Abstract
Background
Treatment options for nontuberculous mycobacteria (NTM) infections are limited by the pathogen’s intrinsic resistance profile and toxicities. Tedizolid and linezolid display in vitro activity against NTM species. However, safety data and treatment outcomes are limited in the solid organ transplant (SOT) population.
Methods
This was a single-center retrospective cohort study of adult SOT recipients receiving linezolid or tedizolid for an NTM infection from January 1, 2010, to August 31, 2019. The primary outcome compared the hematologic safety profiles of tedizolid vs linezolid. We also described nonhematological adverse drug events (ADEs) and therapy discontinuation rates. In an exploratory analysis, we assessed symptomatic microbiologic and clinical outcomes in those receiving tedizolid or linezolid for at least 4 weeks.
Results
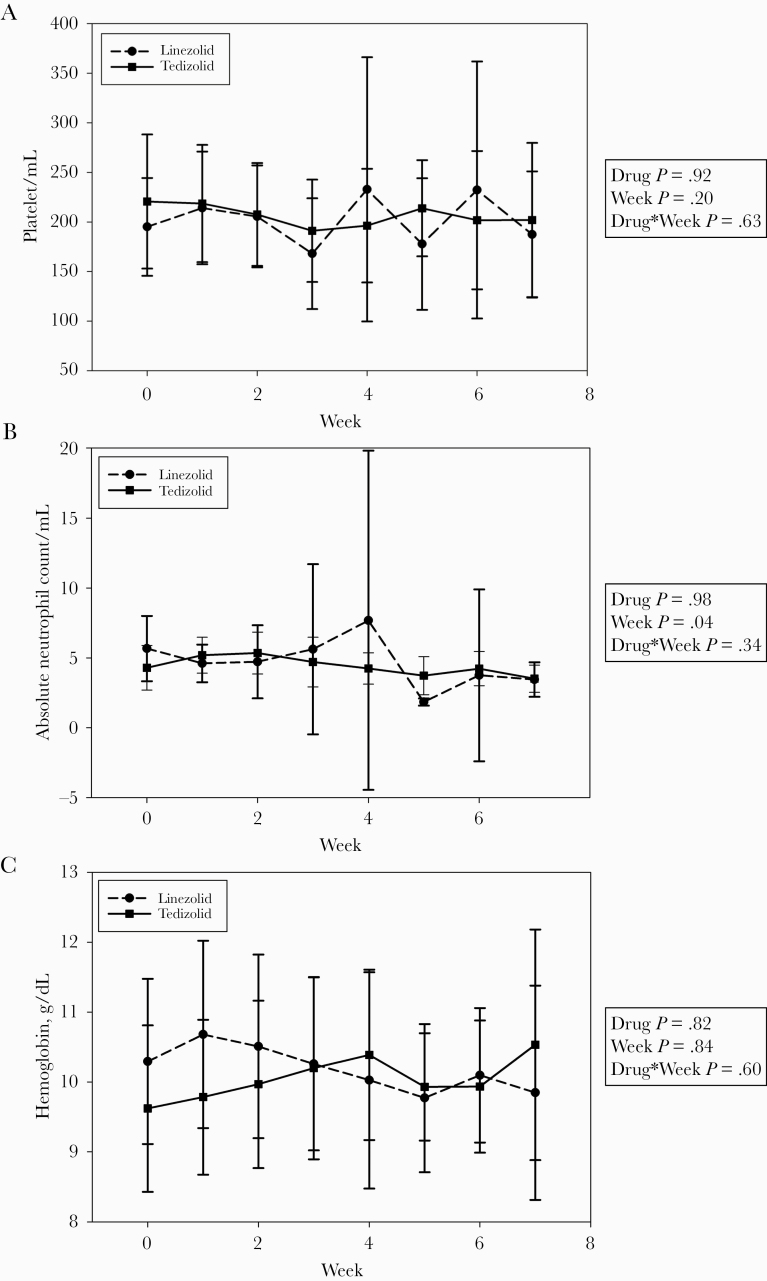
Twenty-four patients were included (15 tedizolid, 9 linezolid). No differences were identified comparing the effects of tedizolid vs linezolid on platelet counts, absolute neutrophil counts (ANCs), and hemoglobin over 7 weeks using mixed-effects analysis of variance models. ANC was significantly decreased in both groups after 7 weeks of therapy (P = .04). Approximately 20% of patients in each arm discontinued therapy due to an ADE. Seven of 12 (58%) and 2 of 3 (67%) patients were cured or clinically cured with tedizolid- and linezolid-containing regimens, respectively.
Conclusions
This study suggests no significant safety benefit of tedizolid over linezolid for the treatment of NTM infections in SOT recipients. Tedizolid or linezolid-containing regimens demonstrated a potential benefit in symptomatic and microbiologic improvement. Larger cohorts are needed to further delineate the comparative role of linezolid and tedizolid for the treatment of NTM infections in SOT recipients.
Keywords: linezolid, Mycobacterium abscessus, nontuberculous mycobacteria, tedizolid
Solid organ transplant (SOT) recipients are at an increased risk for nontuberculous mycobacteria infection due to impaired cell-mediated immunity. Approximately 25 out of 140 nontuberculous mycobacteria (NTM) species identified have been reported to cause infection in SOT patients [1]. These infections are frequently manifested as cutaneous, pleuropulmonary, or disseminated diseases and are often dependent on type of transplant [2]. Although infections caused by NTM are rare, less than half of SOT recipients achieve complete resolution of the infection [3]. Depending on source and species, a triad of interventions, including surgical resection, antimicrobial therapy, and reduction of immunosuppression, may be needed to optimize outcomes [4].
Antimicrobial options for NTM bacteria are limited due to the inherent resistance mechanisms present in these organisms. Furthermore, patients often require several months of treatment, which introduces higher risks for adverse drug events (ADEs). Limited well-designed studies exist to guide optimal therapy for NTM infections; therefore, current guidelines base the selection and length of antimicrobials for SOT recipients on case series and case reports [4].
The oxazolidinones linezolid and tedizolid have in vitro activity against many NTM species [5–7]. The use of these agents is often limited by time-dependent intolerances. Known ADEs of linezolid associated with prolonged durations (>2 weeks) include thrombocytopenia, anemia, neutropenia, and peripheral neuropathy [8, 9]. Tedizolid, approved by the Food and Drug Administration (FDA) 4 years after linezolid’s approval, appears to have a lower incidence rate of hematological (1.3% vs 3.7% for thrombocytopenia, 0.5% vs 0.6% for neutropenia, and 3.1% vs 3.7% for anemia) and neurological toxicities and fewer drug–drug interactions based on initial studies [10–14]. However, tedizolid was initially studied for infections requiring short durations of therapy; therefore, the toxicity associated with prolonged exposures is not well defined. Recent data suggest that the rates of thrombocytopenia in patients taking tedizolid or linezolid were similar (2.4% to 2.7%) based on the FDA adverse event reporting system [15].
With the increasing incidence of NTM infections, it is important to optimize patient tolerability and adherence to therapy [16]. Evaluating the risks vs benefits of linezolid compared with tedizolid for prolonged durations of therapy, especially in a population possessing baseline risks for cytopenias, will help guide safe antimicrobial therapy [17]. The purpose of the study was to compare the safety and tolerability of tedizolid and linezolid throughout the treatment course and to describe the microbiological and clinical outcomes of tedizolid- or linezolid-containing regimens for the treatment of NTM in SOT recipients.
METHODS
This was a single-center, retrospective cohort study in adult SOT recipients (≥18 years of age) who received at least 1 dose of tedizolid or linezolid as part of a multidrug regimen for the treatment of an NTM infection from January 1, 2010, to August 31, 2019. Study subjects were identified using an electronic health record registry [18, 19]. Patients with the following criteria were excluded from the study: absence of at least 1 complete blood count during receipt of tedizolid or linezolid, prior documentation of serious bleeding complications or disseminated intravascular coagulation within 90 days of tedizolid or linezolid initiation, or >72 hours of documented therapy interruption due to medication nonadherence. The study was approved by the University of Texas Southwestern Institutional Review Board.
The primary outcome was the hematologic effects of tedizolid and linezolid from initiation to week 7 of therapy. The time period was chosen based on the median duration of tedizolid therapy. Comparisons of baseline characteristics between tedizolid and linezolid were performed using the Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. A mixed-effects analysis of variance (ANOVA) model was used to assess the effects of tedizolid and linezolid on platelet counts (PLT), absolute neutrophil counts (ANC), and hemoglobin (Hgb) across time. The weekly median value was entered, and subjects were treated as a random effect. The documented nonhematological-related ADEs and oxazolidinone discontinuation rates were recorded. Nonhematological-related ADEs included peripheral neuropathy, serotonin syndrome, and gastrointestinal side effects. A 2-sided value of P < .05 was considered significant. All statistical analyses were performed using SAS, version 9.4 (SAS, Cary, NC, USA).
In exploratory analysis, we described the symptomatic, microbiologic, and clinical outcomes of patients who met the American Thoracic Society/Infectious Diseases Society of America criteria for NTM infection and were treated with a tedizolid- or linezolid-containing regimen for at least 4 weeks [20]. These outcomes were compared from the initiation of the tedizolid- or linezolid-containing regimen to the end of any NTM treatment. The symptomatic, microbiologic, and clinical outcomes were adjudicated by 3 independent reviewers (R.M.L., M.L.M., Y.K.P.) using providers’ documentation, microbiology collected as part of routine medical care, and imaging; discrepancies were resolved by consensus. Cases with microbiologic cultures that were considered contaminants were excluded from the exploratory analysis. Disseminated disease was defined as NTM isolation in blood culture and another independent culture site. Antimicrobial susceptibility testing was performed at Mayo Clinic Laboratories (Rochester, MN, USA). Macrolides underwent inducible resistance screening. Symptomatic improvement was defined as either decreased cough or sputum production for pulmonary infections and decrease in size of the primary lesion for skin and soft tissue or surgical site infections [21–23]. The criteria for a microbiologic response was ≥ 1 negative culture from the site of infection and culture negativity sustained until the end of treatment. Clinical cure was defined as improvement of symptoms without proven negative cultures during and through the end of treatment [24]. A patient was considered cured if both symptomatic (if applicable) and microbiologic (if applicable) criteria were fulfilled [24]. Treatment failure was considered if the criteria for clinical cure or cure were not met. Recurrence was defined as emergence of positive cultures with the same strain of causative pathogen during treatment. Death due to any reason during M. abscessus treatment was recorded [24].
RESULTS
Twenty-four patients were included in the analysis (15 tedizolid, 9 linezolid). Table 1 shows the baseline characteristics of the 2 groups. Mycobacterium abscessus abscessus and Mycobacterium abscessus species were the most common isolates for the tedizolid and linezolid groups, respectively. Pulmonary was the most common source of infection. The tedizolid group had a higher proportion of patients with diabetes (P = .04) and a higher body mass index (P = .04); otherwise, there were no statistically significant differences in baseline characteristics between the 2 groups. The majority of patients in the tedizolid group were initiated and continued on a dose of 200 mg daily (14/15, 93%). All patients in the linezolid group received 600 mg daily or less for the majority of the treatment duration. Five of 9 (56%) patients initiated linezolid with a total daily dose of 1200 mg, and 1 of these patients only received 3 days of therapy. Four patients had dose reduction for linezolid ranging from 4 to 22 days after therapy initiation.
Table 1.
Baseline Characteristics
| Treatment Group | Linezolid (n = 9) | Tedizolid (n = 15) | P Value |
|---|---|---|---|
| Age, median (IQR), y | 66 (61–72) | 64 (49–71) | .34 |
| Male, No. (%) | 8 (89) | 9 (60) | .19 |
| Race, No. (%) | .35 | ||
| White | 8 (89) | 10 (67) | - |
| Other | 1 (11) | 5 (33) | - |
| BMI, median (IQR), kg/m2 | 23 (22–26) | 27 (25–30) | .04 |
| Lung transplant, No. (%) | 9 (100) | 14 (93) | >.99 |
| Days since transplant, median (IQR) | 361 (162–669) | 200 (88–412) | .28 |
| Comorbidities, No. (%) | |||
| Cancer | 1 (11) | 1 (7) | >.99 |
| CHF | 1 (11) | 0 | .37 |
| COPD | 4 (44) | 1 (7) | .05 |
| CrCl,a median (IQR), mL/min | 67 (49–83) | 63 (56–97) | .90 |
| Cystic fibrosis | 0 | 2 (13.3) | .51 |
| Diabetes | 3 (33) | 12 (80) | .04 |
| ESRD | 1 (11) | 1 (7) | >.99 |
| Hypertension | 6 (67) | 11 (73) | >.99 |
| Liver disease | 0 (0) | 1 (7) | >.99 |
| Stroke | 0 (0) | 1 (7) | >.99 |
| Site of infection, No. (%) | - | ||
| Bacteremia | 1 (11) | 4 (27) | - |
| Disseminatedb | 1 (11) | 4 (27) | - |
| Osteomyelitis | 0 | 2 (13) | - |
| Pulmonary | 7 (78) | 12 (80) | - |
| Skin and soft tissue | 2 (22) | 3 (20) | - |
| Surgical site | 0 | 4 (27) | - |
| Species isolated, No. (%) | - | ||
| M. chelonae | 1 (11) | 1 (7) | - |
| M. abscessus complex | 5 (56) | 4 (27) | - |
| M. abscessus abscessus | 2 (22) | 6 (40) | - |
| M. abscessus bolleti | 2 (22) | 2 (13) | - |
| M. abscessus massiliense | 0 | 4 | - |
| Days of therapy, median (IQR) | 24 (19–79) | 48 (25–211) | .31 |
| Baseline platelet count, median (IQR), /µL | 220 (156–253) | 181 (93–304) | .91 |
| Baseline absolute neutrophil count, median (IQR), /µL | 5 (3–8) | 4 (2–5) | .36 |
| Baseline hemoglobin, median (IQR), g/dL | 10 (9–10) | 9 (8–10) | .24 |
| Initial daily linezolid dose, No. (%) | - | ||
| 300 mg | 1 (11) | - | - |
| 600 mg | 3 (33) | - | - |
| 1200 mg | 5 (56) | - | - |
| Initial daily tedizolid dose, No. (%) | - | ||
| 200 mg | - | 14 (93) | - |
| 400 mg | - | 1 (7) | - |
Abbreviations: BMI, body mass index; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; ESRD, end-stage renal disease; IQR, interquartile range.
aDefined by Cockcroft-Gault equation.
bReported NTM isolate from blood culture and another site.
In the mixed-effects ANOVA, the ANC decreased in both groups after 7 weeks of therapy (P = .04). Otherwise, no significant effects for week, treatment group, or interaction between week and treatment group were found (Figure 1). Nonhematologic ADEs occurred in only 1 patient; this patient experienced gastrointestinal side effects while on a multidrug regimen that included tedizolid. Approximately one-fifth of patients in each group discontinued the medication due to ADEs (Table 2). Two patients discontinued linezolid due to ADEs on days 3 (concern for cytopenia) and 19 (thrombocypetnia), respectively. Three patients discontinued tedizolid on days 12 (nausea/vomiting), 25 (cytopenia), and 41 (cytopenia), respectively. Two patients in each group discontinued the medication due to non-ADEs, including cost, hospital shortage, and resistance pattern of the causative pathogen. One patient was lost to follow-up in the linezolid group after 19 days of therapy.
Figure 1.
Effects of linezolid vs tedizolid during the initial 7 weeks of therapy using a mixed-effects analysis of variance model: (a) platelet counts, (b) absolute neutrophil counts, and (c) hemoglobin.
Table 2.
Nonhematological Adverse Effects and Discontinuation of Therapy
| Treatment Group | Linezolid (n = 9) | Tedizolid (n = 15) |
|---|---|---|
| Nonhematological adverse effects, No. (%) | 0 (0) | 1 (7) |
| Gastrointestinal effects (nausea and/or vomiting) | 0 (0) | 1 (7) |
| Peripheral neuropathy | 0 (0) | 0 (0) |
| Serotonin syndrome | 0 (0) | 0 (0) |
| Discontinuation of therapy, No. (%) | 4 (44) | 5 (33) |
| Discontinuation due to ADEs | 2 (22) | 3 (20) |
| Discontinuation due to non-ADEs | 2 (22) | 2 (13) |
| Deceased | 0 (0) | 1 (7) |
| Loss to follow-up | 1 (11) | 0 (0) |
Abbreviations: ADEs, adverse drug events; ANC, absolute neutrophil count; Hgb, hemoglobin; PLT, platelet.
Twelve and 3 cases with at least 4 weeks of tedizolid and linezolid therapy, respectively, were assessed for microbiological and clinical outcomes. Table 3 summarizes the baseline characteristics, comorbidities, microbiology, site of infection, and outcomes data. Mycobacterium abscessus abscessus (7/12, 58%) and M. abscessus species (2/3, 67%) were the most common subspecies in patients receiving a tedizolid- or linezolid-containing regimen, respectively. In patients receiving a tedizolid-containing regimen, the distribution of infections was as follows: 5 (42%) disseminated infections, 5 (42%) pulmonary infections, 5 (42%) surgical site infections, and 4 (33%) skin and soft tissue infections (SSTIs). In patients receiving a linezolid-containing regimen, 2 (67%) patients had pulmonary infection and 1 (33%) had an SSTI. A bilateral lung transplant recipient (case 7) with M. abscessus abscessus infection pretransplant had treatment failure for both the pretransplant pulmonary infection and lung allograft pulmonary infection. The post-transplant course was complicated by M. abscessus abscessus surgical site/sternal osteomyelitis and lung allograft infection. The patient achieved clinical cure for the surgical site infection. A 78-year-old single-lung transplant recipient (case 12) with disseminated M. abscessus abscessus infection had recurrence with new skin lesions ~26 days after therapy initiation. Seven patients receiving a tedizolid-containing regimen were cured or clinically cured for all sites of infection (58%), and 3 patients died (25%) from various causes. In the 3 patients who received a linezolid-containing regimen, 2 patients were cured or clinically cured (67%) and 1 patient (33%) died.
Table 3.
Patient Demographics and Outcomes of M. abscessus Infection
| Pt | Age, y, Sex (Weight) | Transplant Type (Days Since Transplant)a | Comorbidities | TZD or LZD in the Initial Regimen (MIC, µg/mL)b | Companion Drugsc (MIC, µg/mL)b | Macrolide Susceptibility | Site(s) of Infection | Species isolated | Surgical Intervention/Source Removal | Symptomatic | Radiographic/Bronchoscopy | Microbiologic, Days to Negative Culture) | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tedizolid-containing regimen | |||||||||||||
| 1 | 58 F (78 kg) | Bilateral lung (108 d) | DM, HTN | Nok (32) | Imipenem (16), tigecycline (0.25) | R (inducible) | Surgical site | M. abscessus species | No | Yes | NA | NA | Clinical cure |
| 2 | 55 F (70 kg) | Bilateral lung (221 d) | DM, HTN | Yes (32) | Imipenem (8), tigecycline (0.5) | R (inducible) | Bacteremia | M. abscessus abscessus | No | NA | NA | Yes (5 da) | Cure |
| Pulmonary colonization | M. abscessus bolleti | No | No | NA | Yes (171 dd) | NA | |||||||
| SSTI | M. abscessus abscessus | No | Yes | NA | NA | Clinical cure | |||||||
| 3 | 64 F (90 kg) | Heart (38 d) | HTN, ESRD | Yes (16) | Amikacin intravenous (16), imipenem (8) | S | CLABSI | M. abscessus species | Yes | NA | NA | Yes (23 da) | Cure |
| Sternal osteomyelitis | M. abscessus bolleti | Yes | Yes | Improved | Yes (15 de) | Cure | |||||||
| 4 | 43 M (73 kg) | Bilateral lung (177 d) | DM, HTN, stroke | Yes (32) | Imipenem (8), tigecycline (0.5) | S | Bacteremia | M. abscessus massiliense | No | NA | NA | Yes (32 da) | Death |
| SSTI | Yes | Yes | NA | NA | |||||||||
| Pulmonary colonization | No | No | NA | Yes (22 da) | |||||||||
| 5 | 28 M (54 kg) | Bilateral lung (0 di) | DM, CF | No (16) | Amikacin inhaled (32), bedaquiline (NT), clofazimine (>16), imipenem (32), tigecycline (0.12) | R (inducible) | Pulmonary (pretransplant) | M. abscessus species | Yes | Yes | Improved | Yes (NA dh) | Cure |
| Surgical site | Yes | Yes | NA | NA | Clinical cure | ||||||||
| 6 | 58 M (86 kg) | Retransplant, bilateral lung (31 d) | DM, HTN | Yes (32) | Imipenem (8), tigecycline (0.25) | R | Pulmonary (preretransplant) | M. abscessus abscessus | Yesf | No | Improvedj | Nod | Death |
| Surgical site | Yes | No | NA | NA | |||||||||
| 7 | 28 F (59 kg) | Bilateral lung (1178 d) | DM, CF | No (2) | Bedaquiline (NT), imipenem (16), tigecycline (0.25) | R (inducible) | Pulmonary (pretransplant) | M. abscessus abscessus | Yes | No | Improvedj | Nod | Failure |
| Pulmonary (lung allograft) | Yes | No | Improvedj | No | Failure | ||||||||
| Surgical site/sternal osteomyelitis | Yes | Yes | NA | NA | Clinical cure | ||||||||
| 8l | 77 M (77 kg) | Single lung (1045 d) | HTN, COPD | Yes (8) | Azithromycin (0.5), imipenem (64) | S | Bacteremia | M. abscessus abscessus | No | NA | NA | Yes (5 da) | Cure |
| SSTI | No | Yes | No | NA | Clinical cure | ||||||||
| 9 | 66 M (89 kg) | Bilateral lung (233 d) | DM, HTN, liver disease | Yes (>32) | Imipenem (8), tigecycline (0.5) | R (inducible) | Pulmonary colonization | M. abscessus abscessus | No | NAg | NA | Yes (177 dd) | NA |
| Surgical site | Yes | Yes | NA | NA | Clinical cure | ||||||||
| 10 | 74 M (80 kg) | Single lung (600 d) | HTN, chronic anemia | No (8) | Azithromycin (0.5), bedaquiline (NT) | S | Pulmonary | M. abscessus massiliense | No | No | Worsened | Yes (610 da) | Death |
| 11 | 71 M (79 kg) | Single lung (68 d) | DM, HTN, COPD, chronic anemia | Yes (16) | Imipenem (8), tigecycline (0.25) | S | Pulmonary (empyema) | M. abscessus massiliense | Yes | Yes | Improved | NA | Clinical cure |
| 12 | 78 M (91 kg) | Single lung (200 d) | DM, HTN | Yes (32) | Imipenem (16), tigecycline (0.25) | R (inducible) | Bacteremia | M. abscessus abscessus | No | NA | NA | Yes (68 da) | Recurrence |
| SSTI | No | Yes | NA | NA | |||||||||
| Linezolid-containing regimen | |||||||||||||
| 13 | 61 M (74 kg) | Bilateral lungs (361 d) | DM, CHF, COPD, ESRD, chronic anemia | N (16) | Imipenem (8), tigecycline (0.12) | R (inducible) | Pulmonary (lung allograft) | M. abscessus species | No | Yes | Yes | Yes (106 dd) | Cure |
| 14 | 66 M (74 kg) | Bilateral lungs (162 d) | Cancer | Y (16) | Amikacin inhaled (8), azithromycin (8) | R (inducible) | Pulmonary | M. abscessus species | No | No | No | Nod | Deathl |
| 15 | 76 M (74 kg) | Single lung (684 d) | HTN, COPD | Yes (4) | Amikacin topical (16), clarithromycin (1) | S | SSTI | M. abscessus bolletii | No | Yes | NA | NA | Clinical curem |
Abbreviations: BAL, bronchoalveolar lavage; CLABSI, central line-associated bloodstream infection; CF, cystic fibrosis; COPD, chronic obstruction pulmonary disease; DM, diabetes; ESRD, end-stage renal disease; HTN, hypertension; I, intermediate; MIC, minimal inhibitory concentration; NA, not applicable; NT, not tested; R, resistant; S, susceptible; SSTI, skin and soft tissue infection.
aBlood cultures.
bIf there were multiple susceptibility reports, MIC values from the report of the date closest to the initiation of linezolid or tedizolid were used.
cAt the initiation of tedizolid.
dBronchoalveolar lavage or bronchial wash culture.
eSternal wound culture.
fRetransplant.
gAbsence of symptoms initially.
hPositive sputum culture pretransplant and negative BAL cultures post-transplant.
iPatient initiated tedizolid-containing regimen before transplant.
jImprovement was secondary to transplant.
kPatient received tedizolid for 28 days, then switched to linezolid for 23 days due to cost.
lPatient’s post-transplant course was complicated by metastatic adenocarcinoma.
mCases 8 and 15 describe the same patient, who had 2 episodes of NTM infection. Case 8 occurred 5 months after the completion of therapy for case 15.
DISCUSSION
In our study, no significant differences were found comparing the effects of tedizolid vs linezolid for PLT, ANC, and Hgb using mixed-effects ANOVA models over 7 weeks of therapy. However, a significant effect was observed between week of therapy and ANC, suggesting that both agents carry risks of ANC reduction over time. Tedizolid or linezolid-containing regimens demonstrated a potential benefit, resulting in symptomatic and microbiologic improvement in SOT recipients with an M. abscessus infection.
In vitro data support the use of oxazolidinones for NTM infections. Against Mycobacterium abscessus complex, the MIC50 and MIC90 of tedizolid across 3 studies were 1–4 mcg/mL and 4–8 mcg/mL, respectively, several dilutions lower than linezolid [5]. Similar in vitro susceptibility was observed for other rapid growers (Table 4) [6]. The pharmacokinetics of these agents are favorable as they demonstrate excellent oral bioavailability, making them appealing treatment options for NTM infections. Compared with linezolid, tedizolid’s protein binding is higher (70%–90% vs 31%) and its elimination half-life is longer (~12 hours vs ~5 hours), allowing for once-daily dosing. Linezolid is traditionally administered twice daily, but is often reduced to once daily for prolonged durations of therapy as an attempt to reduce the risk of cytopenias [4].
Table 4.
In Vitro Oxazolidinone Activity Against Rapidly Growing Mycobacteria
| Oxazolidinone MIC50, MIC90, µg/mL (No. of Isolates) |
|||
|---|---|---|---|
| Organism | Tedizolid | Linezolid | Reference |
| M. abscessus | 1, 4 (43) | 8, >32 (43) | [5] |
| 4, 8 (81) | 16, 32 (81) | [6] | |
| 2, 8 (15) | 8, 64 (15) | [7] | |
| M. bolletii | 4, 4 (5) | 32, >32 (5) | [5] |
| 2, 4 (14) | 16, 32 (14) | [7] | |
| M. massiliense | 1, 4 (82) | 8, >32 (82) | [5] |
| 2, 4 (12) | 8, 32 (12) | [6] | |
| 4, 8 (15) | 16, 32 (15) | [7] | |
| M. chelonae | 1, 2 (22) | 8, 16 (22) | [6] |
| M. mucogenicum group | 1, NA (9) | 1, NA (9) | [6] |
| M. immunogenum | 1, NA (9) | 8, NA (9) | [6] |
| M. fortuitum | 1, 2 (20) | 2, 4 (20) | [6] |
Abbreviation: MIC, minimal inhibitory concentration.
A retrospective study evaluated the tolerability of linezolid in 102 NTM-infected patients. Forty-five percent of patients experienced linezolid-attributable ADEs, and 87% of them discontinued the therapy over an average of 20 weeks [25]. Most patients (79%) took 600 mg linezolid once daily, and the median linezolid therapy duration was 21.4 weeks. Compared with non-SOT patients, SOT patients who received linezolid had a higher incidence of thrombocytopenia, perhaps due to concurrent bone marrow–suppressive pharmaceuticals [26]. In a retrospective review of prolonged tedizolid use by Kim et al., 24 patients received tedizolid for NTM infections, with a median duration (range) of 101 (15–369) days, and experienced ADEs including peripheral neuropathy (21%), nausea/vomiting (13%), thrombocytopenia (4%), and anemia (4%) [27]. The median therapy duration in our study was shorter compared with the above studies [24, 27]. The discontinuation rate due to ADEs was 20% and 22% for the tedizolid and linezolid groups, respectively. A lower percentage of patients in our study experienced nonhematologic ADEs compared with the Kim et al. study [27]. We did not identify a significant safety benefit of tedizolid over linezolid at 7 weeks of therapy.
There are limited data evaluating treatment outcomes of tedizolid- or linezolid-containing regimen for NTM infections, specifically M. abscessus. The majority of our patients had multiple sites of infection, and treatment required combination antimicrobial therapy and appropriate surgical management. There is no consensus on the definition of cure for M. abscessus infections except for pulmonary infection. Given the difficulty of eradicating M. abscessus complex, treatment goals may vary depending on the treating physician and patient. In this small cohort, tedizolid- or linezolid-containing regimens demonstrated a potential benefit in the majority of patients, resulting in symptomatic and microbiologic improvement in SOT recipients with M. abscessus infection.
Our study highlights a cohort of SOT recipients treated for NTM with oxazolidinones and the effects of tedizolid vs linezolid on PLT, ANC, and Hgb using mixed-effects ANOVA models. The limitations of the study include (1) its retrospective single-center study design, (2) that the study duration was a 9-year period and treatment strategies for M. abscessus infection changed over time, (3) that not all M. abscessus complex subspecies were identified, (4) the short duration of treatment follow-up, and (5) the lack of control for variables associated with the outcomes due to sample size.
Based on the in vitro data, the pharmacokinetics of tedizolid and linezolid, and the results of our study, the comparative safety of these 2 oxazolidinones remains unclear. Our study found no benefit of tedizolid over linezolid. Treatment regimens including tedizolid or linezolid for M. abscessus infection are associated with symptomatic and microbiologic improvement with appropriate surgical interventions. Larger cohort studies are required to compare the hematologic adverse effect profile and efficacy of oxazolidinones for the treatment of NTM infections in SOT recipients.
Acknowledgments
The authors thank the solid organ transplant recipients of UT Southwestern Medical Center for helping us improve care through the use of information collected via our electronic health record system and Donglu Xie for assistance in collecting data.
Financial support. This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH; KL2TR001103). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Potential conflicts of interest. The authors of this manuscript have no conflicts of interest to disclose.
Author contributions. Y.P., R.L., J.S., and M.M. participated in the performance of the research, research design, data analysis, and writing the article. L.H. participated in the performance of data analysis. All authors participated in the development of the final edition of the manuscript.
Patient consent. The design of the work was approved by the local University of Texas Southwestern Institutional Review Board (IRB STU-2019-1289). The study did not include factors necessitating patient consent.
References
- 1. Piersimoni C. Nontuberculous mycobacteria infection in solid organ transplant recipients. Eur J Clin Microbiol Infect Dis 2012; 31:397–403. [DOI] [PubMed] [Google Scholar]
- 2. Abad CL, Razonable RR. Non-tuberculous mycobacterial infections in solid organ transplant recipients: an update. J Clin Tuberc Other Mycobact Dis 2016; 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doucette K, Fishman JA. Nontuberculous mycobacterial infection in hematopoietic stem cell and solid organ transplant recipients. Clin Infect Dis 2004; 38:1428–39. [DOI] [PubMed] [Google Scholar]
- 4. Longworth SA, Daly JS; AST Infectious Diseases Community of Practice . Management of infections due to nontuberculous mycobacteria in solid organ transplant recipients—guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13588. [DOI] [PubMed] [Google Scholar]
- 5. Tang YW, Cheng B, Yeoh SF, et al. . Tedizolid activity against clinical Mycobacterium abscessus complex isolates—an in vitro characterization study. Front Microbiol 2018; 9:2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown-Elliott BA, Wallace RJ Jr. In vitro susceptibility testing of tedizolid against nontuberculous mycobacteria. J Clin Microbiol 2017; 55:1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Compain F, Soroka D, Heym B, et al. . In vitro activity of tedizolid against the Mycobacterium abscessus complex. Diagn Microbiol Infect Dis 2018; 90:186–9. [DOI] [PubMed] [Google Scholar]
- 8. Product Information: ZYVOX(R) Intravenous Injection, Oral Tablets Suspension, Linezolid Intravenous Injection, Oral Tablets Suspension. New York: Pfizer Inc. (per FDA); 2013. [Google Scholar]
- 9. Gerson SL, Kaplan SL, Bruss JB, et al. . Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother 2002; 46:2723–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prokocimer P, Bien P, Surber J, et al. . Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob Agents Chemother 2011; 55:583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lodise TP, Bidell MR, Flanagan SD, et al. . Characterization of the haematological profile of 21 days of tedizolid in healthy subjects. J Antimicrob Chemother 2016; 71:2553–8. [DOI] [PubMed] [Google Scholar]
- 12. Prokocimer P, De Anda C, Fang E, et al. . Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 2013; 309:559–69. [DOI] [PubMed] [Google Scholar]
- 13. Moran GJ, Fang E, Corey GR, et al. . Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2014; 14:696–705. [DOI] [PubMed] [Google Scholar]
- 14. Burdette SD, Trotman R. Tedizolid: the first once-daily oxazolidinone class antibiotic. Clin Infect Dis 2015; 61:1315–21. [DOI] [PubMed] [Google Scholar]
- 15. Lee EY, Caffrey AR. Thrombocytopenia with tedizolid and linezolid. Antimicrob Agents Chemother 2018; 62:e01453-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med 2013; 34:87–94. [DOI] [PubMed] [Google Scholar]
- 17. Barry M, Chandra S, Hymes KB. Cytopenias in transplant patients. Principles and Practice of Transplant Infectious Diseases 2018; 199–207. [Google Scholar]
- 18. La Hoz RM, Liu T, Xie D, et al. . The use of automated data extraction tools to develop a solid organ transplant registry: proof of concept study of bloodstream infections. J Infect 2020; 82:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. La Hoz RM, Wallace A, Barros N, et al. . Epidemiology and risk factors for varicella zoster virus reactivation in heart transplant recipients. Transpl Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Griffith DE, Aksamit T, Brown-Elliott BA, et al. ; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America . An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 21. Philley JV, Wallace RJ Jr, Benwill JL, et al. . Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 2015; 148:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang B, Jhun BW, Moon SM, et al. . Clofazimine-containing regimen for the treatment of Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 2017; 61:e02052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shorr AF, Lodise TP, Corey GR, et al. . Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2015; 59:864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Ingen J, Aksamit T, Andrejak C, et al. . Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J 2018; 51:1800170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Winthrop KL, Ku JH, Marras TK, et al. . The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. Eur Respir J 2015; 45:1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tessier JM, Puzio T, Young A, et al. . Thrombocytopenia associated with linezolid therapy in solid organ transplant recipients: a retrospective cohort study. Surg Infect (Larchmt) 2015; 16:361–7. [DOI] [PubMed] [Google Scholar]
- 27. Kim T, Wills A, Markus A, et al. . Safety and tolerability of long term use of tedizolid for treatment of nontuberculous mycobacterial infections. Open Forum Infect Dis 2016; 3(Suppl 1):577. [Google Scholar]