Abstract
Background
Diabetes mellitus (DM) increases the risk of tuberculosis (TB) disease. Knowledge of the impact of DM on TB treatment outcomes is primarily based on retrospective studies.
Methods
We conducted a prospective cohort study of new pulmonary TB patients with and without DM (TB-DM and TB only) in India. The association of DM with a composite unfavorable TB treatment outcome (failure, recurrence, mortality) over 18 months was determined, and the effect of DM on all-cause mortality and early mortality (death during TB treatment) was assessed.
Results
Of 799 participants, 574 (72%) had TB only and 225 (28%) had TB-DM. The proportion of patients with DM who experienced the composite outcome was 20%, as compared with 21% for TB-only participants (adjusted hazard ratio [aHR], 1.13; 95% CI, 0.75–1.70). Mortality was higher in participants with DM (10% vs 7%), and early mortality was substantially higher among patients with DM (aHR, 4.36; 95% CI, 1.62–11.76).
Conclusions
DM was associated with early mortality in this prospective cohort study, but overall unfavorable outcomes were similar to participants without DM. Interventions to reduce mortality during TB treatment among people with TB-DM are needed.
Keywords: diabetes mellitus, mortality, India, tuberculosis, unfavorable treatment outcomes
Tuberculosis (TB) has emerged as the most fatal infectious disease worldwide [1], and the burden of diabetes mellitus (DM) has risen steeply in low- and middle-income countries (LMICs) [2–4]. India contributes the world’s largest TB burden (>2.7 million cases in 2019) [1, 5, 6] and among the largest burdens of DM (77 million adults) [3, 4, 6]. Convergence of the TB and DM epidemics in India may impede global TB control efforts [7], as it is well accepted that DM increases the risk of TB disease [8–10]. The relationship between DM and TB treatment outcomes remains less certain.
Evidence, mostly from retrospective studies, indicates that persons with TB and DM are at higher risk for unfavorable TB treatment outcomes including delayed sputum conversions, TB treatment failure, recurrence, and death [8, 11–14]. However, the few prospective studies evaluating clinical consequences of DM and pre-DM among TB patients often have methodologic shortcomings (eg, misclassification of DM, nonstandardization of outcome definitions, and no adjustment for confounders), and few have been conducted in high-TB-DM-burden regions [15]. Prospective data from a high-TB-DM-burden setting are needed.
Pune, India has a population of 7 million within the city and surrounding semi-urban/rural areas and a TB notification incidence of 112–132/100 000 person-years (PY) [16]. In this setting, over half of TB cases are dysglycemic, and the mycobacterial burden before TB treatment initiation is 4-fold higher in patients with DM [17]. We hypothesized that, due to higher baseline mycobacterial burden and altered immune response to TB [18], DM would lead to prolonged sputum culture positivity and higher risk of TB treatment failure, recurrence, or death. We further hypothesized that the magnitude of risk of unfavorable outcomes would correlate with the level of hyperglycemia. To investigate these relationships fully, we established a prospective cohort of newly diagnosed pulmonary TB patients with and without DM.
METHODS
Study Design and Study Sites
This prospective cohort study was conducted at the Byramjee-Jeejeebhoy Government Medical College-Sassoon General Hospitals (BJGMC-SGH) clinical research site between December 2013 and May 2019. Dr. D.Y. Patil Medical College (DYPMC) joined the study in 2016. BJGMC-SGH and DYPMC are tertiary care teaching hospitals serving low- and middle-income populations in and around Pune city in India. We conducted a concurrent DM prevalence survey among patients with TB to identify participants for the prospective study [17]. Eligible persons evaluated for TB at 11 Revised National TB Control Program (RNTCP) tuberculosis units (TUs) in greater Pune region, representing >70% coverage of total active TB cases, were referred to study sites [17].
Study Eligibility
Eligibility criteria were age ≥18 years; microbiologically confirmed pulmonary TB by either smear positive for acid-fast bacilli (AFB), GeneXpert (Xpert MTB/RIF assay, Cepheid, Sunnywale, CA, USA), or AFB culture or clinical TB diagnosed using RNTCP clinical criteria; and known DM and HIV status [17]. Persons with prior TB history, rifampin-resistant TB, or multidrug-resistant TB, people with HIV (WH) infection, and pregnant women were excluded. INH monoresistance was not an exclusion criterion. Spot and early morning sputum specimens from individuals with possible TB in our concurrent prevalence study [17] underwent AFB, GeneXpert, and culture using Mycobacterial Growth Indicator Tube (MGIT, Becton Dickinson and Company, Sparks, MD, USA) liquid culture and Löwenstein-Jensen (LJ, EOS laboratories, Mumbai, Maharashtra , India) solid media methods. Baseline fasting or random blood glucose tests (Glucose HK, Roche Diagnostics GmbH, Mannheim, Gemany), HbA1c (Hemoglobin A1c, Bio-Rad Laboratories, Inc, Hercules, CA 94547, USA), and HIV (Determine HIV1/2, Alere Medical Co. Ltd. Chiba,270-2214, Japan) rapid tests were also performed. All microbiologic and blood-based tests were performed at the BJGMC-SGH laboratory.
Study Procedures
Baseline information, including demographics, socioeconomic factors, comorbidities, DM and TB history, current DM medications, and TB risk factors (eg, tobacco exposure history, alcohol use, duration of TB symptoms) were collected via questionnaire. Follow-up visits occurred biweekly in the intensive phase (first 8 weeks) of anti-TB treatment, every 4 weeks during the continuation phase (up to 6 months), and at 12 and 18 months. Spot sputum specimens collected at each visit underwent AFB staining and culture using both MGIT liquid and LJ solid media in the BJGMC-SGH laboratory. Laboratory quality assurance was monitored externally by pSMILE laboratories. Phenotypic drug susceptibility testing was performed when Mtb growth was confirmed and if treatment failure or recurrence was suspected. TUs provided routine TB treatment as per national guidelines. The thrice-weekly regimen via directly observed therapy (DOT) included 450 mg (600 mg for ≥60 kg body weight) of rifampin (R), 600 mg of isoniazid (H), 1200 mg of ethambutol (E), and 1500 mg of pyrazinamide (Z) during the intensive phase followed by rifampin and isoniazid at the same doses during the continuation phase. On April 1, 2017, self-administered daily TB treatment was rolled out in India—weight-based fixed drug combination (FDC) of HRZE (75/150/400/275 mg; 2 tablets for 25–39 kg, 3 tablets for 40–54 kg, 4 tablets for 55–69 kg, and 5 tablets for ≥70 kg) during the intensive phase and weight-banded FDC of HRE in the continuation phase. The study clinician conducted a detailed review of potential causes of death via a questionnaire.
Study Definitions
Microbiologically confirmed TB was defined as a positive sputum smear for AFB, GeneXpert, or culture. DM was defined as HbA1c ≥6.5%, fasting blood glucose ≥126 mg/dL, random blood sugar >200 mg/dL, self-reported DM diagnosis, or current DM medication use. Known DM was defined as DM diagnosis before TB diagnosis and treatment initiation [19]. New DM was defined as DM diagnosis at TB diagnosis and/or treatment initiation.
Study Outcomes
The primary study outcomes were rate of composite unfavorable TB treatment outcome by DM status (TB only and TB-DM) and impact of DM and 1-unit increase of HbA1c on the composite outcome, defined as TB treatment failure (positive smear or culture at month 5 or month 6), recurrence (new TB diagnosis after cure or TB treatment completion), or mortality (all-cause mortality by 18 months) (Supplementary Table 1). Secondary outcomes included individual TB treatment outcomes: failure, recurrence, mortality, and early mortality, defined as mortality during TB treatment, time to culture conversion, and proportion with culture conversion at 2 months of TB treatment. All aforementioned analyses were repeated in subanalyses, defined a priori, by DM subtype, either new or known DM. Post hoc exploratory analyses were conducted to further probe the impact of metformin use on TB treatment outcomes for the entire cohort and among patients with DM.
Sample Size and Statistical Analysis
At the time of study design, the rate of unfavorable TB treatment outcomes in India was 15% [5]. Assuming 15% of patients with TB only and 25% of patients with TB-DM will have unfavorable TB treatment outcomes, a 2-sided alpha of .05, and 10% loss to follow-up, we calculated a sample size of 675 participants (n = 450 TB only and n = 225 TB-DM) to achieve 80% power to assess a 10% difference between groups. All study participants with at least 12 months of follow-up or who died before 12 months were included in the analysis. Baseline characteristics were summarized using proportions and medians with interquartile range (IQR) and compared by DM status using the Fisher exact test and Wilcoxon rank-sum test, respectively. P values <.05 were deemed statistically significant. Risk of composite unfavorable treatment outcome for DM, including subcategories, was estimated using Poisson regression (Supplementary Table 1). Time to culture conversion and proportion of 2-month culture conversion were compared by DM status using the log-rank test and the Fisher exact test, respectively. Predictors of mortality and early mortality were assessed using Cox proportional hazards models, and bootstrap (100x) 95% CIs for hazards rate ratios were estimated. Poisson regression determined the associations of new DM and known DM as predictors of unfavorable treatment outcome. Data were analyzed using Stata, version 14.2 (StataCorp, College Station, TX, USA).
Ethics Approval and Patient Consent Statement
The patients’ written consent was obtained for this study. The design of the work was approved by the Ethics Committees at BJGMC-SGH (FWA00005797) and DYPMC (FWA00027671) and the Institutional Review Board of Johns Hopkins School of Medicine (FWA00005752).
RESULTS
Baseline Characteristics by DM Status
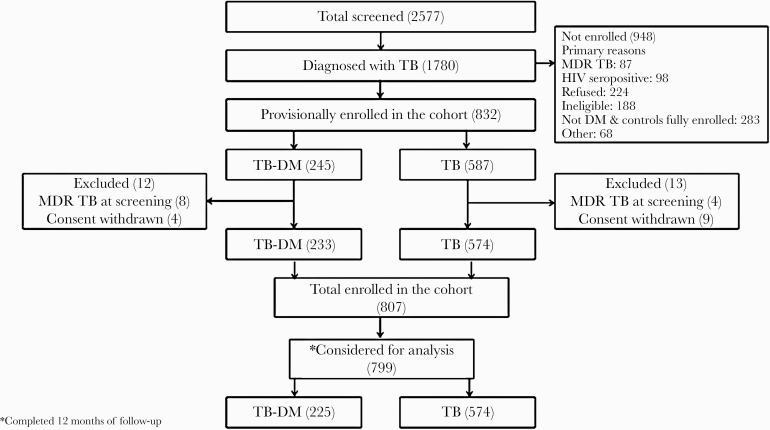
Of 1780 people with TB, 799 (n = 574 TB only and n = 225 TB-DM) completed at least 12 months of follow-up or died before 12 months and were included in this analysis (Figure 1). Compared with TB only, TB-DM participants were more likely to be male (P = .002), above age 40 years (P < .001), anemic (P = .001), to have lower household income (P = .007), and to have normal body mass index (BMI) or be overweight (P < .001) (Table 1). The thrice-weekly DOT regimen was disproportionately received by TB-only patients (488 [85%] vs 131 [58%]; P < .001). Among the 225 TB-DM participants, 155 (69%) were diagnosed with DM before their TB diagnosis, and 70 (31%) were newly diagnosed with DM at TB diagnosis. Of the 70 newly diagnosed with DM, 68 were diagnosed via elevated A1c, and 2 were diagnosed via elevated fasting blood glucose. The median HbA1c (IQR) was 9.7% (7.3%–11.5%) among TB-DM.
Figure 1.
Study flowchart illustrating flow of study participants from screening to enrollment into the prospective tuberculosis cohort by diabetes mellitus status.
Table 1.
Baseline Sociodemographic and Clinical Characteristics of Newly Diagnosed Tuberculosis Patients by Diabetes Mellitus Status in Pune, India
| Characteristic | Overall (n = 799) | TB-only (n = 574) | TB-DM (n = 225) | P value |
|---|---|---|---|---|
| Sociodemographic | ||||
| Sex | ||||
| Female | 269 (34) | 212 (37) | 57 (25) | .002 |
| Male | 530 (66) | 362 (63) | 168 (75) | |
| Age, y | ||||
| <25 | 251 (31) | 242 (42) | 9 (4) | |
| 25–40 | 281 (35) | 231 (40) | 50 (22) | <.001 |
| >40 | 267 (33) | 101 (18) | 166 (74) | |
| Residence | ||||
| Rural | 84 (11) | 54 (9) | 30 (13) | .12 |
| Urban | 715 (89) | 520 (91) | 195 (87) | |
| Family Type | ||||
| Nuclear | 454 (57) | 333 (58) | 121 (54) | .30 |
| Joint | 345 (43) | 241 (42) | 104 (46) | |
| Employment | ||||
| Unemployed | 383 (48) | 272 (48) | 110 (49) | .75 |
| Employed | 416 (52) | 301 (52) | 115 (51) | |
| Household income, Indian rupees | ||||
| >10 000 | 274 (36) | 232 (38) | 42 (26) | .007 |
| <10 000 | 494 (64) | 377 (62) | 117 (74) | |
| Anemiaa | ||||
| No | 678 (86) | 471 (83) | 207 (92) | .001 |
| Yes | 115 (15) | 97 (17) | 18 (8) | |
| Smoking | ||||
| Non-smoker | 648 (81) | 471 (82) | 177 (79) | .27 |
| Smoker | 151 (19) | 103 (18) | 48 (21) | |
| Alcohol | ||||
| No | 561 (70) | 405 (71) | 156 (69) | .73 |
| Yes | 238 (30) | 169 (29) | 69 (31) | |
| Clinical characteristics | ||||
| Smear grade | ||||
| Negative | 236 (30) | 168 (29) | 68 (30) | |
| 1+ | 283 (35) | 203 (35) | 80 (36) | .96 |
| 2+ | 154 (19) | 110 (19) | 44 (20) | |
| 3+ | 126 (16) | 93 (16) | 33 (15) | |
| Body mass indexb | ||||
| Normal | 257 (32) | 140 (24) | 117 (52) | |
| Underweight | 503 (63) | 421 (73) | 82 (36) | <.001 |
| Overweight | 39 (5) | 13 (2) | 26 (12) | |
| Cavity on X-ray | ||||
| Absent | 360 (54) | 262 (55) | 98 (52) | .49 |
| Present | 303 (46) | 213 (45) | 90 (48) | |
| Glycated Hemoglobin (HbA1c) | ||||
| <5.6 | 357 (45) | 354 (62) | 3 (1) | |
| 5.6–6.5 | 238 (30) | 217 (38) | 21 (9) | <.001 |
| ≥6.5 | 200 (25) | 0 | 200 (89) | |
| Diabetes mellitus | ||||
| No DM | 574 (72) | 574 (100) | 0 | |
| New DM | 70 (9) | 0 | 69 (31) | <.001 |
| Known DM | 155 (19) | 0 | 155 (69) | |
| TB Treatment Regimen | ||||
| Intermittent | 619 (77%) | 488 (85%) | 131 (58%) | <.001 |
| Daily | 180 (23%) | 86 (14%) | 94 (42% |
All data are presented as No. (%).
Abbreviations: DM, diabetes mellitus; HH, household; TB, tuberculosis.
aDefined as hemoglobin <8 mg/dL for women and <8.5 mg/dL for men.
bCalculated as weight (kg)/(height (m)2 and categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), or overweight (>25–29.9 kg/m2).
DM and Unfavorable TB Treatment Outcome
Incidence of unfavorable treatment outcome (IQR) was 20.0 (17.1–23.4) per 100 PY overall and was comparable among TB only and TB-DM (20.0 per 100 PY vs 20.1 per 100 PY; P = .29). Neither DM (adjusted relative risk [aRR], 1.13; 95% CI, 0.75–1.70) nor 1-unit increase in HbA1c (aRR, 0.96; 95% CI, 0.88–1.04) was independently associated with unfavorable treatment outcome (Table 2); DM was not associated with unfavorable treatment outcome among patients on the thrice-weekly (n = 619; aRR, 1.06; 95% CI, 0.67–1.67) or daily (n = 180; aRR, 1.23; 95% CI, 0.43–3.52) TB regimen. New DM patients had a higher risk of unfavorable TB treatment outcome than TB only (RR, 1.56; 95% CI, 0.96–2.53), but the association did not reach statistical significance in our adjusted model (aRR, 1.40; 95% CI, 0.83–2.37). Overall, low BMI (aRR, 1.60; 95% CI, 1.07–2.39) and alcohol use (aRR, 1.87; 95% CI, 1.20–2.90) were independently associated with unfavorable TB treatment outcome (Supplementary Table 2). In the stratified analysis by BMI, TB-DM participants with low BMI (RR, 1.24; 95% CI, 0.78–1.97) and normal BMI (RR, 1.66; 95% CI, 0.86–3.20) had a higher likelihood of adverse outcomes while high BMI was protective (RR, 0.24; 95% CI, 0.04–1.29), but none reached statistical significance.
Table 2.
Estimated Risk of Tuberculosis Outcomes by Diabetes Mellitus Status Among a Prospective Tuberculosis Cohort in Pune, India
| Univariable Analysis | Multivariable Analysisa | ||||
|---|---|---|---|---|---|
| Outcome | Rate (95% CI) | Ratiob (95% CI) | P Value | Ratiob (95% CI) | P Value |
| Composite unfavorable outcomec | |||||
| TB only (n = 574) | 20.0 (16.6–24.0) | Ref | Ref | ||
| TB-DM (n = 225) | 20.1 (14.6–27.0) | 1.01 (0.71–1.42) | >.95 | 1.13 (0.75–1.70) | 0.56 |
| HbA1c | – | 0.94 (0.87–1.01) | .10 | 0.96 (0.88–1.04) | 0.31 |
| Treatment failure | |||||
| TB only (n = 574) | 21.8 (16.5–28.3) | Ref | Ref | ||
| TB-DM (n = 225) | 14.0 (7.4–23.8) | 0.56 (0.30–1.06) | .08 | 0.75 (0.36–1.58) | 0.46 |
| Recurrence | |||||
| TB only (n = 424) | 12.2 (8.7–16.5) | Ref | Ref | ||
| TB-DM (n = 159) | 7.5 (3.4–14.2) | 0.62 (0.30–1.27) | .19 | 0.73 (0.31–1.70) | 0.46 |
| Mortality | |||||
| TB only (n = 574) | 6.5 (4.7–8.8) | Ref | Ref | ||
| TB-DM (n = 225) | 9.9 (6.3–14.9) | 1.55 (0.93–2.59 | .09 | 1.54 (0.85–2.79) | 0.16 |
| 2-mo culture conversion | |||||
| TB only (n = 478) | 94.6 (92.5–96.6) | Ref | Ref | ||
| TB-DM (n = 184) | 96.2 (93.4–99.0) | 0.69 (0.29–1.61) | .39 | 0.56 (0.20–1.57) | 0.27 |
| Median time to culture conversion (IQR), d | |||||
| TB only (n = 453) | 1.8 (1.7–2.0) | Ref | Ref | ||
| TB-DM (n = 166) | 2.5 (2.1–2.9) | 1.18 (0.98–1.43) | .08 | 1.15 (0.89–1.48) | 0.29 |
Abbreviations: DM, diabetes mellitus; IQR, interquartile range; TB, tuberculosis.
aAdjusted for sex, age, household income, smoking, alcohol, body mass index, daily vs intermittent TB regimen, and smear grade.
bMeasure of association: relative risk (composite unfavorable treatment outcome); odds ratio (treatment failure); hazard ratio (recurrence, mortality, 2-month culture conversion).
cDefined as treatment failure, recurrence, or all-cause mortality.
Secondary Analyses
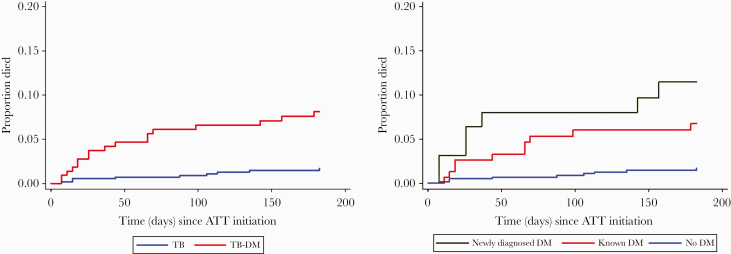
The proportion of 2-month culture conversion was comparable among TB only and TB-DM (95% vs 96%), and median time to culture conversion on solid medium was 31 days in both groups. DM was not associated with delayed time to culture conversion on liquid medium (adjusted hazard ratio [aHR], 1.15; 95% CI, 0.89–1.48) or any individual unfavorable TB outcome (Table 2). Overall, we observed 65 (8%) deaths by 18 months—42 (7%) in TB only and 23 (10%) in TB-DM. Risk of overall mortality was 54% higher among TB-DM compared with TB only (aHR, 1.54; 95% CI, 0.85–2.79), but this finding was not statistically significant (Table 3). Time to mortality was shorter in TB-DM than TB only (66 days vs 88 days; P = .001) (Figure 2A). Respiratory complications of TB were more commonly the cause of death among TB-DM patients compared with TB only (50% vs 27%; P < .001); events related to cardiovascular disease (CVD) were observed in 32% of TB-DM patients who died vs 15% of TB-only patients (P = .09).
Table 3.
Estimated Risk of Mortality and Early Mortality by Diabetes Subtype (New or Known) Among a Prospective Tuberculosis Cohort in Pune, India
| Univariable Analysis | Multivariable Analysisa | ||||
|---|---|---|---|---|---|
| Outcome | Rate (95% CI) | HR (95% CI) | P Value | aHR (95% CI) | P Value |
| All-cause mortality | |||||
| TB only (n = 574) | 6.5 (4.7–8.8) | Ref | Ref | ||
| TB-DM (n = 225) | 9.9 (6.3–14.9) | 1.55 (0.9–2.59) | .09 | 1.54 (0.85–2.79) | .16 |
| New DM (n = 70) | 13.5 (7.0–25.8) | 2.13 (1.04–4.36) | .04 | 1.73 (0.80–3.76) | .17 |
| Known DM (n = 155) | 8.5 (5.0–14.4) | 1.33 (0.72–2.43) | .36 | 1.41 (0.70–2.88) | .34 |
| DM on metformin (n = 117) | 6.22 (3.11–12.43) | 0.96 (0.45–2.05) | .92 | 0.96 (0.40–2.31) | .93 |
| DM no metformin (n = 108) | 14.57 (8.78–24.17) | 2.32 (1.28–4.19) | .005 | 1.99 (1.05–3.78) | .04 |
| Early mortalityb | |||||
| TB only (n = 574) | 3.4 (1.6–6.5) | Ref | Ref | ||
| TB-DM (n = 225) | 17.5 (10.2–28.0) | 5.06 (2.26–11.35) | <.001 | 4.36 (1.62–11.76) | .004 |
| New DM (n = 70) | 24.7 (10.0–51.0) | 7.17 (2.67–19.27) | <.001 | 6.56 (2.18–19.71) | .001 |
| Known DM (n = 155) | 14.53 (6.9–26.7) | 4.20 (1.70–10.33) | .002 | 3.14 (1.03–9.61) | .045 |
| DM on metformin (n = 117) | 11.37 (4.17–24.75) | 3.30 (1.18–9.28) | .02 | 2.32 (0.67–8.08) | .20 |
| DM no metformin (n = 108) | 24.82 (12.39–44.41) | 7.13 (2.96–17.21) | <.001 | 6.17 (2.24–17.04) | <.001 |
| Post-ATT mortalityc | |||||
| TB only (n = 487) | 8.6 (5.9–12.1) | Ref | Ref | ||
| TB-DM (n = 176) | 4.5 (1.6–9.7) | 0.54 (0.22–1.28) | .16 | 0.58 (0.22–1.51) | .27 |
| New DM (n = 49) | 5.3 (0.6–19.1) | 0.64 (0.15–2.69) | .55 | 0.42 (0.10–1.6) | .25 |
| Known DM (n = 126) | 4.2 (1.1–10.7) | 0.50 (0.18–1.41) | .19 | 0.72 (0.23–2.22) | .57 |
| DM on metformin (n = 98) | 2.6 (0.3–9.5) | 0.31 (0.07–1.29) | .11 | 0.47 (0.10–2.17) | .33 |
| DM no metformin (n = 78) | 6.8 (1.9–17.5) | 0.84 (0.30–2.39) | .75 | 0.65 (0.22–1.96) | .45 |
Abbreviations: aHR, adjusted hazards ratio; ATT, antituberculosis treatment; DM, diabetes mellitus; HR, hazards ratio; TB, tuberculosis.
aAdjusted for sex, age, household income, smoking, alcohol, body mass index, daily vs intermittent TB regimen, and smear grade.
bDefined as death during the 6 months of TB treatment.
cParticipants who died on ATT or were lost to follow-up before treatment completion (before 6 months) were not included in this analysis.
Figure 2.
A, Kaplan-Meier curve showing time to early mortality (death during the period of tuberculosis treatment) among patients with tuberculosis (TB) by diabetes mellitus (DM) status. The red line represents patients with DM, and the blue line represents patients without DM. B, Kaplan-Meier curve showing time to early mortality by newly diagnosed diabetes mellitus (DM) and known DM among patients with tuberculosis (TB). The blue line represents patients with TB without DM, the green line represents newly diagnosed DM, and the red line represents known DM.
Early Mortality
Early mortality occurred in 17 (8%) TB-DM and 9 (2%) TB-only patients. DM was independently associated with early mortality (aHR, 4.36; 95% CI, 1.62–11.76) (Table 3), and time to death was shorter among new DM and known DM patients compared with TB-only patients (26 vs 44 vs 88 days; P = .001) (Figure 2B). Both new DM (aHR, 6.56; 95% CI, 2.18–19.71) and known DM (aHR, 3.14; 95% CI, 1.03–9.61) were independently associated with early mortality (Table 3). As shown in Supplementary Table 3, the bootstrapping method did not change the 95% confidence intervals of the associations between early mortality and TB-DM.
Exploratory Analyses
Of the 225 TB-DM patients, 100% of the known DM patients (155) were on DM medication. Of the 70 newly diagnosed DM patients, 17 reported initiating DM medications following TB diagnosis, 14 reported seeking care for DM but did not report medication use, and 39 did not report receiving any DM medications or care. Specific to metformin, 95 of 155 (61%) with DM before TB diagnosis were receiving it, and 10 of 70 (14%) newly diagnosed with DM initiated metformin use after TB diagnosis. Metformin reduced composite unfavorable TB treatment outcome by 50% (aRR, 0.52; 95% CI, 0.26–1.01) among TB-DM patients. Not receiving metformin increased risk of mortality (aHR, 1.99; 95% CI, 1.05–3.78) compared with TB-only patients, and this risk persisted even after further adjustment for HbA1c (aHR, 3.26; 95% CI, 1.45–7.33) (Table 3). Furthermore, not receiving metformin increased risk of early mortality (aHR, 6.17; 95% CI, 2.24–17.04) compared with TB-only patients, and this risk was observed after further adjustment for HbA1c (aHR, 12.69; 95% CI, 4.06–39.67). Moreover, metformin reduced recurrence significantly (aHR, 0.18; 95% CI, 0.04–0.89) but had little impact on treatment failure (HR, 0.59; 95% CI, 0.26–1.33).
DISCUSSION
Recent interest in the synergistic impact of the TB and DM epidemics has led to recommendations for bidirectional screening [20, 21]. The International Union Against Tuberculosis and Lung Disease (Union) and the World Diabetes Foundation (WDF) urge DM-TB co-management during TB treatment [22–24], yet implementation remains uneven, perhaps in part because evidence remains limited and inconsistent [8, 25, 26]. We prospectively followed 799 TB patients with and without DM in a setting with high TB and DM prevalence. In our cohort, DM did not increase risk of our composite unfavorable TB treatment outcome (failure, recurrence, death). However, patients with DM were more likely to die during TB treatment. Furthermore, as compared with TB-only participants, post-TB treatment mortality was lower by nearly one-half among patients with TB and DM (although not statistically significantly so). These results, together with our finding that both newly diagnosed and known DM patients were at higher risk for early mortality, underscore the need for aggressive DM screening among TB patients and early initiation of treatment for newly diagnosed DM [17].
In contrast to several retrospective reports and a systematic review in LMICs [8, 11, 27], our prospective analysis does not indicate an independent association between DM and composite unfavorable TB treatment outcome, consistent with a recent report from South India [28]. Traditional risk factors such as low BMI [29, 30] and alcohol use [31] were associated with adverse outcomes; neither degree of hyperglygemia nor new DM was associated with unfavorable outcomes [28]. We found that low and normal BMI were more common among TB-DM participants than high BMI, a finding explained by studies that find that Indians generally have higher visceral adiposity index than their Western counterparts with the same body weight, leading to a high burden of insulin resistance, even among normal- or low-bodyweight Indians [28, 32]. However, as reported previously, we also found a non–statistically significant directionality between low BMI and DM and adverse treatment outcomes [28]. Moreover, we postulate that metformin use by over half of DM patients in our cohort may have mitigated the previously reported higher risk of unfavorable TB treatment outcomes associated with DM. This is based on our exploratory analyses that TBDM patients not receiving metformin had twice the risk of all-cause mortality (by 18 months) and an increased risk of death during TB treatment by >6-fold compared with patients with TB alone. Furthermore, metformin reduced the risk of recurrence among patients with TB-DM [33]. Metformin, the popular anti-DM drug, is being touted as a potential host-directed adjuvant in TB therapy, following reports of reduced Mycobacterium tuberculosis (Mtb) growth in macrophages in Mtb-infected mice [34, 35]. Furthermore, retrospective studies associate metformin use with reduced TB incidence among DM patients and reversal of DM-associated mortality during TB treatment [11, 36, 37], as well as reduced TB recurrence [33]. Taken together, these findings suggest that TB outcomes might improve with metformin use among TB-DM patients, but this needs further exploration.
Our cohort had 65 deaths during follow-up and 26 during TB treatment, and we further analyzed mortality risk in our cohort, arguably the most important negative outcome. Increased early mortality among patients with TB and DM is our most striking finding and was observed in patients with newly diagnosed and known DM. Respiratory complications were the leading cause of death in TB-DM patients, and CVD events were common. This finding is consistent with prior research that showed higher risk of mortality due to CVD within 3 months of TB diagnosis among TB-DM than patients with TB alone [38, 39]. A South India study showed that endothelial inflammatory markers associated with increased risk of CVD were higher among patients with TB-DM at treatment initiation, providing a plausible biological explanation for early mortality [40–42].
Our study is not without limitations. First, the sample size was powered to measure the independent impact of DM on the composite unfavorable TB treatment outcome, not individual TB treatment outcomes. However, our mortality analyses add depth to our understanding of the impact of DM on TB outcomes even if underpowered. Rollout of the new daily TB regimen in India during the study presents another limitation. Because more TB-DM patients received the daily regimen than TB-only participants, the effect of DM on outcomes may have been underestimated. Although the daily regimen decreased the composite unfavorable outcome in univariable analysis, adjusting for this variable in our primary model did not impact the results. Further, our stratified analysis indicates no association between DM and the composite outcome for either regimen (daily or thrice weekly).
In conclusion, clear evidence from India, a TB-DM epicenter with 27% of TB cases globally (a staggering 2.8 million cases) and high DM prevalence [1], is critical to guide management of DM-associated TB. In our prospective observational TB cohort in India, DM did not increase the risk of composite unfavorable TB treatment outcome but significantly increased the risk of mortality, particularly during TB treatment—the most important outcome for patients and clinicians. Metformin appeared to mitigate this risk. These findings underline the importance of close monitoring and immediate treatment when DM is discovered during screening efforts [43, 44].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the clinic and research staff of BJMC-SGH and Dr. D.Y. Patil Medical College for their immense contributions. We acknowledge the Revised National Tuberculosis Control Program, Maharashtra, for participant referrals and support of the research. We thank Katherine McIntyre for copyediting the manuscript. We would like to acknowledge Trupti Sawant (data manager) for her contribution to the study.
Financial support. This work was supported by the National Institutes of Health under the following awards: R01A1I097494 to J.G.; DAA3-18-64774-1(via CRDF Global) to A.G.; UM1AI069465 to A.G; and D43TW009574 to R.L (via Johns Hopkins University). Support was also received from the Government of India’s Department of Biotechnology as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium.
Disclaimer. The content of this paper is solely the responsibility of the authors and does not necessary represent the official views of the funders.
Potential conflicts of interest. All authors declare no conflict of interest for this work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. V.M., N.G., A.G.1, N.S., D.K., A.K.2, K.E.D., and J.E.G. conceived the study, and J.E.G. obtained funding. V.M., S.G., R.L., S.D.1, D.K., R.B., A.K.1, N.P., S.R., N.S., S.D.2, S.A., T.S., M.B., S.M., A.K.2, S.C., and V.K. ran the study and collected data. N.G. performed data analyses, and V.M., N.G., A.K.2, A.G.1, A.G.2, H.K., K.E.D., and J.E.G. conducted data interpretation. V.M. and J.E.G. drafted the initial manuscript, and all authors assisted in manuscript preparation and approved the manuscript.
References
- 1. World Health Organization. Global tuberculosis report 2020. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed 20 October 2020.
- 2. Djoussé L, Driver JA, Gaziano JM, et al. Association between modifiable lifestyle factors and residual lifetime risk of diabetes. Nutr Metab Cardiovasc Dis 2013; 23:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. International Diabetes Federation. Diabetes Atlas. 9th ed. 2020. Available at: https://www.diabetesatlas.org/data/en/country/93/in.html. Accessed 7 January 2021.
- 4. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018; 138:271–81. [DOI] [PubMed] [Google Scholar]
- 5. Central Tuberculosis Division, Directorate General of Health Sciences. Annual report, TB India 2016_Part1. Available at: http://tbcindia.nic.in/showfile.php?lid=3180. Accessed 23 January 2017.
- 6. Mave V, Nimkar S, Prasad H, et al. Tuberculosis screening among persons with diabetes mellitus in Pune, India. BMC Infect Dis 2017; 17:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Odone A, Houben RM, White RG, Lonnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol 2014; 2:754–64. [DOI] [PubMed] [Google Scholar]
- 8. Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011; 9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harries AD, Kumar AM, Satyanarayana S, et al. Diabetes mellitus and tuberculosis: programmatic management issues. Int J Tuberc Lung Dis 2015; 19:879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeon CY, Harries AD, Baker MA, et al. Bi-directional screening for tuberculosis and diabetes: a systematic review. Trop Med Int Health 2010; 15:1300–14. [DOI] [PubMed] [Google Scholar]
- 11. Degner NR, Wang JY, Golub JE, Karakousis PC. Metformin use reverses the increased mortality associated with diabetes mellitus during tuberculosis treatment. Clin Infect Dis 2018; 66:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruslami R, Aarnoutse RE, Alisjahbana B, et al. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health 2010; 15:1289–99. [DOI] [PubMed] [Google Scholar]
- 13. Dooley KE, Tang T, Golub JE, et al. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg 2009; 80:634–9. [PMC free article] [PubMed] [Google Scholar]
- 14. Huangfu P, Ugarte-Gil C, Golub J, et al. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis 2019; 23:783–96. [DOI] [PubMed] [Google Scholar]
- 15. Huangfu P, Pearson F, Ugarte-Gil C, Critchley J. Diabetes and poor tuberculosis treatment outcomes: issues and implications in data interpretation and analysis. Int J Tuberc Lung Dis 2017; 21:1214–9. [DOI] [PubMed] [Google Scholar]
- 16.Revised National TB Control Programme. India TB report 2019. Available at: https://tbcindia.gov.in/index1.php?lang=1&level=2&sublinkid=5358&lid=3450. Accessed 23 August 2020.
- 17. Mave V, Meshram S, Lokhande R, et al. Prevalence of dysglycemia and clinical presentation of pulmonary tuberculosis in Western India. Int J Tuberc Lung Dis 2017; 21:1280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol 2014; 44:617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kornfeld H, West K, Kane K, et al. High prevalence and heterogeneity of diabetes in patients with TB in South India: a report from the Effects of Diabetes On Tuberculosis Severity (EDOTS) study. Chest 2016; 149:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kapur A, Harries AD, Lönnroth K, et al. Diabetes and tuberculosis co-epidemic: the Bali Declaration. Lancet Diabetes Endocrinol 2016; 4:8–10. [DOI] [PubMed] [Google Scholar]
- 21. Harries AD, Kumar AM, Satyanarayana S, et al. Addressing diabetes mellitus as part of the strategy for ending TB. Trans R Soc Trop Med Hyg 2016; 110:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riza AL, Pearson F, Ugarte-Gil C, et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol 2014; 2:740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Critchley JA, Restrepo BI, Ronacher K, et al. Defining a research agenda to address the converging epidemics of tuberculosis and diabetes: part 1: epidemiology and clinical management. Chest 2017; 152:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin Y, Harries AD, Kumar AMV, et al. Management of Diabetes Mellitus-Tuberculosis: A Guide to The Essential Practice. Paris: International Union Against Tuberculosis and Lung Disease; 2018. [Google Scholar]
- 25. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLoS One 2017; 12:e0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siddiqui AN, Khayyam KU, Sharma M. Effect of diabetes mellitus on tuberculosis treatment outcome and adverse reactions in patients receiving directly observed treatment strategy in India: a prospective study. Biomed Res Int 2016; 2016:7273935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kornfeld H, Sahukar SB, Procter-Gray E, et al. Impact of diabetes and low body mass index on tuberculosis treatment outcomes. Clin Infect Dis 2020; 71:e392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhargava A, Chatterjee M, Jain Y, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS One 2013; 8:e77979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiménez-Corona ME, Cruz-Hervert LP, García-García L, et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax 2013; 68:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health 2009; 9:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wells JC, Pomeroy E, Walimbe SR, et al. The elevated susceptibility to diabetes in India: an evolutionary perspective. Front Public Health 2016; 4:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma Y, Pang Y, Shu W, et al. Metformin reduces the relapse rate of tuberculosis patients with diabetes mellitus: experiences from 3-year follow-up. Eur J Clin Microbiol Infect Dis 2018; 37:1259–63. [DOI] [PubMed] [Google Scholar]
- 34. Singhal A, Jie L, Kumar P, et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med 2014; 6:263ra159. [DOI] [PubMed] [Google Scholar]
- 35. Vashisht R, Brahmachari SK. Metformin as a potential combination therapy with existing front-line antibiotics for tuberculosis. J Transl Med 2015; 13:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marupuru S, Senapati P, Pathadka S, et al. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of South Indian tertiary healthcare facility. Braz J Infect Dis 2017; 21:312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pan SW, Yen YF, Kou YR, et al. The risk of TB in patients with type 2 diabetes initiating metformin vs sulfonylurea treatment. Chest 2018; 153:1347–57. [DOI] [PubMed] [Google Scholar]
- 38. Faurholt-Jepsen D, Range N, PrayGod G, et al. Diabetes is a strong predictor of mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients from Mwanza, Tanzania. Trop Med Int Health 2013; 18:822–9. [DOI] [PubMed] [Google Scholar]
- 39. Reed GW, Choi H, Lee SY, et al. Impact of diabetes and smoking on mortality in tuberculosis. PLoS One 2013; 8:e58044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kumar NP, Moideen K, Sivakumar S, et al. Tuberculosis-diabetes co-morbidity is characterized by heightened systemic levels of circulating angiogenic factors. J Infect 2017; 74:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar NP, Sridhar R, Banurekha VV, et al. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc 2013; 10:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kato M, Natarajan R. Diabetic nephropathy—emerging epigenetic mechanisms. Nat Rev Nephrol 2014; 10:517–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tiberi S, du Plessis N, Walzl G, et al. Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect Dis 2018; 18:e183–98. [DOI] [PubMed] [Google Scholar]
- 44. Wallis RS, Maeurer M, Mwaba P, et al. Tuberculosis—advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect Dis 2016; 16:e34–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.