Abstract
Background:
Lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) are a bothersome frequent symptom in adult males. This systematic review analyzed the available evidence on the pharmacokinetic and pharmacodynamic features of alfuzosin, and its clinical efficacy both as monotherapy and in combination with other drugs for the treatment of male LUTS/BPH.
Methods:
A systematic review of the last 10 years was performed using the MEDLINE, EMBASE and Cochrane libraries in March 2020. The protocol for this systematic review was registered on PROSPERO (Central Registration Depository: CRD42020136120) and is available in full on the University of York website.
Results:
Alfuzosin is a quinazoline derivative and, although a nonspecific α1-blocker, exhibits a selective concentration in the prostate compared with plasma in patients with BPH. Three registration trials assessed the safety and efficacy of alfuzosin. The 10 mg daily formulation has a three-layered matrix containing the active substance between two inactive coats allowing a drug release over 20 h. Alfuzosin showed high tolerability, few vasodilatory effects and a low rate of ejaculation disorders over older alpha-blocking compounds thanks to the high uroselectivity of alfuzosin and its preferential concentration at urinary level. Six randomized clinical trials (RCTs) assessed efficacy and safety of alfuzosin versus other alpha-blockers ± placebo: three studies comparing with tamsulosin, one with doxazosin, and two with silodosin or tamsulosin. One RCT investigated the clinical outcomes of alfuzosin with finasteride, two with propiverine and two with phosphodiesterase-5 inhibitors.
Conclusions:
Alfuzosin is an effective drug for the treatment of LUTS/BPH, with a lower rate of sexual disorders compared with other alpha-blockers. Alfuzosin is also safe with low adverse events in case of concomitant antihypertensive therapy and in patients with cardiovascular morbidity. Safety and efficacy of alfuzosin has been reported also in case of combination therapy with antimuscarinic agents and PDE5i.
Keywords: alfuzosin, alpha adrenoreceptor antagonists, benign prostatic hyperplasia, low urinary tract symptoms, pharmacology
LUTS/BPH: definition, epidemiology and standard of care
Benign prostatic hyperplasia (BPH) is characterized by an increased proliferation of both epithelial and stromal tissue, especially in the periurethral zone of the prostate, and is one of the most common conditions in elderly men.1 The prevalence of BPH increases substantially with advanced age, with a reported prevalence ranging from 8% to 60% in the adult population.2,3 BPH can cause bothersome lower urinary tract symptoms (LUTS), including storage, voiding and post-micturition symptoms variously combined together.4,5 LUTS secondary to BPH (LUTS/BPH) seriously affects quality of life3 and moreover impacts greatly on public health due to the possible consequences of untreated BPH, such as acute urinary retention (AUR), urinary tract infections and worsening of the renal function.6,7
According to current international guidelines,3,4 pharmacological therapy represents the first therapeutic approach in patients with moderate or severe LUTS/BPH. In this context, α1-adrenoceptor antagonists (also known as α1-blockers) play a key role for the treatment of LUTS/BPH. To date, several α1-blockers with different pharmacokinetic and pharmacodynamic profiles have been developed for clinical use.
Aim of this systematic review is to analyze the available evidence on the pharmacokinetic and pharmacodynamic features of alfuzosin, and its clinical efficacy both as monotherapy and in combination with other drugs for the treatment of male LUTS/BPH.
Evidence acquisition
Literature search
A systematic review of the English literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the Cochrane Handbook for Systematic Reviews of Interventions.8,9 A PRISMA checklist is provided as Supplementary Table 1. We searched MEDLINE, Scopus and Web of Science to investigate the efficacy and the treatment profile of alfuzosin in men treated with alfuzosin in monotherapy and in combination therapy for BPH between 1 January 2000 and 28 February 2020. After a first screening based on study title and abstract, all papers were assessed based on full text and excluded with reasons when appropriate. Two reviewers (A.M. and A.M.) carried out this process independently. Disagreement was solved by a third party (C.D.N.). The following string terms were used: [(“alfuzosin”) AND (“BPH” OR “benign prostatic enlargement” OR “benign prostatic hypertrophy” OR “benign prostatic hyperplasia” OR “lower urinary tract symptoms” OR “LUTS”)].
Eligibility criteria
As proposed by the PRISMA guidelines, we used the Population, Intervention, Comparator, Outcome and Study design approach to specify the inclusion criteria. Reports were considered relevant when they included men affected by benign prostatic hyperplasia and lower urinary tract symptoms (P), undergone medical treatment with alfuzosin (I) and compared with patients with comparable symptoms for BPH and treated with other alpha-blockers, finasteride, dutasteride or treated with endoscopic or open or minimally invasive surgery for the removal of the prostatic adenoma (C). These patients were compared to determine the incidence of AUR and BPH-related surgery during the medical treatment, the change in the International Prostate Symptom Score (IPSS), and the secondary outcome measures were changes in individual subjective symptom scores, quality of life score, and peak flow rate (Qmax) from baseline at uroflowmetry. Only randomized clinical trials and cohort prospective studies according to the latest version of the evidence medicine pyramid were included for qualitative analysis.10 Conversely, retrospective studies, low-quality prospective studies, review articles, editorials, comments and meeting abstracts were excluded. References of included manuscripts were scanned for additional studies of interest.
Data extraction
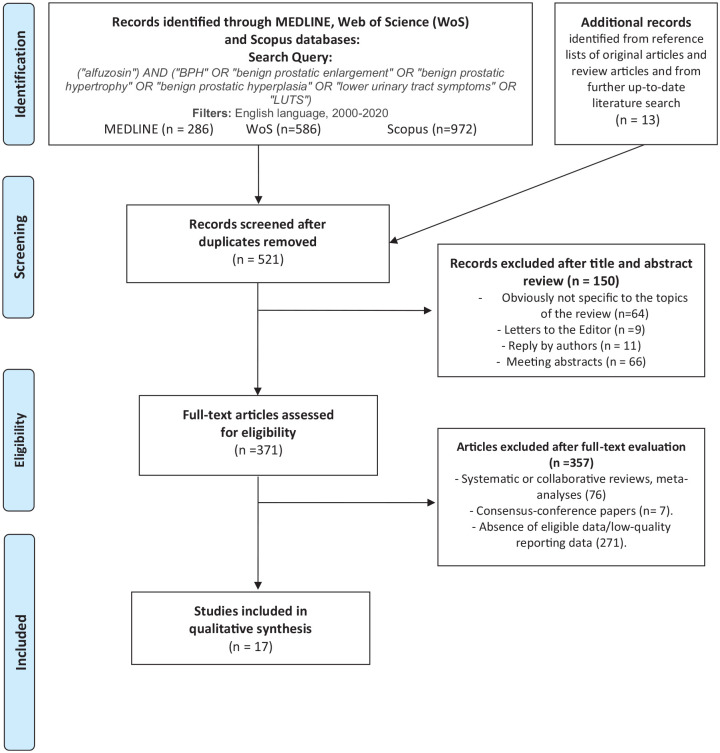
The titles and abstracts of all references retrieved in the searches were screened for potential eligibility, and the full texts of articles identified as possibly being relevant were obtained and evaluated. After full text evaluation, data were independently extracted by two authors (A.M. and A.M.) for further assessment of qualitative analyses. All extracted variables were crosschecked to ensure their reliability. Furthermore, we searched for methods and important confounders to establish comparability. All discrepancies regarding data extraction were generally resolved by consensus or finally decided by a third author (C.D.N.). A flow chart for the article selection process according to PRISMA-P 2015 checklist is detailed in Figure 1. The protocol has also been registered in the International Prospective Register of Systematic Reviews database (PROSPERO: CRD42020136120).
Figure 1.
Flow chart for article selection process to analyze functional urinary outcomes in men affected by benign prostatic hyperplasia and lower urinary tract symptoms undergone medical treatment with alfuzosin.
Alfuzosin: pharmacology and pharmacodynamics
The pharmacology and pharmacodynamic properties of alfuzosin are well established and were extensively analyzed in two reviews in Drugs published in 199311 and in 2002,12 respectively.
Alfuzosin is a quinazoline derivative characterized by the presence of a diaminopropyl spacer and the absence of a piperidine moiety (Figure 2). These biochemical properties determine a selective and competitive antagonist effect on the contraction of prostatic smooth muscle mediated by the α1-adrenoceptors.
Figure 2.
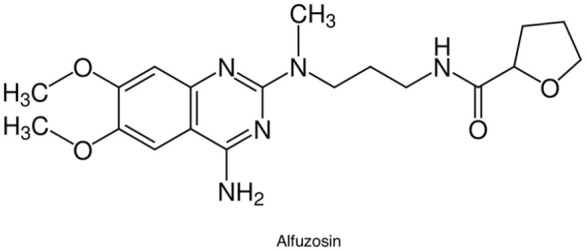
Chemical structure of alfuzosin. Molecular Formula: C19H27N5O4•HCl. Chemical Name: N-[3-[(4-Amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furancarboxamide monohydrochloride.
The receptor selectivity and pharmacokinetics parameter of alfuzosin compared with tamsulosin and silodosin are summarized in Table 1. The different safety and efficacy profile of various α1-blockers depend on their distinct selectivity to the various subtypes of α1-adrenoceptors (α1-ARs) expressed in numerous human tissues. In particular, three different α1-ARs subtypes (α1-A, α1-B and α1-D) with distinctive features in terms of distributions and mediated pharmacological and physiological effects have been identified.13 In detail, α1-A receptors are the predominant subtype expressed in the human prostate tissue with an increased (nearly 9-fold increase) expression in patients with BPH.14
Table 1.
Receptor selectivity and pharmacokinetics parameter of alfuzosin compared with tamsulosin and silodosin.
| Receptor selectivity | Tamsulosin | Alfuzosin | Silodosin |
|---|---|---|---|
| A1 blocker selectivity profile a,b,c | α1A = α1D > α1B | α1A = α1D = α1B | α1A > α1D > α1B |
| A1 blocker selective affinity (α1A:α1B) | 10:1 | 1:1 | 162:1 |
| Pharmacokinetics parameters | Tamsulosin | Alfuzosin | Silodosin |
| Cmax (ng/mL) | 10.1 ± 4.8 | 13.6 ± 5.6 | 27.9 ± 9.6 |
| Bioavailability (%) | 100 | 49 | 32 |
| Protein binding (%) | 94–99 | 82–90 | 97 |
| Excretion in urine (%) | 76 | 24 | 33.5 |
| Excretion in bile/feces (%) | 21 | 69 | 54.9 |
| AUC∞ (ng/h/mL) | 151–199 | 194 ± 75 | 133.7 ± 58 |
| Tmax (h) | 6.67 | 6.69 | 1.36 ± 1.12 |
| T1/2 (h) | 16.13 | 10 | 4.714 ± 3.710 |
Ishiguro M, Futabayashi Y, Ohnuki T, et al. Identification of binding sites of prazosin, tamsulosin and KMD-3213 with alpha(1)-adrenergic receptor subtypes by molecular modeling. Life Sci 2002; 71: 2531–2541.
Schwinn DA and Roehrborn CG. Alpha1-adrenoceptor subtypes and lower urinary tract symptoms. Int J Urol Off J Japanese Urol Assoc 2008; 15: 193–199.
Akiyama K, Hora M, Tatemichi S, et al. KMD-3213, a uroselective and long-acting alpha(1a)-adrenoceptor antagonist, tested in a novel rat model. J Pharmacol Exp Ther 1999; 291: 81–91.
AUC∞, area under the plasma concentration-time curve from time zero to infinity; Cmax, maximum plasma concentration; t1/2, elimination half-life; tmax, time to reach the Cmax; uroselective receptors, α1A and α1D; vascular epithelium, α1b.
Similar to the other α1-blockers, alfuzosin also counteracts the effect of noradrenaline on smooth muscle cells in the prostate and consequently the symptoms due to bladder outlet obstruction.13 It has been largely demonstrated both in human and animal studies that the activity and side effects of α1-blockers rely on their uroselectivity rather than the α1-AR subtype affinity as previously reported.15,16
In this scenario, alfuzosin, although a nonspecific α1-blocker,17 exhibits a selective concentration in the prostate, compared with plasma, in patients with BPH. In detail, the selective concentration of alfuzosin, tamsulosin, doxazosin and terazosin were compared and, in particular, their affinity for prostatic and renal α1-adrenoceptors in intact enzymatically isolated myocytes of the human prostate and renal artery was assessed. Alfuzosin showed a significantly higher concentration in the prostatic tissue compared with other α-blockers.18 Also, in clinical practice, the blood and prostatic concentrations of alfuzosin were determined in patients with BPH treated with alfuzosin 5 mg twice daily prior to surgery in an open trial, and high diffusion of the drug into the prostate in BPH patients was found.19
Several formulations of alfuzosin for the treatment of LUTS/BPH have been developed. Initially, a 2.5 mg immediate-release formulation (administered three times daily) was developed; afterwards, a 5 mg (administered twice daily) sustained-release formulation was produced.20 Lastly, a 10 mg alfuzosin with a controlled release over the dosage interval has been developed. As the drug is highly soluble in water, the major challenge in making a controlled-release formulation was to control its release from the dosage forms. A three-layered matrix containing the active substance between two inactive coats, one swellable and one erodible, was developed allowing a drug release over 20 h with a near constant dissolution rate between 2 and 12 h.20 The absolute bioavailability of 10 mg alfuzosin HCl extended-release tablets under fed conditions is 49%. The time to maximum concentration is 8 h. Cmax and AUC0–24 are 13.6 (SD = 5.6) ng/mL and 194 (SD = 75) ng·h/mL, respectively.20 The absorption extent is 50% lower under fasting conditions. For this reason, it should be taken with food and with the same meal each day.20
The distribution volume in healthy male middle-aged volunteers was 3.2 L/kg after intravenous administration.21 Furthermore, in vitro studies showed that 82–90% of molecules are bound to plasma proteins, with a wide concentration in blood ranging from 5 to 5000 ng/mL.21
Alfuzosin is mostly metabolized by the liver, while only 11% of the molecules of the administered dose are excreted without modifications in the urine.22 Oxidation, O-demethylation and N-dealkylation are the three metabolic processes which alfuzosin undergoes.22 The most important hepatic enzyme isoform involved in its metabolism is the Cytochrome P450 3A4 (CYP3A4).
Although it is not indicated to check the activity of the CYP3A4, repeated administration of 400 mg of ketoconazole, a potent inhibitor of CYP3A4, following a single 10 mg dose of alfuzosin, increased 2.3-fold alfuzosin Cmax.20 Alfuzosin use is also contraindicated in the case of patients taking telaprevir and boceprevir that are used for the treatment of hepatitis C virus infection and induce a potent, reversible inhibition of the CYP3A4/5 enzyme families.23
The properties of once-daily formulation of alfuzosin were studied in an elderly population without finding any pharmacokinetic difference in 16 healthy volunteers between 66 and 78 years.24 Despite 35% greater concentration of alfuzosin in patients aged ⩾75 years,22 clinical trials showed no overall differences in safety or effectiveness between subjects ⩾65 years, ⩾75 years and younger men.22
As regards the pharmacodynamic profile of alfuzosin in special populations, the presence of chronic heart failure has not been found to significantly affect the pharmacokinetics of 10 mg daily alfuzosin. Concerning patients with reduced renal function, an evaluation of the effects of the treatment was performed on 26 volunteers, of which there were eight with normal renal function, six with mild, six with moderate, and six with severe chronic kidney disease.25 The study showed that the mean apparent total body clearance was decreased 30–40% in all groups with renal impairment.25 However, the product monograph highlighted that in clinical trials the safety profile of patients with mild (n = 172) or moderate (n = 56) renal impairment was similar to the patients with normal renal function in phase III clinical trials.22 Nevertheless, alfuzosin should not be administered to patients with severe renal failure (eGFR <30 mL/min) due to the lack of clinical safety data in this group of patients.
Alfuzosin: clinical efficacy as monotherapy
Registration trials
Results from registration trials are summarized in Table 2. The first randomized placebo-controlled trial assessing the safety and efficacy of alfuzosin (2.5 mg TID) as monotherapy for the treatment of LUTS/BPH was published in 1991 by the BPH-ALF group.26 In this trial, alfuzosin was superior to placebo in terms of increased urinary flow parameters [including post-voided residual volume (PVR)] and was also associated with a lower AUR rate (0.4% versus 2.6%) after 6 months of therapy. The overall incidence of adverse events (AEs) was comparable between the two groups.
Table 2.
Description of characteristics and reported outcomes of randomized registration clinical trials of alfuzosin.
| Study (author) | Study type | Duration (weeks) | Treatment (daily dose) | Patients (n) | Change in symptoms (IPSS) (%) | Change in Qmax (mL/s) | PVR change (%) | Adverse events (%) | LE |
|---|---|---|---|---|---|---|---|---|---|
| Jardin et al.26 | RCT | 24 | Placebo | 267 | −32 | +1.3 | −9 | 9.0 | 1b |
| Alfuzosin 3 × 2.5 mg | 251 | −42 | +1.4 | −39 | 10.8 | ||||
| Buzelin et al.27 | RCT | 12 | Placebo | 196 | −18 | +1.1 | 0 | 7.1 | 1b |
| Alfuzosin 2 × 5 mg | 194 | −31 | +2.4 | −17 | 4.6 | ||||
| van Kerrebroeck, et al.28 | RCT | 12 | Placebo | 154 | −27.7 | +1.4 | /// | 1b | |
| Alfuzosin 3 × 2.5 mg | 150 | −38.1 | +3.2 | 9.4 | |||||
| Alfuzosin 1 × 10 mg | 143 | −39.9 | +2.3 | 6.3 |
LE: level of evidence; PVR: post-voided residual; RCT: randomized clinical trial.
Lately, in 1997, the ALGEBI group reported the results of a randomized controlled trial (RCT) comparing the safety and efficacy of alfuzosin 5 mg BID versus placebo; notably, of the 390 patients included, almost half of them had concomitant cardiovascular disease.27 After 12 weeks of therapy, all urinary parameters were significantly improved in the alfuzosin group compared with the placebo one. The AEs rates (including vasodilation-related events) were similar between the two groups.27
In 2000, van Kerrebroek et al.28 published the results of the ALFORTI study group that conducted a randomized double-blind placebo-controlled trial assessing the safety and efficacy of alfuzosin 10 mg once daily and alfuzosin 2.5 mg TID versus placebo. In this study, both alfuzosin formulations significantly improved urinary symptoms and flow rate compared with placebo. Overall, both formulations of alfuzosin were well tolerated in comparison with placebo. In addition, vasodilatory AEs appeared to be fewer with the once-daily than the TID alfuzosin formulation (6.3 versus 9.4%, respectively). Moreover, no specific sexual dysfunctions including ejaculation disorder were reported in the alfuzosin 10 mg once-daily group.
Efficacy
Efficacy of alfuzosin as monotherapy for the treatment of BPH/LUTS has been reported in several prospective series. Lukacs et al.29 reported the results of a 1-year prospective open-label study involving 2829 patients receiving 2.5 mg TID or 5 mg BID alfuzosin formulations. This study confirmed the effectiveness of alfuzosin as reported by aforementioned registration trials. In detail, the IPSS score was reduced by 49.6% and by 53.8% after 6 and 12 months of treatment, respectively. Only 5% of included patients dropped out due to AEs, while 33 (1.2%) patients experienced AUR during the study period. The same authors reported the efficacy and safety profile of alfuzosin up to 36 months of treatment in a large cohort of 7093 patients followed by general practitioners (GPs).30 The percentage of patients/month who dropped out, experienced AEs, AUR and surgery were 0.6–1.6, 0.1–0.5, 0.01–0.03, and 0.1–0.3%, respectively, underlining the long-term efficacy and safety of alfuzosin. Of note, no cases of retrograde ejaculation were reported.
Afterwards, the ALF-ONE study group reported the 2-year results of a large open-label study with alfuzosin 10 mg once daily enrolling 839 European patients followed by GPs.31 At the endpoint, the IPSS improved by 7 points (–38.5%) from baseline (p < 0.001). There were also significant improvements in nocturia (–30%; p < 0.001) and bother score (–43%; p < 0.001) from baseline. Interestingly, most patients (56%) perceived symptom relief within the first 2 weeks of treatment.
In a pooled analysis of the data from the three pivotal phase III registration trials studies conducted to test the efficacy of alfuzosin 10 mg once-daily (n = 473) versus placebo (n = 482), Alfuzosin was also associated with a significant improvement in the absolute change of the nocturia criterion (reduction of the number of micturitions per night – question #7 of the IPSS score) from baseline to study end (84 days) (p = 0.04).32
Overall, alfuzosin 10 mg was well tolerated, and the most common AE related to vasodilatation was dizziness/postural dizziness (3.1%). As previously reported, ejaculatory disorders were uncommon (0.3%) and, importantly, vasodilation-related symptoms were rare also in elderly men and those under antihypertensive therapy. These results were confirmed also at the 3-year follow-up of the same study, emphasizing the lasting effectiveness and safety of alfuzosin 10 mg for the treatment of LUTS/BPH.33
Indeed, the same study group investigated the effect on sexual function of alfuzosin 10 mg in 2434 sexually active patients. After 1 year of therapy in those men with baseline sexual dysfunction, alfuzosin was proved to improve the rigidity of erection, the amount of ejaculate and pain/discomfort on ejaculation (all p < 0.001) over baseline, with the reported improvements being more marked in men with severe LUTS at baseline.34
In another randomized clinical trial conducted in 52 US centers, the effects on sexual function of extended-release alfuzosin 10 mg once daily were evaluated.35 A slight improvement in erectile function after the administration of alfuzosin was found in patients with erectile dysfunction at baseline; conversely, in all the other patients extended-release alfuzosin significantly improved erectile function in men with LUTS/BPH only while maintaining a stable ejaculatory function.35 Indeed, sexual dysfunction and LUTS have a close correlation.36 Although the mechanisms by which alfuzosin decreases sexual dysfunction have not been fully understood, possible explanations could be related to the improvement in the patients’ quality of life,3 to the direct effects of the blockade of the overall α1-adrenergic receptor signal transduction pathway on erectile function,37 and to the limited effects on α1a receptors that induce a decrease of the volume of ejaculate by relaxation of bladder neck and/or reduce seminal vesicle contraction and sperm progression.38
The Italian Alfuzosin Co-Operative Group published the results of a multicentric study on safety and efficacy of alfuzosin 2.5 mg TID administered for 1 year.39 Both objective (uroflowmetry and residual urine volume) and subjective [IPSS and Quality of Life (QoL) questionnaires] outcomes gradually improved from baseline throughout the treatment period and only 4.3% of patients dropped out due to AEs.
Almost in the same period, the results of a large (4018 patients) multicenter observational study conducted in Spain were published; in particular, the authors focused on the cardiovascular safety profile of alfuzosin 5 mg BID for the treatment of LUTS/BPH.40,41 In this study, alfuzosin was well tolerated independently of the age of the patient. In particular, the distribution of vasodilatory/non-vasodilatory AEs was similar in all age groups, and the incidence of asymptomatic orthostatic hypotension was low (0.58%) and not affected by the age of the patients, thus highlighting the uroselectivity of alfuzosin irrespective of patient age.41
Finally, the effectiveness and safety of alfuzosin 10 mg OD has been reported in the open-label extension study of the aforementioned ALFORTI study group.42 Among the 311 patients included, IPSS and uroflowmetry parameters significantly improved from baseline as well as QoL index. Only 4.4% of patients experienced AEs (mainly represented by slight dizziness), and ejaculation disorders were also rare (0.6%).42
Side effects and safety profile: the role of uroselectivity
Beside the excellent cardiovascular profile together with the low rate of ejaculation disorders that emerged from the above reported studies, alfuzosin showed high tolerability and few vasodilatory effects over older alpha-blocking compounds.43 The adverse rate of alfuzosin, tamsulosin and silodosin are summarized in Table 3. A systematic review of the literature (RCTs of alfuzosin versus placebo or versus other drugs for the treatment of LUTS/BPH) reported an incidence of AEs leading to premature withdrawal from the study ranging from 5.8% to 11.8%, similar to the rates of the placebo/control groups.44 In a randomized, double-blind, placebo-controlled study the impact of alfuzosin 10 mg administration on blood pressure (BP) and heart rate (HR) was evaluated in young patients45 and concluded that alfuzosin did not affect BP or HR.
Table 3.
Adverse effects of alfuzosin, tamsulosin and silodosin compared with placebo.
| Adverse effect | Drug | Placebo | |
|---|---|---|---|
| Tamsulosina | Dizziness, % | 14.9 | 10.1 |
| Abnormal ejaculation, % | 8.4 | 0.2 | |
| Asthenia/fatigue, % | 7.8 | 5.5 | |
| Libido decreased, % | 1.0 | 1.2 | |
| Amblyopia, % | 0.2 | 0.4 | |
| Alfuzosinb | Upper respiratory tract infection, % | 3.0 | 0.6 |
| Dizziness, % | 5.7 | 2.8 | |
| Headache, % | 3.0 | 1.8 | |
| Fatigue, % | 2.7 | 1.8 | |
| Silodosinc | Retrograde ejaculation, % range | 14.2–28.1 | 0–1.1 |
| Thirst, % | 10.3 | 4.5 | |
| Loose stool, % | 9.1 | 5.6 | |
| Dizziness, % range | 3.2–5.1 | 4.5 | |
| Orthostatic hypotension, % | 2.6 | 1.5 | |
| Headache, % range | 2.4–5.5 | 0.9–4.7 | |
| Ejaculatory disorders leading to discontinuation of the study, % range | 1.3–2.9 | 0 |
Tamsulosin [package insert] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc, 2003.
Alfuzosin [package insert] Bridgewater, NJ: Sanofi-Synthelabo, Inc, 2004.
Cho HJ and Yoo TK. Silodosin for the treatment of clinical benign prostatic hyperplasia: safety, efficacy, and patient acceptability. Res Rep Urol 2014; 6: 113–119.
Conversely, the ALF-ONE study group examined the impact of age, cardiovascular comorbidity and antihypertensive co-medication on the tolerability of 10 mg alfuzosin;46 among 6523 enrolled patients, 6.4% withdrew from the study mainly for AEs, while 229 (3.5%) experienced serious AEs. Overall, the most commonly reported AE was dizziness (4.8%), whereas hypotension was uncommon (0.7%). Of note, age, cardiovascular comorbidity and antihypertensive co-medication had no impact on the safety profile of 10 mg alfuzosin.
Similarly, in a large multicenter open-label study, 335 patients with LUTS/BPH were randomized to receive alfuzosin 10 mg alone or alfuzosin 10 mg + antihypertensive combination therapy.47 The authors found no differences in terms of efficacy and safety between the two groups, although in patients with uncontrolled or untreated hypertension, alfuzosin 10 mg alone or in combination with antihypertensive therapy appeared to decrease systolic and diastolic BP.
An interesting study by Park et al.48 assessed the potential association of sympathetic activity status (low versus high), based on HR variability measurements, on safety and efficacy of alfuzosin 10 mg in men with LUTS/BPH; the results of the study showed that sympathetic activity status did not influence both safety and efficacy, as well as quality of life, of alfuzosin.
A potential association between presence or absence of metabolic syndrome and response to alfuzosin therapy has been explored in a recent double-blind, randomized, placebo-controlled study;49 after 12 weeks of treatment, all patients did benefit from therapy, independently from baseline characteristics and presence/absence of metabolic syndrome in particular.
Moreover, α1-blockers have been traditionally related to the onset of so-called intraoperative floppy iris syndrome (IFIS) during cataract surgery with potential influences on postoperative outcomes.50 A well-conducted multicenter study evaluated the effects of tamsulosin or alfuzosin versus no medications (control group) on the development of severe IFIS during ocular surgery.51 Severe IFIS was reported in 34.3% of patients who were taking tamsulosin versus 16.3% of alfuzosin and 4.4% of the control group. These results reflect the high uroselectivity of alfuzosin and its preferential concentration at urinary level, thus reducing peripheral side effects.
Comparison with other alpha-blockers
Six RCTs have assessed efficacy and safety of alfuzosin versus other alpha-blockers ± placebo; in detail, three studies compared alfuzosin with tamsulosin (±placebo), one compared alfuzosin with doxazosin, and two trials compared alfuzosin versus silodosin or tamsulosin. The characteristics and the results of these trials are displayed in Table 4.
Table 4.
Characteristics and results of studies assessing the adverse events and outcomes of alfuzosin versus other drugs for the treatment of benign prostatic hyperplasia.
| Study (Author) | Study type | Duration (weeks) | Treatment (daily dose) | Patients (n) | Change in symptoms (IPSS) (% or mean ± SD) | Change in Qmax (mL/s) | PVR change (mL) | Adverse Events (%) |
|---|---|---|---|---|---|---|---|---|
| Nordlinga | RCT | 12 | Alfuzosin 10 mg Alfuzosin 15 mg Tamsulosin 0.4 mg Placebo |
154 158 158 153 |
−6.5 −6.0 −6.5 −4.6 |
1.5 1.5 1.4 0.5 |
/// | 38% 39% 37% 34% |
| Buzelin et al.b | RCT | 12 | Alfuzosin 2.5 mg TID Tamsulosin 0.4 mg |
115 118 |
−3.8 −4.1 (NS) |
1.6 1.6 (NS) |
/// | 48% 53% (NS) |
| de Reijke et al.c | RCT | 14 | Alfuzosin 10 mg Doxazosin 8 mg |
105 105 |
−7.5 (±0.6) −9.2 (±0.6) (<0.05) |
2.9 (0.5) 3.0 (0.4) (NS) |
9.6 (±8.9) −29.2 (±8.6) (<0.05) |
7.5% 12% (NS) |
| Manohar et al.d | RCT | 12 | Alfuzosin 10 mg Tamsulosin 0.4 mg Silodosin 8 mg |
87 89 93 |
11.43 (±6.19) 11.03 (±5.07) 7.97 (±3.84) (NS) |
15.76 (±2.08) 14.33 (±2.15) 16.15 (±4.81) (NS) |
25.80 (±17.99) 24.42 (±14.73) 25.74 (±15.90) (NS) |
12.6% 17.9% 26.9% |
| Manjunatha et al.e | RCT | 12 | Alfuzosin 10 mg Tamsulosin 0.4 mg Silodosin 8 mg |
30 30 30 |
88.2% 72.1% 82.2% (<0.001) |
2.57 (± 4.25) 2.97 (±3.00) 1.33 (±3.00) (0.170) |
/// | /// |
| Senkul et al.f | Observational Prospective | 12 + 12 | Terazosin 5 mg versus Alfuzosin 10 mg | 40* | 18.6% (NS) 15.1% |
12.5 (%) (NS) 9.4 (%) |
/// | 1 patient in the Terazosin group |
| Lapitan et al.g | RCT | 8 | Tamsulosin 0.2 mg versus Alfuzosin 10 mg | 40 36 |
5.9 (± 5.4) (NS) 6.0 (±6.0) |
1.71 (±2.70) (NS) 0.98 (±2.87) |
/// | 25% (NS) 19.4% |
same individuals were treated with two different agents during different time frames.
Nordling J. Efficacy and safety of two doses (10 and 15 mg) of alfuzosin or tamsulosin (0.4 mg) once daily for treating symptomatic benign prostatic hyperplasia. BJU Int 2005; 95: 1006–1012.
Buzelin JM, Fonteyne E, Kontturi M, et al.; The European Tamsulosin Study Group (see appendix) A. Comparison of tamsulosin with alfuzosin in the treatment of patients with lower urinary tract symptoms suggestive of bladder outlet obstruction (symptomatic benign prostatic hyperplasia). Br J Urol 1997; 80: 597–605.
de Reijke TM and Klarskov P. Comparative efficacy of two $α$1;-adrenoreceptor antagonists, doxazosin and alfuzosin, in patients with lower urinary tract symptoms from benign prostatic enlargement. BJU Int 2004; 93: 757–762.
Manohar CMS, Nagabhushana M, Karthikeyan VS, et al. Safety and efficacy of tamsulosin, alfuzosin or silodosin as monotherapy for LUTS in BPH - a double-blind randomized trial. Cent Eur J Urol 2017; 70: 148–153.
Manjunatha R, Pundarikaksha HP, Madhusudhana HR, et al. A randomized, comparative, open-label study of efficacy and tolerability of alfuzosin, tamsulosin and silodosin in benign prostatic hyperplasia. Indian J Pharmacol 2016; 48: 134–140.
Senkul T, Yilmaz O, Iseri C, et al. Comparing the therapeutic outcome of different alpha-blocker treatments for BPH in the same individuals. Int Urol Nephrol 2008; 40: 663–666.
Lapitan MCM, Acepcion V and Mangubat J. A comparative study on the safety and efficacy of tamsulosin and alfuzosin in the management of symptomatic benign prostatic hyperplasia: a randomized controlled clinical trial. J Int Med Res 2005; 33: 562–573.
IPSS: International Prostate Symptom Score; NS: non significant; PVR: post-voided residual; RCT: randomized clinical trial.
Nordling52 reported the results a multicenter experience including 625 patients who were randomized to receive alfuzosin 10 mg or 15 mg, tamsulosin 0.4 mg or placebo. Alfuzosin (10 mg or 15 mg) significantly improved urinary symptoms and uroflowmetry parameters compared with placebo, similarly to tamsulosin, but with a lower rate of sexual function AEs (placebo, 0%; alfuzosin 10 mg, 3%; 15 mg, 1%; tamsulosin, 8%) compared with tamsulosin. Similar results were reported by Buzelin et al.53 who compared tamsulosin 0.4 mg once daily with oral alfuzosin 2.5 mg TID for 12 weeks in 256 patients; both drugs produced similar improvements in urinary parameters, and the rate of AEs was also comparable. De Reijke and Klarskov54 reported the results of a RCT comparing efficacy and safety of alfuzosin and doxazosin for the treatment of LUTS/BPH; the improvements between the two groups were greater in the doxazosin group in terms of PVR and IPSS scores, while the AEs rate was comparable between the two groups. Conversely, a recent single-center double-blind RCT assessed safety and efficacy of alfuzosin compared with silodosin and tamsulosin;55 the three molecules were found to show similar results in terms of amelioration of urinary symptoms with a similar safety profile.
Alfuzosin: clinical efficacy in combination treatment
Several studies have investigated the clinical efficacy and safety of alpha-blockers variously combined with other classes of drugs active on LUTS/BPH. The underlying rationale is based on the possibility of obtaining a synergistic effect from the combination of drugs with different pharmacodynamic and pharmacokinetic characteristics. In detail, seven RCTs have tested the association of alfuzosin and different classes of drugs, as shown in Table 5.
Table 5.
Clinical characteristics and results of studies assessing the adverse events and outcomes of alfuzosin in combination with other drugs for the treatment of benign prostatic hyperplasia.
| Study (author) | Study type | Duration (weeks) | Treatment (drug and daily dose) | Patients (n) | Change in symptoms (IPSS) (% or mean ± SD) | Change in Qmax (mL/s) | PVR change (mL) | Adverse events (%) |
|---|---|---|---|---|---|---|---|---|
| Alfuzosin and 5-α reductase inhibitors | ||||||||
| Debruyne et al.a | RCT | 26 | Alfuzosin 5 mg BID Finasteride 5 mg Alfuzosin 5 mg BID plus Finasteride 5 mg |
358 344 349 |
−41.2% −33.5% −39.1% (p = 0.009) |
1.8 1.8 2.3 (NS) |
/// | 1.7% 2.9% 2.3% (NS) |
| Alfuzosin and muscarinic receptor antagonists | ||||||||
| Bae et al.b | RCT | 10 | Alfuzosin 10 mg Alfuzosin 10 mg plus Propiverine 10 mg |
77 132 |
−7.8 −8.3 (p = 0.031) |
7.1 5.8 (NS) |
11.2 15.6 (NS) |
3.9% 6.8% (NS) |
| Cho et al.c | RCT | 8 | Alfuzosin 10 mg Alfuzosin 10 mg plus Propiverine 10 mg Alfuzosin 10 mg plus Propiverine 20 mg |
43 48 44 |
−4.67 (±5.83) −5.92 (±7.77) −6.56 (±9.60) (NS) |
0.91 (±6.57) 1.95 (±6.29) 3.03 (±10.18) (NS) |
5.44 (±20.15) 1.56 (±27.3) 26.3 (±72.5) (NS) |
2% 8% 18% (NS) |
| Alfuzosin and PDE5 inhibitors | ||||||||
| Kaplan et al.d | RCT | 12 | Alfuzosin 10 mg Sildenafil 25 mg Alfuzosin 10 mg plus Sildenafil 25 mg |
20 21 21 |
15.6% 16.9% 24.1% (p = 0.03) |
11.7% 6.2% 21.1% |
42.6% 26.1% 39.6% |
/// |
| Liguori et al.e | RCT | 12 | Alfuzosin 10 mg Tadalafil 20 mg Alfuzosin 10 mg plus Tadalafil 20 mg |
22 21 23 |
27.2% 8.4% 41.6% |
21.7% 9.5% 29.6% |
/// | /// |
| Ozturk et al.f | RCT | 12 | Alfuzosin 10 mg Alfuzosin 10 mg plus Sildenafil 50 mg |
50 50 |
26.8% 28.2% (NS) |
29.6% 33% (NS) |
23% 26.2% (NS) |
/// |
| Kumar et al.g | RCT | 12 | Alfuzosin 10 mg Tadalafil 10 mg Alfuzosin 10 mg plus Tadalafil 10 mg |
25 25 25 |
−9.5 (±3.5) −6.3 (±1.5) −12.3 (±2.8) (p < 0.004) |
−3.6 (±1.9) −1.9 (±2) −4.1 (±1.4) |
−22.8 (±24.5) −14.6 (±16.4) −56.1 (±46.2) (p > 0.003) |
/// |
Debruyne FM, Jardin A, Colloi D, et al. Sustained-release alfuzosin, finasteride and the combination of both in the treatment of benign prostatic hyperplasia. European ALFIN study group. Eur Urol 1998; 34: 169–175.
Bae JH, Kim SO, Yoo ES, et al. Efficacy and safety of low-dose propiverine in patients with lower urinary tract symptoms/benign prostatic hyperplasia with storage symptoms: a prospective, randomized, single-blinded and multicenter clinical trial. Korean J Urol 2011; 52: 274–278.
Cho HJ, Shin SC, Seo DY, et al. Comparison of alfuzosin 10 mg with or without propiverine 10 mg, 20 mg in men with lower urinary tract symptom and an overactive bladder: randomised, single-blind, prospective study. Int J Clin Pract 2014; 68: 471–477.
Kaplan SA, Gonzalez RR and Te AE. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol 2007; 51: 1717–1723.
Liguori G, Trombetta C, De Giorgi G, et al. Efficacy and safety of combined oral therapy with tadalafil and alfuzosin: An integrated approach to the management of patients with lower urinary tract symptoms and erectile dysfunction. Preliminary report. J Sex Med 2009; 6: 544–552.
Ozturk MI, Kalkan S, Koca O, et al. Efficacy of alfuzosin and sildenafil combination in male patients with lower urinary tract symptoms. Andrologia 2012; 44: 791–795.
Kumar S, Kondareddy C, Ganesamoni R, et al. Randomized controlled trial to assess the efficacy of the combination therapy of alfuzosin and tadalafil in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Low Urin Tract Symptoms 2014; 6: 35–40.
IPSS: International Prostate Symptom Score; NS: non significant; PVR: post-voided residual; RCT: randomized clinical trial.
The ALFIN study group reported the results of a large multicenter RCTs aiming to compare the efficacy and safety of alfuzosin alone versus finasteride versus combination therapy in males aged between 50 and 75 years with an IPSS >7 and a maximum flow rate between 5 and 15 mL/s.56 According to the results reported after 6 months from randomization, alfuzosin was significantly more effective than finasteride in terms of IPSS changes, with no significant additional benefits derived from the combination of both drugs. Of note, finasteride, alone or in combination, may cause erectile dysfunction. Conversely, it seemed that their association did not cause clinically relevant ejaculation disorders.57
Two RCTs have investigated the results of the association of alfuzosin with a muscarinic receptor antagonist (propiverine) in men that met the symptom criteria for overactive bladder with symptomatic outlet obstruction.58,59 In both studies, the association of alfuzosin with propiverine did not provide additional benefits in terms of IPSS voiding symptom score, QoL, Qmax, and PVR; on the contrary, storage symptoms scores significantly improved in case of combination therapy. These results may suggest the use of antimuscarinic agents in combination with an alpha-blocker in patients with marked irritative LUTS.
Moreover, four RCTs have investigated the effects of the combination of alfuzosin with PDE5-inhibitors (PDE5i), two trials with tadalafil (10 or 20 mg)60,61 and two trials with sildenafil (25 or 50 mg).62,63 In general, the results of these trials have demonstrated additional benefits from the association of alfuzosin with PDE5i in terms of both urinary and sexual symptoms without an observed increase rate of AEs. Therefore, alfuzosin + PDE5i may be a safe and effective option in patients with LUTS/BPH and concomitant erectile dysfunction also thanks to the aforementioned uroselectivity of alfuzosin and its low rate of sexual disorders.
Conclusion
Alfuzosin is a safe and effective drug for the treatment of LUTS/BPH, as proven by several clinical trials. Compared with other alpha-blockers, mostly thanks to its high concentration in the prostatic tissue, alfuzosin seems to be associated with a lower rate of sexual dysfunction. Moreover, in terms of safety profile, alfuzosin has been found to be safe also in the case of concomitant antihypertensive therapy and in patients with cardiovascular morbidity. Furthermore, safety and efficacy of alfuzosin has been reported also in case of combination therapy with antimuscarinic agents and PDE5i.
Supplemental Material
Supplemental material, sj-pdf-1-tau-10.1177_1756287221993283 for Alfuzosin for the medical treatment of benign prostatic hyperplasia and lower urinary tract symptoms: a systematic review of the literature and narrative synthesis by Andrea Mari, Alessandro Antonelli, Luca Cindolo, Ferdinando Fusco, Andrea Minervini and Cosimo De Nunzio in Therapeutic Advances in Urology
Footnotes
Contribution: Conception and Design: Mari, Antonelli, Cindolo, Fusco, Minervini, De Nunzio.
Acquisition of data: Mari, Antonelli, Minervini.
Analysis and interpretation of data: Mari, De Nunzio.
Drafting of the manuscript: Mari, Cindolo, Fusco, Minervini
Critical revision of the manuscript for important intellectual content: Antonelli, Cindolo, Fusco, Minervini, De Nunzio
Statistical analysis: NA
Obtaining funding: Antonelli, Cindolo, Fusco, Minervini, De Nunzio
Administrative, technical, or material support: Mari, Antonelli, Cindolo, Fusco, Minervini, De Nunzio
Supervision: De Nunzio
Conflict of interest statement: Ferdinando Fusco: Speaker at symposiums and seminaries for Recordati, Astellas, Glaxo Smith Kline. Cosimo de Nunzio: Consultancy for Pierrefabre, Janssen, Sanofi, Glaxo Smith Kline. Other authors have no additional conflict of interest to declare.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Editorial support in the preparation of this publication was provided and funded by Sanofi. Sanofi’s review of this article has been strictly limited to all references to alfuzosine to enhance consistency within the current labeling of the product and its appropriate use. The authors, individually and collectively, are responsible for all content and editorial decisions.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Andrea Mari  https://orcid.org/0000-0001-9070-5706
https://orcid.org/0000-0001-9070-5706
Contributor Information
Andrea Mari, Department of Urology, University of Florence, Careggi Hospital, San Luca Nuovo, Florence, Italy; Unit of Oncologic Minimally-Invasive Urology and Andrology, Careggi University Hospital, Florence, Italy; Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Alessandro Antonelli, Department of Urology, Azienda Ospedaliera Universitaria Integrata Verona, University of Verona, Italy.
Luca Cindolo, Department of Urology, Villa Stuart Private Hospital, Rome, Italy; “Cure Group”, Hesperia Hospital, Modena, Italy.
Ferdinando Fusco, Department of Woman, Child and General and Specialized Surgery University of Campania “Luigi Vanvitelli”, Naples, Italy.
Andrea Minervini, Unit of Oncologic Minimally-Invasive Urology and Andrology, Careggi University Hospital, Florence, Italy; Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Cosimo De Nunzio, Division of Urology, Ospedale Sant’Andrea, Sapienza University of Rome, Rome, Italy.
References
- 1. Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res 2008; 20(Suppl. 3): S11–S18. [DOI] [PubMed] [Google Scholar]
- 2. Lim KB. Epidemiology of clinical benign prostatic hyperplasia. Asian J Urol 2017; 4: 148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiong Y, Zhang Y, Li X, et al. The prevalence and associated factors of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging males. Aging Male. Epub ahead of print 25 June 2020. DOI: 10.1080/13685538.2020.1781806. [DOI] [PubMed] [Google Scholar]
- 4. Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2015; 67: 1099–1109. [DOI] [PubMed] [Google Scholar]
- 5. Speakman M, Kirby R, Doyle S, et al. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) – focus on the UK. BJU Int 2015; 115: 508–519. [DOI] [PubMed] [Google Scholar]
- 6. Martin S, Lange K, Haren MT, et al. Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. J Urol 2014; 191: 130–137. [DOI] [PubMed] [Google Scholar]
- 7. Fitzpatrick JM. The natural history of benign prostatic hyperplasia. BJU Int 2006; 97(Suppl. 2): 2–3. [DOI] [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011. [Google Scholar]
- 10. Murad MH, Asi N, Alsawas M, et al. New evidence pyramid. Evid Based Med 2016; 21: 125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilde MI, Fitton A, McTavish D., Alfuzosin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in benign prostatic hyperplasia. Drugs 1993; 45: 410–429. [DOI] [PubMed] [Google Scholar]
- 12. McKeage K, Plosker GL. Alfuzosin: a review of the therapeutic use of the prolonged-release formulation given once daily in the management of benign prostatic hyperplasia. Drugs 2002; 62: 633–653. [DOI] [PubMed] [Google Scholar]
- 13. Graham RM, Perez DM, Hwa J, et al. Alpha 1-adrenergic receptor subtypes. Molecular structure, function, and signaling. Circ Res 1996; 78: 737–749. [DOI] [PubMed] [Google Scholar]
- 14. Nasu K, Moriyama N, Kawabe K, et al. Quantification and distribution of alpha 1-adrenoceptor subtype mRNAs in human prostate: comparison of benign hypertrophied tissue and non-hypertrophied tissue. Br J Pharmacol 1996; 119: 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Debruyne FM. Alpha blockers: are all created equal? Urology 2000; 56: 20–22. [DOI] [PubMed] [Google Scholar]
- 16. Martin DJ, Lluel P, Guillot E, et al. Comparative alpha-1 adrenoceptor subtype selectivity and functional uroselectivity of alpha-1 adrenoceptor antagonists. J Pharmacol Exp Ther 1997; 282: 228–235. [PubMed] [Google Scholar]
- 17. Lefevre-Borg F, O’Connor SE, Schoemaker H, et al. Alfuzosin, a selective alpha 1-adrenoceptor antagonist in the lower urinary tract. Br J Pharmacol 1993; 109: 1282–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eckert RE, Utz J, Alloussi S, et al. Prostate selectivity of alpha 1 adrenoceptor blockers. J Urol 1999; 161: 233. [Google Scholar]
- 19. Mottet N, Bressolle F, Delmas V, et al. Prostatic tissual distribution of alfuzosin in patients with benign prostatic hyperplasia following repeated oral administration. Eur Urol 2003; 44: 101–105. [DOI] [PubMed] [Google Scholar]
- 20. U.S. Food and Drug Administration. UROXATRAL® (alfuzosin HCl extended-release tablets). Drug Approval Package, Sanofi-Synthelabo Inc., 2003. [Google Scholar]
- 21. Alfuzosin Hydrochloride ER. FDA prescribing information, side effects and uses. Pulaski, TN: AvKARE, Inc., 2019. [Google Scholar]
- 22. Medical Economics. Physicians desk reference. 58th ed. Montvale, NJ: Thomson PDR, 2004, p. 3036. [Google Scholar]
- 23. Doligalski CT, Tong Logan A, Silverman A. Drug interactions: a primer for the gastroenterologist. Gastroenterol Hepatol (N Y) 2012; 8: 376–383. [PMC free article] [PubMed] [Google Scholar]
- 24. Pinquier JL, Fuder H, Amersdorffer J, et al. Safety and pharmacokinetics of alfuzosin 10 mg once daily formulation comparatively to standard formulation (2.5 mg tid) in elderly subjects. Clin Pharmacol Ther 1999; 65: 202. [Google Scholar]
- 25. Pinquier J-L, Rauch-Desanti C, Miller RP, et al. Effect of renal impairment on the pharmacokinetics and safety of alfuzosin 10 mg OD formulation. Clin Pharmacol Ther 2000; 67: 111. [Google Scholar]
- 26. Jardin A, Bensadoun H, Delauche-Cavallier MC, et al. Alfuzosin for treatment of benign prostatic hypertrophy. Lancet 1991; 337: 1457–1461. [DOI] [PubMed] [Google Scholar]
- 27. Buzelin JM, Roth S, Geffriaud-Ricouard C, et al. Efficacy and safety of sustained-release alfuzosin 5 mg in patients with benign prostatic hyperplasia. ALGEBI study group. Eur Urol 1997; 31: 190–198. [DOI] [PubMed] [Google Scholar]
- 28. van Kerrebroeck P, Jardin A, Laval KU, et al. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI study group. Eur Urol 2000; 37: 306–313. [DOI] [PubMed] [Google Scholar]
- 29. Lukacs B, Grange JC, Comet D. One-year follow-up of 2829 patients with moderate to severe lower urinary tract symptoms treated with alfuzosin in general practice according to IPSS and a health-related quality-of-life questionnaire. BPM group in general practice. Urology 2000; 55: 540–546. [DOI] [PubMed] [Google Scholar]
- 30. Lukacs B, Grange JC, Comet D, et al. History of 7093 patients with lower urinary tract symptoms related to benign prostatic hyperplasia treated with alfuzosin in general practice up to 3 years. Eur Urol 2000; 37: 183–190. [DOI] [PubMed] [Google Scholar]
- 31. Elhilali M, Emberton M, Matzkin H, et al. Long-term efficacy and safety of alfuzosin 10 mg once daily: a 2-year experience in “real-life” practice. BJU Int 2006; 97: 513–519. [DOI] [PubMed] [Google Scholar]
- 32. Roehrborn CG. Safety and efficacy of alfuzosin 10 mg once-daily in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a pooled analysis of three double-blind, placebo-controlled studies – Reply. BJU Int 2003; 92: 1044–1045. [DOI] [PubMed] [Google Scholar]
- 33. Vallancien G, Emberton M, Alcaraz A, et al. Alfuzosin 10 mg once daily for treating benign prostatic hyperplasia: a 3-year experience in real-life practice. BJU Int 2008; 101: 847–852. [DOI] [PubMed] [Google Scholar]
- 34. Van Moorselaar RJA, Hartung R, Emberton M, et al. Alfuzosin 10 mg once daily improves sexual function in men with lower urinary tract symptoms and concomitant sexual dysfunction. BJU Int 2005; 95: 603–608. [DOI] [PubMed] [Google Scholar]
- 35. Rosen R, Seftel A, Roehrborn CG. Effects of alfuzosin 10 mg once daily on sexual function in men treated for symptomatic benign prostatic hyperplasia. Int J Impot Res 2007; 19: 480–485. [DOI] [PubMed] [Google Scholar]
- 36. Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol 2003; 44: 637–649. [DOI] [PubMed] [Google Scholar]
- 37. Becker AJ, Uckert S, Stief CG, et al. Cavernous and systemic plasma levels of norepinephrine and epinephrine during different penile conditions in healthy men and patients with erectile dysfunction. Urology 2002; 59: 281–286. [DOI] [PubMed] [Google Scholar]
- 38. Giuliano F, Bernabe J, Droupy S, et al. A comparison of the effects of tamsulosin and alfuzosin on neurally evoked increases in bladder neck and seminal vesicle pressure in rats. BJU Int 2004; 93: 605–608. [DOI] [PubMed] [Google Scholar]
- 39. Safety, efficacy and impact on patients’ quality of life of a long-term treatment with the alpha(1)-blocker alfuzosin in symptomatic patients with BPH. The Italian Alfuzosin co-operative group. Eur Urol 2000; 37: 680–686. [DOI] [PubMed] [Google Scholar]
- 40. Sanchez-Chapado M, Guil M, Badiella LI, et al. The clinical uroselectivity of alfuzosin is not significantly affected by the age of patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. BJU Int 2000; 86: 432–438. [DOI] [PubMed] [Google Scholar]
- 41. Sanchez-Chapado M, Guil M, Alfaro V, et al. Safety and efficacy of sustained-release alfuzosin on lower urinary tract symptoms suggestive of benign prostatic hyperplasia in 3,095 Spanish patients evaluated during general practice. Eur Urol 2000; 37: 421–427. [DOI] [PubMed] [Google Scholar]
- 42. van Kerrebroec P, Jardin A, van Cangh P, et al. Long-term safety and efficacy of a once-daily formulation of alfuzosin 10 mg in patients with symptomatic benign prostatic hyperplasia: open-label extension study. Eur Urol 2002; 41: 51–54. [DOI] [PubMed] [Google Scholar]
- 43. McVary KT. Alfuzosin for symptomatic benign prostatic hyperplasia: long-term experience. J Urol 2006; 175: 35–42. [DOI] [PubMed] [Google Scholar]
- 44. Wilt TJ, Macdonald R, Rutks I. Alfuzosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. Epub ahead of print 29 June 2013. DOI: 10.1002/14651858.CD002289.pub2. [DOI] [Google Scholar]
- 45. Mondaini N, Giubilei G, Ungar A, et al. Alfuzosin (10 mg) does not affect blood pressure in young healthy men. Eur Urol 2006; 50: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 46. Hartung R, Matzkin H, Alcaraz A, et al. Age, comorbidity and hypertensive co-medication do not affect cardiovascular tolerability of 10 mg alfuzosin once daily. J Urol 2006; 175: 624–628. [DOI] [PubMed] [Google Scholar]
- 47. Zhang LT, Lee SW, Park K, et al. Multicenter, prospective, comparative cohort study evaluating the efficacy and safety of alfuzosin 10 mg with regard to blood pressure in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia with or without antihypertensive med. Clin Interv Aging 2015; 10: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park SG, Chung BH, Lee SW, et al. Alpha-blocker treatment response in men with lower urinary tract symptoms based on sympathetic activity: prospective, multicenter, open-labeled, observational study. Int Neurourol J 2015; 19: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Altin S, Ozan T, Ilhan S, et al. Metabolic syndrome does not impair the response to alfuzosin treatment in men with lower urinary tract symptoms: a double-blind, randomized, placebo-controlled study. Turkish J Urol 2015; 41: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Flach AJ. Intraoperative floppy iris syndrome: pathophysiology, prevention, and treatment. Trans Am Ophthalmol Soc 2009; 107: 234–239. [PMC free article] [PubMed] [Google Scholar]
- 51. Chang DF, Campbell JR, Colin J, et al. Prospective masked comparison of intraoperative floppy iris syndrome severity with tamsulosin versus alfuzosin. Ophthalmology 2014; 121: 829–834. [DOI] [PubMed] [Google Scholar]
- 52. Nordling J. Efficacy and safety of two doses (10 and 15 mg) of alfuzosin or tamsulosin (0.4 mg) once daily for treating symptomatic benign prostatic hyperplasia. BJU Int 2005; 95: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 53. Buzelin JM, Fonteyne E, Kontturi M, et al.; The European Tamsulosin study group (see appendix) A. Comparison of tamsulosin with alfuzosin in the treatment of patients with lower urinary tract symptoms suggestive of bladder outlet obstruction (symptomatic benign prostatic hyperplasia). Br J Urol 1997; 80: 597–605. [DOI] [PubMed] [Google Scholar]
- 54. de Reijke TM, Klarskov P. Comparative efficacy of two α1;-adrenoreceptor antagonists, doxazosin and alfuzosin, in patients with lower urinary tract symptoms from benign prostatic enlargement. BJU Int 2004; 93: 757–762. [DOI] [PubMed] [Google Scholar]
- 55. Manohar CMS, Nagabhushana M, Karthikeyan VS, et al. Safety and efficacy of tamsulosin, alfuzosin or silodosin as monotherapy for LUTS in BPH - a double-blind randomized trial. Cent Eur J Urol 2017; 70: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Debruyne FM, Jardin A, Colloi D, et al. Sustained-release alfuzosin, finasteride and the combination of both in the treatment of benign prostatic hyperplasia. European ALFIN Study Group. Eur Urol 1998; 34: 169–175. [DOI] [PubMed] [Google Scholar]
- 57. Siddique MS, Bashar S, Ahmed F. Efficacy and safety of combine alfuzosin and finasteride in the treatment of symptomatic benign prostatic hyperplasia: a quasi-experimental study. Chattagram Maa-O-Shishu Hosp Med Coll J 2018; 16: 35–39. [Google Scholar]
- 58. Bae JH, Kim SO, Yoo ES, et al. Efficacy and safety of low-dose propiverine in patients with lower urinary tract symptoms/benign prostatic hyperplasia with storage symptoms: a prospective, randomized, single-blinded and multicenter clinical trial. Korean J Urol 2011; 52: 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cho HJ, Shin SC, Seo DY, et al. Comparison of alfuzosin 10 mg with or without propiverine 10 mg, 20 mg in men with lower urinary tract symptom and an overactive bladder: Randomised, single-blind, prospective study. Int J Clin Pract 2014; 68: 471–477. [DOI] [PubMed] [Google Scholar]
- 60. Liguori G, Trombetta C, de Giorgi G, et al. Efficacy and safety of combined oral therapy with tadalafil and alfuzosin: an integrated approach to the management of patients with lower urinary tract symptoms and erectile dysfunction. Preliminary report. J Sex Med 2009; 6: 544–552. [DOI] [PubMed] [Google Scholar]
- 61. Kumar S, Kondareddy C, Ganesamoni R, et al. Randomized controlled trial to assess the efficacy of the combination therapy of alfuzosin and tadalafil in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Low Urin Tract Symptoms 2014; 6: 35–40. [DOI] [PubMed] [Google Scholar]
- 62. Ozturk MI, Kalkan S, Koca O, et al. Efficacy of alfuzosin and sildenafil combination in male patients with lower urinary tract symptoms. Andrologia 2012; 44: 791–795. [DOI] [PubMed] [Google Scholar]
- 63. Kaplan SA, Gonzalez RR, Te AE. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol 2007; 51: 1717–1723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tau-10.1177_1756287221993283 for Alfuzosin for the medical treatment of benign prostatic hyperplasia and lower urinary tract symptoms: a systematic review of the literature and narrative synthesis by Andrea Mari, Alessandro Antonelli, Luca Cindolo, Ferdinando Fusco, Andrea Minervini and Cosimo De Nunzio in Therapeutic Advances in Urology