Abstract
Background
The risk of transmission of Coronavirus Disease 2019 (COVID-19) is increasingly understood to be greatest early after symptom onset, however, factors associated with prolonged and increased risk of transmission remain unclear. In settings where COVID-19 prevalence is low, there may be a benefit of extending the period that patients are isolated to decrease the risk of transmission. This study explored the duration of viral shedding in such a location, in patients with mild-moderate COVID-19 disease in Ballarat, Australia.
Methods
Patients diagnosed with COVID-19 disease using a real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay from oropharyngeal and bilateral deep nasopharyngeal sampling and managed through Ballarat Health Services between March 1 and May 1, 2020 were included. Patients were retested if they were afebrile for >72 hours, asymptomatic and >14 days since symptom onset. If positive on retesting, patients were tested every 3 to 7 days thereafter.
Results
Patients underwent testing a median of 4 days (range 1-12) after initial symptom onset. Duration of symptoms ranged from 1 to 36 days. Positive tests were recorded up to a median of day 21 (range 6-38). Cycle thresholds were inversely correlated with time since symptom onset (P < .0001). Median time to the first negative test was 25 days (range 12-32). Two patients who had remained asymptomatic for >7 days after initial symptom onset had recrudescence of mild symptoms on day 13 and 14; both tested positive on follow-up tests at this time.
Conclusions
This study demonstrates prolonged shedding of COVID-19 in patients with mild-moderate disease. It suggests that some patients with mild disease may have recrudescence of symptoms a week or more after their initial symptoms resolved.
Keywords: COVID-19, SARS-CoV-2, shedding, Australia, mild
Introduction
Since its identification in Wuhan, China in December 2019, the coronavirus disease-2019 (COVID-19) pandemic has led to an unprecedented global shutdown, infecting at least 49.7 million people and causing at least 1.2 million deaths as of November 8, 2020.1,2 The causative organism, SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2), causes a spectrum of disease severity, from no symptoms to severe disease including death.3 Symptoms commonly include fever, cough, dyspnoea, sore throat, myalgia, and anosmia. The duration that patients with COVID-19 should isolate prior to recommencing usual social activities needs to be better understood, to balance the risk of transmission with the individual’s mental health and return to work.
Substantial variability in viral shedding has been reported. Severe disease has a higher viral peak and longer duration of shedding.4 The amount of viral RNA detected is highest in the initial week of symptoms; but shedding has been reported to occur for months after symptom onset and to persist after symptom cessation.5-7
Although the risk of transmission is increasingly understood to be greatest early after symptom onset, in settings such as regional locations with no community transmission, consideration may be given to extending the period that patients are isolated. This retrospective cohort study aimed to explore the duration of viral shedding in laboratory-confirmed COVID-19 patients with mild to moderate disease from the regional town of Ballarat, Australia and to investigate associations with clinical symptoms.
Methods
Patients were included if they were diagnosed with COVID-19 disease using a positive real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) SARS-CoV-2 assay from any clinical specimen and were managed through Ballarat Health Services (BHS) between March 1, 2020 and May 1, 2020. BHS is located in Ballarat, Australia; a regional town of 110 000 people. It was the sole hospital in the town managing COVID-19 patients during this period.
Diagnosis of SARS-CoV-2 was undertaken using a single swab with oropharyngeal and bilateral deep nasopharyngeal sampling. Testing was performed at Dorevitch Pathology (Heidelberg, Australia). The RT-PCR methods for SARS-CoV-2 confirmation have been described elsewhere.8,9 In brief, from March 1 to April 17, RT-PCR was performed using the TIB MolBiol LightMix® Modular E-gene kit as the sole assay. From April 17 onwards, samples were also analyzed using the Roche cobas 6800 SARS-CoV-2 test with targets for both the Sarbecovirus E gene and also the SARS-CoV-2 ORF1. Positive results at a high cycle threshold (Ct) (>34) were quality checked by ensuring an adequate sigmoidal curve was obtained and there were no strong positives in adjacent wells. Samples which were solely positive for the E gene were referred for confirmatory testing at the Victorian Infectious Diseases Reference Laboratory.
Patients who tested positive for SARS-CoV-2 were admitted as inpatients or managed in the community through the Hospital in the Home (HITH) program. Patients in HITH received daily phone calls for symptom monitoring and reported their self-checked temperature and oxygen saturations. Patients were retested for SARS-CoV-2 if they fulfilled the following criteria: afebrile for more than 72 hours, resolution of clinical symptoms other than a dry cough and 14 days or more since symptom onset. If positive on retesting, patients were tested every 3 to 7 days thereafter.
Analysis was undertaken using Stata version 15. Categorical variables were described by frequency and numerical variables described by median and range. The study was approved by the BHS and St John of God Ballarat Ethics Committee (LNR/62865/BHSSJOG-2020-215161(v1)). A requirement for patient consent was waived due to the retrospective nature of this study.
Results
Twelve patients tested positive to SARS-CoV-2 on nasopharyngeal or combined oropharyngeal and deep nasal swabs during the study and were managed by BHS. One of the patients was initially diagnosed by their local medical practitioner before subsequently undergoing repeat testing through BHS. Of the other 11 patients, 2 patients were admitted to hospital with moderate disease and 9 patients were managed through HITH with mild disease. One of the patients with moderate disease was readmitted to hospital after initial discharge with HITH. Patients had a median age of 43.5 years (range 22-76) and 50% were female. All patients were returned travelers with presumed infection overseas or on cruise ships. All patients recovered.
Patients underwent testing a median of 4 days (range 1-12) after initial symptom onset with Cts varying from 13 to 29 (median 21; Figure 1). The most common symptoms at presentation were cough (12/12, 100%), fever (7/12, 58.3%), dyspnoea (5/12, 41.6%), and coryza (4/12, 33.3%). The duration of symptoms ranged from 1 to 36 days.
Figure 1.
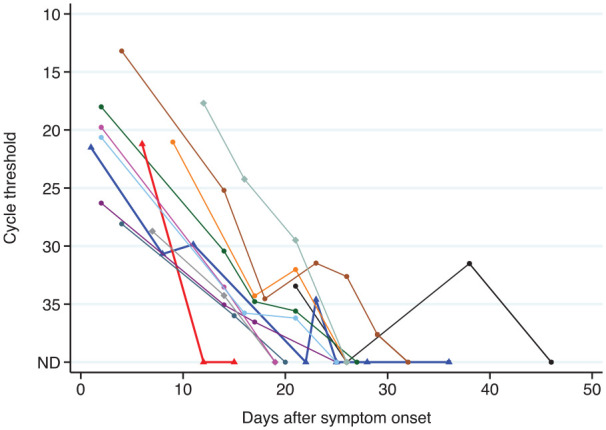
Cycle threshold compared to number of days since symptom onset.
Patients with moderate disease are demonstrated by bold lines with triangles. The blue line with triangles and the black line with circles represent the patients who tested positive again following a negative follow up test. The lines with diamonds represent patients who had recrudescence of symptoms after remaining symptom-free for at least 7 days. Tests performed using the Roche cobas 6800 SARS-CoV-2 test are shown by squares. All other tests were performed using the TIB MolBiol LightMix® Modular E-gene kit.
Repeat testing was undertaken in all patients with a median of 4 (range 3-8) follow-up tests per patient. The first follow-up test occurred at a median of day 14 (range 8-21) after symptom onset, with 92% (11/12) of patients positive at this time. The only patient with a negative first follow up test had moderate disease (Ct = 21.21). Positive tests were recorded up to a median of day 21 (range 6-38) with 58% (7/12) of patients still having a positive test at day 21 or later. The median Ct value at the last day these 7 patients tested positive was 34.62 (range 29.49-37.62). Cts were inversely correlated with time since symptom onset (P < .0001; Figure 1).
The median time to the first negative test was 25 days (range 12-32). Two patients had a first negative test (on day 22 and day 26) before returning positive tests after this (on day 23 [Ct = 34.62] and 38 [Ct = 31.51], respectively). Subsequent tests in these patients were negative. The patient with a positive test at day 38 had a single episode of small volume hemoptysis following exercise the day prior.
Two patients who had remained asymptomatic for more than 7 days after their initial symptom onset (one with anosmia and lethargy only) had recrudescence of very mild symptoms on day 13 and 14 (sore throat and dry cough); both tested positive on follow-up tests on days 12 and 14, respectively. The Ct values of these patients at this time was 17.69 and 34.62, respectively. All patients had isolated on symptom onset or prior to this, on return to the Ballarat region from travel, and there was no evidence of onward transmission from the patients in our study.
Discussion
The current study highlights the prolonged persistence of viral RNA in COVID-19 patients with mild to moderate disease severity, lasting a median of 3 weeks after symptom onset. In addition, it demonstrates that in patients with mild disease, symptoms can potentially recrudesce a week or more after initial symptoms have resolved.
Prolonged detection of viral RNA has been demonstrated in previous studies, although this had been predominantly described in patients with severe disease.6,10 A study of patients with severe disease found positive tests to persist for a median of 31 days.6 A further study of patients with mixed severity had a shorter duration of 20 days detection, with patients who died having persistently positive tests.10 A study of patients with mild disease found viral RNA was rarely detected in upper respiratory tract specimens after day 14 but was more persistent in sputum.5 In contrast, patients in the current study had predominantly mild disease with over half still testing positive at day 21. This study correlates with a recent study from South Korea, that found symptomatic patients treated in the community had 20 days of detection after diagnosis.7
The duration of viral RNA shedding does not necessarily equate to risk of transmission. Studies culturing SARS-CoV-2 have generally been unable to culture live virus beyond day 8 or 9 from symptom onset.5,11,12 In addition, a study of 100 patients from Taiwan that actively followed 2 761 contacts identified transmission only in contacts that were exposed to infected patients during the first 5 days of their symptoms.13 As described previously,5 Ct values in the current study rose rapidly after symptom onset, consistent with increased viral RNA being present early in the illness which may correlate with an increased risk of transmission at this time. This is supported by a number of studies being unable to culture live virus when Ct levels are high.11,12,14
The clinical progress of patients in this study highlights 2 aspects of clinical importance. Two patients had negative tests on follow up, before returning positive tests at later timepoints. This likely reflects low levels of persistent viral RNA, with fluctuation around the limit of detection of the assay, or variation related to differences in specimen collection or transport time. A further 2 patients had asymptomatic periods for at least 7 days before developing symptoms again. Although they were not tested for additional respiratory infections, their isolation over the intervening period makes it unlikely these symptoms were caused by a second virus. Furthermore, secondary bacterial infection was considered unlikely as they did not describe fever, dyspnoea, or significant clinical deterioration.
The current study was limited in its ability to describe the duration of viral RNA shedding due to its small sample size, observational nature preventing daily sample collection and varied laboratory testing methodologies. A lack of viral culture prevents additional conclusions being made about the presence of live virus.
In summary, the current study demonstrates prolonged shedding of SARS-CoV-2 in patients with mild to moderate disease, with a rise in Ct values over time. It remains unclear whether patients in high risk settings should be isolated for a longer period. Isolation is a significant burden on individuals, workplaces, and the healthcare system. Future studies that further evaluate live virus or actively assess contacts are needed to better identify measures that can be used to safely release isolation requirements, including quantitative PCR levels or symptom duration. Furthermore, additional knowledge is required about whether possible symptom recrudescence influences transmission risk.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RJC is supported by an Australian NHMRC Emerging Leader Investigator Grant (1194702).
Declaration of conflicting interest:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Robert J Commons  https://orcid.org/0000-0002-3359-5632
https://orcid.org/0000-0002-3359-5632
References
- 1. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for Global Health Governance. JAMA. 2020;323:709-710. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. COVID-19 Weekly Epidemiological Update – 10 November 2020. World Health Organization; 2020. [Google Scholar]
- 3. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel Coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly. 2020;2:113-122. [PMC free article] [PubMed] [Google Scholar]
- 4. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465-469. [DOI] [PubMed] [Google Scholar]
- 6. Zhou B, She J, Wang Y, Ma X. The duration of viral shedding of discharged patients with severe COVID-19. Clin Infect Dis. 2020;71:2240-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea. JAMA Intern Med. 2020;180:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yip CC, Sridhar S, Cheng AK, et al. Evaluation of the commercially available LightMix(R) Modular E-gene kit using clinical and proficiency testing specimens for SARS-CoV-2 detection. J Clin Virol. 2020;129:104476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roche. Cobas® SARS-CoV-2 Test. 2020. Accessed July 24, 2020. https://diagnostics.roche.com/global/en/products/params/cobas-sars-cov-2-test.html
- 10. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bullard J, Dust K, Funk D, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020;71:2663-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19. Updated July 22, 2020. Accessed September 14, 2020. https://www.cdc.gov/coronavirus/2019-ncov/community/strategy-discontinue-isolation.html
- 13. Cheng HY, Jian SW, Liu DP, et al. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Million M, Lagier JC, Gautret P, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35:101738. [DOI] [PMC free article] [PubMed] [Google Scholar]


