Key Points
Question
Does inhibition of calcium/calmodulin-dependent protein kinase II delta with NP202 after anterior ST-segment elevation myocardial infarction prevent left ventricular (LV) remodeling?
Findings
This randomized clinical trial including 147 patients found no significant difference in the primary end point of change in LV end-systolic volume index over 3 months of treatment with drug NP202 compared with placebo. The drug was safe and well tolerated.
Meaning
Oral NP202 after anterior ST-segment elevation myocardial infarction with residual LV dysfunction does not attenuate LV remodeling.
This randomized clinical trial assesses whether inhibition of calcium/calmodulin-dependent protein kinase II delta after myocardial infarction prevents left ventricular remodeling.
Abstract
Importance
After anterior ST-segment elevation myocardial infarction (STEMI), left ventricular (LV) remodeling results in heart failure and death. Calcium/calmodulin-dependent protein kinase II delta (CaMKIId) is a key molecular mediator of adverse LV remodeling.
Objective
To determine whether NP202, an orally active inhibitor of CaMKIId, prevents LV remodeling in patients after anterior STEMI with early residual LV dysfunction.
Design, Setting, and Participants
A randomized, double-blind, placebo-controlled multicenter clinical trial of NP202 vs placebo in patients after primary percutaneous coronary intervention (PCI) for anterior STEMI was performed from November 19, 2015, to August 1, 2018. The study was performed at 32 sites across the US, Australia, and New Zealand. Patients presenting with anterior STEMI who underwent PCI within 12 hours of symptom onset and left ventricular ejection fraction (LVEF) less than 45% on screening echocardiogram 48 hours after primary PCI were included in the study. Baseline cardiovascular magnetic resonance (CMR) imaging was performed within 5 days of the STEMI and before administration of the study drug. Follow-up CMR was performed after 3 months. Data were analyzed from November 19, 2015, to August 1, 2018.
Interventions
Patients were randomly assigned to NP202, 1000 mg, daily for 3 months vs corresponding placebo.
Main Outcomes and Measures
The primary end point was change in LV end-systolic volume index (LVESVi) on CMR. Secondary end points were change in LV end-diastolic volume index, change in LVEF, change in infarct size, and change in diastolic function. Safety and tolerability were also assessed.
Results
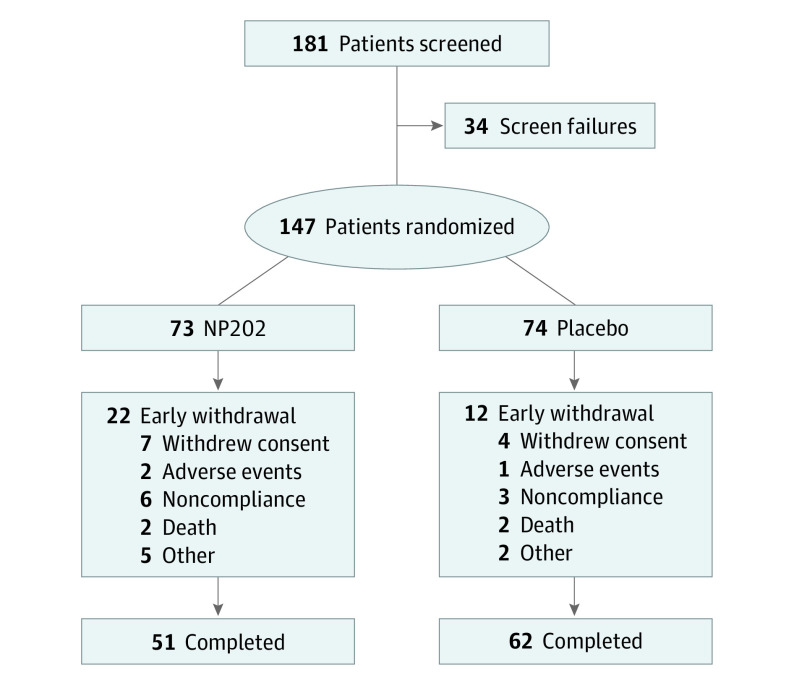
A total of 147 patients (mean [SD] age, 58 [11] years; 129 men [88%]; 130 White patients [88%]) who experienced anterior STEMI treated with primary PCI were randomized to receive NP202 (73 [49.7%]) or placebo (74 [50.3%]). Baseline LVEF was similar between groups. At baseline, patients randomized to NP202 had greater LVESVi (48.2 mL/m2) than that in the placebo group (41.3 mL/m2; P = .03). However, the groups were otherwise well matched. For the primary end point of change in LVESVi from baseline to 3 months, there was no significant difference between the placebo (median [interquartile range] change, −0.60 [−9.28 to 5.99] mL/m2) and NP202 groups (−3.53 [−9.24 to 4.81] mL/m2) (P = .78). There was also no difference in the secondary efficacy end points assessed by CMR. NP202 was well tolerated and demonstrated an acceptable safety profile. Major adverse cardiac and cerebrovascular event rates were similar between groups. Two deaths occurred in each group during the follow-up period.
Conclusions and Relevance
Three months of treatment with NP202 after primary PCI for anterior STEMI with residual LV dysfunction did not improve LV remodeling. The drug was safe and well tolerated.
Trial Registration
ClinicalTrials.gov Identifier: NCT02557217
Introduction
Improvements in the acute treatment of patients with ST-segment elevation myocardial infarction (STEMI) have led to a decline in mortality over the past 4 decades,1 with 1-year cardiac mortality plateauing at around 8%.2 However, morbidity due to post-MI heart failure (HF) remains significant and may even be increasing in some groups.3,4 Furthermore, those patients who are admitted to the hospital with HF after STEMI have a markedly increased mortality, and this also appears to be rising.5
Progressive adverse left ventricular (LV) remodeling may follow STEMI when a significant percentage of the left ventricle is damaged and may result in subsequent HF. Cardiovascular magnetic resonance (CMR) imaging allows noninvasive serial assessment of myocardial function and viability with high spatial resolution. Late gadolinium-enhancement CMR, initially validated in large-animal models, allows for the assessment of the transmural extent of irreversible injury and is superior to single-photon emission computed tomography for the identification of subendocardial MI. Furthermore, it permits quantification of even small areas of myocardial necrosis.6 These techniques are also sensitive enough to detect response to beneficial interventions such as drug therapies, cardiac resynchronization, and stem cell–based interventions. Attenuation or even reversal of this remodeling process can be demonstrated using these techniques.7,8,9
A number of agents have been demonstrated to be beneficial in the post-MI setting with regard to the remodeling process. These agents include angiotensin-converting enzyme (ACE) inhibitors (or angiotensin receptor blockers in patients who are intolerant of ACE inhibitors), β-blockade therapy, and aldosterone receptor antagonists.7 For this reason, all of these agents are recommended as standard medical therapy in patients with LV systolic dysfunction after MI.10 However, there remains an unacceptably high rate of progressive adverse LV remodeling and major cardiovascular events in such patients; hence, there is a clear need for additional therapies to prevent HF after STEMI.
Experimental evidence demonstrates that calcium/calmodulin-dependent protein kinase II (CaMKII), which is expressed in cardiomyocytes, can become constitutively active and contribute to HF, sinus node dysfunction, and atrial fibrillation.11 CaMKII is activated by ischemia-reperfusion injury and contributes to myocyte death and dysfunction by actions in the mitochondria.12 The dominant isoform in the heart is CaMKII delta (CaMKIId).12 Pharmacologic inhibition of CaMKIId using NP202 reduces infarct size13 and attenuates pathologic LV remodeling after experimental MI.14 In this trial, we therefore tested whether NP202 could prevent adverse LV remodeling and reduce infarct size after reperfusion in patients with anterior STEMI.
Methods
Study Design
We performed a randomized, double-blind, placebo-controlled, multicenter, phase II clinical trial from November 19, 2015, to August 1, 2018, including a total of 32 sites located in the US (21 sites), Australia (8 sites), and New Zealand (3 sites) (eTable 7 in Supplement 2). The sites and investigators are listed in the eAppendix in Supplement 2. The Institutional Human Research Ethics Committee of each participating hospital approved the study protocol (Supplement 1), and all patients provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Population
Patients presenting with a confirmed anterior STEMI in the previous 5 days were screened for the following inclusion criteria: (1) age 18 to 80 years, (2) greater than or equal to 0.2-mV ST-segment elevation in 2 or more precordial leads with presentation within a maximum of 12 hours of onset of symptoms, (3) troponin levels greater than 10× the upper limit of normal (local laboratory), (4) successful revascularization by percutaneous coronary intervention (PCI), (5) LV dysfunction post-STEMI as evidenced by LV ejection fraction (LVEF) less than or equal to 45% confirmed by echocardiogram at screening, (6) receipt of guideline-directed medical therapy for acute MI and post-MI LV dysfunction according to the National Cardiology Society/Heart Association guidelines, (7) agreed to use contraception for the duration of the study or were of non–child-bearing potential, and (8) were able to provide written informed consent. A full list of exclusion criteria is listed in the eAppendix in Supplement 2.
Randomization Protocol
Eligible patients were assigned to receive NP202 at a dosage of 1000 mg daily or matching placebo in a 1:1 ratio using a computer-generated algorithm. Randomization was stratified by LVEF (<35% vs ≥35%) as determined by echocardiogram at screening. At each visit when the study drug was dispensed, patients were allocated a bottle number by an interactive web or voice response system. The patient, site, sponsor, and monitoring personnel were blinded as to whether the patient was receiving NP202 or placebo. All patients who were randomized into the study were included in the full analysis set (n = 147). Patients who received at least 1 dose of study medication were included in the safety set (n = 137). Patients who received at least 1 dose of study medication and had an evaluable baseline and postbaseline CMR were included in the modified intention-to-treat (mITT) set (n = 105). All patients from the mITT who completed the study in compliance with the protocol with no major protocol violations were included in the per protocol set (n = 101).
End Points
The prespecified primary study end point was change in LV end-systolic volume index (LVESVi), which was calculated as LVESV divided by body surface area and measured by CMR imaging within 5 days (early) from the acute presentation and again at 90 days post-MI. Secondary efficacy end points included change in LV end-diastolic volume index (LVEDVi), change in infarct size (measured by late gadolinium enhancement on CMR imaging), change in EF, and change in diastolic function as measured by peak filling rate of the left ventricle. CMR images were acquired at the recruiting sites and transmitted to a core laboratory for blinded analysis and were reanalyzed in blinded fashion (MonashHeart). Data were compiled by an independent clinical research organization (Medpace Inc), and statistical analysis was performed by Array Biostatistics LLC and PureCDM independently of the study sponsors (Armaron Bio).
Safety was assessed by recording of adverse events (AEs); major adverse cardiac and cerebral events (MACCE), which were a composite of nonfatal MI, nonfatal stroke, cardiac hospitalization due to HF, and cardiovascular death; vital signs; laboratory parameters; 12-lead electrocardiograms (ECGs); and physical examinations. All end points were adjudicated by a blinded clinical events committee. A data safety monitoring board performed an interim analysis after 50 patients were evaluated. Troponin I and T, high-sensitivity C-reactive protein, and brain natriuretic peptide type B were measured as exploratory end points.
Statistical Analysis
Data were analyzed from November 19, 2015, to August 1, 2018. The study was designed to detect a treatment difference of at least 6.6 mL/m2 in the primary efficacy end point among patients with an evaluable CMR at baseline and at 3 months (day 90). A sample size of at least 90 patients was needed to provide 80% power to detect a treatment difference of at least 6.6 mL/m2 in the primary efficacy end point. Power was calculated for a 2-sided t test with a 5% type I error rate. The SD of the primary end point was assumed to be at most 11 mL/m2.15,16 At least 120 randomized patients were required to ensure at least 90 patients with an evaluable CMR at baseline and at 3 months (day 90) were enrolled. Sample size calculations were performed using SAS software, version 9.3 (SAS Institute Inc). Based on the results of the interim analysis, which showed an SD of 15 mL/m2, recruitment continued up to a maximum of 180 patients.
The analysis of the primary end point was performed using the mITT principle, using an analysis of covariance of the primary end point with terms for treatment, baseline LVESVi, and the randomization stratification factor (LVEF<35% vs LVEF≥35%). The mITT set included patients who had received at least 1 dose of the study drug to which they were randomized and had evaluable early and 3-month CMR films. The F test was used to test for a treatment difference in the primary end point at the 5% α level. Otherwise, normally distributed continuous variables were expressed as mean (SD), and comparisons were made using t tests. Nonnormally distributed data were expressed as medians and interquartile ranges (IQRs) and analyzed with the Wilcoxon signed rank sum test. Categorical variables were compared by the Fisher exact test. Statistical analyses were performed by Array Biostatistics LLC and PureCDM with Stata statistical software, version 11.2 (StataCorp LLC) and SPSS, version 26.0 (IBM Corp).
Results
A total of 147 patients (mean [SD] age, 58 [11] years; 129 men [88%]; 130 White patients [88%]) who experienced anterior STEMI treated with primary PCI were randomized to receive either NP202 (73 [49.7%]) at a dosage of 1000 mg daily or matching placebo (74 [50.3%]) (Figure 1). There was no difference in baseline LVEF between groups. The mean (SD) LVEF on screening echocardiogram was 37 (6%) in the placebo group and 38 (6%) in the treatment group (P = .60). On baseline CMR, the LVEF was 43 (9%) in the placebo group vs 42 (10%) in the treatment group (P = .33). Baseline demographic details are shown in Table 1. The groups were largely well matched. However, baseline weight was higher in the NP202 group than the placebo group (mean [SD], 97 [24] kg vs 89 [17] kg; P = .03), and the NP202 group had larger baseline LVESVi and LVEDVi than the control group. Guideline-directed medical therapy was similar in both groups (Table 2).
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Diagram.
Table 1. Subject Demographic Characteristics.
| Characteristic | No. (%)a | ||
|---|---|---|---|
| NP202 (n = 73) | Placebo (n = 74) | Overall (n = 147) | |
| Age, mean (SD), y | 56 (12) | 59 (10) | 58 (11) |
| Age categories, y | |||
| <65 | 53 (73) | 50 (68) | 103 (70) |
| 65-74 | 16 (22) | 23 (31) | 39 (27) |
| 75-84 | 3 (4) | 1 (1) | 4 (3) |
| ≥85 | 1 (1) | 0 | 1 (1) |
| Sex | |||
| Female | 6 (8) | 12 (16) | 18 (12) |
| Male | 67 (92) | 62 (84) | 129 (88) |
| Race/ethnicity | |||
| Asian | 0 | 4 (5) | 4 (3) |
| Black or African American | 0 | 4 (5) | 4 (3) |
| White | 67 (92) | 63 (85) | 130 (88) |
| Other | 5 (7) | 3 (4) | 7 (5) |
| Multiple | 1 (1) | 0 | 1 (1) |
| Weight, mean (SD), kg | 97 (24) | 89 (17) | 93 (21) |
| Height, mean (SD), cm | 176 (9) | 173 (8) | 175 (9) |
| BSA, mean (SD), m2 | 2.16 (0.29) | 2.06 (0.23) | 2.11 (0.27) |
| BMI, mean (SD) | 31 (7) | 30 (4) | 30 (6) |
| Country | |||
| Australia | 28 (38) | 32 (43) | 60 (41) |
| New Zealand | 11 (15) | 7 (10) | 18 (12) |
| United States | 34 (47) | 35 (47) | 69 (47) |
| Time from onset of symptoms to NP202 treatment, mean (SD), d | 4.9 (1.1) | 5.0 (1.0) | 4.9 (1.1) |
| No. | 66 | 71 | 137 |
| Time from hospital presentation to NP202 treatment, mean (SD), d | 4.7 (1.1) | 4.8 (1.1) | 4.8 (1.1) |
| No. | 66 | 71 | 137 |
| PCI | |||
| No. | 73 | 74 | 147 |
| Door-to-balloon time, mean (SD), h | 2.6 (6.4) | 2.7 (5.5) | 2.6 (5.9) |
| TIMI flow post-PCI | |||
| 0 | 2 (2.7) | 0 | 2 (1.4) |
| 1 | 0 | 2 (2.7) | 2 (1.4) |
| 2 | 2 (3) | 3 (4) | 5 (3) |
| 3 | 69 (95) | 69 (93) | 138 (94) |
| Location of culprit lesion | |||
| LAD proximal | 60 (82) | 62 (84) | 122 (83) |
| LAD distal | 4 (6) | 9 (12) | 13 (9) |
| LAD mid | 9 (12) | 3 (4) | 12 (8) |
| Culprit lesion stented | |||
| Yes | 73 (100) | 73 (99) | 146 (99) |
| No | 0 | 1 (1) | 1 (1) |
| Other stenosis | |||
| Yes, at least one was stented | 10 (14) | 18 (24) | 28 (19) |
| Yes, none were stented | 31 (43) | 30 (41) | 61 (42) |
| No | 32 (44) | 26 (35) | 58 (40) |
| Killip statusb | |||
| I | 63 (90) | 62 (86) | 125 (88) |
| II | 7 (10) | 9 (13) | 16 (11) |
| III | 0 | 1 (1) | 1 (1) |
| Missing | 3 | 2 | 5 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BSA, body surface area; LAD, left anterior descending coronary artery; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Data are presented as No. (%) unless otherwise stated.
Killip class I includes individuals with no clinical signs of heart failure. Class II includes individuals with rales, an S3 gallop, and elevated jugular venous pressure. Class III describes individuals with frank acute pulmonary edema. Class IV describes individuals in cardiogenic shock or with hypotension (ie, systolic blood pressure <90 mm Hg) and evidence of peripheral vasoconstriction (ie, oliguria, cyanosis, or sweating).
Table 2. Medications at Discharge.
| Medication | No. (%) | |
|---|---|---|
| NP202 (n = 66) | Placebo (n = 71) | |
| ACE inhibitors/ARBs | 65 (99) | 63 (89) |
| β-Blockers | 63 (96) | 69 (97) |
| MRAs | 12 (18) | 23 (32) |
| Statins | 65 (99) | 68 (96) |
| SGLT2 inhibitors | 0 | 3 (4) |
| Aspirin | 66 (100) | 69 (97) |
| Clopidogrel | 26 (39) | 24 (34) |
| Prasugrel | 4 (6) | 5 (7) |
| Ticagrelor | 42 (64) | 47 (66) |
| OAC | 11 (17) | 8 (11) |
| Metformin | 11 (17) | 8 (11) |
| Insulin | 9 (14) | 11 (15) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist; OAC, oral anticoagulant; SGLT2, sodium-glucose cotransporter 2.
Primary Efficacy End Point
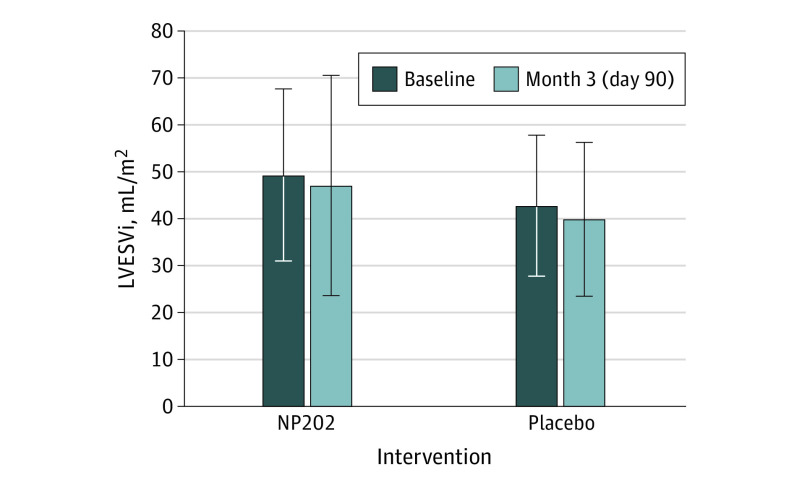
The LVESVi at baseline was larger in the NP202 group (48.2 mL/m2) than the placebo group (41.3 mL/m2; P = .03) (Figure 2). However, LVESVi was similar in both groups at day 90. Although there was a numerical difference in the degree of LV remodeling early post-MI to day 90 in both groups, there was no statistically significant difference between the placebo (median, −0.60 [IQR, −9.28 to 5.99] mL/m2) and NP202 groups (median, −3.53 [IQR, −9.24 to 4.81] mL/m2; P = .78). In a prespecified analysis, we also evaluated the per protocol group. Again, this analysis showed no significant difference between the placebo and NP202 groups (median, −0.6 [IQR, −10.04 to 5.77] mL/m2 vs −3.04 [IQR, −9.13 to 4.92] mL/m2; P = .64).
Figure 2. Primary End Point.
Left ventricular end-systolic volume index (LVESVi) was slightly larger in the NP202 group at baseline. There was no significant difference in the change in LVESVi or the final LVESVi at 3 months.
Subgroup analyses for change in LVESVi at day 90 were performed by infarct size (small infarct [<20% of LV mass] and large infarct [≥20% of LV mass]) and stratified for baseline LVESVi, ischemic duration, and country of origin. These results also showed no significant differences between groups.
Secondary Efficacy End Points
The secondary end points are shown in Table 3. The LVEDVi was larger in the NP202 group at baseline (median, 84 [IQR, 71-90] mL/m2 vs 72 [IQR, 60-84] mL/m2; P = .02). There was no significant difference between groups in the change in LVEF, LVEDVi, diastolic function (measured by LV peak filling rate), or change in infarct size. At baseline, the median absolute infarct size was 34% (IQR, 25%-46%) of the left ventricle in the control group and 29% (IQR, 21%-41%) in the NP202 group (P = .25). At day 90, median infarct size had decreased in both groups to 27% (IQR, 19%-38%) in the control vs 22% (IQR, 14%-28%) in the NP202 group (P = .26).
Table 3. Secondary Efficacy End Pointsa.
| End point | Median (IQR) | P value | ||
|---|---|---|---|---|
| Placebo (n = 59) | NP202 (n = 46) | ANCOVA | Wilcoxon rank sum test | |
| Change in LVEF, % | 5.5 (1.3 to 10.9) | 4.9 (0.4 to 11.1) | .66 | .68 |
| Change in LVEDVi, mL/m2 | 6.0 (−4.4 to 14.3) | 0.8 (−4.1 to 17.8) | .65 | .77 |
| Change in LVPFR | −5.5 (−86.3 to 52.1) | −8.4 (−131.1 to 38.5) | .59 | .39 |
| Change in infarct size | −6.8 (−16.9 to 3.2) | −6.2 (−15.1 to −0.5) | .25 | .97 |
Abbreviations: ANCOVA, analysis of covariance; IQR, interquartile range; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVPFR, left ventricular peak filling rate.
Data were analyzed on modified intention-to-treat principle.
Safety and Tolerability of NP202
All patients enrolled into the study who received at least 1 dose of study medication were included in the safety set. The safety set comprised 66 patients (90.4%) in the NP202 group and 71 patients (95.9%) in the placebo group. Guideline-directed medical therapy at discharge was similar between groups (Table 2). Adverse events leading to study drug discontinuation occurred in 4 (6.1%) of the NP202 group and 4 (5.6%) of the placebo group. The overall incidence of treatment-emergent AEs (TEAEs) was similar in the NP202 group and placebo group for the 137 patients included in the safety population. For example, 2 patients (3.0%) in the NP202 group and 1 patient (1.4%) in the placebo group had palpitations compared with 3 patients (2.2%) overall. The most frequently reported TEAEs during the study were dyspnea and diarrhea, experienced by a total of 15 (10.9%) and 14 patients (10.2%), respectively (eTable 3 in Supplement 2). Both of these TEAEs were reported by numerically more patients in the NP202 group compared with the placebo group (dyspnea, 10 [15.2%] vs 5 [7.0%]; P = .13; diarrhea, 6 [9.1%] vs 2 [2.1%]; P = .12).
The number of patients experiencing MACCE events was similar in the NP202 group and the placebo group (5 [7.6%] vs 7 [9.9%]; P = .32) (eTable 1 in Supplement 2). Four deaths occurred, 2 in each treatment group.
No differences between treatment groups were observed across the study visits for vital signs, ECG results, hematology, brain natriuretic peptide, coagulation, and urinalysis (eTables 2-6 in Supplement 2). There was a trend to reduction in troponin T and troponin I on day 90 (mean [SD], 15.37 [8.96] ng/L) and day 120 (mean [SD], 15.69 [12.18] ng/L) in patients treated with NP202, although the difference was not significant (P = .13 in each case). There were numerically fewer patients with elevated troponin (>15 ng/mL) at day 90 and day 120 post-MI in the NP202 group compared with the placebo group (6 [9%] vs 19 [27%]; P = .05) (eAppendix in Supplement 2).
Discussion
The primary objective of this randomized, double-blind, placebo-controlled multicenter study was to evaluate the efficacy of NP202 compared with placebo in attenuating pathologic LV remodeling in patients post-MI. No effect was observed on the primary end point nor on any of the secondary efficacy end points.
This trial aimed to recruit patients at highest risk for adverse LV remodeling. We only included patients with anterior STEMIs whose LVEF remained depressed more than 48 hours after primary PCI. This protocol selected for moderate-sized infarcts. The baseline infarct size on CMR was 34% of the left ventricle in the control group and 29% of the left ventricle in the NP202 group. The infarct size in this study is in keeping with previous similar studies. A systematic review of randomized trials using acute CMR within days of primary PCI for STEMI showed that the infarct size ranged from 22% to 37% in left anterior descending–territory infarcts.8 Therefore, the negative results of the trial are not due to enrolling patients who are at low risk for remodeling.
There may be several potential explanations for the negative overall trial result. First, the study was effectively underpowered. The mean SD for LVESVi that was used for sample size calculations was 11 mL/m2; however, the mean SD observed for LVESVi in this study was 20 mL/m2. Therefore, despite a numeric trend toward more improvement in the treatment group, the study was significantly underpowered to detect the minimum clinically meaningful difference in LVESVi. Furthermore, the withdrawal rate was higher than anticipated, which could also contribute to effectively underpowering the study.
Another possibility is that the medication was started too late. In preclinical studies, NP202 was administered before reperfusion in sheep13 and on day 2 post-MI in rats,14 and NP202 was efficacious in both of those models. It is possible that there is an early window when NP202 is effective in preventing LV remodeling and, by starting from day 5 post-MI, we started too late. Furthermore, these laboratory studies were not conducted in addition to guideline-directed medical therapy. Standard therapies, such as ACE inhibitors and β-blockers, may have similar effects as NP202, making it redundant in patients on guideline-directed medical therapy. It is also possible that juvenile animals respond to NP202 better than aging humans. This is not the first study to fail in cardioprotection after MI.17 Although preclinical studies targeting CaMKII have yielded mixed results,13,14,18 the strong mechanistic link between CaMKII and cardiac hypertrophy and failure warrants further exploration.
A secondary objective of the study was to assess the safety and tolerability of NP202 compared with placebo when administered once daily for 3 months to patients post-MI. Evaluation of the incidence of TEAEs, changes in vital signs, ECG measurements, and clinical laboratory assessments demonstrated that NP202 was safe and well tolerated compared with placebo. The overall incidence of TEAEs was similar in the NP202 group and placebo group for the 137 patients included in the safety population. The incidence of MACCE events and serious AEs was similar in the NP202 group compared with the placebo group. There were no clinically relevant differences between treatment groups for clinical safety laboratory findings, vital signs, ECGs, or physical examinations. Although treatment assignment was blinded to both patients and treating staff, because of the color change that NP202 causes in semen, it was possible that patients or study staff may have been unblinded. However, as the study efficacy end points were objective and were assessed by blinded reviewers, this factor did not compromise the study objectives.
Limitations
This study has important limitations. First, the baseline groups were not perfectly matched, despite randomization. In particular, the difference in baseline weight and LVESVi may bias the results against the treatment group. Second, we did not calculate the area at risk or the myocardial salvage index. Third, the door-to-balloon time was long at more than 2 hours, which may have altered the LV remodeling. However, there was no difference between groups, and this factor is not likely to alter the interpretation of the findings of the trial. Fourth, the total ischemic time was not recorded. It is assumed that all patients had less than 12 hours between symptom onset and arrival at the hospital, as defined in the inclusion criteria. Differences between groups are unlikely because of randomization, but if there were differences, this could affect the study outcomes. Finally, this study was not powered for clinical events, such as recurrent MI, HF admissions, or mortality, nor was it continued beyond 3 months. Thus, a later beneficial effect on clinical outcomes could not be excluded.
Conclusions
In summary, this randomized clinical trial found that 3 months of treatment with NP202 did not attenuate pathologic LV remodeling after anterior STEMI. NP202 was safe and well tolerated, with a safety profile that was similar between the NP202 and placebo groups. Given the minimal improvements noted in inhibition of the CaMKIId pathways after MI, alternate approaches to minimize LV remodeling should be considered.
Trial Protocol
eAppendix. Exclusion Criteria
eTable 1. Major Adverse Cardiac and Cerebrovascular Events (MACCE)
eTable 2. ECG Findings
eTable 3. Adverse Events Associated With Changes to ECG Parameters
eTable 4. Summary of New York Heart Association Classification
eTable 5. Change in Cardiac Biomarker NT-proBNP
eTable 6. Change in Cardiac Biomarker Troponin T
eTable 7. Investigators and Affiliated Institutions
Data Sharing Statement
References
- 1.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366(1):54-63. doi: 10.1056/NEJMra1112570 [DOI] [PubMed] [Google Scholar]
- 2.Pedersen F, Butrymovich V, Kelbæk H, et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol. 2014;64(20):2101-2108. doi: 10.1016/j.jacc.2014.08.037 [DOI] [PubMed] [Google Scholar]
- 3.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53(1):13-20. doi: 10.1016/j.jacc.2008.08.067 [DOI] [PubMed] [Google Scholar]
- 4.Velagaleti RS, Pencina MJ, Murabito JM, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118(20):2057-2062. doi: 10.1161/CIRCULATIONAHA.108.784215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezad S, Al-Omary MS, Davies AJ, et al. Heart failure admissions following ST segment elevation myocardial infarction. Aust J Rural Health. 2019;27(1):99-100. doi: 10.1111/ajr.12456 [DOI] [PubMed] [Google Scholar]
- 6.Bulluck H, Dharmakumar R, Arai AE, Berry C, Hausenloy DJ. Cardiovascular magnetic resonance in acute ST-segment-elevation myocardial infarction: recent advances, controversies, and future directions. Circulation. 2018;137(18):1949-1964. doi: 10.1161/CIRCULATIONAHA.117.030693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutton MGSJ, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981-2988. doi: 10.1161/01.CIR.101.25.2981 [DOI] [PubMed] [Google Scholar]
- 8.Bulluck H, Hammond-Haley M, Weinmann S, Martinez-Macias R, Hausenloy DJ. Myocardial infarct size by CMR in clinical cardioprotection studies: insights from randomized controlled trials. JACC Cardiovasc Imaging. 2017;10(3):230-240. doi: 10.1016/j.jcmg.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol. 2009;55(1):1-16. doi: 10.1016/j.jacc.2009.06.059 [DOI] [PubMed] [Google Scholar]
- 10.Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation. 2004;110(9):e82-e292. [PubMed] [Google Scholar]
- 11.Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110(12):1661-1677. doi: 10.1161/CIRCRESAHA.111.243956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P. CaMKII: the molecular villain that aggravates cardiovascular disease. Exp Ther Med. 2017;13(3):815-820. doi: 10.3892/etm.2017.4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas CJ, Ng DC, Patsikatheodorou N, et al. Cardioprotection from ischaemia-reperfusion injury by a novel flavonol that reduces activation of p38 MAPK. Eur J Pharmacol. 2011;658(2-3):160-167. doi: 10.1016/j.ejphar.2011.02.041 [DOI] [PubMed] [Google Scholar]
- 14.Kompa A, Khong F, Nguyen C, et al .. NP202 attenuates left ventricular systolic dysfunction and pathological remodelling following myocardial infarction. Heart Lung Circ. 2018;27(2):S117. doi: 10.1016/j.hlc.2018.06.165 [DOI] [Google Scholar]
- 15.Gerbaud E, Montaudon M, Chasseriaud W, et al. Effect of ivabradine on left ventricular remodelling after reperfused myocardial infarction: a pilot study. Arch Cardiovasc Dis. 2014;107(1):33-41. doi: 10.1016/j.acvd.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 16.Najjar SS, Rao SV, Melloni C, et al. ; REVEAL Investigators . Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA. 2011;305(18):1863-1872. doi: 10.1001/jama.2011.592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefer DJ, Marbán E. Is cardioprotection dead? Circulation. 2017;136(1):98-109. doi: 10.1161/CIRCULATIONAHA.116.027039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty A, Pasek DA, Huang T-Q, et al. Inhibition of CaMKII does not attenuate cardiac hypertrophy in mice with dysfunctional ryanodine receptor. PLoS One. 2014;9(8):e104338. doi: 10.1371/journal.pone.0104338 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Exclusion Criteria
eTable 1. Major Adverse Cardiac and Cerebrovascular Events (MACCE)
eTable 2. ECG Findings
eTable 3. Adverse Events Associated With Changes to ECG Parameters
eTable 4. Summary of New York Heart Association Classification
eTable 5. Change in Cardiac Biomarker NT-proBNP
eTable 6. Change in Cardiac Biomarker Troponin T
eTable 7. Investigators and Affiliated Institutions
Data Sharing Statement