H]+. Elemental analysis calculated for C25H24F2N4O5S: C, 56.60; H, 4.56; N, 10.56. Found: C, 56.64; H, 4.58; N, 10.59.
N-(3-(4-Benzhydrylpiperazin-1-yl)-3-oxopropyl)-2,4-dinitrobenzenesulfonamide (7k)
Using 5b and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7k was obtained with yield 29%, off-white solid; mp 82–86 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.22 (4H, br d, 2 × piperazine–CH2), 2.48 (2H, t, J = 7.2, COCH2), 3.14 (2H, q, J = 7.4, CH2NH), 3.38 (4H, br d, 2 × piperazine–CH2), 4.28 (1H, s, CH), 7.18 (2H, t, J = 7.2, 2 × Ar–H), 7.28 (4H, t, J = 7.4, 4 × Ar–H), 7.41 (4H, d, J = 7.4, 4 × Ar–H), 8.24 (1H, d, J = 8.6, Ar–H), 8.35 (1H, t, J = 5.5, NH), 8.61 (1H, dd, J = 8.6 & 2.1, Ar–H), 8.86 (1H, d, J = 2.0, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 32.36, 41.00, 44.64, 51.03, 51.55, 74.64, 120.01, 126.87, 127.19, 127.51, 128.48, 131.26, 137.69, 142.37, 147.58, 168.08; MS (ESI) m/z: 554.16 [M + 1]+. Elemental analysis calculated for C26H27N5O7S: C, 56.41; H, 4.92; N, 12.65. Found: C, 56.44; H, 4.93; N, 12.68.
N-(3-(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl)-3-oxopropyl)-2,4-dinitrobenzene Sulfonamide (7l)
Using 5g and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7l was obtained with yield 53%, yellow solid; 1H NMR (DMSO-d6, 400 MHz): δ 2.21 (4H, br d, 2 × piperazine–CH2), 2.47 (2H, m, COCH2), 3.14 (2H, d, J = 5.8, CH2NH), 3.73 (4H, br d, 2 × piperazine–CH2), 4.37 (1H, s, CH), 7.12 (4H, s, 4 × Ar–H), 7.42 (4H, s, 4 × Ar–H), 8.24 (1H, d, J = 8.6, Ar–H), 8.37 (1H, s, NH), 8.62 (1H, d, J = 8.2, Ar–H), 8.87 (1H, s, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 32.39, 40.88, 44.53, 50.86, 51.34, 72.57, 115.27, 115.48, 120.05, 127.23, 129.38, 129.46, 131.31, 137.71, 138.06, 147.62, 149.60, 159.91, 162.33, 168.12; MS (ESI) m/z: 590.19 [M – 1]+. Elemental analysis calculated for C26H25F2N5O7S: C, 52.97; H, 4.27; N, 11.88. Found: C, 52.99; H, 4.28; N, 11.92.
N-(3-(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl)-3-oxopropyl)-2-nitrobenzene Sulfonamide (7m)
Using 5g and 2-nitrobenzenesulfonyl chloride as starting materials, compound 7m was obtained with yield 53%, off-white solid; mp 142–146 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.20 (4H, brs, 2 × piperazine–CH2), 2.47 (2H, m, COCH2), 3.08 (2H, d, J = 5.5, CH2NH), 3.38 (4H, br d, 2 × piperazine–CH2), 4.37 (1H, s, CH), 7.12 (4H, t, J = 8.3, 4 × Ar–H), 7.42 (4H, s, 4 × Ar–H), 7.85–7.98 (5H, m, SO2NHCH2, 4 × Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 32.34, 40.96, 44.64, 50.81,51.29, 72.52, 115.17, 115.38, 124.40, 129.30, 129.38, 129.46, 132.46, 132.62, 133.96, 147.67, 159.82, 162.24, 168.19; MS (ESI) m/z: 543.43 [M – 1]+. Elemental analysis calculated for C26H26F2N4O5S: C, 57.34; H, 4.81; N, 10.29. Found: C, 57.38; H, 4.85; N, 10.34.
N-(3-(4-(bis(4-fluorophenyl)methyl)piperazin-1-yl)-3-oxopropyl)-4-nitrobenzene Sulfonamide (7n)
Using 5g and 4-nitrobenzenesulfonyl chloride as starting materials, compound 7n was obtained with 48%; off-white solid; mp 68–72 °C; 1H NMR (DMSO-d6, 400 MHz): δ 2.20 (4H, brs, 2 × piperazine–CH2), 2.42 (2H, t, J = 6.7, COCH2), 2.99 (2H, q, J = 6.3, CH2NH), 3.34 (2H, brs, piperazine–CH2), 3.39 (2H, brs, piperidine-CH2), 4.36 (1H, s, CH), 7.12 (4H, t, J = 8.7, 4 × Ar–H), 7.41 (4H, t, J = 8.1, 4 × Ar–H), 7.96 (1H, t, J = 5.5, CH2NHSO2), 8.02 (2H, d, J = 8.6, 2 × Ar–H), 8.40 (2H, d, J = 8.6, 2 × Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 32.42, 40.98, 44.64, 50.84, 51.33, 72.61, 115.17, 115.38, 124.48, 128.02, 129.28, 129.36, 138.86, 146.07, 149.49, 159.84, 162.26, 168.07; MS (ESI) m/z: 545.09 [M + 1]+. Elemental analysis calculated for C26H26F2N4O5S: C, 57.34; H, 4.81; N, 10.29. Found: C, 57.39; H, 4.84; N, 10.33.
(4-Benzhydrylpiperazin-1-yl) (1-((2,4-dinitrophenyl)sulfonyl)piperidin-4-yl)methanone (7o)
Using 5c and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7o was obtained with yield 46%, off-white solid; mp 184–188 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.49 (2H, m, piperidine–CH2), 1.78 (2H, m, piperidine–CH2), 2.23 (4H, brs, 2 × piperazine–CH2), 2.69 (1H, m, piperidine–COCH), 2.84 (2H, t, J = 10.8, piperidine–CH2), 3.43 (4H, br d, 2 × piperazine–CH2), 3.69 (2H, d, J = 12.4, piperidine–CH2), 4.29 (1H, s, CH), 7.17 (2H, t, J = 7.2, 2 × Ar–H), 7.28 (4H, t, J = 7.4, 4 × Ar–H), 7.41 (4H, d, J = 7.3, 4 × Ar–H), 8.26 (1H, d, J = 8.7, Ar–H), 8.56 (1H, dd, J = 2.1, 8.7, Ar–H), 8.97 (1H, d, J = 2.1, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 27.74, 35.62, 41.10, 44.70, 44.97, 51.21, 51.98, 74.62, 119.86, 126.72, 126.87, 127.57, 128.47, 132.15, 134.64, 142.33, 147.61, 150.00, 171.45; MS (ESI positive) m/z: 594.32 [M + H]+. Elemental analysis calculated for C29H31N5O7S: C, 58.67; H, 5.26; N, 11.80. Found: C, 58.66; H, 5.26; N, 11.83.
(4-Benzhydrylpiperazin-1-yl) (1-((2-Nitrophenyl)sulfonyl)piperidin-4-yl)methanone (7p)
Using 5c and 2-nitrobenzenesulfonyl chloride as starting materials, compound 7p was obtained with yield 49%, off-white solid; mp 158–160 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.48 (2H, m, piperidine–CH2), 1.66 (2H, m, piperidine–CH2), 2.22 (4H, brs, 2 × piperazine–CH2), 2.72 (3H, m, piperidine–CH2, COCH), 3.44 (4H, br d, 2 × piperazine–CH2), 3.66 (2H, d, piperidine–CH2), 4.29 (1H, s, CH), 7.17 (2H, t, J = 7.3, 2 × Ar–H), 7.28 (4H, t, J = 7.4, 4 × Ar–H), 7.40 (4H, d, J = 7.5, 4 × Ar–H), 7.85 (2H, m, 2 xAr-H), 7.97 (2H, m, 2 × Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 27.72, 35.72, 41.3, 44.69, 44.96, 51.23, 52.00, 74.64, 124.00, 126.01, 126.87, 127.54, 128.47, 129.20, 130.31, 132.10, 134.66, 142.37, 147.83, 171.55; MS (ESI positive) m/z: 548.9 [M + H]+. Elemental analysis calculated for C29H32N4O5S: C, 63.48; H, 5.88; N, 10.21. Found: C, 63.51; H, 5.93; N, 10.25.
(4-Benzhydrylpiperazin-1-yl) (1-((4-Nitrophenyl)sulfonyl)piperidin-4-yl)methanone (7q)
Using 5c and 4-nitrobenzenesulfonyl chloride as starting materials, compound 7q was obtained with yield 59%, off-white solid; mp 182–186 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.49 (2H, m, piperidine–CH2), 1.64 (2H, m, piperidine–CH2), 2.20 (4H, brs, 2 × piperazine–CH2), 2.37 (2H, m, piperidine–CH2), 2.57 (1H, m, COCH), 3.41 (4H, brs, 2 × piperazine–CH2), 3.62 (2H, m, piperidine–CH2), 4.27 (1H, s, CH), 7.16 (2H, t, J = 7.3, 2 × Ar–H), 7.27 (4H, t, J = 7.4, 4 × Ar–H), 7.39 (4H, d, J = 7.5, 4 × Ar–H), 7.99 (2H, d, J = 6.9, 2 × Ar–H), 8.42 (2H, d, J = 6.9, 2 × Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 27.54, 35.62, 42.00, 44.67, 45.14, 51.23, 51.97, 74.63, 124.56, 126.89, 127.53, 128.48, 128.99, 141.17, 142.34, 149.94, 171.53; MS (ESI positive) m/z: 548.9 [M + H]+. Elemental analysis calculated for C29H32N4O5S: C, 63.48; H, 5.88; N, 10.21. Found: C, 63.50; H, 5.91; N, 10.22.
(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl) (1-((2,4-Nitrophenyl)sulfonyl)piperidin-4-yl)methanone (7r)
Using 5h and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7r was obtained with yield: 45%, off-white solid; mp 238–242 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.50 (2H, m, piperidine–CH2), 1.68 (2H, d, J = 11.9, piperidine–CH2), 2.21 (4H, brs, 2 × piperazine–CH2), 2.70 (1H, m, piperidine–COCH), 2.84 (2H, t, J = 11.8, piperidine–CH2), 3.44 (4H, br d, 2 × piperazine–CH2), 3.69 (2H, d, J = 12.4, piperidine–CH2), 4.38 (1H, s, CH), 7.11 (4H, t, J = 8.7, 4 × Ar–H), 7.41 (4H, t, J = 8.3, 4 × Ar–H), 8.26 (1H, d, J = 8.7, Ar–H), 8.56 (1H, dd, J = 2.1, 8.6, Ar–H), 8.98 (1H, d, J = 2.1, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 27.74, 35.61, 41.09, 44.69, 44.96, 50.99, 51.77, 72.53, 115.15, 115.36, 119.86, 126.71, 129.32, 129.39, 132.14, 134.67, 138.20, 147.61, 150.00, 159.82, 162.24, 171.47; MS (ESI positive) m/z: 630.14 [M + H]+. Elemental analysis calculated for C29H29F2N5O7S: C, 55.32; H, 4.64; N, 11.12. Found: C, 55.31; H, 4.64; N, 11.13.
(4-Benzhydrylpiperazin-1-yl) (1-((2,4-Dinitrophenyl)sulfonyl)piperidin-3-yl)methanone (7s)
Using 5d and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7s was obtained with yield 31%; off-yellow solid; mp 120–126 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.31 (2H, m, piperidine–CH2), 1.74 (2H, m, piperidine–CH2), 2.26 (4H, m, 2 × piperazine–CH2), 2.78 (3H, m, piperidine–CH2 & COCH), 3.49 (4H, m, 2 × piperazine–CH2), 3.67 (2H, m, piperidine–CH2), 4.30 (1H, s, CH) 7.18 (2H, t, J = 7.3, 2 × Ar–H), 7.29 (4H, t, J = 7.4, 4 × Ar–H), 7.42 (4H, d, J = 7.3, 4 × Ar–H), 8.25 (1H, d, J = 8.7, Ar–H), 8.54 (1H, dd, J = 2.0, 8.7, Ar–H), 8.95 (1H, d, J = 1.7, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 24.00, 26.72, 37.69, 41.19, 44.93, 46.03, 48.00, 51.30, 52.03, 74.81, 120.01, 127.00, 127.05, 127.67, 128.65, 132.08, 135.00, 142.50, 147.71, 150.12, 170.37; MS (ESI) m/z: 594.30 [M + 1]+. Elemental analysis calculated for C29H32N4O5S: C, 63.48; H, 5.88; N, 10.21. Found: C, 63.51; H, 5.91; N, 10.25.
(4-Benzhydrylpiperazin-1-yl) (1-((2-Nitrophenyl)sulfonyl)piperidin-3-yl)methanone (7t)
Using 5d and 2-nitrobenzenesulfonyl chloride as starting materials, compound 7t was obtained with yield 31%; off-white solid; mp 120–124 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.30 (1H, m, piperidine–CH), 1.50 (1H, m, piperidine–CH), 1.73 (2H, m, piperidine–CH2), 2.30 (4H, m, 2 × piperazine–CH2), 2.75 (3H, m, piperidine–CH2, COCH), 3.49 (4H, m, 2 × piperazine–CH2), 3.71 (2H, m, piperidine–CH2), 4.30 (1H, s, CH) 7.18 (2H, appt, J = 7.2, Ar–H), 7.29 (4H, appt, J = 7.2, Ar–H), 7.42 (4H, d, J = 7.2, Ar–H) 7.90 (2H, m, Ar–H), 7.97 (2H, m, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 23.92, 26.64, 41.04, 44.78, 45.77, 47.89, 51.18, 51.91, 74.63, 124.10, 126.88, 127.52, 128.49, 129.61, 130.08, 132.25, 134.61, 142.39, 147.75, 170.21; MS (ESI) m/z: 548.9 [M + 1]+. Elemental analysis calculated for C29H32N4O5S: C, 63.48; H, 5.88; N, 10.21. Found: C, 63.53; H, 5.93; N, 10.23.
(4-Benzhydrylpiperazin-1-yl) (1-((4-Nitrophenyl)sulfonyl)piperidin-3-yl)methanone (7u)
Using 5d and 4-nitrobenzenesulfonyl chloride, compound 7u was obtained with 45% yield; off-white solid; mp 218–220 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.22 (1H, m, piperidine–CH), 1.53 (1H, m, piperidine–CH), 1.69 (2H, m, piperidine–CH2), 2.32 (6H, m, 2 × piperazine–CH2), 2.80 (1H, m, COCH), 3.49 (4H, m, 2 × piperazine–CH2), 3.54 (2H, m, piperidine–CH2), 4.30 (1H, s, CH), 7.18 (2H, appt, J = 7.4, Ar–H), 7.30 (4H, appt, J = 7.4, Ar–H), 7.43 (4H, d, J = 7.4, Ar–H), 7.99 (2H, d, J = 9.0), 8.43 (2H, d, J = 9.0); 13C NMR (DMSO-d6, 100 MHz): δ 23.62, 26.60, 41.04, 44.80, 45.91, 48.06, 51.17, 51.92, 74.63, 124.66, 126.90, 127.53, 128.50, 128.84, 141.30, 142.40, 149.98, 170.27; MS (ESI) m/z: 548.9 [M + 1]+. Elemental analysis calculated for C29H32N4O5S: C, 63.48; H, 5.88; N, 10.21. Found: C, 63.52; H, 5.91; N, 10.25.
(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl) (1-((2,4-Dinitrophenyl)sulfonyl)piperidin-3-yl)methanone (7v)
Using 5i and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7v was obtained with 48% yield; yellow solid; mp 122–128 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.33 (1H, m, piperidine–CH), 1.50 (1H, m, piperidine–CH), 1.74 (2H, m, piperidine–CH2), 2.34 (4H, m, 2 × piperazine–CH2), 2.67 (1H, m, COCH), 2.80 (2H, m, piperidine–CH2), 3.48 (4H, m, 2×piperazine–CH2), 3.67 (2H, m, piperidine–CH2), 4.39 (1H, s, CH), 7.12 (4H, t, J = 8.6, Ar–H), 7.43 (4H, t, J = 5.7, Ar–H), 8.25 (1H, d, J = 8.7, Ar–H), 8.54 (1H, d, J = 8.7, Ar–H, 8.96 (1H, d, J = 1.43, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 23.96, 26.68, 44.80, 45.83, 47.92, 51.03, 51.76, 72.60, 115.25, 115.46, 124.15, 129.38, 129.46, 129.60, 130.13, 132.33, 134.70, 138.34, 147.79, 159.88, 162.30, 170.30; MS (ESI) m/z: 630.19 [M + 1]+. Elemental analysis calculated for C29H29F2N5O7S: C, 55.32; H, 4.64; N, 11.12. Found: C, 55.34; H, 4.66; N, 11.15.
(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl) (1-((2-Nitrophenyl)sulfonyl)piperidin-3-yl) Methanone (7w)
Using 5i and 2-nitrobenzenesulfonyl chloride as starting materials, compound 7w was obtained in 57% yield; off-white solid; mp 82–86 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.25 (1H, m, piperidine–CH), 1.50 (1H, m, piperidine–CH), 1.74 (2H, m, piperidine–CH2), 2.26 (4H, m, 2 × piperazine–CH2), 2.63 (1H, m, COCH), 2.75 (2H, m, piperidine–CH2), 3.46 (4H, m, 2 × piperazine–CH2), 3.64 (2H, m, piperidine–CH2), 4.41 (1H, s, CH), 7.12 (4H, t, J = 8.4, Ar–H), 7.43 (4H, m, Ar–H), 7.82–7.97 (4H, m, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 23.92, 26.64, 41.00, 44.76, 45.78, 47.88, 50.98, 51.72, 72.54, 115.19, 116.2 (d, 2JC–F 21), 129.33, 129.40, 129.59, 131.1 (d, 3JC–F 9), 134.63, 138.28, 147.75, 162.1 (d, 1JC–F 242), 170.23; MS (ESI) m/z: 585.24 [M + 1]+. Elemental analysis calculated for C29H30F2N4O5S: C, 59.58; H, 5.17; N, 9.58. Found: C, 59.62; H, 5.19; N, 9.61.
(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl) (1-((4-Nitrophenyl)sulfonyl)piperidin-3-yl) Methanone (7x)
Using 5i and 4-nitrobenzenesulfonyl chloride as starting materials, compound 7x was obtained with 57% yield; off-white solid; 1H NMR (DMSO-d6, 400 MHz): δ 1.15 (1H, m, piperidine–CH), 1.55 (1H, m, piperidine–CH), 1.69 (2H, m, piperidine–CH2), 2.26 (6H, m, 2 × piperazine–CH2), 2.81 (1H, m, COCH), 3.56 (6H, m, 2 × piperazine–CH2), 4.39 (1H, s, CH), 7.12 (4H, t, J = 8.4, Ar–H), 7.43 (4H, m, Ar–H), 7.98 (2H, d J = 9.2, Ar–H) 8.42 (2H, d J = 9.2, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 23.58, 26.56, 41.00, 44.76, 45.87, 48.01, 51.68, 72.51, 115.15, 115.36, 124.61, 128.79, 129.31, 129.37, 138.23, 141.38, 149.96, 159.82, 162.24, 170.25; MS (ESI) m/z: 585.15 [M + 1]+. Elemental analysis calculated for C29H30F2N4O5S: C, 59.58; H, 5.17; N, 9.58. Found: C, 59.60; H, 5.19; N, 9.62.
(4-Benzhydrylpiperazin-1-yl) (1-((2,4-Dinitrophenyl)sulfonyl)pyrrolidin-3-yl)methanone (7y)
Using 5e and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7y was obtained with yield 31%; brown solid; mp 110–116 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.89 (1H, m, pyrrolidine–CH), 2.08 (1H, m, pyrrolidine–CH), 2.29 (4H, br-d, 2 × piperazine–CH2), 3.34–3.56 (9H, m, 2 × piperidine–CH2, 2 × pyrrolidine-CH2 and COCH), 4.29 (1H, s, N–CH), 7.18 (2H, t, J = 7.27, 2 × Ar–H), 7.28 (4H, t, J = 7.42, 4 × Ar–H), 7.41 (4H, d, J = 7.7, 4 × Ar–H), 8.28 (1H, d, J = 8.71, Ar–H), 8.54 (1H, dd, J = 1.9, 8.68, Ar–H),8.95 (1H, d, J = 1.83, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 28.75, 41.40, 44.84, 47.72, 50.08, 51.09, 51.74, 74.60, 119.83, 126.76, 126.91, 127.53, 128.52, 131.90, 134.58, 142.38, 147.79, 149.89, 169.33; MS (ESI) m/z: 580.25 [M + 1]+. Elemental analysis calculated for C28H29N5O7S: C, 58.02; H, 5.04; N, 12.08. Found: C, 58.05; H, 5.05; N, 12.12.
(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl) (1-((4-Nitrophenyl)sulfonyl)pyrrolidin-3-yl) Methanone (7z)
Using 5j and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7z was obtained with 48% yield; brown solid; 1H NMR (DMSO-d6, 400 MHz): δ 1.89 (1H, m, pyrrolidine–CH), 2.08 (1H, m, pyrrolidine–CH), 2.25 (4H, br d, 2 × piperazine–CH2), 3.34–3.51 (8H, m, 2 × piperazine–CH2, 2 × pyrrolidine–CH2), 4.47 (1H, s, CH), 7.12 (4H, m, 4 × Ar–H), 7.42 (4H, t, J = 7.9, 4 × Ar–H), 8.28 (1H, d, J = 8.7, Ar–H), 8.54 (1H, dd, J = 2.0, 8.7, Ar–H), 8.96 (1H, d, J = 2.0, Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 28.83, 41.45, 44.90, 47.79, 50.15, 50.94, 51.59, 72.62, 115.26, 115.47, 119.88, 126.83, 129.40, 129.48, 131.96, 134.72, 138.30, 147.85, 149.94, 159.92, 162.34, 169.49; MS (ESI) m/z: 616.15 [M + 1]+. Elemental analysis calculated for C28H27F2N5O7S: C, 54.63; H, 4.42; N, 11.38. Found: C, 54.62; H, 4.42; N, 11.42.
N-(4-(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl)-4-oxobutyl)-2,4-dinitrobenzene Sulfonamide (7aa)
Using 5k and 2,4-dinitrobenzenesulfonyl chloride as starting materials, compound 7aa was obtained with 60% yield; yellow solid; 1H NMR (DMSO-d6, 400 MHz): δ 1.61 (2H, m, CH2), 2.24 (6H, m, 2 × piperazine–CH2, CH2), 2.93 (2H, m, COCH2), 3.35 (4H, m, 2 × piperazine–CH2), 4.37 (1H, s, CH), 7.12 (4H, t, J = 8.79, 4 × Ar–H), 7.42 (4H, dd, J = 5.7,8.47, 4 × Ar–H), 8.20 (1H, d J = 8.7, Ar–H), 8.45 (1H, brs, Ar–H), 8.62 (1H, dd, J = 2.7, 8.68, Ar–H), 8.87 (1H, d, J = 2.13,Ar–H); 13C NMR (DMSO-d6, 100 MHz): δ 24.71, 28.91, 41.01, 42.35, 44.69, 50.96, 51.44, 72.61, 115.21, 115.42, 120.03, 127.21, 129.33, 129.40, 131.22, 13.22,137.69, 138.30, 147.57, 149.59, 162.27, 169.65; MS (ESI) m/z: 604.21 [M + 1]+. Elemental analysis calculated for C27H27F2N5O7S: C, 53.73; H, 4.51; N, 11.60. Found: C, 53.75; H, 4.52; N, 11.64.
Conclusions
In conclusion, molecular hybridization of benzhydrylpiperazine, amino acids, and nitrobenzenesulfonamide derivatives exhibited excellent anti-TB activity. 2,4-Dinitro groups on benzenesulfonamide can be manipulated further to enhance anti-TB activity. The structure–activity studies of novel hybrid derivatives of first line anti-TB drugs with 2,4-dinitrobenzenesulfonamide can be explored further as a promising drug designing tool for antitubercular therapy.
Acknowledgments
The authors are thankful to the administration, VIT University, Vellore, Birla Institute of Technology and Science Pilani, Hyderabad Campus, Cumming School of Medicine and Hotchkiss Brain Institute, and Alberta Children’s Hospital Research Institute, University of Calgary, for providing facilities to carry out this research work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00369.
List of molecules, 1H NMR and 13C NMR spectra, in vitro MTB MABA assay, nutrient starvation model, in vitro cytotoxicity screening, and electrophysiological measurements (PDF)
Author Present Address
⊥ Chemical Research, Wockhardt Research Centre, D-4, MIDC, Chikalthana, Aurangabad, Maharashtra, India 431006.
The authors declare no competing financial interest.
Supplementary Material
References
- https://www.tballiance.org/why-new-tb-drugs/global-pandemic (accessed March 2, 2021).
- Abubakar I.; Zignol M.; Falzon D.; Raviglione M.; Ditiu L.; Masham S.; Adetifa I.; Ford N.; Cox H.; Lawn S. D.; Marais B. J.; McHugh T. D.; Mwaba P.; Bates M.; Lipman M.; Zijenah L.; Logan S.; McNerney R.; Zumla A.; Sarda K.; Nahid P.; Hoelscher M.; Pletschette M.; Memish Z. A.; Kim P.; Hafner R.; Cole S.; Migliori G. B.; Maeurer M.; Schito M.; Zumla A. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect. Dis. 2013, 13, 529–539. 10.1016/s1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- Konduri S.; Bhargavi D.; Prashanth J.; Krishna V. S.; Sriram D.; Rao K. P. Design and Synthesis of “Chloropicolinate Amides and Urea Derivatives” as Novel Inhibitors for Mycobacterium tuberculosis. ACS Omega 2021, 6, 1657–1667. 10.1021/acsomega.0c05690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P.; Jaiyeola B.; Kerru N.; Ebenezer O.; Bisssessur A. A review of recent advancements in anti-tubercular molecular hybrids. Curr. Med. Chem. 2017, 24, 4180–4212. 10.2174/0929867324666170712164400. [DOI] [PubMed] [Google Scholar]
- Rathi A. K.; Syed R.; Shin H.-S.; Patel R. V. Piperazine derivatives for therapeutic use: a patent review (2010-present). Expert Opin. Ther. Pat. 2016, 26, 777–797. 10.1080/13543776.2016.1189902. [DOI] [PubMed] [Google Scholar]
- Maia R. D. C.; Tesch R.; Fraga C. A. M. Phenylpiperazine derivatives: a patent review (2006–present). Expert Opin. Ther. Pat. 2012, 22, 1169–1178. 10.1517/13543776.2012.719878. [DOI] [PubMed] [Google Scholar]
- Patel R.; Park S. An evolving role of piperazine moieties in drug design and discovery. Mini Rev. Med. Chem. 2013, 13, 1579–1601. 10.2174/13895575113139990073. [DOI] [PubMed] [Google Scholar]
- Gurdal E.; Yarim M.; Durmaz I.; Cetin-Atalay R. Cytotoxic activities of some novel benzhydrylpiperazine derivatives. Drug Res. 2013, 63, 121–128. 10.1055/s-0032-1333275. [DOI] [PubMed] [Google Scholar]
- Roy D.; Panda G. Benzhydryl Amines: Synthesis and Their Biological Perspective. ACS Omega 2019, 5, 19–30. 10.1021/acsomega.9b03090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M.; Poyil A. N.; Joshi S. D.; Patil S. A.; Patil S. A.; Bugarin A. Design, synthesis, and molecular docking study of new piperazine derivative as potential antimicrobial agents. Bioorg. Chem. 2019, 92, 103217. 10.1016/j.bioorg.2019.103217. [DOI] [PubMed] [Google Scholar]
- Ananda Kumar C. S.; Vinaya K.; Chandra J. N. S.; Thimmegowda N. R.; Benaka Prasad S. B.; Sadashiva C. T.; Rangappa K. S. Synthesis and antimicrobial studies of novel 1-benzhydryl-piperazine sulfonamide and carboxamide derivatives. J. Enzyme Inhib. Med. Chem. 2008, 23, 462–469. 10.1080/14756360701654969. [DOI] [PubMed] [Google Scholar]
- Narendra Sharath Chandra JN; Sadashiva CT; Kavitha CV; Rangappa KS Synthesis and in vitro antimicrobial studies of medicinally important novel N-alkyl and N-sulfonyl derivatives of 1-[bis(4-fluorophenyl)-methyl]piperazine. Bioorg. Med. Chem. 2006, 14, 6621–7. 10.1016/j.bmc.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Pudukulatham Z.; Zhang F. X.; Gadotti V. M.; M’Dahoma S.; Swami P.; Tamboli Y.; Zamponi G. W. Synthesis and characterization of a disubstituted piperazine derivative with T-type channel blocking action and analgesic properties. Mol. Pain 2016, 12, 1744806916641678. 10.1177/1744806916641678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A.; Hansch C.; Sammes P.; Taylor J.. Comprehensive Medicinal Chemistry; Pergamon Press: Oxford, 1990. [Google Scholar]
- Ranjith P. K.; Pakkath R.; Haridas K. R.; Kumari S. N. Synthesis and characterization of new N-(4-(4-chloro-1H-imidazole-1-yl)-3-methoxyphenyl) amide/sulfonamide derivatives as possible antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2014, 71, 354–365. 10.1016/j.ejmech.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Saxena A. K.; Singh A. Mycobacterial tuberculosis enzyme targets and their inhibitors. Curr. Top. Med. Chem. 2019, 19, 337–355. 10.2174/1568026619666190219105722. [DOI] [PubMed] [Google Scholar]
- Piton J.; Vocat A.; Lupien A.; Foo C. S.; Riabova O.; Makarov V.; Cole S. T. Structure-based drug design and characterization of sulfonyl-piperazine benzothiazinone inhibitors of DprE1 from Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e00681 10.1128/aac.00681-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yempalla K. R.; Munagala G.; Singh S.; Magotra A.; Kumar S.; Rajput V. S.; Bharate S. S.; Tikoo M.; Singh G. D.; Khan I. A.; Vishwakarma R. A.; Singh P. P. Nitrofuranyl methyl piperazines as new anti-TB agents: identification, validation, medicinal chemistry, and PK studies. ACS Med. Chem. Lett. 2015, 6, 1041–1046. 10.1021/acsmedchemlett.5b00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu K. M.; Nagesh H. N.; Singh M.; Sriram D.; Yogeeswari P.; Sekhar K. V. G. C. Novel amide and sulphonamide derivatives of 6-(piperazin-1-yl) phenanthridine as potent Mycobacterium tuberculosis H37Rv inhibitors. Eur. J. Med. Chem. 2015, 92, 415–426. 10.1016/j.ejmech.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Ghorab M. M.; El-Gaby M. S.; Soliman A. M.; Alsaid M. S.; Abdel-Aziz M. M.; Elaasser M. M. Synthesis, docking study and biological evaluation of some new thiourea derivatives bearing benzenesulfonamide moiety. Chem. Cent. J. 2017, 11, 42–54. 10.1186/s13065-017-0271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R.; Möllmann U.; Cho S.; Franzblau S. G.; Miller P. A.; Miller M. J. Design and syntheses of anti-tuberculosis agents inspired by BTZ043 using a scaffold simplification strategy. ACS Med. Chem. Lett. 2014, 5, 587–591. 10.1021/ml500039g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M.; Malatesta P.; Bosquesi P.; Yamasaki P.; Santos J. L. D.; Vizioli E. Advances in drug design based on the amino acid approach: Taurine analogues for the treatment of CNS diseases. Pharmaceuticals 2012, 5, 1128–1146. 10.3390/ph5101128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Rakesh K. P.; Leng J.; Fang W.-Y.; Ravindar L.; Channe Gowda D.; Qin H.-L. Amino acids/peptides conjugated heterocycles: A tool for the recent development of novel therapeutic agents. Bioorg. Chem. 2018, 76, 113–129. 10.1016/j.bioorg.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Kotha S. The building block approach to unusual α-amino acid derivatives and peptides. Acc. Chem. Res. 2003, 36, 342–351. 10.1021/ar020147q. [DOI] [PubMed] [Google Scholar]
- Berrino E.; Bua S.; Mori M.; Botta M.; Murthy V. S.; Vijayakumar V.; Tamboli Y.; Bartolucci G.; Mugelli A.; Cerbai E.; Supuran C. T.; Carta F. Novel sulfamide-containing compounds as selective carbonic anhydrase I inhibitors. Molecules 2017, 22, 1049. 10.3390/molecules22071049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bua S.; Berrino E.; Del Prete S.; Murthy V. S.; Vijayakumar V.; Tamboli Y.; Capasso C.; Cerbai E.; Mugelli A.; Carta F.; Supuran C. T. Synthesis of novel benzenesulfamide derivatives with inhibitory activity against human cytosolic carbonic anhydrase I and II and Vibrio cholerae α-and β-class enzymes. J. Enzyme Inhib. Med. Chem. 2018, 33, 1125–1136. 10.1080/14756366.2018.1467901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]
- Wang J.; Sánchez-Roselló M.; Aceña J. L.; Del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- Rieder M. J.; Uetrecht J.; Shear N. H.; Spielberg S. P. Synthesis and in vitro toxicity of hydroxylamine metabolites of sulfonamides. J. Pharmacol. Exp. Ther. 1988, 244, 724–8. [PubMed] [Google Scholar]
- Kulkarni A.; Sharma A. K.; Chakrapani H. Redox-guided small molecule antimycobacterials. IUBMB Life 2018, 70, 826–835. 10.1002/iub.1867. [DOI] [PubMed] [Google Scholar]
- Aspatwar A.; Kairys V.; Rala S.; Parikka M.; Bozdag M.; Carta F.; Supuran C. T.; Parkkila S. Mycobacterium tuberculosis β-carbonic anhydrases: Novel targets for developing antituberculosis drugs. Int. J. Mol. Sci. 2019, 20, 5153. 10.3390/ijms20205153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan S.; Sinha C. Sulfonamide derivatives as Mycobacterium tuberculosis inhibitors: in silico approach. Silico Pharmacol. 2018, 6, 4. 10.1007/s40203-018-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzan A.; Willby M. J.; Green K. D.; Gajadeera C. S.; Hou C.; Tsodikov O. V.; Posey J. E.; Garneau-Tsodikova S. Sulfonamide-based inhibitors of aminoglycoside acetyltransferase Eis abolish resistance to kanamycin in Mycobacterium tuberculosis. J. Med. Chem. 2016, 59, 10619–10628. 10.1021/acs.jmedchem.6b01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V.; Dhar N.; Pató J.; Kolly G. S.; Korduláková J.; Forbak M.; Evans J. C.; Székely R.; Rybniker J.; Palčeková Z.; Zemanová J.; Santi I.; Signorino-Gelo F.; Rodrigues L.; Vocat A.; Covarrubias A. S.; Rengifo M. G.; Johnsson K.; Mowbray S.; Buechler J.; Delorme V.; Brodin P.; Knott G. W.; Aínsa J. A.; Warner D. F.; Kéri G.; Mikušová K.; McKinney J. D.; Cole S. T.; Mizrahi V.; Hartkoorn R. C. Identification of aminopyrimidine-sulfonamides as potent modulators of Wag31-mediated cell elongation in mycobacteria. Mol. Microbiol. 2017, 103, 13–25. 10.1111/mmi.13535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.