Abstract

In the past decade, the self-healing elastomers based on multiple hydrogen bonding have attracted ample attention due to their rich chemical structures, adjustable mechanical properties, fast healing speed, and high healing efficiency. Through prolonging the service life and fast recovery of the mechanical properties, self-healing elastomers can be potentially applied in the field of wearable electronics, electronic skins, motion tracking, and health monitoring. In this perspective, we will introduce the concept and classification of self-healing materials first, then the hydrogen bonds, and the corresponding position of hydrogen-bonding units in the polymer structures. We will also conclude the potential application of hydrogen bonding-based elastomers. Finally, a summary and outlook will be provided.
Introduction
Although the concept of self-healing materials has only emerged since forty years,1 the “self-healing” phenomenon in organisms and biological tissues, namely, the recovering ability of living tissues and organisms from the damaged parts to continue their metabolic processes, has existed for thousands of years, which might be even longer than human history.2,3 Therefore, the research on synthetic self-healing materials has been conducted through mimicking and studying the self-healing behaviors in nature during the past decades.4,5 Self-healing materials not only allow themselves to self-repair their internal and external damages and prolong their lifespans but also endow them with better performance compared to their counterparts in many aspects, such as higher Young’s modulus and stress, larger elongation at break, and stronger heat resistance.6−12
Elastomers are one type of material that can deform themselves largely when an external force is applied and can partially or completely recover when the strain is removed. This unique property of elastomers makes them irreplaceable in many fields. However, elastomers usually suffer from environmental or external stress, leading to unexpected damage, crack, and even macroscopic fracture. Especially, internal cracks and injuries are difficult to detect and repair, which could continuously expand and converge, resulting in serious cracks among the internal layers or external parts, then eventually leading to material failure. In addition, failures also make thermoset elastomers difficult to be reprocessed and reshaped due to the formation of the irreversible and cross-linking structures. Such issues would cause many serious environmental problems and waste energy. These problems could be resolved by endowing the elastomers with self-healing properties, namely, self-healing elastomers. The self-healing ability can prolong the service life of elastomers and enhance the use safety of elastomers. Recently, self-healing elastomers have widely demonstrated their application in high-technology fields, with many advantages including being more economic, more convenient, and having higher effectiveness.1,5−7,10,12,13
Several successful approaches have been reported to realize the self-healing function of elastomers over the past decade.6,7,16−18 According to the definition of self-healing, no additional materials beyond the system are required during the repairing process. Depending on the constitution forms of repairing materials, self-healing can be divided into two kinds: extrinsic self-healing and intrinsic self-healing (Figure 1). The self-healing ability of extrinsic self-healing materials comes from the embedded repairing reagents in the matrix in advance, whereas the self-healing ability of intrinsic self-healing materials originated from the rupture and recombination of reversible chemical bonds rather than pre-embedded repairing reagents.14 Because there are a number of well-written reviews to discuss extrinsic self-healing materials, these materials related to extrinsic self-healing will not be the focus of this review.7,10,19,20 Instead, we will summarize the recent progress in the intrinsic self-healing materials.
Figure 1.
Extrinsic (left) and intrinsic self-healing (right) process.14,15 (left) Reproduced with permission. Copyright (2001) Springer Nature. (right) Reproduced with permission. Copyright (2017) National Academy of Sciences.
The repairing process of intrinsic self-healing materials can synchronously proceed at both the macro- and microlevels. During the repairing process, the reconstruction of the polymer network at the microlevel is necessary, and the macroscopic morphological changes resulting from the mechanical damage should also be recovered. Therefore, reversible interaction and the resume of the physical form should be coordinately conducted in time and space. The two or more parts in the damaged region should come into contact with each other in space, where external compress, solvent soak, and heating are necessary. Then, the reversible chemical interaction can help to quickly reconstruct the polymer network and recover its original properties. Also, the self-healing process should be carried out as soon as possible because the chemical activity of the damaged interface may be changed irreversibly by the external environment to lose the repairing function.
As shown in Figure 2, the performance can be maintained in their lifetime (black line in the left picture) for existing materials without damage. However, the performance will sharply decrease when encountering injury (red line in the left picture), and the performance will be lower than the designed safe value for devices (black dash line), whereas the scenario of the self-healing material is a different image. The performance of single self-healing materials will also sharply decrease due to injury, but the performance can be recovered close to its original value as time goes on (green line in the left picture). The behavior of multiple self-healing materials is a little different. The performance of multiple self-healing materials can be recovered during each damage; however, the performance cannot be recovered to its original state after cycling many times (red line in the right picture). Most intrinsically self-healing elastomers have multiple healing properties. Above all, the main target for self-healing materials is to prolong their service life. The three most important factors to evaluate self-healing materials are the following: self-healing efficiency (mechanical, electrical, and other properties), self-healing time, and self-healing temperature (or other extra energy). Mechanical strength, Young’s modulus, and elongation are the three main parameters to express the mechanical self-healing efficiency. For some excellent self-healing elastomers based on multiple hydrogen-bonding interactions, the mechanical self-healing efficiency can be recovered to 80% after 24 h at room temperature, and the electrical self-healing efficiency can almost reach their original values in tens of minutes at room temperature.21−31
Figure 2.
Performance–time relationship in the one-time (left) and multiple self-healing process (right).
The reversible interaction for self-healing elastomers usually includes the rapture and re-formation of chemical bonds (dynamic covalent bonds, reversible radical reaction, and Diels–Alder reaction) and noncovalent bonds (hydrogen bonding, metal–ligand interaction, π–π interaction, and host–guest interaction).6,9,11,16−19 Among these reversible interactions, hydrogen bonding plays a key role due to its dynamic nature, responsiveness to external stimuli, and tunable strength, which will be discussed in detail in the following part. Therefore, in this review, only self-healing elastomers based on hydrogen bonding will be discussed. We will outline the definition, classification, positions in the polymer structures, and the potential application of hydrogen bonding and the elastomers based on hydrogen bonding.
Hydrogen Bonds
Although the exact time in the history of discovering hydrogen bonding is still under debate, it is believed that humans had discovered hydrogen bonding more than 100 years ago.32 Hydrogen bonding is a kind of intermolecular force that widely exists in nature, which plays a crucial role in nature, such as the reproduction of DNA, protein folding, molecular recognition, and so on. Hydrogen bonding is also usually employed to design new materials in recent years, for example, supramolecules, elastomers, and self-healing materials.33−35
The hydrogen bonding between two water molecules can be depicted in Figure 3a. The basic structure of hydrogen bonds can be expressed as X–H···Y, which is composed of two main parts: proton donor (X–H, D for short) and proton acceptor (Y atom with lone pair electrons, A for short). The strength of a single hydrogen bond is relatively weak, and the bond energy is lower than 40 kJ/mol, which is one-tenth of the covalent bonds (∼400 kJ/mol for C–C bonds). Therefore, hydrogen bonding is a weak interaction in traditional cognition, and the force of hydrogen bonding is larger than van der Waals interaction but much less than covalent bonding. However, the strength of hydrogen bonding can vary in a broad range from highly dynamic bonding (association constant Ka < 100 M–1) to quasi-covalent bonding (Ka >106 M–1).36−47 The hydrogen-bonding interaction is susceptible to solvents. The hydrogen-bonding interaction will weaken gradually if the polarity of solvents increases. The creep resistance of one single hydrogen bond as the connection in supramolecules is small and not stable.
Figure 3.
Chemical structures of hydrogen bonding in water molecules (a), single (b), double (c), triple (d), linear array (e), and zigzag array (f) of hydrogen bonding.
Therefore, we can use highly dynamic hydrogen bonding to prepare the polymers with excellent self-healing properties but poor mechanical strength and low Young’s modulus. Although employing strong hydrogen bonding could obtain strong polymers with mechanical properties close to covalent polymers, the hydrogen bonds in these polymers are stable, and the additional energy is necessary to break the previous strong hydrogen bonding and then re-construct a new hydrogen bonding network. Namely, strong polymers can be prepared by strong hydrogen bonding, but the self-healing ability will be weakened. To balance the strength of connection and the self-healing ability, multiple hydrogen bonding with large association constants and directional connection attracts extensive attention.6,9,11,16
Multiple hydrogen bonding can be utilized to prepare various special materials with good environmental responsiveness under specialized conditions. Some examples of double, triple, and linear arrays of hydrogen bonding are shown in Figure 3b–e. Another multiple hydrogen-bonding unit, acylsemicarbazide (ASC), was also employed to construct interesting self-healing polymers.48,49 As a very promising multiple hydrogen-bonding unit, ASC moieties display huge potential in developing self-healing polymer materials.
The association constants can be promoted by the combination of a series of hydrogen bonding (two, three, four, or more) in a sequence of donors and acceptors to form a rigid framework (also called a multiple hydrogen bond combination unit). For example, the association constant of quadruple hydrogen bonding can be up to 107 M–1,37,40,42,46,47 and the supramolecules based on quadruple hydrogen bonding show excellent creep resistance, which can be used as the stable thermoplastic elastomer.
The structure of quadruple hydrogen bonds is very complicated, which can be formed by two different molecules or by the dimerization of a single molecule (self-complementary dimer). The strength of the quadruple hydrogen-bonding interaction is obviously very high. Here, we want to introduce two combinations of self-complementary quadruple hydrogen bonding: ADAD–DADA and AADD–DDAA, as shown in Figure 4.46 There are six mutually exclusive secondary interactions in quadruple hydrogen-bonding interaction by the dimerization of the ADAD-type molecule, resulting in the weak combination strength of the ADAD–DADA quadruple hydrogen bonds, whereas, there are only two mutually exclusive secondary interactions and four mutually attractive secondary interactions in the dimer of DDAA-type molecules. As a result, the strength of the quadruple hydrogen-bonding interaction in a DDAA–AADD dimer is stronger than that in an ADAD–DADA dimer.
Figure 4.
Chemical structures and interactions of DADA and DDAA dimers.46
Several typical self-complementary quadruple hydrogen bonds are shown in Figure 5. The unit of ureidopyrimidinone (UPy) was first discovered by Meijer and co-workers in 1997.50 The self-dimerization of UPy is very easy and the dimerization constants can be up to 107 M–1 (depending on the solvent).39,42,46,47 The high association constant, ease of synthesis, available starting materials, and readily available derivatives for functionalizing polymers of UPy units endow them with promising potential application in many fields. Therefore, the exploration and study of UPy units have attracted numerous researchers recently, especially introducing these units into the polymers as the connection of supramolecules. The supramolecules prepared by UPy units possess various functionalities, strong mechanical strength and creep resistance for the reversibility, selectivity, and high strength of quadruple hydrogen-bonding interaction. Furthermore, the heat stability of the UPy unit is also very good, which starts to degrade from 240 °C (the degraded temperature of the ureido group) and almost completely decompose at 350 °C.51 Other self-complementary quadruple hydrogen bond units with high dimerization constants, such as bis(acylamino)triazine (Figure 3b),47,52 acetylaminotriazine (Figure 3c), ureidotriazine (Figure 3d), acetyl ureidotriazine (Figure 3e)47 and ureidodeazapterin (Figure 3f),44 were also reported to construct high-performance self-healing elastomers. The multiple hydrogen-bonding units can be incorporated in the polymer at the position of chain end, mainchain, sidechain or the ends of the hyperbranched structures.
Figure 5.
Several frequently used quadruple hydrogen-bonding units (a–f).
Positions of Hydrogen-Bonding Units in Elastomers
As discussed above, the dimer of multiple hydrogen-bonding units can affect the properties of the self-healing elastomers, such as stronger mechanical strength, higher extensibility, and more toughness in the resultant polymers. The position of multiple hydrogen-bonding units will determine the density of H-bond cross-linking that will further affect the final properties of the materials. For the multiple hydrogen-bonding polymers, the mechanical performances of the final polymers are strongly dependent on the amounts of multiple hydrogen-bonding units or the density of cross linking.
Hydrogen-Bonding Units as Chain Terminals
Due to the large dimerization constants, the UPy units at chain terminals (or ends) can be deemed to increase the length of the polymer mainchain; therefore, the heat and mechanical properties can be promoted. In addition, the UPy units at chain ends can also endow the polymers with good self-healing properties. The first self-healing block copolymer in a solid state was designed by the Guan group with a novel multiphase using the supramolecular block copolymer architecture (Poly-1,2,3, Figure 6a).53 The microphase-separated supramolecular block copolymers combine the stiffness and the toughness of thermoplastic elastomers through the dynamics and healing capabilities of supramolecular materials, where the effective recovery of mechanical strength and extensibility is observed. Pyun et al. prepared UPy-capped perfluoropolyethers (UPy-PFPEs, Figure 6b) that could recover the storage modulus to its original value within 2 min at 130 °C, which resulted from the formation of hard crystalline UPy domain-based phase separation between the soft polymer backbone and the hydrogen-bonding moieties. However, an increased recovery time of the storage modulus of 18 min at 110 °C was found in the PFPE functionalized with alkylated UPy groups, which showed the suppressed crystallization.54
Figure 6.
Chemical structures of self-healing elastomers with hydrogen-bonding units as chain ends (a–d).
Yao and co-workers reported a three-arm UPy ending siloxane oligomer ((UPy)3T, Figure 6c), which showed water-enhanced healing behavior.55 Good mechanical properties (a high elastic modulus of 47.39 MPa, a tensile strength of >5.0 MPa, and breaking strain of ≈48%) were observed in the polymers with a high content of UPy motifs. The mechanical strength of a (UPy)3T film can recover even after 5 s in water at 70 °C because of the high multiple hydrogen-bonding interaction and the semicrystalline properties. Another self-healing material with multiple hydrogen bonding as terminal units is developed by Loos and co-workers, as shown in Figure 6d.56
Hydrogen-Bonding Units in the Mainchain
The phase separation between hydrophobic hydrogen-bonding domains consisting of UPy moieties and the PEG backbone was observed in the mainchain of PEG functionalized with UPy (Figure 7a), resulting in the formation of solid-like hydrogels.57 When the solid-like hydrogels were heated to 50 °C, self-healing behavior was observed.
Figure 7.
Chemical structures of self-healing elastomers with hydrogen-bonding units in the mainchain (a–f).
Recently, supramolecular polymeric materials (SPMs) including UPy units with the varying amounts of the UPy motif (from 0 to 30 mol %) in the mainchain were synthesized by Bao and co-workers.28 The chemical structures of the SPMs are shown in Figure 7b. A significant effect of the multiple hydrogen-bonding interaction on the mechanical properties of SPMs was observed. Among the four SPMs, SPM-2 (20% UPy motif) showed the maximum breaking strain (170 times) and tensile stress (0.91 MPa) for the moderate density of UPy units. After healing at room temperature for 12 h, the scratches on the SPM-2 film almost totally disappeared, suggesting autonomous self-healing behavior. The authors also evaluated the recovery of mechanical properties of the SPM-2 specimen under ambient conditions, where the healing efficiency was 12% (6 h) and went up to 88% (48 h) along with a fracture strain (150 times). The introduction of UPy units was also found to affect the microphase morphology, mechanical performance, and healing efficiency in the PPG-UPy polymer (Figure 7c),58 and an optimized sample with a good balance of mechanical performance and healing efficiency was synthesized to show 93% in tensile strength and 93% in toughness after healing within 24 h at 80 °C.
A novel strategy of multiple active hydrogen bonds to realize fast and effective self-healing at room temperature or even under harsh conditions was developed by the Fu group.59 Thiourea moieties were incorporated into the microphase-separated polyurea network to form multistrength H-bonds (Figure 7d) at the same time, and the crystallization of hard domains was inserted with the dynamic reversible H-bonds in both hard and soft segments. Another elastomer based on the UPy unit was designed with loosely packed spacer units in a supramolecular elastomer, as shown in Figure 7e,60 which addresses the inherent contradiction of robust strength and room-temperature self-healing. The Aida group reported mechanically robust self-healing materials with a zigzag hydrogen-bonded array formed anomalously by thiourea (Figure 7f).61
Hydrogen-Bonding Units in the Sidechain
Li and co-workers recently prepared a self-healing polyurethane (PU) elastomer with the UPy units as pendant groups (Figure 8a),26 which showed dynamic, supertough, and self-healing properties. When the contents of UPy units increased, all the mechanical properties (strength, modulus, elongation, and toughness) were enhanced synchronously. A high tensile strength (44 MPa) and great toughness (345 MJ m–3) was observed in the PT-HM-U20 elastomer containing 20 mol % of UPy units after healing, and the healing efficiency was 90%. Zhu et al. prepared PU with UPy units in the sidechain.62 They prepared a prepolymer with a carboxyl sidechain and then added glycidol to obtain PU by the UPy with −NCO units. The as-synthesized PU showed self-healing properties under heating for the thermal reversibility of UPy units.
Figure 8.
Chemical structures of self-healing elastomers with hydrogen-bonding units in the sidechain (a–c).
You et al. reported a biodegradable and biocompatible elastomer (bioelastomers) by incorporating UPy units in the sidechain of poly(sebacoyl diglyceride) (PSeD-U, Figure 8b).63 The scratch of a PSeD-U film was significantly healed at 60 °C within 10 min and disappeared after 30 min. The two cut PSeD-U specimens can reconnect after being kept at 60 °C for 30 min, and the healed strip could sustain stretching like a pristine strip.
A self-healing biomass-derived elastomer via the integration of multiple hydrogen bonding (UPy) in the sidechain and covalent cross-linking was synthesized by Lei and co-workers.64 The superior extensibility and self-healing ability resulted from the dynamic nature and soft characteristics of the elastomer, where the covalent cross-links can assist the re-association of ruptured H-bonds (Figure 8c). Therefore, the rough elastomer showed an elongation of 2600% at break and toughness of 42.76 MJ m–3. In addition, the elastomer exhibited a good self-healing ability with the complete recovery of scratch and with good mechanical recovery with 1900% of elongation and 24.1 MJ m–3 of toughness after healing at 60 °C for 24 h.
Hydrogen-Bonding Units in Branched Polymers
As shown in Figure 9, a series of random hyperbranched polymers (RHPs) using a high density of hydrogen bonds for the self-healing process at room temperature were realized in the Wu group.65 The internal molecular fragments in glassy hyperbranched polymers are highly restricted and have low molecular mobility, while the external branching units and the end groups have high mobility. Therefore, the authors introduced a variety of hydrogen bond complementary groups that can realize self-healing through controlling the external sidechain and the terminal group of the polymer. These polymers have a large number of amino, amide, and other groups and can form high-density hydrogen bonds. After being in contact with the material at room temperature for 1 min, the tensile strength can be restored to 5.5 MPa.
Figure 9.
Design concept and synthesis of RHP. (A) Chemical route to the synthesis of RHP through Michael addition between MBA and BDA at 30 °C. (B) Schematic diagram of interactions between RHP molecules. (C) Multiple hydrogen bonds of RHP molecules. (Inset) A photograph of RHP-1.65 Reproduced with permission. Copyright (2020) National Academy of Sciences.
Potential Applications
Unlike traditional metallic materials and inorganic nonmetallic materials, polymeric materials possess more convenient preparation technology, abundant kinds, various functions, and are lightweight, resulting in irreplaceable roles in industrial fabrication and our daily life. Among the polymeric materials, self-healing elastomers can not only enhance the safety and stability of devices, prolong the service life, promote the development of the industry, and have huge economic value but also play an important role in facilitating the efficient utilization of resources and sustainable development of the human society. The reported self-healing elastomers with a special function have inspired broad interests, suggesting the prospect of vigorous development. According to their functional features, self-healing elastomers can be applied in various fields, such as self-healing conductive films, self-healing electronic skins, self-healing stretchable triboelectric nanogenerators (TENG), self-healing actuators, and other fields. In these potential applications, self-healing elastomers with sensing properties are the key component to develop smart electronic devices, which is one of the hottest topics in the self-healing field.
Self-Healing Conductive Films
Aging and mechanical damage could inevitably take place during the usage of materials. If such a situation occurs on conductive materials, it will often result in serious safety problems for people and property. Therefore, more and more researchers have begun to combine the self-healing property into the conductive materials. The self-healing conductive materials can be repaired in time after being damaged and can maintain the safety and stability of the materials, save the cost, and reduce unnecessary hazards.
A universal method was developed by the Bao group to prepare a self-healing conductive material with good stretchability.66 They sprayed carbon nanotubes on octadecyltrimethoxysilane-functionalized Si wafer and then got a conductive network. A self-healing PU elastomer was coated on the conductive network. After drying and tearing off the polymer layer, an elastic conductive material embedded with a carbon nanotube (CNT) layer on its surface was obtained. Thanks to the excellent self-healing behavior of the PU, the conductivity can be restored in a short time, and the electric and mechanical properties can be fully recovered after healing for 12 h (Figure 10a). This preparative method of conductive materials can be applied to construct various conductive networks, such as Ag nanowires. These conductive materials as electrodes can be linked to many different sensors, and the electronic skin integrated with multiple functions was obtained in the end.
Figure 10.
(a) Proposed recovery mechanism for CNTs embedded in a self-healing polymer matrix.66 (b) I–V curves of the conductive film after several cutting–healing cycles. (c) Demonstration of electric conductivity retention capability under various situations (i) original, (ii) cut, (iii) after healing, (iv) healed specimen with a notch, (v) healed specimen with a notch was stretched to 300% strain, (vi) stretched specimen with a notch was unloaded and then rested for 12 h, (vii) when the specimen was stretched to 300% strain again, the conductivity remained. Surprisingly, the notch vanished.67 (a) Reproduced with permission. Copyright (2018) Springer Nature. (b,c) Reproduced with permission. Copyright (2020) Wiley-VCH.
By the rational design of the hard domains with dynamic hydrogen bonding, a transparent PU-urea supramolecular elastomer (PPGTD-IDA) was synthesized with decent mechanical strength, extreme toughness, outstanding notch-sensitivity, and room-temperature self-healing. The PPGTD-IDA shows distinguishing electrical self-healing features (Figure 10b).67 A circuit composing of a light-emitting diode (LED) bulb, the PPGTD-IDA/GaInSnP conductive film, and dry batteries was set up to evaluate the distinguishing electrical self-healing features of PPGTD-IDA, as shown in Figure 10c. The LED lit up once again after the conductive film was cut into two-halves and brought into contact. The complete electrical healing can be realized within 30 s. After three repeated cutting–healing cycles at the same location, the electrical healing efficiency could still maintain 90.2% of the original conductivity. The conductive film can be stretched to 300% with a healing time of 2 h, and the brightness of the LED was hardly changed for the rapid recovery of the conductive network. In addition, the conductive film was also notch-insensitive.
E-Skin
In 2012, the Bao group incorporated conductive micro-nickel (μ-Ni) particles into self-healing polymers to prepare a self-healing stress sensor that can realize the stress detection in the range of 0–400 kPa (Figure 11a).68 After being cut into two-halves and put together again, this self-healing sensor can recover its electrical property up to 90% of the original value in 15 s and restore its mechanical properties fully in 10 min at 50 °C (Figure 11b). In addition, they also attached this sensor onto a robot model and realized the detection of the robot joint motion and the stress.
Figure 11.
(a) Tactile sensor response at increasing peak pressure values (inset: sensor schematic). Equation represents least-squares fit relationship (dotted line) of resistance and applied pressure. (b) Repeated electrical healing for three cuts at the same severed location.68 Reproduced with permission. Copyright (2012) Springer Nature.
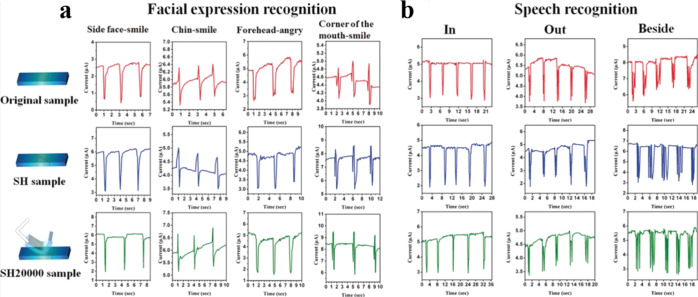
One group in Sichuan University reported a supramolecular reversible cross-linking network with the self-healing property based on a dynamic ionic bond, metal–ligand bonding, and multiple hydrogen bonding, namely, the cross-linking network was constructed between the conductive filler network and rubber substrates.25,69 As shown in Figure 12, this cross-linking network can realize real-time (≤30 s) and multiple self-healing (the healing efficiency after three self-healing cycles is 93%) at room temperature. Self-healing and flexible sensors with ultra-high sensitivity and the ability to detect tiny physiological motion (such as Adam’s apple pronunciation, facial expressions, chewing, cough, and swallowing) can be prepared by a layer-by-layer manufacturing method. Key materials and technology can be supplied for the application in wearable electronics. Based on the excellent mechanical behavior, electric reliability, and response sensitivity of the flexible sensor material, the research group designed and developed a robot prototype system to control facial expression and a synchronous pronunciation prototype system. They also fulfilled the integrated application between flexible sensors and electronic control systems and then realized the man–machine interaction. Another self-healing sensor with tunable piezoresistivity was developed by the same group via the construction of a hierarchical structure connected through supramolecular metal–ligand coordination bonds.70 Multiple tiny signals, such as pronunciation, coughing, and deep breathing, can be detected by this sensor after being attached on the human body. Very fast (2 min), autonomous, and repeatable self-healing with a high healing efficiency (88.6% after the third healing process) was observed in the healed samples, which still possessed flexibility, high sensitivity, and accurate detection capability, even after bending over 10,000 cycles.
Figure 12.
Current responses of the original, self-healed (SH), and SH20000 strain sensors in (a) facial expression recognition at different facial parts and (b) vocal-cord vibrations with the tester pronouncing different words. SH sample and SH sample after bending over 20,000 times (SH20000).25 Reproduced with permission. Copyright (2017) Wiley-VCH.
Based on a tough and water insensitive self-healing elastomer (PDMS–MPU0.4–IU0.6), the Bao group prepared self-healing electrodes to fabricate a capacitive strain-sensing e-skin by inserting a self-healing polymer film between two electrodes.23 The e-skin can be recovered to operate after being damaged by cutting, scratching, or notching at room temperature (Figure 13a,b). The e-skin was highly resistant to the constant mechanical damage. The operation of the e-skin was not interrupted because of its excellent self-healing ability. The authors demonstrated a wafer-scale 7 × 7 strain sensor array that can detect the strain deformation induced by external stimuli, proving that this approach is scalable.
Figure 13.
(a) Capacitance change of the strain sensor by stretching with an original sensor and 9 h healed sensor after damage.23 (b) Optical images of the cut strain sensor (inset), and the healed strain sensor maintains high stretchability. (c) Capacitance change of the strain sensor by stretching with the original sensor and 9 h of healing the sensor after damage.71 Reproduced with permission. Copyright (2017 (a), 2020 (b,c)) Wiley-VCH.
Haick et al. synthesized another water insensitive self-healing PU for the preparation of stretchable and self-healing electrodes.71 Soft electronic devices made from this elastomer are highly robust and can recover their original electrical properties after being damaged in both ambient and aqueous conditions. As shown in Figure 13c,d, the self-healing capability leads to the elimination of significant electric leakage in extreme wet or underwater conditions. An underwater self-healing strain sensor with carbon black as conductive fillers and the PBPUU as substrates was fabricated. Highly efficient electrical recovery after making a surface cut was observed.
Triboelectric Nanogenerator
A single-electrode mode TENG (s-TENG) device with a close sandwich structure was fabricated by a super tough and self-healable poly(dimethylsiloxane) elastomer via hydrogen-bonding association (PAMPS-U10).72 The s-TENG device is a tough (16,500 J/m2), self-healable (efficiency ∼97%), and transparent TENG (Figure 14a). Then, a self-powered system with the s-TENG was demonstrated by the authors.
Figure 14.
(a) Output voltage (VOC) of s-TENG after self-healing at ambient for 72 h.72 (b) Digital photographs (top) and optical images (bottom) of polymer composites with 0.2 wt % o-CNT before and after healing at 90 °C and 80% humidity condition. (c). Triboelectric output voltage of the self-healable polymer composite with 0.2 wt % o-CNT before and after healing.73 Copyright (2020) ACS.
A mechanically strong and self-healable poly(hindered urea) (PHU) network was developed, which is not only flexible but also shows a good mechanical property of high tensile strength (1.7 MPa at break).73 The network can be SH quickly and repeatedly and re-processable under mild conditions. The authors employed PHU to fabricate self-healing TENGs that can recover their triboelectric performance after the complete healing of the damaged surfaces (Figure 14b). The self-healing TENG exhibited the highest triboelectric output performance (169.9 V/cm2) among all the reported healable TENGs for the interfacial polarization-induced enhancement of the dielectric constant.
The human skin can be deemed as a natural infrared radiation emitter that supplies favorable conditions for the device to function efficiently. The infrared radiation from the skin can be employed to promote the healing efficiency of wearable self-healing materials and devices. Based on this design concept, a self-healing, flexible, and tailorable TENG was designed as a wearable sensor to monitor the motion of the human body (Figure 15).74 In this self-healing TENG, the self-healable electrification layer was constructed by the introduction of reversible imine bonds and quadruple hydrogen-bonding UPy moieties into a polymer network. The conductive nanocomposite as electrodes was obtained by incorporating UPy-functionalized multiwalled carbon nanotubes into the healable polymer. The materials in both layers showed excellent self-healing and shape-tailorable features for the UPy dynamic bonds and resulted in a robust interface bonding that existed in the TENG device. When the device is damaged, the output electric performances of the self-healing TENG devices can almost restore their original state. This work contributes to the community of wearable electronics with a novel strategy for flexible devices and sustainable energy.
Figure 15.
Output characteristics of IU-TENG under varying healing conditions. (a) Open-circuit voltage and (b) short-circuit current of the original, broken, and SH IU-TENG after three different healing times from 3 to 6 h and then to 9 h at 34 °C. (c) Voltage and current changes of IU-TENG after different healing times at 34 °C. (d) Open-circuit voltage and (e) short-circuit current of SH IU-TENG at different temperatures (15, 25, 34, 45, and 55 °C) for 9 h. (f) Voltage and current changes of IU-TENG at different healing temperatures for 9 h. (g) Open-circuit voltage and (h) short-circuit voltage of the original and SH IU-TENG after one to four cycles under NIR irradiation. (i) Voltage and current changes of IU-TENG after healing under NIR irradiation for several cycles (0 represent the original).74 Reproduced with permission from ref (74). Copyright (2020) Wiley-VCH.
A transparent, three-dimensional (3D) printable, highly stretchable, and healable thermoplastic elastomer (polyurethane acrylate, PUA) was developed by the Lee group.75 They used PUA, liquid metal, and silver flakes to fabricate a highly conductive (6250 S·cm–1), extremely stretchable, and healable composite as the stretchable conductor for TENGs. The PUA elastomer acted as the matrix for the conductor and as the triboelectric layer. Because of the supramolecular hydrogen bonding of the PUA, the nanogenerator exhibited stretchability (2500%) and self-healing (efficiency 96%) properties after extreme mechanical damage. After severe mechanical damage and healing, the energy-harvesting performance of the self-TENG can be recovered to its original values.
Actuator
Inspired by living systems, a synergistic metal coordination cross-linked supramolecular elastomer with a unique 3D interconnected network structure design was developed by the Zhang group to simultaneously realize multifunctional properties.76 Based on this supramolecular elastomer, they prepared a novel self-healable multistimuli responsive actuator (Figure 16a,b). This biomimetic actuator exhibited high photothermal efficiency (η = 79.1%) and thermal conductivity (31.92 W m–1 K–1). A superfast actuating response (near-infrared light: 0.44 s; magnetic field: 0.36 s) was observed. Excellent self-healing performance in both mechanical and actuating properties could be endowed by the supramolecular cross-linking. The biomimetic actuator may provide potential applications in artificial muscles, soft robotics, and biomedical microdevices.
Figure 16.
(a) Actuation force as a function of the original and SH sample. (b) Photographs showing the cutting-healing process following with the puncturing healing process of the butterfly robot and the resultant actuator vibrates wings rapidly with the NIR light irradiation on and off. Scale bar: 1 cm.76 (c) Single-chamber actuator achieves 400% strains and 5 N force output, with no distinguishable difference in the performance between pristine and puncture-healed actuators. Error bars, standard deviation (n = 5).77 (d) Actuating stress of original and SH actuators under NIR light. (e) Time dependent bending angle of the original and SH samples when exposed to NIR light.21 (a,b) Reproduced with permission ref (76). Copyright (2019) Wiley-VCH. (c) Reproduced with permission from ref (77). Copyright (2020) Springer Nature. (d,e) Reproduced with permission from ref (21). Copyright (2020) ACS.
By introducing high-strength synthetic proteins together with the hydrogen-bonding nanostructure and network morphology, the self-healing of micro- and macroscale mechanical damage with 1 s by local heating was realized in the Max-Planck group.77 The authors made an actuator based on this excellent self-healing protein. In the original and healed actuators, almost the same performance was observed with a maximum displacement of 10 mm (400% actuation strain) and a force output of 5 N for a single-chamber actuator (Figure 16c). Such a self-healing performance could extend the operational lifetime of the actuators beyond unpredictable damage and address current limitations in self-healing materials for soft actuators and personal protective equipment.
A near-infrared light-responsive soft actuator based on a hydrogen-bonding supramolecular network was reported, which showed outstanding performance including the fast and reliable light-responsive behavior of the bending angle over 90° within 1.6 s, a robust mechanical strength of 12.52 MPa, superfast self-healing speed of 2 s, and satisfactory self-healing efficiency in both mechanical (87.68%) and actuating (99.50%) performances (Figure 16d,e).21 Furthermore, the actuators can be conveniently fabricated and reconfigured through a mild-temperature molding strategy to obtain various 3D structures with diverse actuating locomotion. The healed “butterfly” and “gripper” were demonstrated to execute actuating instructions upon NIR irradiation.
Other Applications
An elastomer with dopamine moieties as pendent groups was synthesized and showed good mechanical strength (1.9 MPa stress at break and 5100% fracture strain) and adhesion strength (≈62 kPa in air and ≈16 kPa underwater) at the same time.78 Because of the excellent adhesive strength of the elastomer with an epithelial tissue, a stretchable, self-healable, and highly adhesive bio-interfacial electrode for electromyogram measurement was fabricated by spray-coating silver nanowires on the elastic substrate. After cutting the electrode, the electromyogram signal immediately became weak with a low signal/noise ratio due to the resistance increasing. As shown in Figure 17a, the signal/noise ratio almost increased to the original level after healing for 4 h, indicating that this electrode had a good response to breakage and could keep working smoothly during the application. Furthermore, no residue was observed after the electromyogram monitoring and the electrode was peeled off, which is important to keep the human skin clear and healthy.
Figure 17.
(a) Ambulatory EMG monitoring when the DAE electrode was cut off and after healing.78 (b) Photographs showing the HELIOS device healed immediately after a puncture.79 (c) Comparative gas-separation performance of original polymer membranes (blue) and polymer membranes after being cut and healing (orange), CO2 permeability (up), and CO2/N2 selectivity (bottom).80 (d) Self-healing polymeric (SHP–PEG) binder enabled by multiple H-bond interactions for maintaining the integrity of the Si electrodes during charge–discharge cycles. (e) Cycle performances and coulombic efficiency of the Si electrode with a varied SHP–PEG binder.81 (a) Reproduced with permission from ref (78). Copyright 2018, Wiley-VCH. (b) Reproduced with permission from ref (79). Copyright 2020 Springer Nature. (c–e) Reproduced with permission from ref (80). Copyright 2018, Wiley-VCH.
A low-field illuminating optoelectronic stretchable (HELIOS) device was prepared by introducing the transparent, high permittivity polymeric dielectric.79 As shown in Figure 17b, the HELIOS device also SH mechanically and electronically from punctures or when damaged because rich hydrogen-fluorine intermolecular bonds exist in the fluoroelastomer.
Self-healing H-bond-cross-linked polymeric elastomers (U-PDMS-Es) can be used as gas separation membranes. After being healed at 40 °C in 20 min or 120 min at room temperature, the damage on the membrane can be completely recovered because of the reversible multiple hydrogen-bonding network (Figure 17c).80 The CO2 permeability and CO2/N2 selectivity of the original and healed membranes are almost at the same level. The gas permeability and gas-separation properties can be tuned by the cross-linking density and CO2 philicity.
Another interesting application of self-healing H-bond-cross-linked polymeric elastomers is reported by Bao and co-workers through incorporating poly(ethylene glycol) (SHP–PEG) as binders into Si anodes, as shown in Figure 17d.81 By optimizing the chemical structures, the excellent self-healing capability of SHP–PEG could effectively keep the interface completeness of Si nanoparticles and electrolytes. The as-prepared Li-ion battery exhibited a high discharge capability (2600 mA h g–1) and good cyclic rate performance (Figure 17e). After the binder film with hydrogen-bonding units was scratched by a razor blade and then left to heal at room temperature, a significant self-healing area was observed. This result should be utilized for the next-generation batteries with high capacity materials that suffer from a large volume change or damage during cycling.
It can be expected that more and more interesting and promising applications of self-healing polymers with hydrogen-bonding interaction will be witnessed in various interdisciplinary fields soon.
Summary and Outlook
We have summarized the recent progress in the design, synthesis, characterization, and application of self-healing elastomers with the hydrogen-bonding units, especially multiple hydrogen bonding. This review describes the definition and classification of self-healing materials, and then discusses the structures, mutual effect, and examples of multiple hydrogen bonds. The positions of multiple hydrogen bonding in the polymer chain are also summarized and discussed to clarify the structure–property relationship. In the last part, the potential applications of self-healing elastomers based on multiple hydrogen bonding, especially in the e-skins, stretchable TENG and stretchable actuators, have been presented.
However, two main existing challenges impede the way to a bright future of self-healing elastomers: (1) external energy is necessary during the healing process of almost all the reported self-healing materials. For example, the healing process can only start after the damaged parts dabbed the individual cut specimens and are pressed under a plumb at a specified time. However, it is either impossible or not convenient to supply extra energy for the healing process in a real environment or in real devices and (2) the healing speed and efficiency will be dramatically decreased after several healing cycles. The water molecules and dust particles will take up the positions where the reversible bonding among polymer chains will be formed and fractured.
With the advance on material science and chemistry, new multiple hydrogen-bonding units for self-healing elastomers could be designed and synthesized. Ideal self-healing elastomers with a quick healing time (<1 s), high efficiency (∼100% or higher), and completely autonomous healing (totally without external energy, such as in the atmosphere, underwater, or at a low temperature) will be realized in the near future by the effort of the members of the community of chemistry, physics, and materials. We believe that diverse high-performance self-healing elastomers with various properties should have a bright future in stretchable and wearable electronics.
Acknowledgments
B.L.H. acknowledges support from Ningbo Institute of Materials Technology and Engineering and the CAS Pioneer Hundred Talents Program. Q.Z. thanks the support from starting funds from City University of Hongkong and 111 Project (D20015).
The authors declare no competing financial interest.
References
- Jud K.; Kausch H. H.; Williams J. G. Fracture mechanics studies of crack healing and welding of polymers. J. Mater. Sci. 1981, 16, 204–210. 10.1007/bf00552073. [DOI] [Google Scholar]
- Han R.; Campbell K. P. Dysferlin and muscle membrane repair. Curr. Opin. Cell Biol. 2007, 19, 409–416. 10.1016/j.ceb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- París R.; Lamattina L.; Casalongué C. A. Nitric oxide promotes the wound-healing response of potato leaflets. Plant Physiol. Biochem. 2007, 45, 80–86. 10.1016/j.plaphy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Kushner A. M.; Williams G. A.; Guan Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. 10.1038/nchem.1314. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Urban M. W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. 10.1039/c3cs60109a. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Ding X.; Urban M. W. Chemical and physical aspects of self-healing materials. Prog. Polym. Sci. 2015, 49–50, 34–59. 10.1016/j.progpolymsci.2015.06.001. [DOI] [Google Scholar]
- Wu D. Y.; Meure S.; Solomon D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. 10.1016/j.progpolymsci.2008.02.001. [DOI] [Google Scholar]
- Wei Z.; Yang J. H.; Zhou J.; Xu F.; Zrínyi M.; Dussault P. H.; Osada Y.; Chen Y. M. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem. Soc. Rev. 2014, 43, 8114–8131. 10.1039/c4cs00219a. [DOI] [PubMed] [Google Scholar]
- Roy N.; Bruchmann B.; Lehn J.-M. DYNAMERS: dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. 10.1039/c5cs00194c. [DOI] [PubMed] [Google Scholar]
- Murphy E. B.; Wudl F. The world of smart healable materials. Prog. Polym. Sci. 2010, 35, 223–251. 10.1016/j.progpolymsci.2009.10.006. [DOI] [Google Scholar]
- Hou R.; Li G. Q.; Zhang Y.; Li M. J.; Zhou G. M.; Chai X. M. Self-Healing Polymers Materials Based on Dynamic Supramolecular Motifs. Prog. Chem. 2019, 31, 690–698. 10.7536/PC180928. [DOI] [Google Scholar]
- de Espinosa L. M.; Fiore G. L.; Weder C.; Johan Foster E.; Simon Y. C. Healable supramolecular polymer solids. Prog. Polym. Sci. 2015, 49–50, 60–78. 10.1016/j.progpolymsci.2015.04.003. [DOI] [Google Scholar]
- Burattini S.; Greenland B. W.; Chappell D.; Colquhoun H. M.; Hayes W. Healable polymeric materials: a tutorial review. Chem. Soc. Rev. 2010, 39, 1973–1985. 10.1039/b904502n. [DOI] [PubMed] [Google Scholar]
- White S. R.; Sottos N. R.; Geubelle P. H.; Moore J. S.; Kessler M. R.; Sriram S. R.; Brown E. N.; Viswanathan S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- Fu F.; Chen Z.; Zhao Z.; Wang H.; Shang L.; Gu Z.; Zhao Y. Bio-inspired self-healing structural color hydrogel. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 5900. 10.1073/pnas.1703616114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P.; Wang H. High-Performance Polymeric Materials through Hydrogen-Bond Cross-Linking. Adv. Mater. 2020, 32, 1901244. 10.1002/adma.201901244. [DOI] [PubMed] [Google Scholar]
- Hillewaere X. K. D.; Du Prez F. E. Fifteen chemistries for autonomous external self-healing polymers and composites. Prog. Polym. Sci. 2015, 49–50, 121–153. 10.1016/j.progpolymsci.2015.04.004. [DOI] [Google Scholar]
- Zhang Z. P.; Rong M. Z.; Zhang M. Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. 10.1016/j.progpolymsci.2018.03.002. [DOI] [Google Scholar]
- An S.; Lee M. W.; Yarin A. L.; Yoon S. S. A review on corrosion-protective extrinsic self-healing: Comparison of microcapsule-based systems and those based on core-shell vascular networks. Chem. Eng. J. 2018, 344, 206–220. 10.1016/j.cej.2018.03.040. [DOI] [Google Scholar]
- Zhu D. Y.; Rong M. Z.; Zhang M. Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. 10.1016/j.progpolymsci.2015.07.002. [DOI] [Google Scholar]
- Qiu X.; Guo Q.; Wang Y.; Huang X.; Cao J.; Zheng Z.; Zhang X. Self-Healing and Reconfigurable Actuators Based on Synergistically Cross-Linked Supramolecular Elastomer. ACS Appl. Mater. Interfaces 2020, 12, 41981–41990. 10.1021/acsami.0c11708. [DOI] [PubMed] [Google Scholar]
- Li X.; Yu R.; He Y.; Zhang Y.; Yang X.; Zhao X.; Huang W. Self-Healing Polyurethane Elastomers Based on a Disulfide Bond by Digital Light Processing 3D Printing. ACS Macro Lett. 2019, 8, 1511–1516. 10.1021/acsmacrolett.9b00766. [DOI] [PubMed] [Google Scholar]
- Kang J.; Son D.; Wang G. J. N.; Liu Y.; Lopez J.; Kim Y.; Oh J. Y.; Katsumata T.; Mun J.; Lee Y.; Jin L.; Tok J. B. H.; Bao Z. Tough and Water-Insensitive Self-Healing Elastomer for Robust Electronic Skin. Adv. Mater. 2018, 30, 1706846. 10.1002/adma.201706846. [DOI] [PubMed] [Google Scholar]
- Kim S.-M.; Jeon H.; Shin S.-H.; Park S.-A.; Jegal J.; Hwang S. Y.; Oh D. X.; Park J. Superior Toughness and Fast Self-Healing at Room Temperature Engineered by Transparent Elastomers. Adv. Mater. 2018, 30, 1705145. 10.1002/adma.201705145. [DOI] [PubMed] [Google Scholar]
- Cao J.; Lu C.; Zhuang J.; Liu M.; Zhang X.; Yu Y.; Tao Q. Multiple Hydrogen Bonding Enables the Self-Healing of Sensors for Human-Machine Interactions. Angew. Chem., Int. Ed. 2017, 56, 8795–8800. 10.1002/anie.201704217. [DOI] [PubMed] [Google Scholar]
- Song Y.; Liu Y.; Qi T.; Li G. L. Towards Dynamic but Supertough Healable Polymers through Biomimetic Hierarchical Hydrogen-Bonding Interactions. Angew. Chem., Int. Ed. 2018, 57, 13838–13842. 10.1002/anie.201807622. [DOI] [PubMed] [Google Scholar]
- Li T.; Zheng T.-Z.; Guo Z.-X.; Xu J.; Guo B.-H. A Well-defined Hierarchical Hydrogen Bonding Strategy to Polyureas with Simultaneously Improved Strength and Toughness. Chin. J. Polym. Sci. 2019, 37, 1257–1266. 10.1007/s10118-019-2275-3. [DOI] [Google Scholar]
- Yan X.; Liu Z.; Zhang Q.; Lopez J.; Wang H.; Wu H.-C.; Niu S.; Yan H.; Wang S.; Lei T.; Li J.; Qi D.; Huang P.; Huang J.; Zhang Y.; Wang Y.; Li G.; Tok J. B.-H.; Chen X.; Bao Z. Quadruple H-Bonding Cross-Linked Supramolecular Polymeric Materials as Substrates for Stretchable, Antitearing, and Self-Healable Thin Film Electrodes. J. Am. Chem. Soc. 2018, 140, 5280–5289. 10.1021/jacs.8b01682. [DOI] [PubMed] [Google Scholar]
- Söntjens S. H. M.; Renken R. A. E.; van Gemert G. M. L.; Engels T. A. P.; Bosman A. W.; Janssen H. M.; Govaert L. E.; Baaijens F. P. T. Thermoplastic Elastomers Based on Strong and Well-Defined Hydrogen-Bonding Interactions. Macromolecules 2008, 41, 5703–5708. 10.1021/ma800744c. [DOI] [Google Scholar]
- Zhang L.; Liang J.; Jiang C.; Liu Z.; Sun L.; Chen S.; Xuan H.; Lei D.; Guan Q.; Ye X.; You Z. Peptidoglycan-inspired autonomous ultrafast self-healing bio-friendly elastomers for bio-integrated electronics. Natl. Sci. Rev. 2020, 10.1093/nsr/nwaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Lu X.; Sun S.; Yu C.; Xia H. Preparation, characterization and properties of intrinsic self-healing elastomers. J. Mater. Chem. B 2019, 7, 4876–4926. 10.1039/c9tb00831d. [DOI] [PubMed] [Google Scholar]
- Gibb B. C. The centenary (maybe) of the hydrogen bond. Nat. Chem. 2020, 12, 665–667. 10.1038/s41557-020-0524-2. [DOI] [PubMed] [Google Scholar]
- Kitagawa S.; Uemura K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 2005, 34, 109–119. 10.1039/b313997m. [DOI] [PubMed] [Google Scholar]
- Liu C. H.; Niazi M. R.; Perepichka D. F. Strong Enhancement of π-Electron Donor/Acceptor Ability by Complementary DD/AA Hydrogen Bonding. Angew. Chem., Int. Ed. 2019, 58, 17312–17321. 10.1002/anie.201910288. [DOI] [PubMed] [Google Scholar]
- Irimia-Vladu M.; Kanbur Y.; Camaioni F.; Coppola M. E.; Yumusak C.; Irimia C. V.; Vlad A.; Operamolla A.; Farinola G. M.; Suranna G. P.; González-Benitez N.; Molina M. C.; Bautista L. F.; Langhals H.; Stadlober B.; Głowacki E. D.; Sariciftci N. S. Stability of Selected Hydrogen Bonded Semiconductors in Organic Electronic Devices. Chem. Mater. 2019, 31, 6315–6346. 10.1021/acs.chemmater.9b01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Wang X.-Z.; Jiang X.-K.; Chen Y.-Q.; Li Z.-T.; Chen G.-J. Hydrazide-Based Quadruply Hydrogen-Bonded Heterodimers. Structure, Assembling Selectivity, and Supramolecular Substitution. J. Am. Chem. Soc. 2003, 125, 15128–15139. 10.1021/ja037312x. [DOI] [PubMed] [Google Scholar]
- van der Lubbe S. C. C.; Zaccaria F.; Sun X.; Fonseca Guerra C. Secondary Electrostatic Interaction Model Revised: Prediction Comes Mainly from Measuring Charge Accumulation in Hydrogen-Bonded Monomers. J. Am. Chem. Soc. 2019, 141, 4878–4885. 10.1021/jacs.8b13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Cate A. T.; Kooijman H.; Spek A. L.; Sijbesma R. P.; Meijer E. W. Conformational Control in the Cyclization of Hydrogen-Bonded Supramolecular Polymers. J. Am. Chem. Soc. 2004, 126, 3801–3808. 10.1021/ja039408x. [DOI] [PubMed] [Google Scholar]
- Söntjens S. H. M.; Sijbesma R. P.; van Genderen M. H. P.; Meijer E. W. Stability and Lifetime of Quadruply Hydrogen Bonded 2-Ureido-4[1H]-pyrimidinone Dimers. J. Am. Chem. Soc. 2000, 122, 7487–7493. 10.1021/ja000435m. [DOI] [Google Scholar]
- Ligthart G. B. W. L.; Ohkawa H.; Sijbesma R. P.; Meijer E. W. Complementary Quadruple Hydrogen Bonding in Supramolecular Copolymers. J. Am. Chem. Soc. 2005, 127, 810–811. 10.1021/ja043555t. [DOI] [PubMed] [Google Scholar]
- Folmer B. J. B.; Sijbesma R. P.; Kooijman H.; Spek A. L.; Meijer E. W. Cooperative Dynamics in Duplexes of Stacked Hydrogen-Bonded Moieties. J. Am. Chem. Soc. 1999, 121, 9001–9007. 10.1021/ja991409v. [DOI] [Google Scholar]
- de Greef T. F. A.; Ercolani G.; Ligthart G. B. W. L.; Meijer E. W.; Sijbesma R. P. Influence of Selectivity on the Supramolecular Polymerization of AB-Type Polymers Capable of Both A·A and A·B Interactions. J. Am. Chem. Soc. 2008, 130, 13755–13764. 10.1021/ja8046409. [DOI] [PubMed] [Google Scholar]
- Corbin P. S.; Zimmerman S. C.; Thiessen P. A.; Hawryluk N. A.; Murray T. J. Complexation-Induced Unfolding of Heterocyclic Ureas. Simple Foldamers Equilibrate with Multiply Hydrogen-Bonded Sheetlike Structures1. J. Am. Chem. Soc. 2001, 123, 10475–10488. 10.1021/ja010638q. [DOI] [PubMed] [Google Scholar]
- Corbin P. S.; Zimmerman S. C. Self-Association without Regard to Prototropy. A Heterocycle That Forms Extremely Stable Quadruply Hydrogen-Bonded Dimers. J. Am. Chem. Soc. 1998, 120, 9710–9711. 10.1021/ja981884d. [DOI] [Google Scholar]
- Brielle E. S.; Arkin I. T. Quantitative Analysis of Multiplex H-Bonds. J. Am. Chem. Soc. 2020, 142, 14150–14157. 10.1021/jacs.0c04357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijer F. H.; Sijbesma R. P.; Kooijman H.; Spek A. L.; Meijer E. W. Strong Dimerization of Ureidopyrimidones via Quadruple Hydrogen Bonding. J. Am. Chem. Soc. 1998, 120, 6761–6769. 10.1021/ja974112a. [DOI] [Google Scholar]
- Beijer F. H.; Kooijman H.; Spek A. L.; Sijbesma R. P.; Meijer E. W. Self-Complementarity Achieved through Quadruple Hydrogen Bonding. Angew. Chem., Int. Ed. 1998, 37, 75–78. . [DOI] [Google Scholar]
- Wang S.; Fu D.; Wang X.; Pu W.; Martone A.; Lu X.; Lavorgna M.; Wang Z.; Amendola E.; Xia H. High performance dynamic covalent crosslinked polyacylsemicarbazide composites with self-healing and recycling capabilities. J. Mater. Chem. A 2021, 9, 4055–4065. 10.1039/D0TA11251H. [DOI] [Google Scholar]
- Fu D.; Pu W.; Escorihuela J.; Wang X.; Wang Z.; Chen S.; Sun S.; Wang S.; Zuilhof H.; Xia H. Acylsemicarbazide Moieties with Dynamic Reversibility and Multiple Hydrogen Bonding for Transparent, High Modulus, and Malleable Polymers. Macromolecules 2020, 53, 7914–7924. 10.1021/acs.macromol.0c01667. [DOI] [Google Scholar]
- Sijbesma R. P.; Beijer F. H.; Brunsveld L.; Folmer B. J. B.; Hirschberg J. H. K. K.; Lange R. F. M.; Lowe J. K. L.; Meijer E. W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604. 10.1126/science.278.5343.1601. [DOI] [PubMed] [Google Scholar]
- Armstrong G.; Buggy M. Thermal stability of a ureidopyrimidinone model compound. Mater. Sci. Eng., C 2001, 18, 45–49. 10.1016/s0928-4931(01)00359-9. [DOI] [Google Scholar]
- Beijer F. H.; Sijbesma R. P.; Vekemans J. A. J. M.; Meijer E. W.; Kooijman H.; Spek A. L. Hydrogen-Bonded Complexes of Diaminopyridines and Diaminotriazines: Opposite Effect of Acylation on Complex Stabilities. J. Org. Chem. 1996, 61, 6371–6380. 10.1021/jo960612v. [DOI] [PubMed] [Google Scholar]
- Hentschel J.; Kushner A. M.; Ziller J.; Guan Z. Self-Healing Supramolecular Block Copolymers. Angew. Chem., Int. Ed. 2012, 51, 10561–10565. 10.1002/anie.201204840. [DOI] [PubMed] [Google Scholar]
- Li G.; Wie J. J.; Nguyen N. A.; Chung W. J.; Kim E. T.; Char K.; Mackay M. E.; Pyun J. Synthesis, self-assembly and reversible healing of supramolecular perfluoropolyethers. J. Polym. Sci., Part A: Polym. Chem. 2013, 51, 3598–3606. 10.1002/pola.26777. [DOI] [Google Scholar]
- Liu M.; Liu P.; Lu G.; Xu Z.; Yao X. Multiphase-Assembly of Siloxane Oligomers with Improved Mechanical Strength and Water-Enhanced Healing. Angew. Chem., Int. Ed. 2018, 57, 11242–11246. 10.1002/anie.201805206. [DOI] [PubMed] [Google Scholar]
- Golkaram M.; van Ruymbeke E.; Portale G.; Loos K. Supramolecular Polymer Brushes: Influence of Molecular Weight and Cross-Linking on Linear Viscoelastic Behavior. Macromolecules 2020, 53, 4810–4820. 10.1021/acs.macromol.0c00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gemert G. M. L.; Peeters J. W.; Söntjens S. H. M.; Janssen H. M.; Bosman A. W. Self-Healing Supramolecular Polymers In Action. Macromol. Chem. Phys. 2012, 213, 234–242. 10.1002/macp.201100559. [DOI] [Google Scholar]
- Fan C.-J.; Huang Z.-C.; Li B.; Xiao W.-X.; Zheng E.; Yang K.-K.; Wang Y.-Z. A robust self-healing polyurethane elastomer: From H-bonds and stacking interactions to well-defined microphase morphology. Sci. China Mater. 2019, 62, 1188–1198. 10.1007/s40843-019-9422-7. [DOI] [Google Scholar]
- Xu J.; Chen P.; Wu J.; Hu P.; Fu Y.; Jiang W.; Fu J. Notch-Insensitive, Ultrastretchable, Efficient Self-Healing Supramolecular Polymers Constructed from Multiphase Active Hydrogen Bonds for Electronic Applications. Chem. Mater. 2019, 31, 7951–7961. 10.1021/acs.chemmater.9b02136. [DOI] [Google Scholar]
- Wu B.; Liu Z.; Lei Y.; Wang Y.; Liu Q.; Yuan A.; Zhao Y.; Zhang X.; Lei J. Mutually-complementary structure design towards highly stretchable elastomers with robust strength and autonomous self-healing property. Polymer 2020, 186, 122003. 10.1016/j.polymer.2019.122003. [DOI] [Google Scholar]
- Yanagisawa Y.; Nan Y.; Okuro K.; Aida T. Mechanically robust, readily repairable polymers via tailored noncovalent cross-linking. Science 2018, 359, 72–76. 10.1126/science.aam7588. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Hu J.; Liu Y. Shape memory effect of thermoplastic segmented polyurethanes with self-complementary quadruple hydrogen bonding in soft segments. Eur. Phys. J. E 2009, 28, 3–10. 10.1140/epje/i2008-10395-2. [DOI] [PubMed] [Google Scholar]
- Chen S.; Bi X.; Sun L.; Gao J.; Huang P.; Fan X.; You Z.; Wang Y. Poly(sebacoyl diglyceride) Cross-Linked by Dynamic Hydrogen Bonds: A Self-Healing and Functionalizable Thermoplastic Bioelastomer. ACS Appl. Mater. Interfaces 2016, 8, 20591–20599. 10.1021/acsami.6b05873. [DOI] [PubMed] [Google Scholar]
- Wu B.; Lei Y.; Xiao Y.; Wang Y.; Yuan Y.; Jiang L.; Zhang X.; Lei J. A Bio-Inspired and Biomass-Derived Healable Photochromic Material Induced by Hierarchical Structural Design. Macromol. Mater. Eng. 2020, 305, 1900539. 10.1002/mame.201900539. [DOI] [Google Scholar]
- Wang H.; Liu H.; Cao Z.; Li W.; Huang X.; Zhu Y.; Ling F.; Xu H.; Wu Q.; Peng Y.; Yang B.; Zhang R.; Kessler O.; Huang G.; Wu J. Room-temperature autonomous self-healing glassy polymers with hyperbranched structure. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 11299–11305. 10.1073/pnas.2000001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son D.; Kang J.; Vardoulis O.; Kim Y.; Matsuhisa N.; Oh J. Y.; To J. W.; Mun J.; Katsumata T.; Liu Y.; McGuire A. F.; Krason M.; Molina-Lopez F.; Ham J.; Kraft U.; Lee Y.; Yun Y.; Tok J. B.-H.; Bao Z. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol. 2018, 13, 1057–1065. 10.1038/s41565-018-0244-6. [DOI] [PubMed] [Google Scholar]
- Wang D.; Xu J.; Chen J.; Hu P.; Wang Y.; Jiang W.; Fu J. Transparent, Mechanically Strong, Extremely Tough, Self-Recoverable, Healable Supramolecular Elastomers Facilely Fabricated via Dynamic Hard Domains Design for Multifunctional Applications. Adv. Funct. Mater. 2020, 30, 1907109. 10.1002/adfm.201907109. [DOI] [Google Scholar]
- Tee B. C.-K.; Wang C.; Allen R.; Bao Z. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol. 2012, 7, 825–832. 10.1038/nnano.2012.192. [DOI] [PubMed] [Google Scholar]
- Liu X.; Lu C.; Wu X.; Zhang X. Self-healing strain sensors based on nanostructured supramolecular conductive elastomers. J. Mater. Chem. A 2017, 5, 9824–9832. 10.1039/c7ta02416a. [DOI] [Google Scholar]
- Liu X.; Su G.; Guo Q.; Lu C.; Zhou T.; Zhou C.; Zhang X. Hierarchically Structured Self-Healing Sensors with Tunable Positive/Negative Piezoresistivity. Adv. Funct. Mater. 2018, 28, 1706658. 10.1002/adfm.201706658. [DOI] [Google Scholar]
- Khatib M.; Zohar O.; Saliba W.; Srebnik S.; Haick H. Highly Efficient and Water-Insensitive Self-Healing Elastomer for Wet and Underwater Electronics. Adv. Funct. Mater. 2020, 30, 1910196. 10.1002/adfm.201910196. [DOI] [Google Scholar]
- Chen H.; Koh J. J.; Liu M.; Li P.; Fan X.; Liu S.; Yeo J. C. C.; Tan Y.; Tee B. C. K.; He C. Super Tough and Self-Healable Poly(dimethylsiloxane) Elastomer via Hydrogen Bonding Association and Its Applications as Triboelectric Nanogenerators. ACS Appl. Mater. Interfaces 2020, 12, 31975–31983. 10.1021/acsami.0c08213. [DOI] [PubMed] [Google Scholar]
- Patel T.; Kim M. P.; Park J.; Lee T. H.; Nellepalli P.; Noh S. M.; Jung H. W.; Ko H.; Oh J. K. Self-Healable Reprocessable Triboelectric Nanogenerators Fabricated with Vitrimeric Poly(hindered Urea) Networks. ACS Nano 2020, 14, 11442–11451. 10.1021/acsnano.0c03819. [DOI] [PubMed] [Google Scholar]
- Dai X.; Huang L. B.; Du Y.; Han J.; Zheng Q.; Kong J.; Hao J. Self-Healing, Flexible, and Tailorable Triboelectric Nanogenerators for Self-Powered Sensors based on Thermal Effect of Infrared Radiation. Adv. Funct. Mater. 2020, 30, 1910723. 10.1002/adfm.201910723. [DOI] [Google Scholar]
- Parida K.; Thangavel G.; Cai G.; Zhou X.; Park S.; Xiong J.; Lee P. S. Extremely stretchable and self-healing conductor based on thermoplastic elastomer for all-three-dimensional printed triboelectric nanogenerator. Nat. Commun. 2019, 10, 2158. 10.1038/s41467-019-10061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Guo Q.; Su G.; Cao J.; Liu J.; Zhang X. Hierarchically Structured Self-Healing Actuators with Superfast Light- and Magnetic-Response. Adv. Funct. Mater. 2019, 29, 1906198. 10.1002/adfm.201906198. [DOI] [Google Scholar]
- Pena-Francesch A.; Jung H.; Demirel M. C.; Sitti M. Biosynthetic self-healing materials for soft machines. Nat. Mater. 2020, 19, 1230–1235. 10.1038/s41563-020-0736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Chen L.; Lu L.; Du R.; Ma W.; Cai Y.; An X.; Wu H.; Luo Q.; Xu Q.; Zhang Q.; Jia X. A Highly-Adhesive and Self-Healing Elastomer for Bio-Interfacial Electrode. Adv. Funct. Mater. 2021, 31, 2006432. 10.1002/adfm.202006432. [DOI] [Google Scholar]
- Tan Y. J.; Godaba H.; Chen G.; Tan S. T. M.; Wan G.; Li G.; Lee P. M.; Cai Y.; Li S.; Shepherd R. F.; Ho J. S.; Tee B. C. K. A transparent, self-healing and high-κ dielectric for low-field-emission stretchable optoelectronics. Nat. Mater. 2020, 19, 182–188. 10.1038/s41563-019-0548-4. [DOI] [PubMed] [Google Scholar]
- Cao P.-F.; Li B.; Hong T.; Townsend J.; Qiang Z.; Xing K.; Vogiatzis K. D.; Wang Y.; Mays J. W.; Sokolov A. P.; Saito T. Superstretchable, Self-Healing Polymeric Elastomers with Tunable Properties. Adv. Funct. Mater. 2018, 28, 1800741. 10.1002/adfm.201800741. [DOI] [Google Scholar]
- Munaoka T.; Yan X.; Lopez J.; To J. W. F.; Park J.; Tok J. B.-H.; Cui Y.; Bao Z. Ionically Conductive Self-Healing Binder for Low Cost Si Microparticles Anodes in Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703138. 10.1002/aenm.201703138. [DOI] [Google Scholar]