Key Points
Question
Is intravenous administration of tranexamic acid associated with thromboembolic events in patients of all ages and of any medical discipline?
Findings
In this systematic review and meta-analysis of 216 studies of 125 550 patients undergoing surgical procedures and receiving either intravenous administration of tranexamic acid or placebo or no treatment, 1020 (2.1%) thromboembolic events in the tranexamic acid group and 900 (2.0%) total thromboembolic events in the control group were found. There was no increased risk of any thromboembolic event in patients of all medical disciplines.
Meaning
These results clarify whether vascular occlusive events are associated with administration of tranexamic acid.
Abstract
Importance
Tranexamic acid (TXA) is an efficient antifibrinolytic agent; however, concerns remain about the potential adverse effects, particularly vascular occlusive events, that may be associated with its use.
Objective
To examine the association between intravenous TXA and total thromboembolic events (TEs) and mortality in patients of all ages and of any medical disciplines.
Data Source
Cochrane Central Register of Controlled Trials and MEDLINE were searched for eligible studies investigating intravenous TXA and postinterventional outcome published between 1976 and 2020.
Study Selection
Randomized clinical trials comparing intravenous TXA with placebo/no treatment. The electronic database search yielded a total of 782 studies, and 381 were considered for full-text review. Included studies were published in English, German, French, and Spanish. Studies with only oral or topical tranexamic administration were excluded.
Data Extraction and Synthesis
Meta-analysis, subgroup and sensitivity analysis, and meta-regression were performed. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Main Outcomes and Measures
Vascular occlusive events and mortality.
Results
A total of 216 eligible trials including 125 550 patients were analyzed. Total TEs were found in 1020 (2.1%) in the group receiving TXA and 900 (2.0%) in the control group. This study found no association between TXA and risk for total TEs (risk difference = 0.001; 95% CI, −0.001 to 0.002; P = .49) for venous thrombosis, pulmonary embolism, venous TEs, myocardial infarction or ischemia, and cerebral infarction or ischemia. Sensitivity analysis using the risk ratio as an effect measure with (risk ratio = 1.02; 95% CI, 0.94-1.11; P = .56) and without (risk ratio = 1.03; 95% CI, 0.95-1.12; P = .52) studies with double-zero events revealed robust effect size estimates. Sensitivity analysis with studies judged at low risk for selection bias showed similar results. Administration of TXA was associated with a significant reduction in overall mortality and bleeding mortality but not with nonbleeding mortality. In addition, an increased risk for vascular occlusive events was not found in studies including patients with a history of thromboembolism. Comparison of studies with sample sizes of less than or equal to 99 (risk difference = 0.004; 95% CI, −0.006 to 0.014; P = .40), 100 to 999 (risk difference = 0.004; 95% CI, −0.003 to 0.011; P = .26), and greater than or equal to 1000 (risk difference = −0.001; 95% CI, −0.003 to 0.001; P = .44) showed no association between TXA and incidence of total TEs. Meta-regression of 143 intervention groups showed no association between TXA dosing and risk for venous TEs (risk difference, −0.005; 95% CI, −0.021 to 0.011; P = .53).
Conclusions and Relevance
Findings from this systematic review and meta-analysis of 216 studies suggested that intravenous TXA, irrespective of dosing, is not associated with increased risk of any TE. These results help clarify the incidence of adverse events associated with administration of intravenous TXA and suggest that TXA is safe for use with undetermined utility for patients receiving neurological care.
This systematic review and meta-analysis with meta-regression of 216 randomized clinical trials assesses the association between intravenous administration of tranexamic acid and vascular occlusive events in patients undergoing surgery or experiencing bleeding.
Introduction
Major surgery is commonly associated with substantial blood loss, subsequent anemia, and the need for blood transfusion. The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study investigators1 reported that among 40 004 patients undergoing noncardiac surgical procedures, the most common complications leading to death were major bleeding followed by myocardial injury and infection. Tranexamic acid (TXA) is an antifibrinolytic agent and widely used for prophylaxis and treatment of bleeding caused by hyperfibrinolysis. Poeran and colleagues2 reported that the use of TXA increased in orthopedic surgery from almost 0% in 2006 to 11.2% in 2012, and the effectiveness to reduce surgical blood loss and associated complications has been reported.
Ker and colleagues3 performed a meta-analysis including 129 trials encompassing more than 10 000 patients suggesting that administration of TXA is associated with reductions in allogeneic blood transfusion by 38% and that further trials assessing this effect are unlikely to add new insights. However, TXA is only applied with caution because antifibrinolytic therapy may be associated with an increased risk of seizures,4,5 myocardial infarction,6 and other thrombotic complications.7,8,9 Nevertheless, the association of vascular occlusive events with TXA administration is controversial. Overall, vascular occlusive events are rare, and studies with 0 events are often excluded from meta-analysis because the assumption is that these studies may not be relevant to the treatment effect. Of 115 investigated trials in the meta-analysis by Ker and colleagues,3 72 examined the rate of pulmonary embolism (PE) and 66 studies examined the rate of deep vein thrombosis (DVT). However, trials with 0 events were excluded from analysis. Overall, TXA was not associated with an increased risk of PE in 10 trials (risk ratio [RR] = 0.61; 95% CI, 0.25-1.47) (event rate in TXA group: 4 of 449 vs control: 8 of429) and DVT in 19 trials (RR = 0.86; 95% CI, 0.53-1.39) (event rate in TXA group: 25 of 785 vs control: 29 of 785). Based on the low number of included event rates, the issue of use of TXA and vascular occlusive events remains unaddressed.3 Several guidelines recommend the use of TXA in patients with excessive bleeding.10,11,12,13 However, little is known about the incidence of vascular occlusive events in patients with substantial comorbidities or a history of thromboembolic events (TEs). In addition, debate is ongoing about the optimal perioperative dosing of intravenous TXA, which varies widely, ranging from 0.5 to 5 g or 10 to 100 mg/kg and might also explain the different observed incidences of vascular occlusive events.
To further explore the possible associations between intravenous TXA and vascular occlusive events in patients undergoing surgery or experiencing bleeding, we performed a comprehensive meta-analysis. We included randomized clinical trials (RCTs) irrespective of event rate and dosing regimen comparing intravenous TXA with a control group (placebo or no treatment) in our analysis. These data might help to clarify the safety of intravenous TXA and elucidate a possible dosing effect.
Methods
Search Strategy and Study Selection
We systematically searched Cochrane Central Register of Controlled Trials and MEDLINE via PubMed for eligible RCTs investigating the effect of intravenous TXA on postinterventional outcome published between 1976 and 2018, followed by a manual search through September 20, 2019. To identify RCTs published after completion of the meta-analysis, another systematic review was performed for eligible studies published between July 1, 2018, and December 31, 2020. Search terms used and additional details are available in eAppendix 1 in the Supplement.
Outcome Measures
End points were venous thrombosis (VT), PE, venous thromboembolic events (VTEs), myocardial infarction or ischemia (MI), cerebral infarction or ischemia (CII), limb ischemia, mesenteric ischemia, hepatic artery thrombosis, and composite of all vascular occlusive events (total thromboembolic events [total TEs]). In addition, we assessed overall mortality, bleeding mortality, and any nonbleeding mortality rate (eAppendix 2 in the Supplement).
Data Extraction and Statistical Analyses
This study was conducted in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for cohort studies.14 Because many studies showed 0 events in both groups, we assessed the risk difference (RD) to provide accurate results. The fixed-effect analysis was used for meta-analysis because we assumed that the true effect size was the same in all studies because TEs are rare and the only reason the effect size varied between studies could be caused by the number of recruited patients. However, we performed sensitivity analysis using a random-effects model to estimate whether our results were robust. Furthermore, we performed a sensitivity analysis using the RR as an effect size measure with either including (continuity correction of 0.5) or excluding studies with 0 cell frequencies. An additional analysis of total TEs with subgroups by study size was conducted. Heterogeneity was assessed by using I2 statistics. A meta-regression was performed to investigate a relation between the event rate and the dosage of intravenous TXA.
We also investigated whether intravenous TXA was associated with increased risk for TE, VT, PE, VTEs, and overall mortality in patients with risks for TEs. Therefore, secondary analyses were performed only with studies including patients with a history of any TE, coronary artery disease, thrombophilia, or contraindication for TXA. Two of us (I.T. and S.C.) independently assessed the methodologic quality of included studies based on the Cochrane Risk of Bias tool. A sensitivity analysis with studies judged at low risk of selection bias was assessed for total TEs. Funnel plots were generated to detect possible evidence for small-study bias. Discrepancies were resolved by group discussion (I.T., S.C., P.M., E.H., and S.W.). Two-sided P < .05 was considered statistically significant. The Review Manager (RevMan) program, version 5.3 (The Nordic Cochrane Centre) and R software, version 3.6.1 (R Foundation for Statistical Computing) were used for analysis and graphic illustrations. Continuity corrections were performed with the R package, meta version 4.12-0. Further details appear in eAppendix 3 in the Supplement.
Results
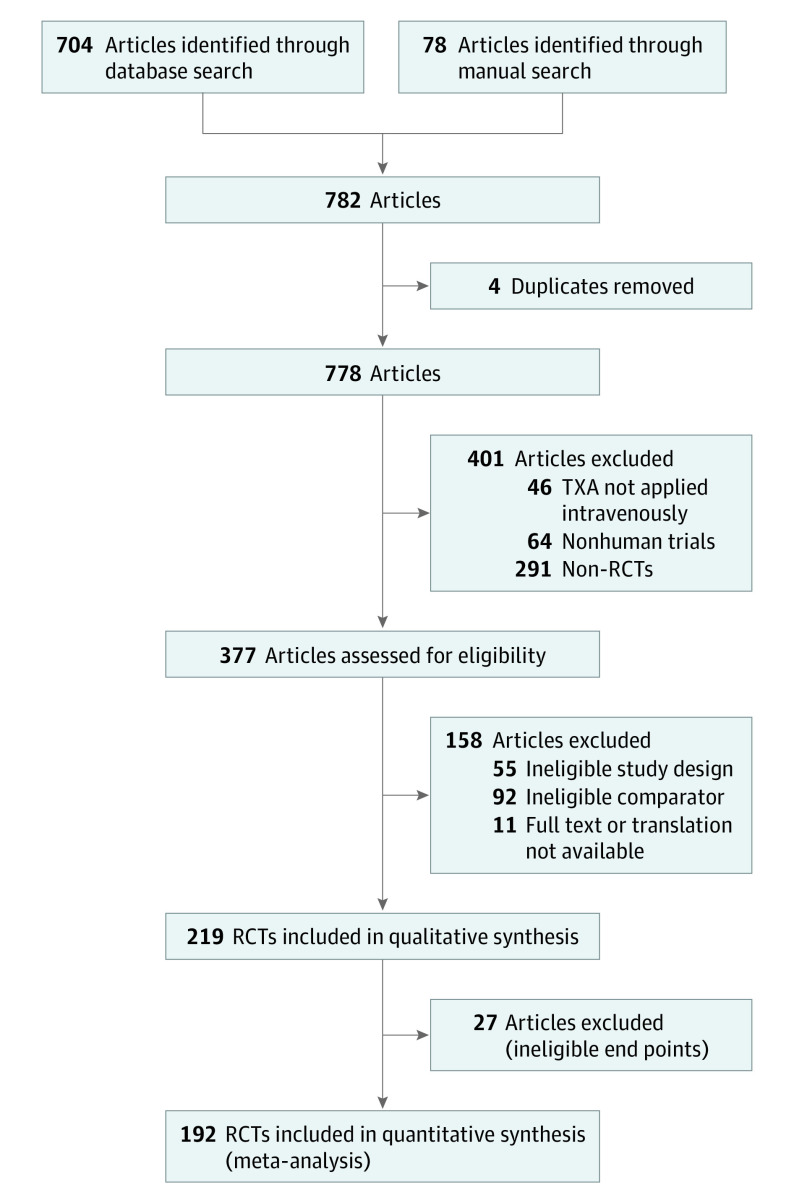
The electronic database and manual search yielded a total of 782 studies published between 1976 and 2018. In total, 381 articles were considered for full-text review, of which 189 were excluded because of ineligible study designs (n = 55) or control groups (n = 92), ineligible end points (n = 27), missing or nontranslatable full text (n = 11), and duplicates (n = 4), leaving 192 RCTs6,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205 for final analysis (Figure). Included studies were published in English, German, French, and Spanish. Twenty-three authors were contacted, of whom 2 provided clarification113 or data,185 whereas 21 did not reply. In total, 68 118 patients of this search were included in this meta-analysis (34 610 TXA group and 33 508 control group). Demographic data and subgroup characteristics are reported in eTable 1 and eAppendix 4 in the Supplement.
Figure. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Flow Diagram Showing Study Selection.
The database search was conducted for articles published between 1976 and 2018, and the manual search was conducted through September 20, 2019. An updated search for studies published between July 1, 2018, and December 31, 2020, resulted in 72 potential additional studies of which 48 studies were excluded leaving 24 additional studies. Overall, 216 eligible studies underwent analysis. RCT indicates randomized clinical trial; TXA, tranexamic acid.
Total Thromboembolic Events
In total, 176 studies (eAppendix 11 in the Supplement) provided data on total TEs in 65 900 patients (n = 33 487 TXA group vs n = 32 413 control group). We found 779 total TEs (2.3%) in the TXA group and 706 total TEs (2.2%) in the control group. Overall, administration of intravenous TXA was not associated with an increased risk for total TEs (RD = 0.001; 95% CI, −0.002 to 0.003; P = .66) (Table 1; eFigure 1 in the Supplement). However, the subgroup analysis showed that TXA was associated with a significantly increased risk for total TEs in the group of patients with neurological conditions (RD = 0.026; 95% CI, 0.007-0.045; P = .007). Sensitivity analysis using a random-effects model showed robust effect estimates for total TEs (RD = −0.001; 95% CI, −0.002 to 0.001; P = .39), but no significantly increased risk for total TEs in the subgroup of patients with neurological conditions was shown (RD = 0.018; 95% CI, −0.013 to 0.048; P = .26) (Table 1; eFigure 2 in the Supplement). Analysis of patients with neurological conditions showed a significant heterogeneity (I2 = 57%). To investigate whether the heterogeneity was associated with sample size, we performed a sensitivity analysis including studies with sample sizes with more than 1000 patients. Overall, the heterogeneity remained high (I2 = 73%). A sensitivity analysis was performed using the RR as an effect measure with and without studies with double-zero events revealed robust effect estimates for all subgroups (RR = 1.01; 95% CI, 0.92-1.11; P = .77 and RR = 1.02; 95% CI, 0.93-1.12; P = .71) (eFigure 3 and eFigure 4 in the Supplement). To elucidate whether incidence of total TEs increases with sample size, we performed a sensitivity analysis of studies including less than or equal to 99 patients, 100 to 999 patients, or greater than or equal to 1000 patients. Overall, administration of intravenous TXA was not associated with an increased risk for total TEs in studies with less than or equal to 99 patients (RD = 0.004; 95% CI, −0.006 to 0.014; P = .40), 100 to 999 patients (RD = 0.004; 95% CI, −0.003 to 0.011; P = .26), and greater than or equal to 1000 patients (RD = −0.001; 95% CI, −0.003 to 0.001; P = .44) (eFigure 5 in the Supplement). The results remained robust using a random-effects model (eFigure 6 in the Supplement).
Table 1. TXA and Total Thromboembolic Events.
| Medical discipline | No. of included studies | TXA | Control | Model | Risk difference (95% CI) | P value | I2, % | ||
|---|---|---|---|---|---|---|---|---|---|
| Events | No. of included patients | Events | No. of included patients | ||||||
| Cardiothoracic | 16 | 72 | 3171 | 74 | 3009 | Fixed effect | −0.001 (−0.009 to 0.007) | .83 | 0 |
| Random effects | −0.001 (−0.007 to 0.008) | .91 | |||||||
| Neurological | 12 | 282 | 2007 | 230 | 2000 | Fixed effect | 0.026 (0.007 to 0.045) | .01 | 57 |
| Random effects | 0.018 (−0.013 to 0.048) | .26 | |||||||
| Gynecological | 26 | 35 | 12 356 | 41 | 12 286 | Fixed effect | −0.001 (−0.002 to 0.001) | .53 | 0 |
| Random effects | −0.001 (−0.002 to 0.001) | .50 | |||||||
| Orthopedic | 101 | 172 | 4787 | 113 | 4149 | Fixed effect | 0.001 (−0.007 to 0.009) | .79 | 0 |
| Random effects | 0.001 (−0.004 to 0.007) | .64 | |||||||
| Major trauma | 1 | 204 | 10 060 | 233 | 10 067 | Fixed effect | −0.003 (−0.007 to 0.001) | .16 | NA |
| Random effects | −0.003 (−0.007 to 0.001) | .16 | |||||||
| Maxillofacial | 6 | 0 | 265 | 0 | 192 | Fixed effect | 0.000 (−0.023 to 0.023) | >.99 | 0 |
| Random effects | 0.000 (−0.019 to 0.019) | >.99 | |||||||
| Pediatric | 2 | 0 | 42 | 0 | 40 | Fixed effect | 0.000 (−0.067 to 0.067) | >.99 | 0 |
| Random effects | 0.000 (−0.064 to 0.064) | >.99 | |||||||
| Other | 12 | 14 | 799 | 15 | 670 | Fixed effect | −0.004 (−0.021 to 0.013) | .62 | 0 |
| Random effects | −0.004 (−0.018 to 0.011) | .63 | |||||||
| Total | 176 | 779 | 33 487 | 706 | 32 413 | Fixed effect | 0.001 (−0.002 to 0.003) | .66 | 0 |
| Random effects | −0.001 (−0.002 to 0.001) | .39 | |||||||
Abbreviations: NA, not applicable; TXA, tranexamic acid.
Venous Thrombosis, Pulmonary Embolism, and Venous Thromboembolic Events
In total, 163 studies (eAppendix 12 in the Supplement) provided data on VTs in 59 666 patients (n = 30 334 TXA group vs n = 29 332 control group). We found 272 VTs (0.9%) in the TXA group and 213 VTs (0.7%) in the control group. Overall, administration of intravenous TXA was not associated with an increased risk for VT (RD = −0.000; 95% CI, −0.002 to 0.002; P > .99) (eTable 2, eFigure 7 in the Supplement). Sensitivity analysis using a random-effects model showed robust effect estimates for PE (RD = −0.000; 95% CI, −0.001 to 0.000; P = .26) (eTable 2; eFigure 8 in the Supplement).
In total, 129 studies (eAppendix 13 in the Supplement) provided data on PE events in 61 562 patients (n = 31 155 TXA group vs n = 30 407 control group). We found 152 PE events (0.5%) in the TXA group and 153 PE events (0.5%) in the control group. Overall, administration of intravenous TXA was not associated with an increased risk for PE (RD = −0.000; 95% CI, −0.001 to 0.001; P = .89) (eTable 3; eFigure 9 in the Supplement). Sensitivity analysis using a random-effects model showed robust effect estimates for VT (RD = −0.000; 95% CI, −0.001 to 0.001; P = .68) (eTable 3; eFigure 10 in the Supplement).
To assess the total number of VTEs, PE and VT were combined and analyzed. In total, 123 studies (eAppendix 14 in the Supplement) provided data on VTEs in 56 126 patients (n = 28 438 TXA group vs n = 27 688 control group). We found 348 VTEs (1.2%) in the TXA group and 304 VTEs (1.1%) in the control group. Overall, administration of intravenous TXA was not associated with an increased risk for VTEs (RD = −0.000; 95% CI, −0.002 to 0.002; P = .71) (eTable 4; eFigure 11 in the Supplement). Sensitivity analysis using a random-effects model showed robust effect estimates for VTE (RD = −0.001; 95% CI, −0.002 to 0.001; P = .39) (eTable 4; eFigure 12 in the Supplement).
Myocardial Infarction or Ischemia, Cerebral Infarction or Ischemia, and Other Thromboembolic Events
Overall, administration of intravenous TXA was not associated with an increased risk for MI (RD = −0.000; 95% CI, −0.001 to 0.001; P = .56) (eTable 5; eFigure 13 and eFigure 14 in the Supplement). Detailed analyses are reported in eAppendix 5 in the Supplement.
Overall, administration of intravenous TXA was not associated with an increased risk for CII (RD = −0.000; 95% CI, −0.001 to 0.000; P = .90) (eTable 6; eFigure 15 in the Supplement) or other TEs (eFigures 16-18 in the Supplement). Detailed analyses are reported in eAppendix 6 in the Supplement.
Overall Mortality
In total, 63 studies (eAppendix 15 in the Supplement) assessed the overall mortality in 55 305 patients (n = 27 865 TXA group vs n = 27 440 control group). Death occurred in 2218 patients (8%) in the TXA group and 2456 patients (9%) in the control group. Overall, administration of intravenous TXA was associated with significant reductions in overall mortality in patients of the TXA group (RD = −0.011; 95% CI, −0.015 to −0.007; P < .001) (Table 2) (eFigure 19 in the Supplement). Subgroup analysis showed a significantly decreased overall mortality in patients with trauma (RD = −0.015; 95% CI, −0.022 to −0.008; P = .004) and patients receiving care from other disciplines (RD = −0.038; 95% CI, −0.06 to −0.015; P = .001) for the TXA groups, whereas no significant differences could be detected within the remaining medical disciplines. Sensitivity analysis using a random-effects model did not show robust effect estimates for overall mortality (RD = −0.004; 95% CI, −0.008 to 0.000; P = .05) and for the subgroup of patients receiving care from other disciplines (RD = −0.024; 95% CI, −0.058 to 0.009; P = .15) (Table 2; eFigure 20 in the Supplement). Analysis of patients of other disciplines showed a significant heterogeneity (I2 = 78%).
Table 2. TXA and Overall Mortality.
| Medical discipline | No. of included studies | TXA | Control | Model | Risk difference (95% CI) | P value | I2, % | ||
|---|---|---|---|---|---|---|---|---|---|
| Events | No. of included patients | Events | No. of included patients | ||||||
| Cardiothoracic | 15 | 32 | 3006 | 46 | 2970 | Fixed effect | −0.005 (−0.011 to 0.002) | .12 | 0 |
| Random effects | −0.003 (−0.009 to 0.003) | .30 | |||||||
| Neurological | 13 | 426 | 2017 | 449 | 2002 | Fixed effect | −0.013 (−0.032 to 0.005) | .31 | 27 |
| Random effects | −0.016 (−0.041 to 0.02) | .28 | |||||||
| Gynecological | 8 | 227 | 10 871 | 256 | 10 814 | Fixed effect | −0.003 (−0.007 to 0.001) | .17 | 0 |
| Random effects | −0.002 (−0.005 to 0.002) | .31 | |||||||
| Orthopedic | 16 | 18 | 844 | 17 | 652 | Fixed effect | 0.001 (−0.018 to 0.019) | .94 | 0 |
| Random effects | −0.002 (−0.015 to 0.011) | .76 | |||||||
| Major trauma | 1 | 1463 | 10 060 | 1613 | 10 067 | Fixed effect | −0.015 (−0.022 to −0.008) | .004 | NA |
| Random effects | −0.015 (−0.022 to −0.008) | .004 | |||||||
| Pediatric | 1 | 0 | 40 | 0 | 42 | Fixed effect | 0.000 (−0.046 to 0.046) | >.99 | NA |
| Random effects | 0.000 (−0.047 to 0.047) | >.99 | |||||||
| Other | 9 | 52 | 1027 | 75 | 893 | Fixed effect | −0.038 (−0.06 to −0.015) | .001 | 78 |
| Random effects | −0.024 (−0.058 to 0.009) | .15 | |||||||
| Total | 63 | 2218 | 27 865 | 2456 | 27 440 | Fixed effect | −0.011 (−0.015 to −0.007) | <.001 | 16 |
| Random effects | −0.004 (−0.008 to 0.000) | .05 | |||||||
Abbreviations: NA, not applicable; TXA, tranexamic acid.
Nonbleeding Mortality and Bleeding Mortality
In total, 48 studies (eAppendix 16 in the Supplement) assessed nonbleeding mortality in 46 619 patients (n = 23 458 TXA group vs n = 23 161 control group). Death occurred in 1180 patients (5%) in the TXA group and 1228 patients (5%) in the control group. Overall, administration of intravenous TXA was not associated with a decreased risk for nonbleeding mortality (RD = −0.002; 95% CI, −0.006 to 0.002; P = .29) (eTable 7; eFigure 21 in the Supplement). However, subgroup analysis showed a significant increase for nonbleeding mortality in patients with neurological conditions of the TXA group (RD = 0.044; 95% CI, 0.007-0.081; P = .02), whereas the subgroup analysis of cardiothoracic surgery showed a significant decrease for nonbleeding mortality in patients of the TXA group (RD = −0.025; 95% CI, −0.045 to −0.005; P = .02). Sensitivity analysis using a random-effects model showed robust effect estimates for nonbleeding mortality (RD = −0.000; 95% CI, −0.002 to 0.001; P = .92) but not for the subgroup of patients with neurological conditions (RD = 0.021; 95% CI, −0.014 to 0.057; P = .24) and cardiothoracic surgery (RD = −0.015; 95% CI, −0.031 to 0.002; P = .10) (eTable 7; eFigure 22 in the Supplement).
In total, 49 studies (eAppendix 17 in the Supplement) assessed the bleeding mortality in 46 702 patients (n = 23 501 TXA group vs n = 23 201 control group). Death occurred in 692 patients (3%) in the TXA group and 874 patients (4%) in the control group. Overall, administration of intravenous TXA was associated with an overall significant decrease of bleeding mortality (RD = −0.008; 95% CI, −0.011 to −0.005; P < .001) (Table 3; eFigure 23 in the Supplement). Subgroup analysis showed a significantly decreased bleeding mortality in patients with neurological conditions (RD = −0.071; 95% CI, −0.102 to −0.041; P < .001), patients with trauma (RD = −0.008; 95% CI, −0.015 to −0.002; P = .008), and patients of any other disciplines of the TXA group (RD = −0.018; 95% CI, −0.033 to −0.004; P = .02), whereas no significant differences could be detected within the remaining medical disciplines (cardiothoracic, gynecological, orthopedic, and pediatric). Sensitivity analysis using a random-effects model showed robust effect estimates for bleeding mortality (RD = −0.004; 95% CI, −0.008 to −0.001; P = .02) but not for the subgroup of other disciplines (RD = −0.01; 95% CI, −0.028 to −0.009; P = .30) (Table 3; eFigure 24 in the Supplement). Significant heterogeneity was detected for patients with neurological conditions (I2 = 60%).
Table 3. TXA and Bleeding Mortality.
| Medical discipline | No. of included studies | TXA | Control | Model | Risk difference (95% CI) | P value | I2, % | ||
|---|---|---|---|---|---|---|---|---|---|
| Events | No. of included patients | Events | No. of included patients | ||||||
| Cardiothoracic | 12 | 0 | 543 | 1 | 478 | Fixed effect | −0.002 (−0.016 to −0.012) | .77 | 0 |
| Random effects | −0.004 (−0.012 to 0.011) | .94 | |||||||
| Neurological | 8 | 43 | 685 | 91 | 678 | Fixed effect | −0.071 (−0.102 to −0.041) | <.001 | 60 |
| Random effects | −0.056 (−0.11 to −0.002) | .04 | |||||||
| Gynecological | 8 | 155 | 10 871 | 191 | 10 814 | Fixed effect | −0.003 (−0.007 to −0.000) | .05 | 0 |
| Random effects | −0.002 (−0.005 to 0.001) | .12 | |||||||
| Orthopedic | 13 | 0 | 647 | 0 | 461 | Fixed effect | 0.000 (−0.014 to 0.014) | .77 | 0 |
| Random effects | 0.000 (−0.013 to 0.013) | >.99 | |||||||
| Major trauma | 1 | 489 | 10 060 | 574 | 10 067 | Fixed effect | −0.008 (−0.015 to −0.002) | .01 | NA |
| Random effects | −0.008 (−0.015 to −0.002) | .01 | |||||||
| Pediatric | 1 | 0 | 40 | 0 | 42 | Fixed effect | 0.000 (−0.046 to 0.046) | >.99 | NA |
| Random effects | 0.000 (−0.046 to 0.046) | >.99 | |||||||
| Other | 6 | 5 | 655 | 17 | 661 | Fixed effect | −0.018 (−0.033 to −0.004) | .02 | 53 |
| Random effects | −0.01 (−0.028 to −0.009) | .30 | |||||||
| Total | 49 | 692 | 23 501 | 874 | 23 201 | Fixed effect | −0.008 (−0.011 to −0.005) | <.001 | 9 |
| Random effects | −0.004 (−0.008 to −0.001) | .02 | |||||||
Abbreviations: NA, not applicable; TXA, tranexamic acid.
Patients With Risks for Thromboembolic Events
Overall, administration of intravenous TXA was not associated with an increased risk for total TEs (RD = −0.000; 95% CI, −0.008 to 0.009; P > .99) (eFigure 25 in the Supplement), for VT (RD = 0.003; 95% CI, −0.007 to 0.013; P = .57) (eFigure 26 in the Supplement), for PE (RD = −0.001; 95% CI, −0.009 to 0.007; P = .73) (eFigure 27 in the Supplement), or for VTEs (RD = −0.000; 95% CI, −0.012 to 0.01; P = .89) (eFigure 28 in the Supplement). Administration of intravenous TXA was associated with significant reductions in overall mortality (RD = −0.038; 95% CI, −0.057 to −0.018; P < .001). Detailed analyses are reported in eAppendix 7, eFigure 29 and eFigure 30, and eTable 8 in the Supplement.
Meta-regression
A meta-regression of 143 intervention groups was conducted to assess a possible association between different intravenous dosages of TXA and VTE rate. Results from this analysis showed no association between total dosing (RD = −0.005; 95% CI, −0.021 to 0.011; P = .53), single dosing (RD = 0.018; 95% CI, −0.053 to 0.09; P = .60), or any dose of intravenous TXA (RD = −0.005; 95% CI, −0.013 to 0.003; P = .21) and incidence of VTEs. Detailed analyses are reported in eAppendix 8 in the Supplement.
Risk of Bias
Overall, 139 studies (72%) were judged with low risk and 10 (5%) at high risk for random sequence generation. The risk of bias in the remaining 43 studies (22%) was unclear because of insufficient information. Allocation was adequately concealed in 68 studies (35%), whereas 4 studies (2%) were judged at high risk because patients were not randomly assigned to intravenous TXA or the control group. Sensitivity analysis with studies judged at low risk for selection bias was performed for total TEs and showed that results remained robust (RD = -0.001, 95% CI, −0.002 to 0.003, P = .89) (eFigure 33 in the Supplement). Detailed analysis including funnel plots is reported in eAppendix 9 and in eFigures 31-36 in the Supplement.
Updated Meta-analysis
A systematic search was performed to identify RCTs published between July 1, 2018, and December 31, 2020. In total, 72 studies were considered for full-text review, of which 48 were excluded because of ineligible study designs or control groups (n = 35), ineligible end points (n = 5), missing or nontranslatable full text (n = 4), and duplicates (n = 4), leaving 24 RCTs206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229 including 27 888 patients (14 242 TXA group and 13 646 control group) for analysis (eTable 9 in the Supplement).
Of all trials (n = 216) comprising 125 550 patients, we found 1020 total TEs (2.1%) in the TXA group and 900 total TEs (2.0%) in the control group. The addition of 24 trials published after our analysis was completed showed that effect estimates for total TEs (RD = 0.001; 95% CI, −0.001 to 0.002; P = .49), VT (RD = −0.000; 95% CI, −0.001 to 0.001; P = .85), PE (RD = 0.000; 95% CI, −0.001 to 0.001; P = .74), VTE (RD = 0.000; 95% CI, −0.001 to 0.002; P = .85), and overall mortality (RD = −0.007; 95% CI, −0.012 to −0.004; P < .001) remained robust (eTables 10-14; eFigures 37-46 in the Supplement). Sensitivity analysis for total TEs using the RR as an effect measure including studies with (RR = 1.02; 95% CI, 0.94-1.11; P = .56) and without (RR = 1.03; 95% CI, 0.95-1.12; P = .52) double-zero events showed similar results.Detailed analyses are reported in eAppendix 10 and eFigures 37-48 in the Supplement.
Discussion
Tranexamic acid is efficient to reduce bleeding by inhibiting the enzymatic breakdown of existing fibrin blood clots and is therefore widely used in anesthesia and surgery. The utility of TXA was supported by the results of 3 trials (CRASH-2, WOMAN and CRASH-3), reporting its efficiency in reducing bleeding-associated deaths in patients with trauma,41 postpartum hemorrhage,40 and traumatic brain injury.206 However, a significant survival benefit was achieved only when TXA was administered within the first 3 hours after injury or delivery.40,41 On the basis of the CRASH-2 results, intravenous TXA was included in the World Health Organization list of essential medicine in 2011.230 Along with the positive effect, concerns about potential adverse effects, in particular vascular occlusive events, were raised. Controversial results were published, reporting from no incidence of TE40 up to 12-fold higher rates for DVT7 after intravenous TXA administration. Yates and colleagues231 performed a meta-analysis including RCTs with different application methods of TXA. Analysis of 20 679 patients revealed no increased risk for VTEs after administration of intravenous TXA compared with placebo or no intervention. No subgroup analysis was performed by the authors. In orthopedic patients undergoing surgery with or without intravenous TXA, a systematic review by Franchini and colleagues232 encompassing 67 RCTs revealed no significant difference of VTEs measured as RD including studies with 0 events (RD, 0.0008) and RR excluding these studies (RR, 1.0411).
To assess any risk of TEs associated with administration of intravenous TXA, we performed a meta-analysis including studies from all medical disciplines that assessed and provided data for TEs. Analyses were performed using RD including studies with 0 events to allow a general conclusion. Although this method yields wide 95% CIs when events are rare, this approach provides precise information when analyzing all available evidence instead of excluding a large proportion of studies that provided double-zero events. Excluding these trials from analysis generates the risk of inflating the magnitude of the pooled treatment effect. However, we performed a sensitivity analysis using the RR as an effect measure with and without studies with double-zero events and revealed robust effect estimates. In total, 216 studies published between 1976 and 2020 encompassing 125 500 patients were included in the meta-analysis, and overall we found 1020 total TEs (2.1%) in the TXA group and 900 total TEs (2.0%) in the control group. There was no association between intravenous TXA and increased risk for vascular occlusive events. The bleeding-associated mortality was significantly reduced in intravenous TXA–treated patients compared with controls, whereas no difference in nonbleeding mortality was detected. Particularly, the use of intravenous TXA in patients experiencing major trauma and in patients with neurological conditions was associated with significant survival benefit concerning bleeding-related mortality. Notably, we found only 1 study providing data for patients with major trauma treated with intravenous TXA. An updated meta-analysis was performed to identify RCTs published recently. Our results remain unchanged when data from these trials for total TEs, VT, PE, VTE, and overall mortality were included in the meta-analysis.
Of all investigated subgroups, we found inconclusive results for patients with neurological conditions. In this subgroup, results obtained with the fixed-effect model did not remain robust when using the random-effects model for the end points of TE and nonbleeding mortality. However, we found a decrease in bleeding-associated mortality in patients of the TXA group compared with the control group. In total, 12 studies provided data for total TEs in patients with neurological conditions; study size varied between 24 and 2235. Heterogeneity remained high after omitting studies with more than 1000 patients. Overall, increased heterogeneity and asymmetry in funnel plots indicate that further trials are necessary to solve the uncertainty for patients with neurological conditions.
It is commonly held that trials with low numbers of recruited patients might be underpowered to detect an intervention effect. To address this possibility, we performed a sensitivity analysis and found that administration of intravenous TXA was not associated with an increased risk for total TEs in studies with less than or equal to 99 patients, 100 to 999 patients, and greater than or equal to 1000 patients. Patients with an increased risk for thromboembolism were often excluded from RCTs. Given the short elimination half-life of intravenous TXA, patients with a risk factor may benefit from treatment during surgery. We found that the risk for vascular occlusive events or overall mortality rate was not increased in studies including patients with increased risks for thromboembolism. To assess a possible effect of study size or risk of bias, we conducted sensitivity analyses for the primary endpoint of total TEs, with results remaining robust.
Overall, the administration dose of intravenous TXA varied widely from 0.5 to 5 g or 10 to 100 mg/kg. Moving away from one-dose-fits-all to weight-adapted dosing might be associated with the different treatment regimens applied worldwide in the context of trauma and surgery. Notably, we did not detect any dose-dependent association of TEs.
Limitations
Although this meta-analysis provides substantial data, this study has limitations. We cannot exclude that additional references might have been missed by our systematic search of databases. However, we believe that inclusion of further studies had no impact on our main findings regarding vascular occlusive events. The follow-up varied between trials, ranging from 24 hours to several months. However, the half-life of intravenous TXA is 1.9-2.7 hours.233,234 Considering that postoperative thrombotic events occur 6 to 8 days after surgery,235 we hypothesized that intravenous TXA–related adverse events would be detected within even in a short period of follow-up. Furthermore, TEs were not examined using ultrasonographic screening; therefore, asymptomatic thrombosis might not have been detected in all cases, and the incidence of TEs might be underestimated in some studies. Low incidence of VTEs with an approximate rate of 1 per 1000 patients236 and the routine use of postoperative thrombosis prophylaxis might also be associated with a low detection rate in patients with and without administration of intravenous TXA. Many of the included studies did not provide sufficient information about thrombosis prophylaxis; therefore, the association of postoperative care and vascular occlusive events was not analyzed further.
Conclusions
Taken as a whole, this systematic review and meta-analysis did not find that intravenous treatment with TXA in patients of any medical discipline was associated with a significant increased risk for TEs irrespective of administered dose. The results of this study suggest that use of intravenous TXA may have utility in all medical fields, with some uncertainty for patients with neurological conditions.
eAppendix 1. Methods
eAppendix 2. Outcome Measure
eAppendix 3. Statistical Analyses
eAppendix 4. Demographic Data and Subgroup Characteristics
eAppendix 5. Effect of Tranexamic Acid on Myocardial Infarction or Ischaemia
eAppendix 6. Effect of Tranexamic Acid on Cerebral Infarction or Ischaemia and on Other Thromboembolic Events
eAppendix 7. Effect of Tranexamic Acid in Patients with risks for Thromboembolic Events
eAppendix 8. Meta-Regression
eAppendix 9. Risk of Bias.
eAppendix 10. Updated Meta-Analysis
eTable 1. Study Characteristics
eTable 2. Venous Thrombosis
eTable 3. Pulmonary Embolism
eTable 4. Venous Thrombosis + Pulmonary Embolism
eTable 5. Myocardial Infarction or Ischaemia
eTable 6. Cerebral Infarction or Ischaemia
eTable 7. Non-Bleeding Mortality
eTable8. Summary of Sensitivity Analysis including Patients with increased Risk for total Thromboembolic Events
eTable 9. Study Characteristics of Updated Meta-Analysis
eTable 10. Total Thromboembolic Events of Updated Meta-Analysis
eTable 11. Venous Thrombosis of Updated Meta-Analysis
eTable 12. Pulmonary Embolism of Updated Meta-Analysis
eTable 13. Venous Thrombosis + Pulmonary Embolism of Updated Meta-Analysis
eTable 14. Overall Mortality of Updated Meta-Analysis
eAppendix 11. References on Total TE
eAppendix 12. References on VT Events
eAppendix 13. References on PE Events
eAppendix 14. References on VTE
eAppendix 15. References on Overall Mortality
eAppendix 16. References on Non-bleeding Mortality
eAppendix 17. References on Bleeding Mortality
eFigure 1. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 2. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 3. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control analysed with Risk Ratio in Studies with Zero Events
eFigure 4. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control analysed with Risk Ratio in Studies without Zero Events
eFigure 5. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control according to Sample Size using the Fixed-Effect Model
eFigure 6. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control according to Sample Size using the Random-Effects Model
eFigure 7. Venous Thrombosis Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 8. Venous Thrombosis Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 9: Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect model
eFigure 10: Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 11. Venous Thromboembolic and Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 12. Venous Thromboembolic and Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 13. Myocardial Infarction Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 14. Myocardial Infarction Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 15. Cerebral Ischaemia Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 16. Limb Ischaemia Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 17. Mesenteric Ischaemia Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 18. Hepatic Artery Thrombosis Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 19. Overall Mortality Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 20. Overall Mortality Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 21. Non-Bleeding associated Mortality Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 22. Non-Bleeding associated Mortality Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 23. Bleeding associated Mortality Rate in Patients receiving TXA compared to Control using the Fixed-Effect model
eFigure 24. Bleeding associated Mortality Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 25. Total Thromboembolic Event Rate in High Risk Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 26. Venous Thrombosis Event Rate in High Risk Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 27. Pulmonary Embolism Event Rate in High Risk Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 28. Venous Thromboembolic Event Rate in High Risk Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 29. Overall Mortality Rate in High Risk Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 30. Overall Mortality Rate in High Risk Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 31. Risk of Bias Summary
eFigure 32. Risk of bias Graph
eFigure 33. TotalThromboembolic Event Rate in Patients receiving TXA compared to Control of Studies Judged with Low Selection Bias using the Fixed-Effect Model
eFigure 34. Funnel Plots for Total Thromboembolic Event, Venous Thrombosis, Cerebral Infarction, and Bleeding Mortality
eFigure 35. Funnel Plots for Pulmonary Embolism, Venous Thromboembolic Events, and Myocardial Infarction
eFigure 36. Funnel Plots for Overall and Bleeding associated Mortality
eFigure 37. TotalThromboembolic Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model of updated Meta-Analysis
eFigure 38. TotalThromboembolic Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model updated Meta-Analysis
eFigure 39. Venous Thrombosis Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model of updated Meta-Analysis
eFigure 40. Venous Thrombosis Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model of updated Meta-Analysis
eFigure 41. Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model of updated Meta-Analysis
eFigure 42. Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model of updated Meta-Analysis
eFigure 43. Venous Thromboembolic and Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model of updated Meta-Analysis
eFigure 44. Venous Thromboembolic and Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model of updated Meta-Analysis
eFigure 45. Overall Mortality Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model of updated Meta-Analysis
eFigure 46: Overall Mortality Rate in Patients receiving TXA compared to Control using the Random-Effects Model of updated Meta-Analysis
eFigure 47. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control analysed with Risk Ratio in Studies with Zero Events of updated Meta-Analysis
eFigure 48. TotalThromboembolic Event Rate in Patients receiving TXA compared to Control analysed with Risk Ratio in Studies without Zero Events of updated Meta-Analysis
eReferences
Reference
- 1.Spence J, LeManach Y, Chan MTV, et al. ; Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators . Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019;191(30):E830-E837. doi: 10.1503/cmaj.190221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829. doi: 10.1136/bmj.g4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ker K, Edwards P,Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi: 10.1136/bmj.e3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: a meta-analysis. Seizure. 2016;36:70-73. doi: 10.1016/j.seizure.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 5.Lier H, Maegele M, Shander A. Tranexamic acid for acute hemorrhage: a narrative review of landmark studies and a critical reappraisal of its use over the last decade. Anesth Analg. 2019;129(6):1574-1584. doi: 10.1213/ANE.0000000000004389 [DOI] [PubMed] [Google Scholar]
- 6.Sprigg N, Flaherty K, Appleton JP, et al. ; TICH-2 Investigators . Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391(10135):2107-2115. doi: 10.1016/S0140-6736(18)31033-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg. 2012;147(2):113-119. doi: 10.1001/archsurg.2011.287 [DOI] [PubMed] [Google Scholar]
- 8.Gruen RL, Jacobs IG, Reade MC; PATCH-Trauma Study . Trauma and tranexamic acid. Med J Aust. 2013;199(5):310-311. doi: 10.5694/mja13.10747 [DOI] [PubMed] [Google Scholar]
- 9.Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Ann Surg. 2015;261(2):390-394. doi: 10.1097/SLA.0000000000000717 [DOI] [PubMed] [Google Scholar]
- 10.Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. 2017;34(6):332-395. doi: 10.1097/EJA.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 11.American Society of Anesthesiologists Task Force on Perioperative Blood Management . Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology. 2015;122(2):241-275. doi: 10.1097/ALN.0000000000000463 [DOI] [PubMed] [Google Scholar]
- 12.Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016. 12;20:100. doi: 10.1186/s13054-016-1265-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spahn DR, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. Published online March 27, 2019. doi: 10.1186/s13054-019-2347-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celebi N, Celebioglu B, Selcuk M, Canbay O, Karagoz AH, Aypar U. The role of antifibrinolytic agents in gynecologic cancer surgery. Saudi Med J. 2006;27(5):637-641. [PubMed] [Google Scholar]
- 16.Gohel M, Patel P, Gupta A, Desai P. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: a randomized case controlled prospective study. J Obstet Gynaecol India. 2007;57(3):227-230. https://jogi.co.in/may_june_2007/07_oa_efficacy.pdf [Google Scholar]
- 17.Caglar GS, Tasci Y, Kayikcioglu F, Haberal A. Intravenous tranexamic acid use in myomectomy: a prospective randomized double-blind placebo controlled study. Eur J Obstet Gynecol Reprod Biol. 2008;137(2):227-231. doi: 10.1016/j.ejogrb.2007.04.003 [DOI] [PubMed] [Google Scholar]
- 18.Sekhavat L, Tabatabaii A, Dalili M, Farajkhoda T, Tafti AD. Efficacy of tranexamic acid in reducing blood loss after cesarean section. J Matern Fetal Neonatal Med. 2009;22(1):72-75. doi: 10.1080/14767050802353580 [DOI] [PubMed] [Google Scholar]
- 19.Rashmi PS, Sudha TR, Prema P, Rajashri P, Vijayanath V. Role of tranexamic acid in reducing blood loss during and after cesarean section: a randomized case control prospective study. J Med Res Pract. 2012;1(2):40-43. [Google Scholar]
- 20.Ducloy-Bouthors AS, Jude B, Duhamel A, et al. ; EXADELI Study Group . High-dose tranexamic acid reduces blood loss in postpartum haemorrhage. Crit Care. 2011;15(2):R117. doi: 10.1186/cc10143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gungorduk K, Yıldırım G, Asıcıoğlu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double-blind, placebo-controlled study. Am J Perinatol. 2011;28(3):233-240. doi: 10.1055/s-0030-1268238 [DOI] [PubMed] [Google Scholar]
- 22.Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. Int J Gynaecol Obstet. 2011;115(3):224-226. doi: 10.1016/j.ijgo.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 23.Gupta K, Rastogi B, Krishan A, Gupta A, Singh VP, Agarwal S. The prophylactic role of tranexamic acid to reduce blood loss during radical surgery: a prospective study. Anesth Essays Res. 2012;6(1):70-73. doi: 10.4103/0259-1162.103378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Aleem H, Alhusaini TK, Abdel-Aleem MA, Menoufy M, Gülmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: randomized clinical trial. J Matern Fetal Neonatal Med. 2013;26(17):1705-1709. doi: 10.3109/14767058.2013.794210 [DOI] [PubMed] [Google Scholar]
- 25.Goswami U, Sarangi S, Gupta S, Babbar S. Comparative evaluation of two doses of tranexamic acid used prophylactically in anemic parturients for lower segment cesarean section: a double-blind randomized case control prospective trial. Saudi J Anaesth. 2013;7(4):427-431. doi: 10.4103/1658-354X.121077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gungorduk K, Asıcıoğlu O, Yıldırım G, Ark C, Tekirdağ AI, Besımoglu B. Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labor in vaginal delivery? a randomized controlled study. Am J Perinatol. 2013;30(5):407-413. doi: 10.1097/01.ogx.0000436756.05118.cd [DOI] [PubMed] [Google Scholar]
- 27.Sentürk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: a double-blind, placebo-controlled, randomized clinical trial. Arch Gynecol Obstet. 2013;287(4):641-645. doi: 10.1007/s00404-012-2624-8 [DOI] [PubMed] [Google Scholar]
- 28.Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. J Coll Physicians Surg Pak. 2013;23(7):459-462. [PubMed] [Google Scholar]
- 29.Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double-blind randomization trial. Arch Gynecol Obstet. 2013;287(3):463-468. doi: 10.1007/s00404-012-2593-y [DOI] [PubMed] [Google Scholar]
- 30.Ghosh A, Chaudhuri P, Muhuri B. Efficacy of intravenous tranexamic acid before cesarean section in preventing post-partum hemorrhage-a prospective randomized double blind placebo controlled study. Int J Biomed Res. 2014;5(4):4461-4464. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.680.9525&rep=rep1&type=pdf [Google Scholar]
- 31.Lundin ES, Johansson T, Zachrisson H, et al. Single-dose tranexamic acid in advanced ovarian cancer surgery reduces blood loss and transfusions: double-blind placebo-controlled randomized multicenter study. Acta Obstet Gynecol Scand. 2014;93(4):335-344. doi: 10.1111/aogs.12333 [DOI] [PubMed] [Google Scholar]
- 32.Gobbur VR, Shiragur SS, Jhanwar UR, Tehalia MJ. Efficacy of tranexamic acid in reducing blood loss during lower segment caesarean section. Int J Reprod Contracept Obstet Gynecol. 2014;3(2):414-417. doi: 10.5455/2320-1770.ijrcog20140626 [DOI] [Google Scholar]
- 33.Yehia AH, Koleib MH, Abdelazimab IA, Atik A. Tranexamic acid reduces blood loss during and after cesarean section: a double blinded, randomized, controlled trial. Asian Pac J Reprod. 2014;3(1):53-56. doi: 10.1016/S2305-0500(14)60002-6 [DOI] [Google Scholar]
- 34.Ahmed MR, Sayed Ahmed WA, Madny EH, Arafa AM, Said MM. Efficacy of tranexamic acid in decreasing blood loss in elective caesarean delivery. J Matern Fetal Neonatal Med. 2015;28(9):1014-1018. doi: 10.3109/14767058.2014.941283 [DOI] [PubMed] [Google Scholar]
- 35.Maged AM, Helal OM, Elsherbini MM, et al. A randomized placebo-controlled trial of preoperative tranexamic acid among women undergoing elective cesarean delivery. Int J Gynaecol Obstet. 2015;131(3):265-268. doi: 10.1016/j.ijgo.2015.05.027 [DOI] [PubMed] [Google Scholar]
- 36.Ngichabe S, Obura T, Stones W. Intravenous tranexamic acid as an adjunct haemostat to ornipressin during open myomectomy: a randomized double blind placebo controlled trial. Ann Surg Innov Res. 2015;9:10. doi: 10.1186/s13022-015-0017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray I, Bhattacharya R, Chakraborty S, Bagchi C, Mukhopadhyay S. Role of intravenous tranexamic acid on caesarean blood loss: a prospective randomised study. J Obstet Gynaecol India. 2016;66(suppl 1):347-352. doi: 10.1007/s13224-016-0915-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaaban MM, Ahmed MR, Farhan RE, Dardeer HH. Efficacy of tranexamic acid on myomectomy-associated blood loss in patients with multiple myomas: a randomized controlled clinical trial. Reprod Sci. 2016;23(7):908-912. doi: 10.1177/1933719115623646 [DOI] [PubMed] [Google Scholar]
- 39.Topsoee MF, Bergholt T, Ravn P, et al. Anti-hemorrhagic effect of prophylactic tranexamic acid in benign hysterectomy-a double-blinded randomized placebo-controlled trial. Am J Obstet Gynecol. 2016;215(1):72.e1-72.e8. doi: 10.1016/j.ajog.2016.01.184 [DOI] [PubMed] [Google Scholar]
- 40.WOMAN Trial Collaborators . Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10084):2105-2116. doi: 10.1016/S0140-6736(17)30638-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shakur H, Roberts I, Bautista R, et al. ; CRASH-2 Trial Collaborators . Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23-32. doi: 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 42.Choi WS, Irwin MG, Samman N. The effect of tranexamic acid on blood loss during orthognathic surgery: a randomized controlled trial. J Oral Maxillofac Surg. 2009;67(1):125-133. doi: 10.1016/j.joms.2008.08.015 [DOI] [PubMed] [Google Scholar]
- 43.Alimian M, Mohseni M. The effect of intravenous tranexamic acid on blood loss and surgical field quality during endoscopic sinus surgery: a placebo-controlled clinical trial. J Clin Anesth. 2011;23(8):611-615. doi: 10.1016/j.jclinane.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 44.Sankar D, Krishnan R, Veerabahu M, Vikraman B. Evaluation of the efficacy of tranexamic acid on blood loss in orthognathic surgery: a prospective, randomized clinical study. Int J Oral Maxillofac Surg. 2012;41(6):713-717. doi: 10.1016/j.ijom.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 45.Dakir A, Ramalingam B, Ebenezer V, Dhanavelu P. Efficacy of tranexamic acid in reducing blood loss during maxillofacial trauma surgery-a pilot study. J Clin Diagn Res. 2014;8(5):ZC06-ZC08. doi: 10.7860/JCDR/2014/8680.4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuhi S, Goljanian Tabrizi A, Zarkhah L, Rashedi Ashrafi B. Impact of intravenous tranexamic acid on hemorrhage during endoscopic sinus surgery. Iran J Otorhinolaryngol. 2015;27(82):349-354. [PMC free article] [PubMed] [Google Scholar]
- 47.Apipan B, Rummasak D, Narainthonsaenee T. The effect of different dosage regimens of tranexamic acid on blood loss in bimaxillary osteotomy: a randomized, double-blind, placebo-controlled study. Int J Oral Maxillofac Surg. 2018;47(5):608-612. doi: 10.1016/j.ijom.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 48.Hiippala S, Strid L, Wennerstrand M, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth. 1995;74(5):534-537. doi: 10.1093/bja/74.5.534 [DOI] [PubMed] [Google Scholar]
- 49.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440. doi: 10.1302/0301-620X.78B3.0780434 [DOI] [PubMed] [Google Scholar]
- 50.Hiippala ST, Strid LJ, Wennerstrand MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg. 1997;84(4):839-844. doi: 10.1213/00000539-199704000-00026 [DOI] [PubMed] [Google Scholar]
- 51.Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601. doi: 10.1093/bja/83.4.596 [DOI] [PubMed] [Google Scholar]
- 52.Benoni G, Lethagen S, Nilsson P, Fredin H. Tranexamic acid, given at the end of the operation, does not reduce postoperative blood loss in hip arthroplasty. Acta Orthop Scand. 2000;71(3):250-254. doi: 10.1080/000164700317411834 [DOI] [PubMed] [Google Scholar]
- 53.Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130. doi: 10.1213/00000539-200011000-00014 [DOI] [PubMed] [Google Scholar]
- 54.Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi: 10.1080/000164701753532754 [DOI] [PubMed] [Google Scholar]
- 55.Engel JM, Hohaus T, Ruwoldt R, Menges T, Jürgensen I, Hempelmann G. Regional hemostatic status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesth Analg. 2001;92(3):775-780. doi: 10.1213/00000539-200103000-00041 [DOI] [PubMed] [Google Scholar]
- 56.Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. 2001;93(1):82-87. doi: 10.1097/00000539-200107000-00018 [DOI] [PubMed] [Google Scholar]
- 57.Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705. doi: 10.1302/0301-620X.83B5.0830702 [DOI] [PubMed] [Google Scholar]
- 58.Veien M, Sørensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211. doi: 10.1034/j.1399-6576.2002.461007.x [DOI] [PubMed] [Google Scholar]
- 59.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599. doi: 10.1093/bja/aeg111 [DOI] [PubMed] [Google Scholar]
- 60.Husted H, Blønd L, Sonne-Holm S, Holm G, Jacobsen TW, Gebuhr P. Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty: a prospective randomized double-blind study in 40 patients. Acta Orthop Scand. 2003;74(6):665-669. doi: 10.1080/00016470310018171 [DOI] [PubMed] [Google Scholar]
- 61.Garneti N, Field J. Bone bleeding during total hip arthroplasty after administration of tranexamic acid. J Arthroplasty. 2004;19(4):488-492. doi: 10.1016/j.arth.2003.12.073 [DOI] [PubMed] [Google Scholar]
- 62.Lemay E, Guay J, Côté C, Roy A. Tranexamic acid reduces the need for allogenic red blood cell transfusions in patients undergoing total hip replacement. Can J Anaesth. 2004;51(1):31-37. doi: 10.1007/BF03018543 [DOI] [PubMed] [Google Scholar]
- 63.Yamasaki S, Masuhara K, Fuji T. Tranexamic acid reduces blood loss after cementless total hip arthroplasty-prospective randomized study in 40 cases. Int Orthop. 2004;28(2):69-73. doi: 10.1007/s00264-003-0511-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zohar E, Ellis M, Ifrach N, Stern A, Sapir O, Fredman B. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg. 2004;99(6):1679-1683. doi: 10.1213/01.ANE.0000136770.75805.19 [DOI] [PubMed] [Google Scholar]
- 65.Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319. doi: 10.1080/00016470510030751 [DOI] [PubMed] [Google Scholar]
- 66.Niskanen RO, Korkala OL. Tranexamic acid reduces blood loss in cemented hip arthroplasty: a randomized, double-blind study of 39 patients with osteoarthritis. Acta Orthop. 2005;76(6):829-832. doi: 10.1080/17453670510045444 [DOI] [PubMed] [Google Scholar]
- 67.Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576-582. doi: 10.1093/bja/ael057 [DOI] [PubMed] [Google Scholar]
- 68.Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi: 10.1016/j.knee.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 69.Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401. doi: 10.1080/00015458.2007.11680081 [DOI] [PubMed] [Google Scholar]
- 70.Molloy DO, Archbold HA, Ogonda L, McConway J, Wilson RK, Beverland DE. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. J Bone Joint Surg Br. 2007;89(3):306-309. doi: 10.1302/0301-620X.89B3.17565 [DOI] [PubMed] [Google Scholar]
- 71.Sadeghi M, Mehr-Aein A. Does a single bolus dose of tranexamic acid reduce blood loss and transfusion requirements during hip fracture surgery? a prospective randomized double blind study in 67 patients. Acta Med Iran. 2007;45(6):437-442. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.514.38&rep=rep1&type=pdf [Google Scholar]
- 72.Alvarez JC, Santiveri FX, Ramos I, Vela E, Puig L, Escolano F. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion. 2008;48(3):519-525. doi: 10.1111/j.1537-2995.2007.01564.x [DOI] [PubMed] [Google Scholar]
- 73.Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976). 2008;33(24):2577-2580. doi: 10.1097/BRS.0b013e318188b9c5 [DOI] [PubMed] [Google Scholar]
- 74.Wong J, El Beheiry H, Rampersaud YR, et al. Tranexamic acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg. 2008;107(5):1479-1486. doi: 10.1213/ane.0b013e3181831e44 [DOI] [PubMed] [Google Scholar]
- 75.Kakar PN, Gupta N, Govil P, Shah V. Efficacy and safety of tranexamic acid in control of bleeding following TKR: a randomized clinical trial. Indian J Anaesth. 2009;53(6):667-671. [PMC free article] [PubMed] [Google Scholar]
- 76.Jalaeian TR, Mashhadinezhad H, Sharifian Attar A, Peivandi A. The effect of intravenous tranexamic acid on blood loss in lumbar hernial disc resection under inhalation and total intravenous anesthesia. Iran Red Crescent Med J. 2009;11(3):265-270. https://www.researchgate.net/profile/Arash-Peivandi-Yazdi/publication/26627438_The_Effect_of_Intravenous_Tranexamic_Acid_on_Blood_Loss_in_Lumbar_Hernial_Disc_Resection_under_Inhalation_and_Total_Intravenous_Anesthesia/links/004635247e26dc347d000000/The-Effect-of-Intravenous-Tranexamic-Acid-on-Blood-Loss-in-Lumbar-Hernial-Disc-Resection-under-Inhalation-and-Total-Intravenous-Anesthesia.pdf [Google Scholar]
- 77.Kazemi SM, Mosaffa F, Eajazi A, et al. The effect of tranexamic acid on reducing blood loss in cementless total hip arthroplasty under epidural anesthesia. Orthopedics. 2010;33(1):17. doi: 10.3928/01477447-20091124-30 [DOI] [PubMed] [Google Scholar]
- 78.Zufferey PJ, Miquet M, Quenet S, et al. ; Tranexamic Acid in Hip-Fracture Surgery (THIF) Study . Tranexamic acid in hip fracture surgery: a randomized controlled trial. Br J Anaesth. 2010;104(1):23-30. doi: 10.1093/bja/aep314 [DOI] [PubMed] [Google Scholar]
- 79.Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clin Orthop Relat Res. 2011;469(10):2874-2880. doi: 10.1007/s11999-011-1874-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farrokhi MR, Kazemi AP, Eftekharian HR, Akbari K. Efficacy of prophylactic low dose of tranexamic acid in spinal fixation surgery: a randomized clinical trial. J Neurosurg Anesthesiol. 2011;23(4):290-296. doi: 10.1097/ANA.0b013e31822914a1 [DOI] [PubMed] [Google Scholar]
- 81.Lin PC, Hsu CH, Chen WS, Wang JW. Does tranexamic acid save blood in minimally invasive total knee arthroplasty? Clin Orthop Relat Res. 2011;469(7):1995-2002. doi: 10.1007/s11999-011-1789-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacGillivray RG, Tarabichi SB, Hawari MF, Raoof NT. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty. 2011;26(1):24-28. doi: 10.1016/j.arth.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 83.Malhotra R, Kumar V, Garg B. The use of tranexamic acid to reduce blood loss in primary cementless total hip arthroplasty. Eur J Orthop Surg Traumatol. 2011;21:101-104. doi: 10.1007/s00590-010-0671-z [DOI] [Google Scholar]
- 84.Suksamosorn P, Suarjui J, Lewsirirat S. Tranexamic acid in reducing perioperative blood loss in lumbar spinal stenosis surgery: a double-blind randomized controlled trial. Thai J Ortho Surg. 2011;35(3-4):1-7. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.985.6372&rep=rep1&type=pdf [Google Scholar]
- 85.Tsutsumimoto T, Shimogata M, Ohta H, Yui M, Yoda I, Misawa H. Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine (Phila Pa 1976). 2011;36(23):1913-1918. doi: 10.1097/BRS.0b013e3181fb3a42 [DOI] [PubMed] [Google Scholar]
- 86.Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124. doi: 10.1186/1471-2474-13-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Imai N, Dohmae Y, Suda K, Miyasaka D, Ito T, Endo N. Tranexamic acid for reduction of blood loss during total hip arthroplasty. J Arthroplasty. 2012;27(10):1838-1843. doi: 10.1016/j.arth.2012.04.024 [DOI] [PubMed] [Google Scholar]
- 88.Lin PC, Hsu CH, Huang CC, Chen WS, Wang JW. The blood-saving effect of tranexamic acid in minimally invasive total knee replacement: is an additional pre-operative injection effective? J Bone Joint Surg Br. 2012;94(7):932-936. doi: 10.1302/0301-620X.94B7.28386 [DOI] [PubMed] [Google Scholar]
- 89.Raviraj A, Anand A, Chakravarthy M, Kumarswamy S, Prabhu A, Pai S. Tranexamic acid reduces blood loss in simultaneous bilateral total knee arthroplasty: a randomized control trial. Eur J Orthop Surg Traumatol. 2012;22(5):381-386. doi: 10.1007/s00590-011-0845-3 [DOI] [Google Scholar]
- 90.Xu C, Wu A, Yue Y. Which is more effective in adolescent idiopathic scoliosis surgery: batroxobin, tranexamic acid or a combination? Arch Orthop Trauma Surg. 2012;132(1):25-31. doi: 10.1007/s00402-011-1390-6 [DOI] [PubMed] [Google Scholar]
- 91.Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007. doi: 10.2106/JBJS.L.01182 [DOI] [PubMed] [Google Scholar]
- 92.Gautam VK, Sambandam B, Singh S, Gupta P, Gupta R, Maini L. The role of tranexamic acid in reducing blood loss in total knee replacement. J Clin Orthop Trauma. 2013;4(1):36-39. doi: 10.1016/j.jcot.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2611-2617. doi: 10.1007/s00167-012-2213-1 [DOI] [PubMed] [Google Scholar]
- 94.Lee YC, Park SJ, Kim JS, Cho CH. Effect of tranexamic acid on reducing postoperative blood loss in combined hypotensive epidural anesthesia and general anesthesia for total hip replacement. J Clin Anesth. 2013;25(5):393-398. doi: 10.1016/j.jclinane.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 95.Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and intravenous tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874. doi: 10.1007/s00167-012-2079-2 [DOI] [PubMed] [Google Scholar]
- 96.Vijay BS, Bedi V, Mitra S, Das B. Role of tranexamic acid in reducing postoperative blood loss and transfusion requirement in patients undergoing hip and femoral surgeries. Saudi J Anaesth. 2013;7(1):29-32. doi: 10.4103/1658-354X.109803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Q, Liu J, Fan R, et al. Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Eur Spine J. 2013;22(9):2035-2038. doi: 10.1007/s00586-013-2836-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bidolegui F, Arce G, Lugones A, Pereira S, Vindver G. Tranexamic acid reduces blood loss and transfusion in patients undergoing total knee arthroplasty without tourniquet: a prospective randomized controlled trial. Open Orthop J. 2014;8:250-254. doi: 10.2174/1874325001408010250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Emara WM, Moez KK, Elkhouly AH. Topical versus intravenous tranexamic acid as a blood conservation intervention for reduction of post-operative bleeding in hemiarthroplasty. Anesth Essays Res. 2014;8(1):48-53. doi: 10.4103/0259-1162.128908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim TK, Chang CB, Kang YG, et al. Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1870-1878. doi: 10.1007/s00167-013-2492-1 [DOI] [PubMed] [Google Scholar]
- 101.Oremus K, Sostaric S, Trkulja V, Haspl M. Influence of tranexamic acid on postoperative autologous blood retransfusion in primary total hip and knee arthroplasty: a randomized controlled trial. Transfusion. 2014;54(1):31-41. doi: 10.1111/trf.12224 [DOI] [PubMed] [Google Scholar]
- 102.Sarzaeem MM, Razi M, Kazemian G, Moghaddam ME, Rasi AM, Karimi M. Comparing efficacy of three methods of tranexamic acid administration in reducing hemoglobin drop following total knee arthroplasty. J Arthroplasty. 2014;29(8):1521-1524. doi: 10.1016/j.arth.2014.02.031 [DOI] [PubMed] [Google Scholar]
- 103.Verma K, Errico T, Diefenbach C, et al. The relative efficacy of antifibrinolytics in adolescent idiopathic scoliosis: a prospective randomized trial. J Bone Joint Surg Am. 2014;96(10):e80. doi: 10.2106/JBJS.L.00008 [DOI] [PubMed] [Google Scholar]
- 104.Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116. doi: 10.1016/j.arth.2014.07.019 [DOI] [PubMed] [Google Scholar]
- 105.Digas G, Koutsogiannis I, Meletiadis G, Antonopoulou E, Karamoulas V, Bikos CH. Intra-articular injection of tranexamic acid reduce blood loss in cemented total knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(7):1181-1188. doi: 10.1007/s00590-015-1664-8 [DOI] [PubMed] [Google Scholar]
- 106.Hsu CH, Lin PC, Kuo FC, Wang JW. A regime of two intravenous injections of tranexamic acid reduces blood loss in minimally invasive total hip arthroplasty: a prospective randomised double-blind study. Bone Joint J. 2015;97-B(7):905-910. doi: 10.1302/0301-620X.97B7.35029 [DOI] [PubMed] [Google Scholar]
- 107.Jaszczyk M, Kozerawski D, Kołodziej Ł, Kazimierczak A, Sarnecki P, Sieczka Ł. Effect of single preoperative dose of tranexamic acid on blood loss and transfusion in hip arthroplasty. Ortop Traumatol Rehabil. 2015;17(3):265-273. doi: 10.5604/15093492.1162426 [DOI] [PubMed] [Google Scholar]
- 108.Karaaslan F, Karaoğlu S, Yurdakul E. Reducing intra-articular hemarthrosis after arthroscopic anterior cruciate ligament reconstruction by the administration of intravenous tranexamic acid: a prospective, randomized controlled trial. Am J Sports Med. 2015;43(11):2720-2726. doi: 10.1177/0363546515599629 [DOI] [PubMed] [Google Scholar]
- 109.Kundu R, Das A, Basunia SR, Bhattacharyya T, Chattopadhyay S, Mukherjee A. Does a single loading dose of tranexamic acid reduce perioperative blood loss and transfusion requirements after total knee replacement surgery? a randomized, controlled trial. J Nat Sci Biol Med. 2015;6(1):94-99. doi: 10.4103/0976-9668.149099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin SY, Chen CH, Fu YC, Huang PJ, Chang JK, Huang HT. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty. 2015;30(5):776-780. doi: 10.1016/j.arth.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 111.Motififard M, Tahririan MA, Saneie M, Badiei S, Nemati A. Low dose perioperative intravenous tranexamic acid in patients undergoing total knee arthroplasty: a double-blind randomized placebo controlled clinical trial. J Blood Transfus. 2015;2015:948304. doi: 10.1155/2015/948304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Öztaş S, Öztürk A, Akalin Y, et al. The effect of local and systemic application of tranexamic acid on the amount of blood loss and allogeneic blood transfusion after total knee replacement. Acta Orthop Belg. 2015;81(4):698-707. [PubMed] [Google Scholar]
- 113.Peters A, Verma K, Slobodyanyuk K, et al. Antifibrinolytics reduce blood loss in adult spinal deformity surgery: a prospective, randomized controlled trial. Spine (Phila Pa 1976). 2015;40(8):E443-E449. doi: 10.1097/BRS.0000000000000799 [DOI] [PubMed] [Google Scholar]
- 114.Raksakietisak M, Sathitkarnmanee B, Srisaen P, et al. Two doses of tranexamic acid reduce blood transfusion in complex spine surgery: a prospective randomized study. Spine (Phila Pa 1976). 2015;40(24):E1257-E1263. doi: 10.1097/BRS.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 115.Shen PF, Hou WL, Chen JB, Wang B, Qu YX. Effectiveness and safety of tranexamic acid for total knee arthroplasty: a prospective randomized controlled trial. Med Sci Monit. 2015;21:576-581. doi: 10.12659/MSM.892768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shinde A, Sobti A, Maniar S, Mishra A, Gite R, Shetty V. Tranexamic acid reduces blood loss and need of blood transfusion in total knee arthroplasty: a prospective, randomized, double-blind study in Indian population. Asian J Transfus Sci. 2015;9(2):168-172. doi: 10.4103/0973-6247.154251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xie B, Tian J, Zhou DP. Administration of tranexamic acid reduces postoperative blood loss in calcaneal fractures: a randomized controlled trial. J Foot Ankle Surg. 2015;54(6):1106-1110. doi: 10.1053/j.jfas.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 118.Barrachina B, Lopez-Picado A, Remon M, et al. Tranexamic acid compared with placebo for reducing total blood loss in hip replacement surgery: a randomized clinical trial. Anesth Analg. 2016;122(4):986-995. doi: 10.1213/ANE.0000000000001159 [DOI] [PubMed] [Google Scholar]
- 119.Baruah RK, Borah PJ, Haque R. Use of tranexamic acid in dynamic hip screw plate fixation for trochanteric fractures. J Orthop Surg (Hong Kong). 2016;24(3):379-382. doi: 10.1177/1602400322 [DOI] [PubMed] [Google Scholar]
- 120.Chen X, Cao X, Yang C, Guo K, Zhu Q, Zhu J. Effectiveness andsafety of fixed-dose tranexamic acid in simultaneous bilateral total knee arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2016;31(11):2471-2475. doi: 10.1016/j.arth.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 121.Drosos GI, Ververidis A, Valkanis C, et al. A randomized comparative study of topical versus intravenous tranexamic acid administration in enhanced recovery after surgery (ERAS) total knee replacement. J Orthop. 2016;13(3):127-131. doi: 10.1016/j.jor.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keyhani S, Esmailiejah AA, Abbasian MR, Safdari F. Which route of tranexamic acid administration is more effective to reduce blood loss following total knee arthroplasty? Arch Bone Jt Surg. 2016;4(1):65-69. [PMC free article] [PubMed] [Google Scholar]
- 123.Seviciu A, Gross I, Fathima S, Walsh SM. Effects of tranexamic acid and bipolar sealer alone or in combination in primary total knee arthroplasty: a prospective, randomized, controlled trial. Arthroplast Today. 2016;2(2):77-82. doi: 10.1016/j.artd.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tzatzairis TK, Drosos GI, Kotsios SE, Ververidis AN, Vogiatzaki TD, Kazakos KI. Intravenous vs topical tranexamic acid in total knee arthroplasty without tourniquet application: a randomized controlled study. J Arthroplasty. 2016;31(11):2465-2470. doi: 10.1016/j.arth.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 125.Volquind D, Zardo RA, Winkler BC, Londero BB, Zanelatto N, Leichtweis GP. Use of tranexamic acid in primary total knee replacement: effects on perioperative blood loss. Braz J Anesthesiol. 2016;66(3):254-258. doi: 10.1016/j.bjan.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 126.Wang C, Kang P, Ma J, Yue C, Xie J, Pei F. Single-dose tranexamic acid for reducing bleeding and transfusions in total hip arthroplasty: a double-blind, randomized controlled trial of different doses. Thromb Res. 2016;141:119-123. doi: 10.1016/j.thromres.2016.02.027 [DOI] [PubMed] [Google Scholar]
- 127.Yi Z, Bin S, Jing Y, Zongke Z, Pengde K, Fuxing P. Tranexamic acid administration in primary total hip arthroplasty: a randomized controlled trial of intravenous combined with topical versus single-dose intravenous administration. J Bone Joint Surg Am. 2016;98(12):983-991. doi: 10.2106/JBJS.15.00638 [DOI] [PubMed] [Google Scholar]
- 128.Zekcer A, Del Priori R, Tieppo C, da Silva RS, Severino NR. Topical vs intravenous administration of tranexamic acid in knee arthroplasty and prevalence of deep venous thrombosis: a randomized clinical trial. J Vasc Bras. 2016;15(2):120-125. doi: 10.1590/1677-5449.007515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Y, Zhang L, Ma X, et al. What is the optimal approach for tranexamic acid application in patients with unilateral total hip arthroplasty? Orthopade. 2016;45(7):616-621. doi: 10.1007/s00132-016-3252-y [DOI] [PubMed] [Google Scholar]
- 130.Fraval A, Effeney P, Fiddelaers L, Smith B, Towell B, Tran P. OBTAIN A: Outcome Benefits of Tranexamic Acid in Hip Arthroplasty: a randomized double-blinded controlled trial. J Arthroplasty. 2017;32(5):1516-1519. doi: 10.1016/j.arth.2016.11.045 [DOI] [PubMed] [Google Scholar]
- 131.Huang Z, Xie X, Li L, et al. Intravenous and topical tranexamic acid alone are superior to tourniquet use for primary total knee arthroplasty: a prospective, randomized controlled trial. J Bone Joint Surg Am. 2017;99(24):2053-2061. doi: 10.2106/JBJS.16.01525 [DOI] [PubMed] [Google Scholar]
- 132.Lacko M, Cellar R, Schreierova D, Vasko G. Comparison of intravenous and intra-articular tranexamic acid in reducing blood loss in primary total knee replacement. Eklem Hastalik Cerrahisi. 2017;28(2):64-71. doi: 10.5606/ehc.2017.54914 [DOI] [PubMed] [Google Scholar]
- 133.Melo GLR, Lages DS, Madureira Junior JL, Pellucci GP, Pellucci JWJ. The use of tranexamic acid in patients submitted to primary total hip arthroplasty: an evaluation of its impact in different administration protocols. Rev Bras Ortop. 2017;52(suppl 1):34-39. doi: 10.1016/j.rbo.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prakash J, Seon JK, Park YJ, Jin C, Song EK. A randomized control trial to evaluate the effectiveness of intravenous, intra-articular and topical wash regimes of tranexamic acid in primary total knee arthroplasty. J Orthop Surg (Hong Kong). 2017;25(1):2309499017693529. doi: 10.1177/2309499017693529 [DOI] [PubMed] [Google Scholar]
- 135.Song EK, Seon JK, Prakash J, Seol YJ, Park YJ, Jin C. Combined administration of intravenous and topical tranexamic acid is not superior to either individually in primary navigated TKA. J Arthroplasty. 2017;32(1):37-42. doi: 10.1016/j.arth.2016.06.052 [DOI] [PubMed] [Google Scholar]
- 136.Sun Q, Yu X, Nie X, Gong J, Cai M. The efficacy comparison of tranexamic acid for reducing blood loss in total knee arthroplasty at different dosage time. J Arthroplasty. 2017;32(1):33-36. doi: 10.1016/j.arth.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 137.Sun Q, Yu X, Wu J, Ge W, Cai M, Li S. Efficacy of a single dose and an additional dose of tranexamic acid in reduction of blood loss in total knee arthroplasty. J Arthroplasty. 2017;32(7):2108-2112. doi: 10.1016/j.arth.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 138.Uğurlu M, Aksekili MA, Çağlar C, Yüksel K, Şahin E, Akyol M. Effect of topical and intravenously applied tranexamic acid compared to control group on bleeding in primary unilateral total knee arthroplasty. J Knee Surg. 2017;30(2):152-157. doi: 10.1055/s-0036-1583270 [DOI] [PubMed] [Google Scholar]
- 139.Vara AD, Koueiter DM, Pinkas DE, Gowda A, Wiater BP, Wiater JM. Intravenous tranexamic acid reduces total blood loss in reverse total shoulder arthroplasty: a prospective, double-blinded, randomized, controlled trial. J Shoulder Elbow Surg. 2017;26(8):1383-1389. doi: 10.1016/j.jse.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 140.Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi: 10.1016/j.arth.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 141.Wang JW, Chen B, Lin PC, Yen SH, Huang CC, Kuo FC. The efficacy of combined use of rivaroxaban and tranexamic acid on blood conservation in minimally invasive total knee arthroplasty: a double-blind randomized, controlled trial. J Arthroplasty. 2017;32(3):801-806. doi: 10.1016/j.arth.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 142.Watts CD, Houdek MT, Sems SA, Cross WW, Pagnano MW. Tranexamic acid safely reduced blood loss in hemi- and total hip arthroplasty for acute femoral neck fracture: a randomized clinical trial. J Orthop Trauma. 2017;31(7):345-351. doi: 10.1097/BOT.0000000000000837 [DOI] [PubMed] [Google Scholar]
- 143.Yen SH, Lin PC, Chen B, Huang CC, Wang JW. Topical tranexamic acid reduces blood loss in minimally invasive total knee arthroplasty receiving rivaroxaban. Biomed Res Int. 2017;2017:9105645. doi: 10.1155/2017/9105645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yuan X, Li B, Wang Q, Zhang X. Comparison of 3 routes of administration of tranexamic acid on primary unilateral total knee arthroplasty: a prospective, randomized, controlled study. J Arthroplasty. 2017;32(9):2738-2743. doi: 10.1016/j.arth.2017.03.059 [DOI] [PubMed] [Google Scholar]
- 145.Zekcer A, Priori RD, Tieppo C, Silva RSD, Severino NR. Comparative study of topical vs intravenous tranexamic acid regarding blood loss in total knee arthroplasty. Rev Bras Ortop. 2017;52(5):589-595. doi: 10.1016/j.rbo.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeng Y, Si HB, Shen B, et al. Intravenous combined with topical administration of tranexamic acid in primary total hip arthroplasty: a randomized controlled trial. Orthop Surg. 2017;9(2):174-179. doi: 10.1111/os.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu W, Yang C, Huang X, Liu R. Tranexamic acid reduces occult blood loss, blood transfusion, and improves recovery of knee function after total knee arthroplasty: a comparative study. J Knee Surg. 2018;31(3):239-246. doi: 10.1055/s-0037-1602248 [DOI] [PubMed] [Google Scholar]
- 148.López-Hualda Á, Dauder-Gallego C, Ferreño-Márquez D, Martínez-Martín J. Efficacy and safety of topical tranexamic acid in knee arthroplasty. Med Clin (Barc). 2018;151(11):431-434. doi: 10.1016/j.medcle.2018.01.039 [DOI] [PubMed] [Google Scholar]
- 149.Painter TW, Daly DJ, Kluger R, et al. Intravenous tranexamic acid and lower limb arthroplasty-a randomised controlled feasibility study. Anaesth Intensive Care. 2018;46(4):386-395. doi: 10.1177/0310057X1804600407 [DOI] [PubMed] [Google Scholar]
- 150.Zhao H, Xiang M, Xia Y, Shi X, Pei FX, Kang P. Efficacy of oral tranexamic acid on blood loss in primary total hip arthroplasty using a direct anterior approach: a prospective randomized controlled trial. Int Orthop. 2018;42(11):2535-2542. doi: 10.1007/s00264-018-3846-6 [DOI] [PubMed] [Google Scholar]
- 151.Coffey A, Pittmam J, Halbrook H, Fehrenbacher J, Beckman D, Hormuth D. The use of tranexamic acid to reduce postoperative bleeding following cardiac surgery: a double-blind randomized trial. Am Surg. 1995;61(7):566-568. [PubMed] [Google Scholar]
- 152.Horrow JC, Van Riper DF, Strong MD, Grunewald KE, Parmet JL. The dose-response relationship of tranexamic acid. Anesthesiology. 1995;82(2):383-392. doi: 10.1097/00000542-199502000-00009 [DOI] [PubMed] [Google Scholar]
- 153.Karski JM, Teasdale SJ, Norman P, et al. Prevention of bleeding after cardiopulmonary bypass with high-dose tranexamic acid: double-blind, randomized clinical trial. J Thorac Cardiovasc Surg. 1995;110(3):835-842. doi: 10.1016/S0022-5223(95)70118-4 [DOI] [PubMed] [Google Scholar]
- 154.Speekenbrink RG, Vonk AB, Wildevuur CR, Eijsman L. Hemostatic efficacy of dipyridamole, tranexamic acid, and aprotinin in coronary bypass grafting. Ann Thorac Surg. 1995;59(2):438-442. doi: 10.1016/0003-4975(94)00865-5 [DOI] [PubMed] [Google Scholar]
- 155.Katsaros D, Petricevic M, Snow NJ, Woodhall DD, Van Bergen R. Tranexamic acid reduces postbypass blood use: a double-blinded, prospective, randomized study of 210 patients. Ann Thorac Surg. 1996;61(4):1131-1135. doi: 10.1016/0003-4975(96)00022-7 [DOI] [PubMed] [Google Scholar]
- 156.Shore-Lesserson L, Reich DL, Vela-Cantos F, Ammar T, Ergin MA. Tranexamic acid reduces transfusions and mediastinal drainage in repeat cardiac surgery. Anesth Analg. 1996;83(1):18-26. doi: 10.1213/00000539-199607000-00005 [DOI] [PubMed] [Google Scholar]
- 157.Brown RS, Thwaites BK, Mongan PD. Tranexamic acid is effective in decreasing postoperative bleeding and transfusions in primary coronary artery bypass operations: a double-blind, randomized, placebo-controlled trial. Anesth Analg. 1997;85(5):963-970. doi: 10.1213/00000539-199711000-00003 [DOI] [PubMed] [Google Scholar]
- 158.Dryden PJ, O’Connor JP, Jamieson WR, et al. Tranexamic acid reduces blood loss and transfusion in reoperative cardiac surgery. Can J Anaesth. 1997;44(9):934-941. doi: 10.1007/BF03011964 [DOI] [PubMed] [Google Scholar]
- 159.Hardy JF, Bélisle S, Dupont C, et al. Prophylactic tranexamic acid and epsilon-aminocaproic acid for primary myocardial revascularization. Ann Thorac Surg. 1998;65(2):371-376. doi: 10.1016/S0003-4975(97)01016-3 [DOI] [PubMed] [Google Scholar]
- 160.Nuttall GA, Oliver WC, Ereth MH, et al. Comparison of blood-conservation strategies in cardiac surgery patients at high risk for bleeding. Anesthesiology. 2000;92(3):674-682. doi: 10.1097/00000542-200003000-00010 [DOI] [PubMed] [Google Scholar]
- 161.Casati V, Gerli C, Franco A, et al. Tranexamic acid in off-pump coronary surgery: a preliminary, randomized, double-blind, placebo-controlled study. Ann Thorac Surg. 2001;72(2):470-475. doi: 10.1016/S0003-4975(01)02802-8 [DOI] [PubMed] [Google Scholar]
- 162.Zabeeda D, Medalion B, Sverdlov M, et al. Tranexamic acid reduces bleeding and the need for blood transfusion in primary myocardial revascularization. Ann Thorac Surg. 2002;74(3):733-738. doi: 10.1016/S0003-4975(02)03784-0 [DOI] [PubMed] [Google Scholar]
- 163.Pleym H, Stenseth R, Wahba A, Bjella L, Karevold A, Dale O. Single-dose tranexamic acid reduces postoperative bleeding after coronary surgery in patients treated with aspirin until surgery. Anesth Analg. 2003;96(4):923-928. doi: 10.1213/01.ANE.0000054001.37346.03 [DOI] [PubMed] [Google Scholar]
- 164.Andreasen JJ, Nielsen C. Prophylactic tranexamic acid in elective, primary coronary artery bypass surgery using cardiopulmonary bypass. Eur J Cardiothorac Surg. 2004;26(2):311-317. doi: 10.1016/j.ejcts.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 165.Diprose P, Herbertson MJ, O’Shaughnessy D, Deakin CD, Gill RS. Reducing allogeneic transfusion in cardiac surgery: a randomized double-blind placebo-controlled trial of antifibrinolytic therapies used in addition to intra-operative cell salvage. Br J Anaesth. 2005;94(3):271-278. doi: 10.1093/bja/aei044 [DOI] [PubMed] [Google Scholar]
- 166.Karski J, Djaiani G, Carroll J, et al. Tranexamic acid and early saphenous vein graft patency in conventional coronary artery bypass graft surgery: a prospective randomized controlled clinical trial. J Thorac Cardiovasc Surg. 2005;130(2):309-314. doi: 10.1016/j.jtcvs.2004.11.015 [DOI] [PubMed] [Google Scholar]
- 167.Vanek T, Jares M, Fajt R, et al. Fibrinolytic inhibitors in off-pump coronary surgery: a prospective, randomized, double-blind TAP study (tranexamic acid, aprotinin, placebo). Eur J Cardiothorac Surg. 2005;28(4):563-568. doi: 10.1016/j.ejcts.2005.06.027 [DOI] [PubMed] [Google Scholar]
- 168.Murphy GJ, Mango E, Lucchetti V, et al. A randomized trial of tranexamic acid in combination with cell salvage plus a meta-analysis of randomized trials evaluating tranexamic acid in off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2006;132(3):475-480, 480.e1-480.e8. doi: 10.1016/j.jtcvs.2006.01.064 [DOI] [PubMed] [Google Scholar]
- 169.Santos AT, Kalil RA, Bauemann C, Pereira JB, Nesralla IA. A randomized, double-blind, and placebo-controlled study with tranexamic acid of bleeding and fibrinolytic activity after primary coronary artery bypass grafting. Braz J Med Biol Res. 2006;39(1):63-69. doi: 10.1590/S0100-879X2006000100007 [DOI] [PubMed] [Google Scholar]
- 170.Jimenez JJ, Iribarren JL, Lorente L, et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care. 2007;11(6):R117. doi: 10.1186/cc6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chakravarthy M, Muniraj G, Patil S, Suryaprakash S, Mitra S, Shivalingappa B. A randomized prospective analysis of alteration of hemostatic function in patients receiving tranexamic acid and hydroxyethyl starch (130/0.4) undergoing off pump coronary artery bypass surgery. Ann Card Anaesth. 2012;15(2):105-110. doi: 10.4103/0971-9784.95072 [DOI] [PubMed] [Google Scholar]
- 172.Myles PS, Smith JA, Forbes A, et al. ; ATACAS Investigators of the ANZCA Clinical Trials Network . Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376(2):136-148. doi: 10.1056/NEJMoa1606424 [DOI] [PubMed] [Google Scholar]
- 173.Chandra B. Treatment of subarachnoid hemorrhage from ruptured intracranial aneurysm with tranexamic acid: a double-blind clinical trial. Ann Neurol. 1978;3(6):502-504. doi: 10.1002/ana.410030607 [DOI] [PubMed] [Google Scholar]
- 174.Fodstad H, Liliequist B, Schannong M, Thulin CA. Tranexamic acid in the preoperative management of ruptured intracranial aneurysms. Surg Neurol. 1978;10(1):9-15. [PubMed] [Google Scholar]
- 175.Maurice-Williams RS. Prolonged antifibrinolysis: an effective non-surgical treatment for ruptured intracranial aneurysms? BMJ. 1978;1(6118):945-947. doi: 10.1136/bmj.1.6118.945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kaste M, Ramsay M. Tranexamic acid in subarachnoid hemorrhage: a double-blind study. Stroke. 1979;10(5):519-522. doi: 10.1161/01.STR.10.5.519 [DOI] [PubMed] [Google Scholar]
- 177.Fodstad H, Forssell A, Liliequist B, Schannong M. Antifibrinolysis with tranexamic acid in aneurysmal subarachnoid hemorrhage: a consecutive controlled clinical trial. Neurosurgery. 1981;8(2):158-165. doi: 10.1227/00006123-198102000-00004 [DOI] [PubMed] [Google Scholar]
- 178.Vermeulen M, Lindsay KW, Murray GD, et al. Antifibrinolytic treatment in subarachnoid hemorrhage. N Engl J Med. 1984;311(7):432-437. doi: 10.1056/NEJM198408163110703 [DOI] [PubMed] [Google Scholar]
- 179.Tsementzis SA, Hitchcock ER, Meyer CH. Benefits and risks of antifibrinolytic therapy in the management of ruptured intracranial aneurysms: a double-blind placebo-controlled study. Acta Neurochir (Wien). 1990;102(1-2):1-10. doi: 10.1007/BF01402177 [DOI] [PubMed] [Google Scholar]
- 180.Roos Y; STAR Study Group . Antifibrinolytic treatment in subarachnoid hemorrhage: a randomized placebo-controlled trial. Neurology. 2000;54(1):77-82. doi: 10.1212/WNL.54.1.77 [DOI] [PubMed] [Google Scholar]
- 181.Hillman J, Fridriksson S, Nilsson O, Yu Z, Saveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. 2002;97(4):771-778. doi: 10.3171/jns.2002.97.4.0771 [DOI] [PubMed] [Google Scholar]
- 182.Yutthakasemsunt S, Kittiwatanagul W, Piyavechvirat P, Thinkamrop B, Phuenpathom N, Lumbiganon P. Tranexamic acid for patients with traumatic brain injury: a randomized, double-blinded, placebo-controlled trial. BMC Emerg Med. 2013;13:20. doi: 10.1186/1471-227X-13-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sprigg N, Renton CJ, Dineen RA, Kwong Y, Bath PM. Tranexamic acid for spontaneous intracerebral hemorrhage: a randomized controlled pilot trial (ISRCTN50867461). J Stroke Cerebrovasc Dis. 2014;23(6):1312-1318. doi: 10.1016/j.jstrokecerebrovasdis.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 184.Arumugam A, A Rahman NA, Theophilus SC, Shariffudin A, Abdullah JM. Tranexamic acid as antifibrinolytic agent in non traumatic intracerebral hemorrhages. Malays J Med Sci. 2015;22(Spec Issue):62-71. [PMC free article] [PubMed] [Google Scholar]
- 185.Vel R, Udupi BP, Satya Prakash MV, Adinarayanan S, Mishra S, Babu L. Effect of low dose tranexamic acid on intra-operative blood loss in neurosurgical patients. Saudi J Anaesth. 2015;9(1):42-48. doi: 10.4103/1658-354X.146304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hooda B, Chouhan RS, Rath GP, Bithal PK, Suri A, Lamsal R. Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci. 2017;41:132-138. doi: 10.1016/j.jocn.2017.02.053 [DOI] [PubMed] [Google Scholar]
- 187.Zonis Z, Seear M, Reichert C, Sett S, Allen C. The effect of preoperative tranexamic acid on blood loss after cardiac operations in children. J Thorac Cardiovasc Surg. 1996;111(5):982-987. doi: 10.1016/S0022-5223(96)70374-4 [DOI] [PubMed] [Google Scholar]
- 188.Dadure C, Sauter M, Bringuier S, et al. Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery: a randomized double-blind study. Anesthesiology. 2011;114(4):856-861. doi: 10.1097/ALN.0b013e318210f9e3 [DOI] [PubMed] [Google Scholar]
- 189.Goobie SM, Meier PM, Pereira LM, et al. Efficacy of tranexamic acid in pediatric craniosynostosis surgery: a double-blind, placebo-controlled trial. Anesthesiology. 2011;114(4):862-871. doi: 10.1097/ALN.0b013e318210fd8f [DOI] [PubMed] [Google Scholar]
- 190.Biggs JC, Hugh TB, Dodds AJ. Tranexamic acid and upper gastrointestinal haemorrhage–a double-blind trial. Gut. 1976;17(9):729-734. doi: 10.1136/gut.17.9.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Engqvist A, Broström O, von Feilitzen F, et al. Tranexamic acid in massive haemorrhage from the upper gastrointestinal tract: a double-blind study. Scand J Gastroenterol. 1979;14(7):839-844. doi: 10.3109/00365527909181413 [DOI] [PubMed] [Google Scholar]
- 192.Barer D, Ogilvie A, Henry D, et al. Cimetidine and tranexamic acid in the treatment of acute upper-gastrointestinal-tract bleeding. N Engl J Med. 1983;308(26):1571-1575. doi: 10.1056/NEJM198306303082606 [DOI] [PubMed] [Google Scholar]
- 193.Auvinen O, Baer GA, Nordback I, Saaristo J. Antifibrinolytic therapy for prevention of hemorrhage during surgery of the thyroid gland. Klin Wochenschr. 1987;65(6):253-255. doi: 10.1007/BF01773442 [DOI] [PubMed] [Google Scholar]
- 194.von Holstein CC, Eriksson SB, Källén R. Tranexamic acid as an aid to reducing blood transfusion requirements in gastric and duodenal bleeding. BMJ (Clin Res Ed). 1987;294(6563):7-10. doi: 10.1136/bmj.294.6563.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yassen K, Bellamy MC, Sadek SA, Webster NR. Tranexamic acid reduces blood loss during orthotopic liver transplantation. Clin Transplant. 1993;7(5):453-458. [Google Scholar]
- 196.Boylan JF, Klinck JR, Sandler AN, et al. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology. 1996;85(5):1043-1048. doi: 10.1097/00000542-199611000-00012 [DOI] [PubMed] [Google Scholar]
- 197.Kaspar M, Ramsay MA, Nguyen AT, Cogswell M, Hurst G, Ramsay KJ. Continuous small-dose tranexamic acid reduces fibrinolysis but not transfusion requirements during orthotopic liver transplantation. Anesth Analg. 1997;85(2):281-285. doi: 10.1097/00000539-199708000-00007 [DOI] [PubMed] [Google Scholar]
- 198.Dalmau A, Sabaté A, Acosta F, et al. Comparative study of antifibrinolytic drugs in orthotopic liver transplantation. Transplant Proc. 1999;31(6):2361-2362. doi: 10.1016/S0041-1345(99)00378-4 [DOI] [PubMed] [Google Scholar]
- 199.Ramezani AR, Ahmadieh H, Ghaseminejad AK, Yazdani S, Golestan B. Effect of tranexamic acid on early postvitrectomy diabetic haemorrhage; a randomised clinical trial. Br J Ophthalmol. 2005;89(8):1041-1044. doi: 10.1136/bjo.2004.062638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu CC, Ho WM, Cheng SB, et al. Perioperative parenteral tranexamic acid in liver tumor resection: a prospective randomized trial toward a “blood transfusion”-free hepatectomy. Ann Surg. 2006;243(2):173-180. doi: 10.1097/01.sla.0000197561.70972.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Crescenti A, Borghi G, Bignami E, et al. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: double blind, randomised, placebo controlled trial. BMJ. 2011;343:d5701. doi: 10.1136/bmj.d5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kumsar S, Dirim A, Toksöz S, Sağlam HS, Adsan O. Tranexamic acid decreases blood loss during transurethral resection of the prostate (TUR -P). Cent European J Urol. 2011;64(3):156-158. doi: 10.5173/ceju.2011.03.art13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jendoubi A, Malouch A, Bouzouita A, et al. Safety and efficacy of intravenous tranexamic acid in endoscopic transurethral resections in urology: prospective randomized trial. Article in French. Prog Urol. 2017;27(16):1036-1042. doi: 10.1016/j.purol.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 204.Mohammadi-Sichani M, Kazemi R, Nouri-Mahdavi K, Gholipour F. Reevaluation of the efficacy of tranexamic acid in reducing blood loss in percutaneous nephrolithotomy: a randomized clinical trial. Minerva Urol Nefrol. 2019;71(1):55-62. doi:10.23736/S0393-2249.18.03151-X [DOI] [PubMed] [Google Scholar]
- 205.Tavakoli N, Mokhtare M, Agah S, et al. Comparison of the efficacy of intravenous tranexamic acid with and without topical administration versus placebo in urgent endoscopy rate for acute gastrointestinal bleeding: a double-blind randomized controlled trial. United European Gastroenterol J. 2018;6(1):46-54. doi: 10.1177/2050640617714940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.CRASH-3 Trial Collaborators . Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394(10210):1713-1723. doi: 10.1016/S0140-6736(19)32233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.HALT-IT Trial Collaborators . Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10241):1927-1936. doi: 10.1016/S0140-6736(20)30848-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Abdul IF, Amadu MB, Adesina KT, Olarinoye AO, Omokanye LO. Adjunctive use of tranexamic acid to tourniquet in reducing haemorrhage during abdominal myomectomy: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2019;242:150-158. doi: 10.1016/j.ejogrb.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 209.Batıbay SG, Türkmen İ, Duman S, Çamur S, Sağlam N, Batıbay S. Is tranexamic acid safe and reliable during tibial intramedullary nailing? Ulus Travma Acil Cerrahi Derg. 2018;24(6):575-580. doi: 10.5505/tjtes.2018.42147 [DOI] [PubMed] [Google Scholar]
- 210.Chen F, Jiang Z, Li M, Zhu X. Efficacy and safety of perioperative tranexamic acid in elderly patients undergoing trochanteric fracture surgery: a randomised controlled trial. Hong Kong Med J. 2019;25(2):120-126. doi: 10.12809/hkmj187570 [DOI] [PubMed] [Google Scholar]
- 211.Clavé A, Gérard R, Lacroix J, et al. A randomized, double-blind, placebo-controlled trial on the efficacy of tranexamic acid combined with rivaroxaban thromboprophylaxis in reducing blood loss after primary cementless total hip arthroplasty. Bone Joint J. 2019;101-B(2):207-212. doi: 10.1302/0301-620X.101B2.BJJ-2018-0898.R1 [DOI] [PubMed] [Google Scholar]
- 212.Cuff DJ, Simon P, Gorman RA II. Randomized prospective evaluation of the use of tranexamic acid and effects on blood loss for proximal humeral fracture surgery. J Shoulder Elbow Surg. 2020;29(8):1627-1632. doi: 10.1016/j.jse.2020.04.016 [DOI] [PubMed] [Google Scholar]
- 213.Felli L, Revello S, Burastero G, et al. Single intravenous administration of tranexamic acid in anterior cruciate ligament reconstruction to reduce postoperative hemarthrosis and increase functional outcomes in the early phase of postoperative rehabilitation: a randomized controlled trial. Arthroscopy. 2019;35(1):149-157. doi: 10.1016/j.arthro.2018.07.050 [DOI] [PubMed] [Google Scholar]
- 214.Fenger-Eriksen C, D’Amore Lindholm A, Nørholt SE, et al. Reduced perioperative blood loss in children undergoing craniosynostosis surgery using prolonged tranexamic acid infusion: a randomised trial. Br J Anaesth. 2019;122(6):760-766. doi: 10.1016/j.bja.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 215.Karampinas PK, Megaloikonomos PD, Lampropoulou-Adamidou K, et al. Similar thromboprophylaxis with rivaroxaban and low molecular weight heparin but fewer hemorrhagic complications with combined intra-articular and intravenous tranexamic acid in total knee arthroplasty. Eur J Orthop Surg Traumatol. 2019;29(2):455-460. doi: 10.1007/s00590-018-2307-7 [DOI] [PubMed] [Google Scholar]
- 216.Lei Y, Xie J, Huang Q, Huang W, Pei F. Additional benefits of multiple-dose tranexamic acid to anti-fibrinolysis and anti-inflammation in total knee arthroplasty: a randomized controlled trial. Arch Orthop Trauma Surg. 2020;140(8):1087-1095. doi: 10.1007/s00402-020-03442-2 [DOI] [PubMed] [Google Scholar]
- 217.Levack AE, McLawhorn AS, Dodwell E, DelPizzo K, Nguyen J, Sink E. Intravenous tranexamic acid reduces blood loss and transfusion requirements after periacetabular osteotomy. Bone Joint J. 2020;102-B(9):1151-1157. doi: 10.1302/0301-620X.102B9.BJJ-2019-1777.R1 [DOI] [PubMed] [Google Scholar]
- 218.Liu YF, Hong CK, Hsu KL, et al. Intravenous administration of tranexamic acid significantly improved clarity of the visual field in arthroscopic shoulder surgery: a prospective, double-blind, and randomized controlled trial. Arthroscopy. 2020;36(3):640-647. doi: 10.1016/j.arthro.2019.10.020 [DOI] [PubMed] [Google Scholar]
- 219.Meretoja A, Yassi N, Wu TY, et al. Tranexamic Acid in Patients With Intracerebral Haemorrhage (STOP-AUST): a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2020;19(12):980-987. doi: 10.1016/S1474-4422(20)30369-0 [DOI] [PubMed] [Google Scholar]
- 220.Monaco F, Nardelli P, Pasin L, et al. Tranexamic acid in open aortic aneurysm surgery: a randomised clinical trial. Br J Anaesth. 2020;124(1):35-43. doi: 10.1016/j.bja.2019.08.028 [DOI] [PubMed] [Google Scholar]
- 221.Mu X, Wei J, Wang C, et al. Intravenous administration of tranexamic acid significantly reduces visible and hidden blood loss compared with its topical administration for double-segment posterior lumbar interbody fusion: a single-center, placebo-controlled, randomized trial. World Neurosurg. 2019;122:e821-e827. doi: 10.1016/j.wneu.2018.10.154 [DOI] [PubMed] [Google Scholar]
- 222.Opoku-Anane J, Vargas MV, Marfori CQ, Moawad G, Maasen MS, Robinson JK. Intraoperative tranexamic acid to decrease blood loss during myomectomy: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2020;223(3):413.e1-413.e7. doi: 10.1016/j.ajog.2020.02.019 [DOI] [PubMed] [Google Scholar]
- 223.Rowell SE, Meier EN, McKnight B, et al. Effect of out-of-hospital tranexamic acid vs placebo on 6-month functional neurologic outcomes in patients with moderate or severe traumatic brain injury. JAMA. 2020;324(10):961-974. doi: 10.1001/jama.2020.8958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sentilhes L, Winer N, Azria E, et al. ; Groupe de Recherche en Obstétrique et Gynécologie . Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med. 2018;379(8):731-742. doi: 10.1056/NEJMoa1800942 [DOI] [PubMed] [Google Scholar]
- 225.Sidelmann JJ, Gram JB, Godtfredsen ACM, Thorn JJ, Ingerslev J; Pinholt EM. Orthognathic surgery-induced fibrinolytic shutdown is amplified by tranexamic acid. J Oral Maxillofac Surg. 2020;78(7):1183-1189. doi: 10.1016/j.joms.2020.02.026 [DOI] [PubMed] [Google Scholar]
- 226.Xu X, Jiang J, Liu W, Li X, Lu H. Application of thromboelastography to evaluate the effect of different routes administration of tranexamic acid on coagulation function in total hip arthroplasty. J Orthop Surg Res. 2019;14(1):430. doi: 10.1186/s13018-019-1497-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zaman SU, Zakir I, Faraz Q, Akhtar S, Nawaz A, Adeel M. Effect of single-dose intravenous tranexamic acid on postoperative nasal bleed in septoplasty. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136(6):435-438. doi: 10.1016/j.anorl.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 228.Zhou KD, Wang HY, Wang Y, Liu ZH, He C, Feng JM. Is topical or intravenous tranexamic acid preferred in total hip arthroplasty? a randomized, controlled, noninferiority clinical trial. PLoS One. 2018;13(10):e0204551. doi: 10.1371/journal.pone.0204551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhou XD, Zhang Y, Jiang LF, et al. Efficacy and safety of tranexamic acid in intertrochanteric fractures: a single-blind randomized controlled trial. Orthop Surg. 2019;11(4):635-642. doi: 10.1111/os.12511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.World Health Organization . 18th Expert Committee on the Selection and Use of Essential Medicines. Published 2011. Accessed March 8, 2021. https://www.who.int/selection_medicines/committees/expert/18/en/
- 231.Yates J, Perelman I, Khair S, et al. Exclusion criteria and adverse events in perioperative trials of tranexamic acid: a systematic review and meta-analysis. Transfusion. 2019;59(2):806-824. doi: 10.1111/trf.15030 [DOI] [PubMed] [Google Scholar]
- 232.Franchini M, Mengoli C, Cruciani M, et al. Safety and efficacy of tranexamic acid for prevention of obstetric haemorrhage: an updated systematic review and meta-analysis. Blood Transfus. 2018;16(4):329-337. doi: 10.2450/2018.0026-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Eriksson O, Kjellman H, Pilbrant A, Schannong M. Pharmacokinetics of tranexamic acid after intravenous administration to normal volunteers. Eur J Clin Pharmacol. 1974;7(5):375-380. doi: 10.1007/BF00558210 [DOI] [PubMed] [Google Scholar]
- 234.Pilbrant A, Schannong M, Vessman J. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol. 1981;20(1):65-72. doi: 10.1007/BF00554669 [DOI] [PubMed] [Google Scholar]
- 235.Schiff RL, Kahn SR, Shrier I, et al. Identifying orthopedic patients at high risk for venous thromboembolism despite thromboprophylaxis. Chest. 2005;128(5):3364-3371. doi: 10.1378/chest.128.5.3364 [DOI] [PubMed] [Google Scholar]
- 236.Encke A, Haas S, Kopp I. The prophylaxis of venous thromboembolism. Dtsch Arztebl Int. 2016;113(31-32):532-538. doi: 10.3238/arztebl.2016.0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Methods
eAppendix 2. Outcome Measure
eAppendix 3. Statistical Analyses
eAppendix 4. Demographic Data and Subgroup Characteristics
eAppendix 5. Effect of Tranexamic Acid on Myocardial Infarction or Ischaemia
eAppendix 6. Effect of Tranexamic Acid on Cerebral Infarction or Ischaemia and on Other Thromboembolic Events
eAppendix 7. Effect of Tranexamic Acid in Patients with risks for Thromboembolic Events
eAppendix 8. Meta-Regression
eAppendix 9. Risk of Bias.
eAppendix 10. Updated Meta-Analysis
eTable 1. Study Characteristics
eTable 2. Venous Thrombosis
eTable 3. Pulmonary Embolism
eTable 4. Venous Thrombosis + Pulmonary Embolism
eTable 5. Myocardial Infarction or Ischaemia
eTable 6. Cerebral Infarction or Ischaemia
eTable 7. Non-Bleeding Mortality
eTable8. Summary of Sensitivity Analysis including Patients with increased Risk for total Thromboembolic Events
eTable 9. Study Characteristics of Updated Meta-Analysis
eTable 10. Total Thromboembolic Events of Updated Meta-Analysis
eTable 11. Venous Thrombosis of Updated Meta-Analysis
eTable 12. Pulmonary Embolism of Updated Meta-Analysis
eTable 13. Venous Thrombosis + Pulmonary Embolism of Updated Meta-Analysis
eTable 14. Overall Mortality of Updated Meta-Analysis
eAppendix 11. References on Total TE
eAppendix 12. References on VT Events
eAppendix 13. References on PE Events
eAppendix 14. References on VTE
eAppendix 15. References on Overall Mortality
eAppendix 16. References on Non-bleeding Mortality
eAppendix 17. References on Bleeding Mortality
eFigure 1. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 2. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 3. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control analysed with Risk Ratio in Studies with Zero Events
eFigure 4. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control analysed with Risk Ratio in Studies without Zero Events
eFigure 5. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control according to Sample Size using the Fixed-Effect Model
eFigure 6. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control according to Sample Size using the Random-Effects Model
eFigure 7. Venous Thrombosis Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 8. Venous Thrombosis Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 9: Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect model
eFigure 10: Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 11. Venous Thromboembolic and Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 12. Venous Thromboembolic and Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 13. Myocardial Infarction Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 14. Myocardial Infarction Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 15. Cerebral Ischaemia Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 16. Limb Ischaemia Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 17. Mesenteric Ischaemia Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 18. Hepatic Artery Thrombosis Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 19. Overall Mortality Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 20. Overall Mortality Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 21. Non-Bleeding associated Mortality Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 22. Non-Bleeding associated Mortality Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 23. Bleeding associated Mortality Rate in Patients receiving TXA compared to Control using the Fixed-Effect model
eFigure 24. Bleeding associated Mortality Rate in Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 25. Total Thromboembolic Event Rate in High Risk Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 26. Venous Thrombosis Event Rate in High Risk Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 27. Pulmonary Embolism Event Rate in High Risk Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 28. Venous Thromboembolic Event Rate in High Risk Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 29. Overall Mortality Rate in High Risk Patients receiving TXA compared to Control using the Fixed-Effect Model
eFigure 30. Overall Mortality Rate in High Risk Patients receiving TXA compared to Control using the Random-Effects Model
eFigure 31. Risk of Bias Summary
eFigure 32. Risk of bias Graph
eFigure 33. TotalThromboembolic Event Rate in Patients receiving TXA compared to Control of Studies Judged with Low Selection Bias using the Fixed-Effect Model
eFigure 34. Funnel Plots for Total Thromboembolic Event, Venous Thrombosis, Cerebral Infarction, and Bleeding Mortality
eFigure 35. Funnel Plots for Pulmonary Embolism, Venous Thromboembolic Events, and Myocardial Infarction
eFigure 36. Funnel Plots for Overall and Bleeding associated Mortality
eFigure 37. TotalThromboembolic Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model of updated Meta-Analysis
eFigure 38. TotalThromboembolic Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model updated Meta-Analysis
eFigure 39. Venous Thrombosis Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model of updated Meta-Analysis
eFigure 40. Venous Thrombosis Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model of updated Meta-Analysis
eFigure 41. Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model of updated Meta-Analysis
eFigure 42. Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model of updated Meta-Analysis
eFigure 43. Venous Thromboembolic and Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model of updated Meta-Analysis
eFigure 44. Venous Thromboembolic and Pulmonary Embolism Event Rate in Patients receiving TXA compared to Control using the Random-Effects Model of updated Meta-Analysis
eFigure 45. Overall Mortality Rate in Patients receiving TXA compared to Control using the Fixed-Effect Model of updated Meta-Analysis
eFigure 46: Overall Mortality Rate in Patients receiving TXA compared to Control using the Random-Effects Model of updated Meta-Analysis
eFigure 47. Total Thromboembolic Event Rate in Patients receiving TXA compared to Control analysed with Risk Ratio in Studies with Zero Events of updated Meta-Analysis
eFigure 48. TotalThromboembolic Event Rate in Patients receiving TXA compared to Control analysed with Risk Ratio in Studies without Zero Events of updated Meta-Analysis
eReferences