Abstract
Background
Pyomyositis is a bacterial infection of skeletal muscle that classically leads to abscess formation. A related, but distinct, entity is infectious myositis. The epidemiology of these infections has changed in recent years.
Methods
To better characterize both pyomyositis and infectious myositis, we conducted a retrospective study at our tertiary care institution. We identified 43 cases of pyomyositis and 18 cases of infectious myositis treated between January 2012 and May 2020.
Results
The mean age of patients was 48 years, and 66% were male. Diabetes mellitus affected one third of patients, and 16% had other immunocompromising comorbidities. Staphylococcal species accounted for 46% of all infections, and common symptoms included muscle pain (95%) and subjective fever (49%). Altered mental status was a presenting symptom in 16% of cases. Approximately half of all patients received >1 class of antibiotic, and the median length of antimicrobial therapy was 18 days. Open and percutaneous drainage procedures figured prominently in the management of these infections, with 28% of patients requiring multiple procedures. Pathology specimens were available for 12 of 61 cases. Overall, the treatment success rate was 84%.
Conclusions
Gram-positive bacteria accounted for most infections at our institution, and management commonly involved open or percutaneous drainage procedures. Future studies that prospectively evaluate treatment strategies for pyomyositis and infectious myositis are warranted.
Keywords: infectious myositis, muscle, pyomyositis, Staphylococcus aureus
We report 61 cases of pyomyositis and infectious myositis. Gram-positive bacteria accounted for most infections, and management commonly involved percutaneous or open drainage procedures. Overall, successful outcomes occurred in 84% of cases.
Pyomyositis, or Myositis tropicans [1], is a bacterial infection of skeletal muscle that classically leads to abscess formation, and its pathogenesis is thought to involve hematogenous seeding of muscle [2]. Staphylococcus aureus is the most frequent culprit, and group A streptococcus or other streptococcal species are occasionally isolated [3]. A related, but distinct, entity is infectious myositis. This is generally defined as muscle inflammation caused by an infectious agent, and cases involving bacterial, fungal, parasitic, and viral pathogens have been reported [3].
As its alternate name implies, pyomyositis was first described in tropical climates and mainly affected young adult males or pediatric patients [2, 3]. Pyomyositis was previously rare in temperate climates [2, 3], and only 98 cases were reported in North America between 1971 and 1992 [1]. The epidemiology has since changed. A recent population-based study demonstrated a 3-fold increase in the incidence of pyomyositis-related hospitalizations in the United States between 2002 and 2014 [4]. Staphylococcus aureus was found to account for approximately half of all pyomyositis cases in a recent retrospective study conducted at 3 institutions [5].
For infectious myositis, the epidemiology is less well defined due to the extensive list of causative agents [3]. Fungal pathogens have been found to preferentially affect immunocompromised hosts, and the spectrum of parasitic pathogens is informed by patients’ travel histories or environmental exposures. The rising prevalence and complexity of immunocompromising conditions [6] warrant further studies on infectious myositis.
Several large studies on pyomyositis have been conducted in subtropical or tropical climates [7–10], and most evidence from temperate climates is confined to small case series or case reports [2], with some notable exceptions [5, 11, 12]. To better characterize both pyomyositis and infectious myositis, we conducted a retrospective study at our institution. We chose to analyze both pyomyositis and infectious myositis because they represent infectious syndromes in which muscle is the primary site of involvement. To our knowledge, this is the largest single-center dataset on both pyomyositis and infectious myositis in a temperate climate. We reviewed all cases treated between January 2012 and May 2020 and aimed to (1) characterize clinical presentations and microbiology, (2) describe management strategies and outcomes, and (3) analyze the pathology of pyomyositis and infectious myositis.
METHODS
Study Overview
We retrospectively reviewed the electronic medical records of all patients with pyomyositis or infectious myositis who received their care in the Yale New Haven Health System between January 2012 and May 2020. Cases were identified after searching for all adult patients with 1 or more of the following International Classification of Diseases (ICD)-9 or ICD-10 diagnoses: infective myositis (728.0), tropical pyomyositis (040.81), infective myositis (M60.000–M60.078), infective myositis (M60.00–M60.09), tropical pyomyositis (M60.0). The pathology and microbiology databases at our institution were also queried. Yale University Institutional Review Board approved our study and waved the need for informed consent. Deidentified data were stored in a secure, encrypted fashion over the course of this study.
Definitions and Data Collection
We defined pyomyositis as an intramuscular collection attributed to a suspected or proven infectious etiology. To be included, cases had to have a collection demonstrated on imaging studies and/or found during surgical exploration, drainage, or aspiration of the involved site. We defined infectious myositis as inflammation of 1 or more skeletal muscle groups with suspected or proven infectious etiology appreciated on imaging studies and/or surgical exploration of the site. For both pyomyositis and infectious myositis, cases had to be accompanied by symptoms and clinical data congruent with an infectious process. We excluded cases resulting from contiguous spread (eg, intrabdominal infection, osteomyelitis, diabetic foot wound, etc), retained foreign bodies, or surgical intervention at the involved site within the preceding month. Cases resulting from contiguous spread were excluded because they represent a secondary process as opposed to a primary infection. In addition, classic descriptions do not consider genuine pyomyositis to arise from contiguous spread [2, 3]. Consistent with Infectious Diseases Society of America guidelines [13], cases resulting from hematogenous spread or minor trauma were included.
Medical records were individually reviewed. We collected in-depth information on preceding events, presenting symptoms, laboratory abnormalities, microbiology, antimicrobial therapy, surgical management, and pathology reports. For antimicrobial therapy, we recorded length of therapy, identity of antimicrobial agents, and whether patients had documented nonadherence or episodes of leaving the hospital against medical advice (AMA). Microbiology results were used to divide cases into 4 groups: staphylococcal, streptococcal, culture-negative, and other. The “Other” category encompasses Gram-negative bacteria, polymicrobial infections, Actinomyces spp, Nocardia spp, fungi, and viruses.
Demographic data on age, sex, body mass index, and select comorbidities were also collected. We labeled cases as having an immunocompromised status if their medical history included 1 or more of the following: active malignancy, cirrhosis, end stage renal disease, person with human immunodeficiency virus (HIV), recent use of immunosuppressive therapies, hematopoietic stem cell transplant, or solid organ transplant.
Treatment outcomes of pyomyositis and infectious myositis cases were categorized as successes or failures. Success was defined as resolution of the infection without need to restart antimicrobial therapy or perform additional surgical interventions. Failure was defined as the need to restart antimicrobial therapy or perform additional surgical interventions during a case’s follow-up period at our institution. All deaths attributable to pyomyositis or infectious myositis were also categorized as failures. Medical records were reviewed until death, transition of care to an outside institution, loss to follow up, or the end of this study’s review period in September 2020.
Statistical Analyses
Demographic data were summarized with descriptive statistics. One-way analysis of variance was used to investigate the difference between groups for continuous variables among patients who had staphylococcal species infections, streptococcal species infections, culture-negative infections, and infections in the “Other” group. Fisher’s exact test was used for categorical variables. Statistical analyses were performed using Stata statistical software 15.1 (StataCorp, College Station, TX). P < .05 was considered statistically significant.
RESULTS
Demographics, Clinical Presentation, and Microbiology of Pyomyositis and Infectious Myositis
We identified 43 cases of pyomyositis and 18 cases of infectious myositis treated at our institution between January 2012 and May 2020. Table 1 summarizes demographic information and presenting symptoms for all patients in our study. The mean age was 48 years, and 34% of patients were female. Staphylococcal cases had a preponderance of males (86% males), and differences in the distribution of male patients between groups were significant (P = .02). In total, 33% (20 of 61) were affected by diabetes mellitus. There was a significant difference in the distribution of immunocompromising comorbidities between groups (P = .03), with 50% of patients in the Other group being affected.
Table 1.
Demographics and Clinical Presentation of Pyomyositis and Infectious Myositis
| Demographics and Clinical Information | Staphylococcal Species (N = 28) | Streptococcal Species (N = 7) | Culture-Negative (N = 16) | Other (N = 10) | Total (N = 61) | P Value |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 48 ± 16 | 47 ± 15 | 47 ± 21 | 52 ± 22 | 48 ± 18 | .94a |
| Male sex (No., %) | 24 (86%) | 3 (43%) | 8 (50%) | 5 (50%) | 40 (66%) | .02b |
| Body mass indexc (kg/m2) (mean ± SD) | 29 ± 4 | 27 ± 2 | 27 ± 3 | 27 ± 5 | 28 ± 4 | .49a |
| Diabetes mellitus (No., %) | 11 (39%) | 2 (29%) | 4 (25%) | 3 (30%) | 20 (33%) | .81b |
| Immunocompromising comorbidityd (No., %) | 3 (11%) | 1 (14%) | 1 (6%) | 5 (50%) | 10 (16%) | .03b |
| Clinical Presentation | ||||||
| Pyomyositis cases (No., %) | 24 (86%) | 5 (71%) | 8 (50%) | 6 (60%) | 43 (70%) | .06b |
| Myositis cases (No., %) | 4 (14%) | 2 (29%) | 8 (50%) | 4 (40%) | 18 (30%) | .06b |
| Intravenous drug use in past month (No., %) | 3 (11%) | 3 (43%) | 2 (13%) | 0 | 8 (13%) | .10b |
| Rigorous exercise in past month (No., %) | 3 (11%) | 0 | 1 (6%) | 0 | 4 (7%) | .88b |
| No. of days between symptom onset and presentation (median, range) | 7 (1–30) | 4 (2–21) | 3 (1–21) | 8 (1–30) | 5 (1–30) | .19a |
| Muscle pain (No., %) | 27 (96%) | 6 (86%) | 16 (100%) | 9 (90%) | 58 (95%) | .26b |
| Tenderness to palpation (No., %) | 18 (64%) | 5 (71%) | 13 (81%) | 6 (60%) | 42 (69%) | .60b |
| Strength or range of motion change (No., %) | 7 (25%) | 2 (29%) | 7 (44%) | 4 (40%) | 20 (33%) | .61b |
| Area of induration or palpable mass (No., %) | 9 (32%) | 2 (29%) | 3 (19%) | 3 (30%) | 17 (28%) | .86b |
| Subjective fever (No., %) | 13 (46%) | 5 (71%) | 7 (44%) | 5 (50%) | 30 (49%) | .70b |
| Malaise (No., %) | 11 (39%) | 3 (43%) | 5 (31%) | 6 (60%) | 25 (41%) | .57b |
| Night sweats (No., %) | 3 (11%) | 1 (14%) | 2 (13%) | 1 (10%) | 7 (11%) | 1.00b |
| Weight loss (No., %) | 3 (11%) | 0 | 0 | 1 (10%) | 4 (7%) | .55b |
| Altered mental status (No., %) | 6 (21%) | 0 | 2 (13%) | 2 (20%) | 10 (16%) | .66b |
| Temperature (mean ± SD, °F) | 100.0 ± 1.6 | 101.0 ± 2.2 | 100.0 ± 1.8 | 100.2 ± 2.3 | 100.1 ± 1.8 | .62a |
| White blood cell count (103/μL) (mean ± SD) | 15 ± 6 | 14 ± 4 | 12 ± 3 | 13 ± 6 | 14 ± 5 | .17a |
| ≥5% bands (No., %) | 6 (21%) | 3 (43%) | 1 (6%) | 1 (10%) | 11 (18%) | .18b |
| Blood culture positive (No., %) | 13 (46%) | 1 (14%) | 0 | 3 (30%) | 17 (28%) | .003b |
Abbreviations: SD, standard deviation.
aOne-way analysis of variance.
bFisher’s exact test.
cOne patient’s body mass index is missing from the streptococcal species group’s data.
dCirrhosis, person with human immunodeficiency virus, hematopoietic stem cell transplant, solid organ transplant, end stage renal disease, active malignancy, or receipt of immunosuppressive regimen.
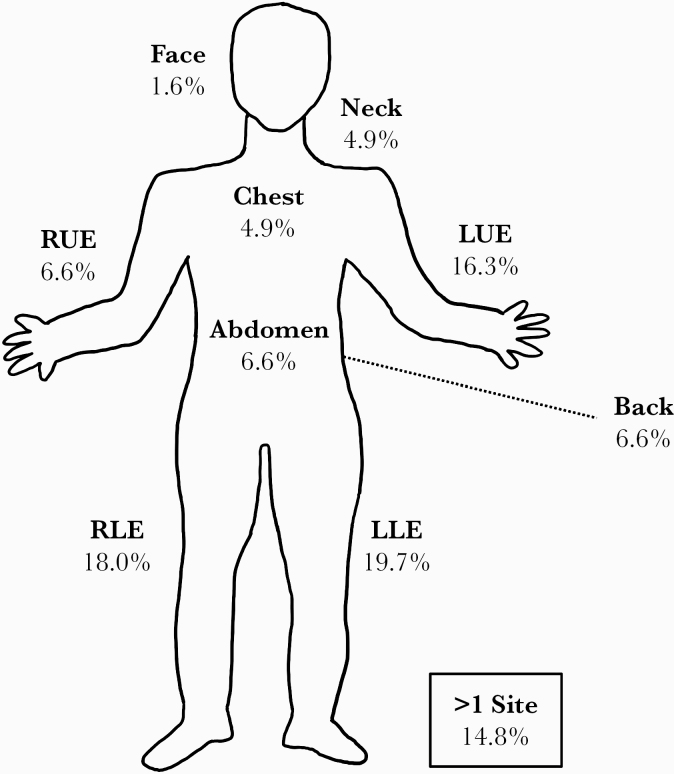
Figure 1 displays the body regions involved in pyomyositis and infectious myositis cases. Upper and lower extremities were most commonly affected. The duration of symptoms before presentation ranged from 1 to 30 days with a median of 5 days. Common complaints were muscle pain (95%), tenderness to palpation (69%), and subjective fevers (49%). It is interesting to note that 16% of patients presented with altered mental status. All cases had abnormal imaging studies at 1 or more times during their hospital course. In total, 11 of 61 patients (18%) had history of intravenous drug use, with 8 of 11 reporting intravenous drug use less than 1 month before admission. Seven patients (7 of 61; 11%) had reported cocaine, opioid, or marijuana use at some point in their past. Approximately one fifth (11 of 61) of patients had bandemia, and blood cultures were positive in 28% of cases. Culture positivity varied between groups (P = .003), with staphylococcal cases having the highest rate (46%).
Figure 1.
Sites of involvement for pyomyositis and infectious myositis cases. LLE, left lower extremity; LUE, left upper extremity; RLE, right lower extremity; RUE, right upper extremity.
Staphylococcal species accounted for the majority of culture-positive cases (62%), with methicillin-sensitive S aureus (MSSA) in 61% of staphylococcal cases and methicillin-resistant S aureus in 29% of cases (Supplementary Figure S1). Streptococcal species were identified in 7 cases. One fourth (16 of 61) of pyomyositis and infectious myositis cases were culture-negative, with 67% of these cases receiving empiric antibiotics before cultures of blood, wound, and/or body fluids were collected. Two of five polymicrobial cases were caused by a mix of staphylococcal and streptococcal species, and 1 case of pyomyositis was caused by Gram-negative bacteria (Escherichia coli). We also identified 1 fungal (Cryptococcus neoformans) case and 1 viral (influenza A) case of infectious myositis.
Treatment and Outcome of Pyomyositis and Infectious Myositis
Table 2 summarizes the treatment modalities and outcomes of pyomyositis and infectious myositis cases treated at our institution. The median length of hospital stay was 9 days (range, 0–57 days), and the median length of antimicrobial therapy was 18 days (range, 2–221 days). Statistically significant differences in length of hospital stay (P = .05) and antimicrobial therapy length (P = .04) existed between the groups, with staphylococcal cases having the longest median hospital stay (13 days) and treatment duration (32 days). Beta-lactam antibiotics and vancomycin were commonly used, and half of all patients (30 of 61) received more than 1 class of antibiotic. Differences in the use of vancomycin were statistically significant (P < .001), and culture-negative cases most frequently received it. Eighty-eight percent of the empiric treatment regimens for culture-negative cases included vancomycin, and 31% of patients in the culture-negative group received vancomycin and an antipseudomonal beta-lactam within 24 hours of presentation. Adverse events complicated the treatment course of 10 patients, with a morbilliform rash and fever occurring in 2 patients who received trimethoprim-sulfamethoxazole. Eight percent of patients had documented nonadherence as an outpatient or left the hospital AMA during their treatment course.
Table 2.
Treatment and Outcome of Pyomyositis and Infectious Myositis
| Treatment and Outcome Information | Staphylococcal Species (N = 28) | Streptococcal Species (N = 7) | Culture-Negative (N = 16) | Other (N = 10) | Total (N = 61) | P Value |
|---|---|---|---|---|---|---|
| Length of hospital stay in days (median, range) | 13 (2–57) | 10 (0–24) | 6 (1–13) | 8 (5–46) | 9 (0–57) | .05a |
| Length of antimicrobial therapy in days (median, range) | 32 (3–183) | 9 (6–28) | 15 (2–43) | 22 (5–221) | 18 (2–221) | .04a |
| Antimicrobial Therapy | ||||||
| Beta-lactams (No., %) | 18 (64%) | 6 (86%) | 12 (75%) | 4 (40%) | 40 (66%) | .24b |
| Vancomycin (No., %) | 11 (39%) | 1 (14%) | 14 (88%) | 2 (20%) | 28 (46%) | <.00b |
| Fluoroquinolones (No., %) | 0 | 1 (14%) | 2 (13%) | 1 (10%) | 4 (7%) | .11b |
| Tetracyclines (No., %) | 3 (11%) | 0 | 2 (13%) | 1 (10%) | 6 (10%) | 1.00b |
| Trimethoprim-sulfamethoxazole (No., %) | 9 (32%) | 1 (14%) | 2 (13%) | 4 (40%) | 16 (26%) | .35b |
| Received >1 class of antibiotic (No., %) | 11 (39%) | 2 (29%) | 14 (88%) | 3 (30%) | 30 (49%) | .003b |
| Other class of antimicrobial agentc (No., %) | 5 (18%) | 1 (14%) | 6 (38%) | 4 (40%) | 16 (26%) | .32b |
| Adverse events related to antimicrobial therapy (No., %) | 7 (25%) | 0 | 2 (13%) | 1 (10%) | 10 (16%) | .49b |
| Documented nonadherence or left hospital AMA (No., %) | 2 (7%) | 1 (14%) | 1 (6%) | 1 (10%) | 5 (8%) | .84b |
| Surgical Intervention | ||||||
| Incision and drainage (No., %) | 13 (46%) | 3 (43%) | 2 (13%) | 4 (40%) | 22 (36%) | .13b |
| Interventional radiology drainage (No., %) | 15 (54%) | 2 (29%) | 1 (6%) | 4 (40%) | 22 (36%) | .01b |
| Multiple procedures required (No., %) | 11 (39%) | 2 (29%) | 1 (6%) | 3 (30%) | 17 (28%) | .10b |
| Treatment Outcomes | ||||||
| Success (No., %) | 25 (89%) | 6 (86%) | 15 (94%) | 5 (50%) | 51 (84%) | .03b |
Abbreviations: AMA, against medical advice.
aOne-way analysis of variance.
bFisher’s exact test.
cIncludes other antibiotic classes (eg, daptomycin), antivirals, antifungals, etc.
Incision and drainage surgical procedures (22 of 61) and interventional radiology (IR) drainage procedures (22 of 61) were equally common in the treatment of our study population. Significant differences in the prevalence of IR procedures were noted (P = .01), with only 1 culture-negative case undergoing IR drainage. Three patients required fasciotomies. Approximately one fourth of all patients underwent multiple procedures.
Overall, 84% of all pyomyositis and infectious myositis cases were successfully treated. Differences in outcomes between groups were statistically significant (P = .03), with the Other group having the lowest success rate (50%). Death occurred in 3 of 7 cases classified as treatment failures, and chronic osteomyelitis affected 2 of 7 cases with treatment failure. Three patients in our study population were lost to follow up shortly after discharge and were not classified as treatment successes due to an inadequate amount of follow-up time.
Pathology of Pyomyositis and Infectious Myositis
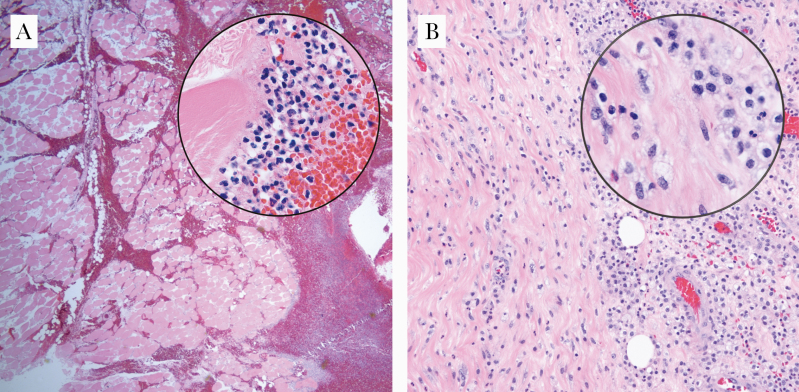
Pathology specimens were available for 12 of 61 cases treated at our institution, with 83% (10 of 12) demonstrating acute inflammation, myonecrosis, abscess formation, or a combination of these findings. Most specimens (11 of 12) were obtained from open or percutaneous procedures, with the exception of 1 specimen collected on autopsy. Figure 2 depicts representative slides from the influenza A myositis and Actinomyces spp pyomyositis cases.
Figure 2.
Influenza A myositis and Actinomyces spp pyomyositis. (A) Photomicrograph of the lower extremity of a 35-year-old female with influenza A infection (×40, hematoxylin and eosin [H&E]; inset: ×1000, H&E). Histologically, the muscle cells are devoid of nuclei, and there is an interstitial infiltrate composed of acute and chronic inflammatory cells. (B) Photomicrograph of the rectus muscle of an 80-year-old male with an intramuscular abscess, the culture of which grew Actinomyces spp (×100, H&E; inset: ×1000, H&E). Histologically, the muscle fibers are infiltrated by acute and chronic inflammatory cells.
Polymicrobial and Other Pyomyositis and Infectious Myositis Cases
In total, there were 10 cases of culture-positive pyomyositis and infectious myositis not caused by solely staphylococcal or streptococcal species. Table 3 summarizes these cases. Fifty percent of patients had immunocompromising comorbidities, and 1 patient had hereditary hemorrhagic telangiectasia (HHT). Polymicrobial infections accounted for 5 of 10 cases, and the following pathogens were identified in the remaining cases: Actinomyces spp, C neoformans, E coli, influenza A, and Nocardia farcinica. The majority (8 of 10) required surgical intervention. Five cases had successful treatment courses, 3 cases were fatal, and 2 patients were lost to follow up shortly after discharge.
Table 3.
Polymicrobial and Other Pyomyositis and Infectious Myositis Cases
| Infection | Age/Sex | Pathogen | Relevant Comorbid Conditions | Area of Involvement | Blood Cultures | Initial Pathogen-Specific Antimicrobial Therapy | Surgical Intervention | Outcome |
|---|---|---|---|---|---|---|---|---|
| Pyomyositis | 80/male | Actinomyces spp | Renal cell carcinoma | Right rectus muscle | Negative | TMP-SMX | IR drainage and biopsy followed by I&D | Success |
| Pyomyositis | 62/male | Eikenella corrodens, viridans Streptococcus | Hereditary hemorrhagic telangiectasia | Right masseter muscle | Negative | Moxifloxacin | Interventional neuroradiology aspiration and biopsy | Success |
| Pyomyositis | 75/female | Escherichia coli | Hemodialysis-dependent end stage renal disease | Left lower extremity | E coli | Ceftriaxone | I&D followed by IR drainage | Clinically stable at discharge then lost to follow up |
| Pyomyositis | 24/female | MSSA, MAC | person with HIV with CD4+ count 3/μL | Left upper extremity | MAC | Nafcillin then clarithromycin, amikacin, ethambutol (added when AFB culture returned) | None | Died months after initial presentation from failure to thrive, disseminated MAC infection, and other complications |
| Pyomyositis | 63/male | MSSA, viridans Streptococcus | Diabetes mellitus, history of intravenous drug use, HBV, HCV | Left upper extremity | Negative | Vancomycin | I&D | Success |
| Pyomyositis | 75/female | Nocardia farcinica | Renal transplant recipient on immunosuppressive therapy, diabetes mellitus | Right lower extremity | Negative | TMP-SMX | IR drainage | Success |
| Myositis | 22/male | CoNS, Cutibacterium acnes | None | Left upper extremity and chest wall | Negative | Cefazolin | I&D | Success |
| Myositis | 36/male | CoNS, Group A Streptococcus | None | Right lower extremity | Negative | Empiric therapy | Fasciotomy | Clinically stable at discharge then lost to follow up |
| Myositis | 43/female | Cryptococcus neoformans | Renal transplant recipient on immunosuppressive therapy, diabetes mellitus | Left lower extremity | C neoformans | Fluconazole, liposomal amphotericin B | None | Readmitted shortly after discharge and died from sepsis |
| Myositis | 35/female | Influenza A | None | Diffuse | Stenotrophomonas maltophilia (from autopsy) | Oseltamivir | Numerous fasciotomy procedures | Death |
Abbreviations: AFB, acid-fast bacillus; CoNS, coagulase negative Staphylococcus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; I&D, incision and drainage; IR, interventional radiology; MAC, Mycobacterium avium complex; MSSA, methicillin-sensitive Staphylococcus aureus; TMP-SMX, trimethoprim-sulfamethoxazole.
Patient Consent Statement
The study protocol was reviewed and approved by Yale University Institutional Review Board (protocol no. 2000028148). All work performed during the study period was in accordance with the ethical standards of our institution as well as those detailed by the 1964 Helsinki declaration and its later amendments. Need for inform consent was waived by Yale University Institutional Review Board.
DISCUSSION
We conducted a retrospective study at our institution and identified 61 cases of pyomyositis and infectious myositis caused by a wide array of pathogens. Staphylococcal species accounted for 46% of all infections, and common symptoms included muscle pain and fever. Approximately half of all patients received >1 class of antibiotic, with open and percutaneous drainage procedures figuring prominently in the management of these infections. Overall, the treatment success rate was 84%.
Maravelas et al [4] reported an association between male sex and pyomyositis, and we also found a preponderance of males in our study (66%). It is interesting to note that differences in the distribution of male cases between groups in our study was statistically significant (P = .02), with 86% of staphylococcal infections found in men. Pyomyositis has been associated with HIV [14]; however, we only identified 1 case of pyomyositis in a person with HIV. Four patients in our study had active malignancies, and 2 patients had received solid organ transplants. Diabetes mellitus was common among pyomyositis and infectious myositis cases in our study, and the epidemiology of these infections in the United States is likely impacted by that of metabolic syndrome [15, 16]. Highly vascularized skeletal muscle is resistant to infection [3], but the risk of infectious syndromes affecting muscle may be heightened by the impact of diabetes mellitus on both microvasculature and immune responses [17]. The rising incidence of pyomyositis may also be affected by better care for patients with compromised immune systems, which translates to larger numbers of hosts susceptible to atypical infectious syndromes. Finally, extensive imaging studies are often obtained for patients in resource-rich settings, and previously unrecognized sources of infection are now brought to light.
The clinical presentation of pyomyositis or infectious myositis involved a range of symptoms, with most being expected. Muscle pain was almost universal (95%), and this has been previously reported [5]. Most notably, we found that altered mental status was part of the symptom constellation in 16% of cases, and patients with fulminant infections are known to develop varying degrees of encephalopathy [18]. Given that history taking is limited in altered patients, pyomyositis or infectious myositis may merit consideration when evaluating febrile patients with an absence of localizing symptoms. At best, screening for pyomyositis or infectious myositis is ill defined, but point-of-care ultrasound may be a safe place to start.
We were not surprised to find that staphylococcal species accounted for 56% of the 43 pyomyositis cases in our study. This high prevalence is consistent with other retrospective studies in temperate climates [5, 11], and Maravelas et al [4] reported methicillin-sensitive and methicillin-resistant S aureus as the most common bacteria implicated in pyomyositis cases in the United States. We found 8 of 43 (19%) pyomyositis cases to be culture-negative, and Burdette et al [5] similarly reported 27% of cases as culture-negative. The notable proportion of culture-negative cases likely results from administration of broad-spectrum, empiric antibiotics before obtaining specimens for laboratory evaluation. Inciting pathogens in culture-negative cases were likely staphylococcal species or polymicrobial, and the negative cultures simply result from preceding antimicrobial therapy as opposed to fastidious or atypical pathogens that fail to grow on typical media. Contrary to several reports [2–4], our series only identified 1 culture-positive case of group A streptococcus, which suggests that the epidemiology of these infections is a changing landscape.
We report several atypical cases of pyomyositis and infectious myositis. One case of masseter muscle pyomyositis occurred in a 62-year-old man with HHT. We are not aware of any reports of pyomyositis in patients with HHT, but it is known that these patients are at increased risk for cerebral abscesses and other infections due to arteriovenous malformations [19]. We also report a case of Mycobacterium avium complex (MAC) and MSSA pyomyositis in a woman with HIV. She was nonadherent to prescribed medications and ultimately died from disseminated MAC infection and other complications. The C neoformans, influenza A, and Streptococcus pneumoniae cases in our series have been reported elsewhere [20–22].
Our study demonstrates that pyomyositis and infectious myositis is caused by a diverse assemblage of pathogens, and treatment outcomes can be excellent. Nonetheless, there are limitations inherent to the retrospective design of our study. We searched extensively for cases at our institution; however, we are unable to determine the true incidence of infections given that cases may not have been captured by our search strategy. In addition, the clinical judgement behind treatment decisions was that of individual providers, so we are unable to ascertain which criteria were applied when selecting length of therapy or deciding on need for surgical intervention. Prospective studies that stratify patients based on inciting pathogen or infection severity and formally assess the outcomes of treatment modalities are warranted. Finally, infectious complications of opioid use disorder are notable [23], and 13% of our cohort reported recent intravenous drug use, with all patients who left AMA having histories of intravenous drug use. Further exploration of the barriers to care and treatment strategies for patients with substance use disorders is needed [24].
CONCLUSIONS
The incidence of pyomyositis in the United States is rising, [4] and we found staphylococcal species to be the culprits in the majority of cases at our institution. We also report a large number of infectious myositis cases, and increased vigilance for atypical pathogens is warranted based on the results of our study. Future studies that prospectively evaluate treatment strategies for pyomyositis and infectious myositis are warranted.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. C. R. decided study concept and design, collected data, and wrote the manuscript. S. G. assisted with data collection and helped write the manuscript. Y. S. N. performed the statistical analyses and helped write the manuscript. D. P. assisted with data collection and helped revise the manuscript. S. D. assisted with data collection and helped revise the manuscript. M. G. decided study concept and design, revised the manuscript, and conducted study supervision. All authors contributed to the manuscript and its review.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis 1992; 15:668–77. [DOI] [PubMed] [Google Scholar]
- 2. Small LN, Ross JJ. Tropical and temperate pyomyositis. Infect Dis Clin North Am 2005; 19:981–9, x-xi. [DOI] [PubMed] [Google Scholar]
- 3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev 2008; 21:473–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maravelas R, Melgar TA, Vos D, et al. Pyomyositis in the United States 2002-2014. J Infect 2020; 80:497–503. [DOI] [PubMed] [Google Scholar]
- 5. Burdette SD, Watkins RR, Wong KK, et al. Staphylococcus aureus pyomyositis compared with non-Staphylococcus aureus pyomyositis. J Infect 2012; 64:507–12. [DOI] [PubMed] [Google Scholar]
- 6. Dropulic LK, Lederman HM. Overview of infections in the immunocompromised host. Microbiol Spectr 2016; doi: 10.1128/microbiolspec.DMIH2,0026-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma A, Kumar S, Wanchu A, et al. Clinical characteristics and predictors of mortality in 67 patients with primary pyomyositis: a study from North India. Clin Rheumatol 2010; 29:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martinez-de Jesus FR, Mendiola-Segura I. Clinical stage, age and treatment in tropical pyomyositis: a retrospective study including forty cases. Arch Med Res 1996; 27:165–70. [PubMed] [Google Scholar]
- 9. Yu CW, Hsiao JK, Hsiao JK, et al. Bacterial pyomyositis: MRI and clinical correlation. Magn Reson Imaging 2004; 22:1233–41. [DOI] [PubMed] [Google Scholar]
- 10. Borges AH, Faragher B, Lalloo DG. Pyomyositis in the upper Negro River Basin, Brazilian Amazonia. Trans R Soc Trop Med Hyg 2012; 106:532–7. [DOI] [PubMed] [Google Scholar]
- 11. Zalavras CG, Rigopoulos N, Poultsides L, Patzakis MJ. Increased oxacillin resistance in thigh pyomyositis in diabetic patients. Clin Orthop Relat Res 2008; 466:1405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soler R, Rodríguez E, Aguilera C, Fernández R. Magnetic resonance imaging of pyomyositis in 43 cases. Eur J Radiol 2000; 35:59–64. [DOI] [PubMed] [Google Scholar]
- 13. IDSA. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Available at: https://www.idsociety.org/practice-guideline/skin-and-soft-tissue-infections/. Accessed 29 November 2020.
- 14. Crum NF. Bacterial pyomyositis in the United States. Am J Med 2004; 117:420–8. [DOI] [PubMed] [Google Scholar]
- 15. Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988-2012. Prev Chronic Dis 2017; 14:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020. [Google Scholar]
- 17. Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev 2007; 23:3–13. [DOI] [PubMed] [Google Scholar]
- 18. Green R, Scott LK, Minagar A, Conrad S. Sepsis associated encephalopathy (SAE): a review. Front Biosci 2004; 9:1637–41. [DOI] [PubMed] [Google Scholar]
- 19. Dupuis-Girod S, Giraud S, Decullier E, et al. Hemorrhagic hereditary telangiectasia (Rendu-Osler disease) and infectious diseases: an underestimated association. Clin Infect Dis 2007; 44:841–5. [DOI] [PubMed] [Google Scholar]
- 20. Lier AJ, Virmani S, Ilagan-Ying Y, et al. Unilateral leg pain caused by cryptococcal myositis: an unusual presentation of disseminated cryptococcosis in a kidney transplant recipient. Transpl Infect Dis 2020; e13491. [DOI] [PubMed] [Google Scholar]
- 21. Odio CD, Mandimika C, Jabuonski TA, Malinis M. Severe influenza A(H1N1) virus infection complicated by myositis, refractory rhabdomyolysis, and compartment syndrome. Case Rep Med 2019; 2019:1540761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howard F, Sankey C. Pneumococcal bacteremia complicated by hemophagocytic lymphohistiocytosis. J Gen Intern Med 2019; 34:1653–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood) 2016; 35:832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seval N, Eaton E, Springer SA. Beyond antibiotics: a practical guide for the infectious disease physician to treat opioid use disorder in the setting of associated infectious diseases. Open Forum Infect Dis 2020; 7:ofz539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.