Abstract
An atypical femoral fracture (AFF) is a rare complication associated with excessive inhibition of osteoclast expression during treatment of osteoporosis. We herein describe a patient who had been treated with alendronate for more than 10 years and subsequently developed an AFF that healed after treatment with vitamin K2 (VK2). We also discuss the potential beneficial effects of VK2 on the healing of AFFs. A 48-year-old Asian man with secondary osteoporosis was treated with alendronate for more than 10 years. The patient underwent surgical treatment for a complete AFF of the right femur. Six months postoperatively, he complained of pain in his left thigh. X-ray examination revealed an incomplete AFF of the left femoral shaft. He was then treated with VK2. After 4 months of VK2 treatment, the patient reported that the pain in his left thigh had decreased, and follow-up X-ray examination demonstrated healing of the left AFF line. This case report indicates that VK2 may be a potential direction for pharmacological treatment of AFFs in future research.
Keywords: Vitamin K2, atypical femoral fracture, bisphosphonate, secondary osteoporosis, radiographic examination, case report
Introduction
Bisphosphonates (BPs) are widely prescribed as a treatment for osteoporosis and work by inhibiting bone resorption.1 Although their use may increase bone mineral density (BMD) and effectively prevent osteoporotic fracture, a low incidence of atypical femoral fractures (AFFs), such as subtrochanteric and diaphyseal femoral fractures, has emerged as a potential complication of long-term BP treatment within the past decade.2 A main symptom of an AFF is early-onset thigh pain with minor trauma or even no trauma.3 Radiographic characteristics of AFFs include a transverse to oblique fracture with a thickened lateral cortex and spiking of the lateral cortex.4
An AFF may be associated with over-suppression of bone turnover from prolonged BP therapy,5 or it may occur independent of BP use. In addition, a reduced bone turnover rate may lead to delayed bone healing at the fracture site in patients with osteoporosis.6 The use of anabolic agents, such as parathyroid hormone, to reverse low bone turnover and stimulate new bone formation is a common method of addressing this problem.
Vitamin K2 (VK2), an anabolic agent, has positive effects on bone formation and bone mineralization as well as the ability to prevent bone loss in patients with osteoporosis.7 Azuma et al.8 analyzed the bone tissue of PXR-deficient mice and found that the osteoprotective effect of vitamin K is partly mediated by SXR/PXR-dependent signal transduction. They identified genes induced by vitamin K in an SXR-dependent manner, including Tsukiji (Tsk), matrlin-2 (Matn2), and CD14, through comprehensive transcriptome analysis using microarrays.8 Several case reports and case series have described the benefits of using VK2 to prevent AFFs, including combined treatment9 or prior administration of VK2.10 However, no cases or studies have focused on the use of VK2 to treat patients with AFFs.
The present case report describes a patient with secondary osteoporosis who, during postoperative follow-up after treatment of a right femoral fracture, developed a low-energy AFF of the left femur. He had a >10-year history of treatment with alendronate without regular drug holidays. We found that VK2 achieved a considerable treatment effect and has potential as a conservative treatment modality for AFFs.
Case report
A 48-year-old man had been diagnosed with Addison’s disease 37 years previously based on juvenile-onset skin hyperpigmentation and an increased plasma adrenocorticotropic hormone concentration. He was also subsequently diagnosed with gonadotropic hypogonadism. He therefore underwent hormone replacement therapy with hydrocortisone acetate (20 mg at 7:00 am, 10 mg at 6:00 pm) and gonadotropin acetate (2000 IU twice a week). In 2006, the patient presented to our hospital for further treatment of his Addison’s disease. The endocrinologist had diagnosed the patient with secondary osteoporosis, which might have been caused by either Addison’s disease or long-term use of hormones. Since then, he had taken alendronate at 70 mg per week until September 2017 with irregular drug holidays. Vitamin D3 and calcium supplementation were continued. As shown in Table 1, the patient had normal results for a series of follow-up hormone assay indexes (e.g., osteocalcin, calcitonin, and parathormone) and bone metabolite indexes (e.g., 25-hydroxy vitamin D, β-collagen degradation product, and total type I collagen amino terminal propeptide). In August 2015, his BMD as measured by a dual-energy X-ray absorptiometry bone densitometer machine (Norland XR-600; Norland CooperSurgical, Trumbull, CT, USA) revealed osteoporosis of the lumbar spine (BMD of 0.81 g/cm2, T-score of −3.6, Z-score of −2.3) and left femur (BMD of 0.61 g/cm2, T-score of −3.6, Z-score of −2.6). Moreover, he underwent an intramedullary nail procedure for a complete AFF of his right femoral diaphysis in October 2018 and continued vitamin D3 and calcium supplementation. This fracture showed satisfactory healing on plain films during regular follow-up.
Table 1.
Patient’s laboratory findings before and after vitamin K2 treatment.
| Characteristics | Before treatment | 4 months after treatment |
|---|---|---|
| 25-hydroxy vitamin D (ng/mL) | 38.19 | 38.29 |
| β-collagen degradation product (pg/mL) | 527.5 | 541.2 |
| Total type I collagen amino terminal propeptide (ng/mL) | 134.60 | 66.67 |
| N-terminal molecular fragment of osteocalcin (ng/mL) | 33.55 | 21.71 |
| Calcitonin (pg/mL) | – | 2.55 |
| Bone-specific alkaline phosphatase (μg/L) | 27.6 | 27.2 |
| Parathormone (pg/mL) | 26.7 | 21.6 |
| Alkaline phosphatase (U/L) | 131 | 124 |
| Alanine transaminase (U/L) | 16 | 29 |
| Total calcium (mmol/L) | 2.55 | 2.62 |
| Phosphorus (mmol/L) | 1.12 | 1.37 |
| Creatinine (μmol/L) | 49.3 | 63.8 |
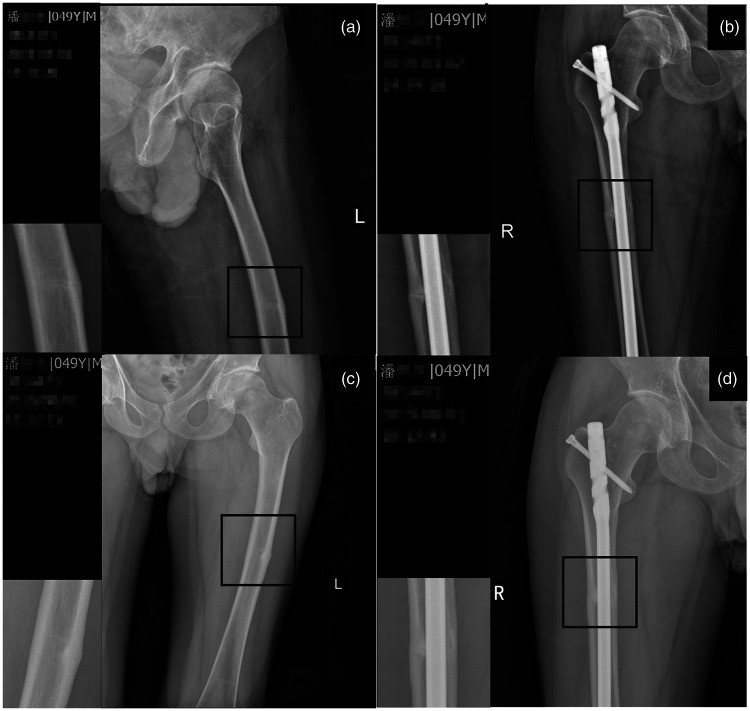
The patient complained of pain in his left thigh 6 months after the surgery on his right femur. Physical examination revealed left lower limb paresthesia, local soft tissue tenderness, and longitudinal percussion pain along the femur. Plain films indicated that the right femoral fracture was in good linear and positional alignment (Figure 1(b)) but also revealed a cortical linear fracture of the left femur (Figure 1(a)). We accordingly diagnosed the patient with a linear AFF of the left femur.
Figure 1.
X-ray examination conducted at the time of diagnosis showed (a) an atypical fracture of the left femur and (b) a right femoral fracture (bar). X-ray examination after 4 months of conservative management showed healing of the (c) atypical fracture of the left femur and (d) right femoral fracture (bar).
The patient refused treatment with parathyroid hormone for financial reasons; therefore, we recommended that he take 15 mg of VK2 (menatetrenone soft capsules; Eisai Co., Tokyo, Japan) three times a day while continuing his other prescriptions. After 4 months, the patient reported reduced pain in his left femur, obtained normal laboratory findings as shown in Table 1, and showed improvement in the healing of his left femoral fracture on follow-up imaging. In particular, the X-ray examination showed local thickening in the middle of the adjacent cortex of the left femur without an obvious fracture line (Figure 1(c)). This improvement in fracture healing occurred with no adverse drug reactions. In October 2019, repeat measurement of his BMD using dual-energy X-ray absorptiometry still revealed osteoporosis of the lumbar spine (BMD of 0.81 g/cm2, T-score of −3.3, Z-score of −2.0) and left femur (BMD of 0.61 g/cm2, T-score of −3.7, Z-score of −2.7).
Discussion
AFFs are most commonly associated with prolonged BP use. Although the mechanism is unclear, they probably develop because of gradual accumulation of bone microdamage in patients with osteoporosis, causing the bone to become brittle.2 The incidence of AFFs increases with prolonged use of BPs, especially with treatment regimens lasting more than 5 years.6 Independent risk factors for developing AFFs include osteopenia or osteoporosis, rheumatoid arthritis, increased anterior and lateral femoral curvature, and a thickened lateral femoral cortex.11 Complete AFFs are predominantly fixed by locked intramedullary nailing, which is favored over plate fixation because of its higher load-sharing capacity and less bending movement across the fracture site.12 However, there are no definite treatments or diagnostic guidelines for early incomplete AFFs despite an increase in their incidence. In the present case, we consider that VK2 may have played a vital role in supporting healing of the AFF.
The therapeutic effects of VK2 on osteoporosis (i.e., inhibiting osteoclast-induced bone resorption and/or increasing the activity of osteoblasts) have been confirmed by a large number of experiments. VK2 has been shown to have a protective effect on bone metabolism; it can also promote bone formation by stimulating the differentiation of osteoblasts and inhibiting the formation of osteoclasts.13,14 However, these studies were limited to the cellular level and lack the support of clinical trials. A meta-analysis of 13 studies in which VK2 was administered at 45 mg/day, the optimal dose, revealed that VK2 has a protective effect on BMD and reduces the risk of hip, vertebral, and non-vertebral fractures.15 Furthermore, it has been reported that combination treatment of VK2 and BPs might be more effective for the prevention of new vertebral fractures than treatment with a BP alone in postmenopausal women with osteoporosis.16 Although the role of VK2 in reducing the overall fracture risk needs to be confirmed in larger randomized controlled trials, VK2 still has beneficial effects in current clinical practice. One study showed that VK2 combined with calcium and vitamin D3 increased the BMD of the lumbar vertebrae in postmenopausal women, whereas the combination of calcium and vitamin D3 did not. In addition, the combination of VK2 and alendronate reduced the levels of bone turnover markers and undercarboxylated osteocalcin in postmenopausal women.17 Capozzi et al.18 showed that VK2 supplementation is an important way to enhance the link between calcium and vitamin D. VK2 can increase the BMD and maintain the overall bone strength in patients with osteoporosis. However, another study showed that treatment with VK2 as an add-on to calcium and vitamin D did not improve the bone microarchitecture or BMD in postmenopausal women, although VK2 can enhance the carboxylation of osteocalcin.19 Alternatively, Cockayne et al.15 suggested that supplementation with VK2 can reduce bone loss. Patients at risk of fracture should be encouraged to eat foods rich in vitamin K, including leafy greens and certain vegetable oils.15 Meanwhile, pretreatment with VK2 before zoledronic acid therapy might partially prevent the inhibition of bone formation caused by zoledronic acid.10 Cases of atypical fractures due to long-term use of alendronate have been reported, but in these cases, the patients were cured after being treated with teriparatide.20,21 No case reports to date have shown that VK2 can be used to treat AFFs, and the precise mechanisms behind this effect have not been elucidated. Therefore, we can only speculate on the following points. VK2 has a positive effect on transcription of extracellular matrix-related genes as well as collagen accumulation by activation of the xenobiotic steroid receptor in osteoblastic cells.22 Another mechanism underlying the treatment of osteoporosis with VK2 is that VK2 inhibits apoptotic cell death of osteoblasts by reducing the expression of the proapoptotic agents Fas and Bax in a dose-dependent manner in osteoblasts.23 VK2 also suppresses NF-κB activation to reduce osteoclastogenesis.24 In a biochemical context, VK2 may affect bone metabolism by participating in the carboxylation of vitamin K-dependent glutenin, mainly osteocalcin,25 which converts the glutamic acid (Glu) residue in Gla protein to γ-carboxyglutamic acid (Gla), which has a positive effect on regulating bone mineralization and bone maturation.26 Thus, VK2, alone or in combination with other medications, might reverse the suppression of bone turnover that results from long-term BP use.
In this case, successful bone union was observed in imaging studies at the 4-month follow-up in a patient who started VK2 after development of an AFF. Further studies with larger patient numbers are necessary to determine whether VK2 can enhance bone formation and improve fracture healing in patients with AFFs.
Footnotes
Ethics approval: This study was approved by the Research Ethics Committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (2017 Clinical Research Ethics Approval No. 18).
Consent to participate: Signed consent for treatment and written informed consent for publication of this report were obtained from the patient.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by the National Key Research and Development Program of China (grant number 2017YFB1304200).
ORCID iD: Lei Yang https://orcid.org/0000-0003-2454-4208
References
- 1.Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357: 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schilcher J, Michaëlsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364: 1728–1737. [DOI] [PubMed] [Google Scholar]
- 3.Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br 2007; 89: 349–353. [DOI] [PubMed] [Google Scholar]
- 4.Neviaser AS, Lane JM, Lenart BA, et al. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma 2008; 22: 346–350. [DOI] [PubMed] [Google Scholar]
- 5.Kwek EB, Goh SK, Koh JS, et al. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury 2008; 39: 224–231. [DOI] [PubMed] [Google Scholar]
- 6.Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305: 783–789. [DOI] [PubMed] [Google Scholar]
- 7.Schwalfenberg GK. Vitamins K1 and K2: the emerging group of vitamins required for human health. J Nutr Metab 2017; 2017: 6254836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azuma K, Ouchi Y, Inoue S. Vitamin K: novel molecular mechanisms of action and its roles in osteoporosis. Geriatr Gerontol Int 2014; 14: 1–7. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Miyazaki T, Uemura Y, et al. Design of a randomized clinical trial of concurrent treatment with vitamin K2 and risedronate compared to risedronate alone in osteoporotic patients: Japanese Osteoporosis Intervention Trial-03 (JOINT-03). J Bone Miner Metab 2014; 32: 298–304. [DOI] [PubMed] [Google Scholar]
- 10.Zhao B, Zhao W, Wang Y, et al. Prior administration of vitamin K2 improves the therapeutic effects of zoledronic acid in ovariectomized rats by antagonizing zoledronic acid-induced inhibition of osteoblasts proliferation and mineralization. PLoS One 2018; 13: e0202269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim SJ, Yeo I, Yoon PW, et al. Incidence, risk factors, and fracture healing of atypical femoral fractures: a multicenter case-control study. Osteoporos Int 2018; 29: 2427–2435. [DOI] [PubMed] [Google Scholar]
- 12.Giannoudis PV, Ahmad MA, Mineo GV, et al. Subtrochanteric fracture non-unions with implant failure managed with the “Diamond” concept. Injury 2013; 44: S76–S81. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto J. Vitamin K2 therapy for postmenopausal osteoporosis. Nutrients 2014; 6: 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbari S, Rasouli-Ghahroudi AA. Vitamin K and bone metabolism: a review of the latest evidence in preclinical studies. Biomed Res Int 2018; 2018: 4629383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 2006; 166: 1256–1261. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto J, Takeda T, Ichimura S. Combined treatment with vitamin K2 and bisphosphonate in postmenopausal women with osteoporosis. Yonsei Med J 2003; 44: 751–756. [DOI] [PubMed] [Google Scholar]
- 17.Capozzi A, Scambia G, Lello S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas 2020; 140: 55–63. [DOI] [PubMed] [Google Scholar]
- 18.Capozzi A, Scambia G, Migliaccio S, et al. Role of vitamin K(2) in bone metabolism: a point of view and a short reappraisal of the literature. Gynecol Endocrinol 2020; 36: 285–288. [DOI] [PubMed] [Google Scholar]
- 19.Rønn SH, Harsløf T, Oei L, et al. The effect of vitamin MK-7 on bone mineral density and microarchitecture in postmenopausal women with osteopenia, a 3-year randomized, placebo-controlled clinical trial. Osteoporos Int 2021; 32: 185–191. [DOI] [PubMed] [Google Scholar]
- 20.Gomberg SJ, Wustrack RL, Napoli N, et al. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J Clin Endocrinol Metab 2011; 96: 1627–1632. [DOI] [PubMed] [Google Scholar]
- 21.Huang HT, Kang L, Huang PJ, et al. Successful teriparatide treatment of atypical fracture after long-term use of alendronate without surgical procedure in a postmenopausal woman: a case report. Menopause 2012; 19: 1360–1363. [DOI] [PubMed] [Google Scholar]
- 22.Horie-Inoue K, Inoue S. Steroid and xenobiotic receptor mediates a novel vitamin K2 signaling pathway in osteoblastic cells. J Bone Miner Metab 2008; 26: 9–12. [DOI] [PubMed] [Google Scholar]
- 23.Urayama S, Kawakami A, Nakashima T, et al. Effect of vitamin K2 on osteoblast apoptosis: vitamin K2 inhibits apoptotic cell death of human osteoblasts induced by Fas, proteasome inhibitor, etoposide, and staurosporine. J Lab Clin Med 2000; 136: 181–193. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi M, Weitzmann MN. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int J Mol Med 2011; 27: 3–14. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Hara K, Akiyama Y. [ Effect of menatetrenone (V.K2) on bone mineral density and bone strength in Ca/Mg deficient rats]. Nihon Yakurigaku Zasshi 2002; 120: 195–204. [DOI] [PubMed] [Google Scholar]
- 26.Weng SJ, Yan DY, Gu LJ, et al . Combined treatment with vitamin K2 and PTH enhanced bone formation in ovariectomized rats and increased differentiation of osteoblast in vitro. Chem Biol Interact 2019; 300: 101–110. [DOI] [PubMed] [Google Scholar]