Abstract
Background
Our objective is to describe the prevalence of patients with internal anal sphincter achalasia (IASA) without Hirschsprung disease (HD) among children undergoing anorectal manometry (ARM) and their clinical characteristics.
Methods
We performed a retrospective review of high‐resolution ARM studies performed at our institution and identified patients with an absent rectoanal inhibitory reflex (RAIR). Clinical presentation, medical history, treatment outcomes, and results of ARM and other diagnostic tests were collected. We compared data between IASA patients, HD patients, and a matched control group of patients with functional constipation (FC).
Key results
We reviewed 1,072 ARMs and identified 109 patients with an absent RAIR, of whom 28 were diagnosed with IASA. Compared to patients with FC, patients with IASA had an earlier onset of symptoms and were more likely to have abnormal contrast enema studies. Compared to patients with HD, patients with IASA were more likely to have had a normal timing of meconium passage, a later onset of symptoms, and were diagnosed at an older age. At the latest follow‐up, the majority of patients diagnosed with IASA (54%) were only using oral laxatives. Over half of patients with IASA had been treated with anal sphincter botulinum toxin injection, and 55% reported a positive response.
Conclusions and Inferences
Patients diagnosed with IASA may represent a more severe patient population compared to patients with FC, but have a later onset of symptoms compared to patients with HD. They may require different treatments for their constipation and deserve further study.
Keywords: anorectal manometry, children, constipation, functional constipation, Hirschsprung's disease, internal anal sphincter achalasia
Internal anal sphincter achalasia (IASA) is a finding in children with intractable constipation that has not been well‐recognized. Children diagnosed with IASA had an earlier onset of symptoms and were more likely to have an abnormal contrast enema compared to children with intractable functional constipation. They may represent a more severe patient population and deserve further study.

Key message.
Anorectal manometry is regularly performed in children with constipation to evaluate for the presence of the recto‐anal inhibitory reflex (RAIR). Children with internal anal sphincter achalasia (IASA) have been minimally described.
In our retrospective review, an absent RAIR was not uncommon and children with IASA represented a more severe patient population compared to those with functional constipation, with a later onset of symptoms compared to those with Hirschsprung Disease.
Children with IASA may require different treatments for their constipation and deserve further study.
1. INTRODUCTION
Anorectal manometry (ARM) testing evaluates the neuromuscular function of the anus and rectum. It allows assessment of anal sphincter characteristics and function, defecation dynamics, rectal sensation, and the presence or absence of the rectoanal inhibitory reflex (RAIR). 1 It is the most commonly performed motility test in children. 1 Traditionally, the primary indication for ARM has been to evaluate for the presence of the RAIR and help exclude Hirschsprung disease (HD) in children with constipation. 1 The RAIR is an anal reflex mediated by a complex intramural neuronal plexus that results in relaxation of the internal anal sphincter following distention of the rectum by gas, feces, or, as is the case during ARM testing, inflation of a rectal balloon. 2 In patients with HD, the RAIR is absent due to the absence of ganglion cells in the distal gastrointestinal tract, which causes colonic dysmotility of a variable length and a risk of developing enterocolitis. 3 , 4 Patients with HD undergo surgery to remove the affected bowel and bring the ganglionic bowel down to the anus. Studies have shown that children with HD have a significantly lower quality of life compared with healthy children. 5 The gold standard for diagnosing HD is a full‐thickness rectal biopsy demonstrating absence of ganglion cells. 6 Studies have shown that ARM has a high sensitivity (91%) and specificity (94%) for diagnosing HD. 7 These values depend on the criteria used to diagnose or exclude HD with ARM, a recent study using rather strict values to exclude HD, found a positive predictive value of just 74%. 8 This would indicate that up to 26% of children who have an absent RAIR on ARM are eventually not diagnosed with HD.
There are multiple reasons why an ARM can show an absent RAIR in the presence of rectal ganglion cells. Examination‐related reasons include technical problems, such as air leakage, displacements of catheters, artifacts, or insufficient volume of rectal balloon inflations. In addition, distressed children may contract their external anal sphincter during balloon inflations and/or not allow adequate balloon filling, which may limit the ability to detect a RAIR. Non‐examination‐related causes of an absent RAIR include neuronal intestinal dysplasia or possible immaturity of the anorectal canal, as the literature is inconsistent in whether the RAIR is already present at birth or may develop later on in life. 9 , 10 , 11 In children with HD with a very short aganglionic segment, it is possible that a rectal biopsy misses the affected segment. 12 However, it may also be possible that children with an absent RAIR and present rectal ganglion cells may represent a different population and diagnosis with its own pathophysiology. Currently, the diagnosis of internal anal sphincter achalasia (IASA) is made when the RAIR is absent during ARM but ganglion cells are present on rectal biopsy. 13 Children with IASA have not been thoroughly described in the literature. 9 , 13 , 14 , 15 , 16 , 17
Our objective was therefore to evaluate the prevalence of IASA among children with constipation undergoing ARM, to describe the patient and clinical characteristics of children with IASA, and to compare them with children with HD and FC.
2. MATERIALS AND METHODS
We performed a retrospective review of all high‐resolution ARM studies performed in children ≤18 years of age at Nationwide Children's Hospital between August 2010 and April 2019, this period was chosen to respect the start of using a solid‐state manometry catheter at our institution.
A pediatrician with training in interpreting manometry testing assessed each ARM study for the presence or absence of a RAIR. For studies in which the second assessment differed from the original report by a pediatric gastroenterologist, another pediatric gastroenterologist with advanced training in motility disorders performed a third assessment. The study was considered inconclusive if rectal balloon inflation was not performed, if rectal balloon volumes were limited, or if adequate measurement of the RAIR was not possible due to low anal sphincter resting pressure. After identifying the studies with an absent RAIR, we identified a matched control group among the studies with a present RAIR. We matched patients based on age at time of the ARM, study condition (awake or asleep) and sex. We recorded outcomes of ARM testing, demographic information, medical and surgical history, and results of other relevant diagnostic testing. Patients with an absent RAIR were then grouped into four categories: diagnosis of HD (known or diagnosed with rectal biopsy), diagnosis of anorectal malformation, diagnosis of IASA, or unknown diagnosis. We diagnosed children with IASA if they 1) had an absent RAIR on ARM with adequate balloon inflation (at least 20 ml in infants, until reported sensation (generally pain or discomfort) in awake children, or at least 60 ml in asleep children 1 , 18 ) and 2) had a rectal biopsy (full‐thickness or suction) that showed the presence of ganglion cells. Among patients with an absent RAIR, we compared gender, medical history, symptom history, and age at diagnosis of those diagnosed with HD to those diagnosed with IASA. In addition, we compared gender, medical history, symptom history, symptoms, and treatment at time of ARM between those diagnosed with IASA and FC. We reviewed the treatment at latest follow‐up of IASA patients, including the effect of anal sphincter botulinum toxin injections.
2.1. Manometry protocol
ARM studies were performed using a solid‐state catheter (UniTip High Resolution Catheter, model number K12959‐L5‐1038‐D from Unisensor AG) according to our institutional protocol. Indications for ARM testing at our institution include evaluation for HD and measurement of anal sphincter resting pressure, pelvic floor dynamics, and rectal sensation. The study was performed awake if possible, with the patient lying on their left side. For those unable to tolerate an awake study, the study was performed with sedation or anesthesia while lying supine. All studies examined the resting pressure of the anal sphincter and involved graduated rectal balloon inflations to evaluate for a RAIR, until reported sensation, or until a maximum based on age and size if a child was not able to report sensation. If the study was performed awake, squeeze and push (or bear down) maneuvers were evaluated, in addition to evaluation of rectal sensory thresholds during rectal balloon inflations.
2.2. Analysis of manometric data
All analyses of manometric data were performed using a commercially available manometric system (Solar GI HRM v9.1, Medical Measurement Systems (MMS), Enschede, the Netherlands). We concluded that a RAIR was present when we observed a drop of >15% in internal anal sphincter pressure during a balloon inflation. 19 We measured this percentage by calculating the mean percent relaxation during the three balloon inflations with the highest volumes. If during one of those three balloon inflations the resting pressure was extremely low or the catheter seemed to migrate, we used prior measurements with smaller balloon volumes instead. The resting anal sphincter pressure was calculated as the mean pressure during a resting period of at least 20 seconds. This was usually measured at the beginning of the study. However, if a child was awake and very nervous, a more accurate measurement was obtained at the end of the study. For patients with an absent RAIR, we also recorded the presence or absence of a "pressure column." We noticed in our practice that in some patients with an absent RAIR, not only there is no decrease in internal anal sphincter pressure, but also a rise in pressure extending proximally from the anal canal, as shown in Figure 1. We compared the prevalence of the pressure column in patients with IASA and HD. Other manometry outcomes were only compared between patients with IASA and FC.
Figure 1.
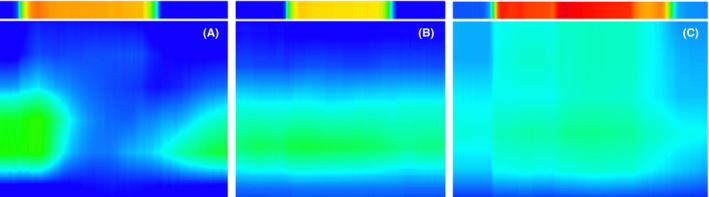
Anorectal manometry studies during balloon inflation. Upper column shows pressure of the balloon inflation, and lower part shows effect on anorectal canal. Pressures are visualized gradually by colors from dark blue (lowest pressure = 0 mm Hg) to red (highest pressure = 200 mm Hg). A: present rectoanal inhibitory reflex (RAIR), B: absent RAIR, C: absent RAIR with "pressure column"
2.3. Statistical analyses
Normally distributed continuous data are presented as mean and standard deviation; non‐normally distributed continuous data are presented as medians and interquartile ranges; and categorical data are presented as frequencies and percentages. Comparison of normally distributed continuous variables was conducted using t‐tests; comparison of non‐normally distributed variables was conducted using Mann–Whitney U test; and comparison of categorical data was conducted using Fisher's exact test. P‐values were corrected for multiple comparisons using Holm–Bonferroni correction. P‐values less than 0.05 were considered statistically significant. Statistical analyses were conducted with SPSS for Windows, version 24.0.0.0 (SPSS, Inc).
3. RESULTS
We reviewed ARM studies of 1072 patients (50% female, median age 7 years at time of study, IQR 4‐11 years, range 0‐18 years). Twenty‐nine studies were inconclusive for presence or absence of a RAIR and were therefore excluded, leaving the total number of studies included at 1043. As shown in Figure 2, 111 patients (11%) had an absent RAIR on ARM. Of the 111 patients, 60 (54%) had been previously diagnosed with either HD (51/60) or an anorectal malformation (9/60). All patients who were previously diagnosed with HD or an anorectal malformation had undergone a surgical intervention prior to the manometry study. The patients with known HD or anorectal malformations had their diagnosis established in the first year of life except in 8 cases (13%). Of the 51/111 (46%) patients with an absent RAIR without a prior diagnosis, 8 (16%) had a repeat ARM within months of the first study, of whom two were found to have a RAIR the second time around. Both patients had their first ARM performed awake and were reported to be severely distressed during the study, resulting in maximum balloon inflations of only 20 and 40 ml. The repeat ARM done under general anesthesia allowed for larger balloon inflations which resulted in demonstration of a RAIR (at 30 and 60 ml respectively). None of the other patients with absent RAIR and awake manometry were reported to be severely distressed or uncooperative during the study. Out of the 49 patients with an absent RAIR, a rectal biopsy pathology report was available to us in 31 (63%) cases. The pathology report of 28 patients noted a presence of ganglion cells, and a diagnosis of IASA was made. For the remaining three patients, two had biopsies with no ganglion cells and they were therefore diagnosed with HD and one patient's biopsies were inconclusive. One of the newly diagnosed HD patients was a 9‐month‐old infant with severe constipation, and the second one was an 18‐year‐old with autism spectrum disorder. There were 18 patients out of the total 49 (37%) without an available rectal biopsy result. Some had biopsies performed at outside institutions, and some were waiting to have their biopsies performed at the time of data collection or had not yet had a biopsy ordered. The prevalence of IASA in children without a prior diagnosis of HD or anorectal malformation who underwent an ARM study was therefore 2.8‐4.8% (28‐47/983).
Figure 2.
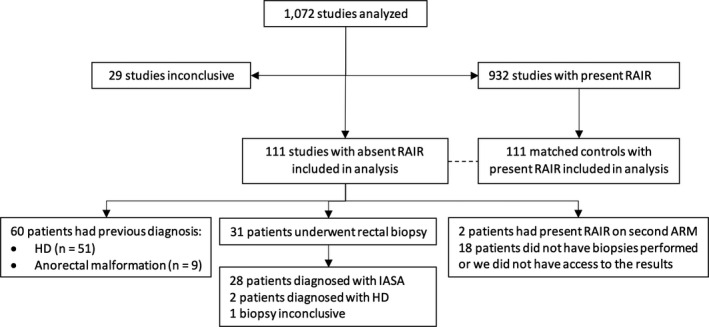
Patient flow diagram.
Abbreviations: ARM, anorectal manometry; HD, Hirschsprung Disease; IASA, internal anal sphincter achalasia; RAIR, rectoanal inhibitory reflex
3.1. Anorectal manometry findings
Comparison of ARM findings among groups is shown in Table 1. Since rectal sensation of children with IASA was only reported in a limited number of children, we did not compare rectal sensation between patient groups. Of the patients with IASA, seven reported first sensation (median 20 ml [IQR 10‐40]), four reported urge sensation (median 30 ml [IQR 25‐35]), and five reported discomfort (median 150 ml [IQR 90‐150]). No statistically significant differences were found in ARM outcomes between patients with IASA and FC. In addition, we found no significant difference in the presence of a pressure column between patients with HD and IASA.
Table 1.
Anorectal manometry results by diagnosis
| IASA (n = 28) | HD (n = 53) | FC (n = 111) | |
|---|---|---|---|
| Age at procedure, mean in years (SD) | 7.21 (5.0) | 8.6 (4.3) | 8.15 (4.3) |
| Procedure awake, n (%) | 10 (35.7%) | 31 (58.5%) | 61 (54.9%) |
| Resting pressure in mmHg, mean (SD) | 48.9 (19.9) | 52.3 (22.9) | 62.5 (20.7) |
| Maximum squeeze pressure in mmHg, median (IQR) | 132 (94‐250) | 227 (162‐282) | 213 (174‐276) |
| Duration squeeze in seconds, median (IQR) | 12.6 (10.4‐15.7) | 12.1 (9.5‐14.9) | 13.5 (11.9‐17.5) |
| Pressure column, n (%) | 8 (28.6%) | 10 (18.9%) | n/a |
| Abnormal push test, n (%) | 5/8 (62.5%) | 16/26 (61.5%) | 25/54 (46.3%) |
Abbreviations: FC, functional constipation; HD, Hirschsprung Disease; IASA, internal anal sphincter achalasia.
3.2. Comparison of patient populations
As shown in Table 2, compared to patients with HD, patients with IASA were more likely to have a later onset of symptoms (p = 0.013), a normal meconium passage (p = 0.005), and were older at diagnosis (p < 0.001). Compared to patients with FC, patients with IASA were more likely to have an earlier onset of symptoms (p = 0.043). As shown in Table 3, patients with IASA were more likely to have abnormal contrast enemas, mainly with redundancy or segmental dilation of the colon, when compared to patients with FC (p = 0.003). Colonic manometry findings did not differ between patients with IASA and FC.
Table 2.
Comparison of clinical characteristics by diagnosis
| IASA (n = 28) | HD (n = 53) | FC (n = 111) | |
|---|---|---|---|
| Male | 15 (54%) | 38 (71%) | 73 (66%) |
| Medical history | |||
| Prematurity <37 weeks, n/N (%) | 6/26 (23%) | 7/48 (15%) | 25/104 (24%) |
| Extreme (<28 weeks), n/N (%) | 2/26 (7.7%) | 0/48 (0%) | 4/104 (3.8%) |
| Very preterm (28‐32 weeks), n/N (%) | 2/26 (7.7%) | 0/48 (0%) | 4/104 (3.8%) |
| Moderate preterm (32‐37 weeks), n/N (%) | 2/26 (7.7%) | 7/48 (15%) | 17/104 (16%) |
| Trisomy 21, n (%) | 2 (7.1%) | 6 (11%) | 0 (0%) |
| Spinal cord disorder, n (%) | 1 (3.6%) | 1 (1.9%) | 0 (0%) |
| Developmental delay, n (%) | 7 (25%) | 5 (9.4%) | 21 (19%) |
| Behavioral disorders, n (%) | 5 (17%) | 6 (11%) | 39 (35%) |
| Autism, n (%) | 4 (14%) | 3 (5.7%) | 8 (7.1%) |
| Symptom history | |||
| Age at start symptoms in years, median (IQR) | 0 (0‐0) | 0 (0‐0)* | 2 (0‐4)* |
| Meconium <24 hours, n (%) | 6 (33%) | 2 (5%) | 14 (31%) |
| Meconium >48 hours, n (%) | 1 (5.6%) | 30 (75%)** | 4 (9.1%) |
| Previous admissions for clean‐out, n (%) | 15 (52%) | n/a | 24 (21%) |
| Age at diagnosis in years, median (IQR) | 6 (4‐12) | 0 (0‐0)*** | n/a |
| Symptoms at time of ARM | |||
| Constipation, n (%) | 28 (100%) | 29 (55%) | 108 (97%) |
| Fecal incontinence, n (%) | 13 (68%) | 44 (88%) | 70 (75%) |
| Treatment at time of ARM | |||
| No medication, n (%) | 3 (11%) | 12 (23%) | 8 (7.2%) |
| Oral laxatives, n (%) | 24 (86%) | 22 (42%) | 92 (83%) |
| Rectal suppositories, n (%) | 5 (18%) | 7 (13%) | 8 (7.2%) |
| Rectal enemas, n (%) | 0 (0%) | 4 (7.5%) | 4 (3.6%) |
| Antegrade continence enemas, n (%) | 0 (0%) | 5 (9.4%) | 10 (9.0%) |
| Loperamide, n (%) | 1 (3.6%) | 8 (15%) | 1 (0.9%) |
| Anal sphincter botulinum toxin injections, n (%) | 2 (7.1%) | 10 (20%) | 2 (1.8%) |
Abbreviations: ARM, anorectal manometry; FC, functional constipation; HD, Hirschsprung Disease; IASA, internal anal sphincter achalasia.
Denotes P‐value <0.05 when compared to IASA; **P‐value <0.01, ***P‐value <0.001.
Table 3.
Comparison of colonic manometry and contrast enema results of IASA and FC patients.
| IASA (n = 28) | FC (n = 111) | |
|---|---|---|
| Colonic manometry, total, n | 13 | 39 |
| Normal, n (%) | 7 (54%) | 27 (69%) |
| Colonic dysmotility, n (%) | 6 (46%) | 12 (31%) |
| Contrast enema, total, n | 22 | 55 |
| Normal, n (%) | 7 (32%) | 45 (82%)** |
| Redundant/distended colon, n (%) | 13 (59%) | 7 (13%)** |
| Concern for HD, n (%) | 2 (9.1%) | 3 (5.5%) |
Abbreviations: IASA, internal anal sphincter achalasia; FC, functional constipation; HD, Hirschsprung Disease.
Denotes P‐value <0.01.
3.3. Internal anal sphincter achalasia patients
At the latest follow‐up (mean follow‐up time of 1.3 years), ongoing medication treatment consisted of only oral laxatives in 15 (54%) patients, antegrade continence enemas in 7 (25%) patients, oral and rectal medications in 3 (11%) patients, and only rectal suppositories in 1 (3.6%) patient. Two patients (7.1%) were not using any constipation‐related medications but had an ostomy. The first patient had distal colonic dysmotility with a redundant and dilated sigmoid colon and had a transverse loop colostomy performed. The second patient presented with septic shock, a proximal sigmoid stricture of unknown etiology and diffuse colitis, and underwent a laparoscopic sigmoid colectomy with end colostomy. Anal sphincter botulinum toxin injections were performed in 15/28 (54%) of IASA patients, ranging from one to seven injections per patient. Most (53%) had had only one injection. Patients with more than one injection had these three to nine months apart. We had follow‐up data of nine of the 15 patients, and five (55%) reported an improvement in bowel movement frequency ranging in duration from one week up to three months.
4. DISCUSSION
To our knowledge, this is the largest study evaluating the likelihood of children having an absent RAIR on ARM and describing subgroups of children diagnosed with IASA. In our cohort, 5.1% of the patients without a previous diagnosis undergoing ARM had an absent RAIR, and 2.9% were eventually diagnosed with IASA. The only other study reporting a prevalence of IASA was done by Caluwé et al., and instead of reviewing all patients undergoing ARM, they reviewed all patients undergoing rectal biopsy and found a prevalence of 15/332 (4.5%) in six years. 14 At our institution, there may be a lower threshold to perform an ARM, since we use it not only to evaluate for HD, but also to evaluate for hypertensive anal sphincter, pelvic floor dyssynergia, and rectal hypersensitivity. This may explain the lower prevalence we found compared to Caluwé et al.
When comparing ARM parameters between patients with IASA and patients with FC, we did not find any statistically significant differences. In addition to traditional ARM measurements, we examined the presence of a "pressure column" as visualized in Figure 1. This increase in pressure expanding more proximally into the rectum was visible in some patients with absent RAIR but was not specific for HD or IASA. We speculate that this could be an artifact, it could be the result of an increase in pressure between a non‐relaxing anal sphincter and the rectal balloon with limited rectal compliance, or it could be the manifestation of a tonic anal wall contraction or spasm mediated by nitrergic nerve depletion. 15 , 20 Because of the limited available data on rectal sensation in the IASA group, we did not compare their rectal sensory thresholds to patients with FC. It is however possible that due to the high frequency of abnormal contrast enemas and prolonged symptom history, patients in the IASA group have increased rectal sensory threshold as described in another study by Ciamarra et al. 13 However, the limited amount of data on rectal sensation that we collected in patients with IASA did not indicate the presence of extremely increased rectal sensory thresholds.
Clinically, we found that patients with IASA had a later onset of symptoms, were more likely to have had normal meconium passage, and were diagnosed at an older age compared to patients with HD. Compared to patients with FC, patients with IASA had an earlier onset of symptoms and were more likely to have an abnormal contrast enema. We found no difference in frequency of fecal incontinence. Although we did not systematically collect information about stool withholding in our population, we did not find a difference in the likelihood of an abnormal push test in patients with IASA and FC, a finding that some consider the manometric equivalent of stool withholding. 21 We compared our findings with a previous study by Ciamarra et al. 13 Ciamarra et al. described a population of 20 children with IASA and also found that patients with IASA had an earlier onset of symptoms than those with FC. However, in their cohort, patients with IASA were less likely to have fecal incontinence and less likely to show withholding behavior. These differences may have resulted from the smaller and younger control group Ciamarra et al. used. Withholding behavior is known to be a major contributing factor to the development of constipation and is especially seen in younger children. 22 , 23 , 24
At follow‐up, the majority of patients diagnosed with IASA (54%) were only using oral laxatives to treat their constipation. More than half of the patients with IASA had been treated with anal sphincter botulinum toxin injections, and among the nine patients with follow‐up data, 55% reported a positive response. We found a much lower response rate and for a shorter duration compared to that reported in other studies, which ranged from 92 to 95% and a response duration that ranged from one week to more than 18 months. 13 , 25 This may be because of the retrospective nature of our study design and incomplete follow‐up data, or other studies may have overestimated the effect of the injections. Other studies investigating the effects of anal sphincter botulinum toxin injections found it to be a safe intervention with a positive response in children regardless of anal sphincter dynamics, including the presence or absence of a RAIR. 26 , 27 If response to anal sphincter botulinum toxin injections is similar between children with FC or IASA, it may be worth revisiting the clinical significance of the diagnosis of IASA. If the diagnosis has no effect on response to treatment, management would then still consist of constipation treatment according to severity regardless of the presence or absence of a RAIR. Another treatment which has been studied in children with IASA is a posterior internal anal sphincter myectomy and a meta‐analysis found it to have better outcomes than anal sphincter botulinum toxin injections. 16 However, potential detrimental effects of a procedure that weakens the anal sphincter may not become apparent for years and some experts advise to avoid this procedure if possible. 28
These findings allow us to have a better understanding of the significance of the internal anal sphincter and the diagnosis of IASA. Patients with IASA appear to represent a more severe patient population compared to patients with FC (earlier onset of symptoms, more likely to have redundancy or segmental dilation of the colon), but with later symptom onset compared to patients with HD. Our data raise the question of whether the internal anal sphincter can lose its ability to relax due to longstanding constipation. If this were true, we would expect IASA to be more common in patients with a longer history of constipation. However, IASA is very rare in adults, who in general have a longer history of defecatory complaints than children. 29 The pathophysiology of IASA is not fully understood and thought to be multifactorial. 15 Altered intramuscular innervation, specifically nitrergic nerve depletion, defective innervation of the neuromuscular junction, and an altered distribution of the c‐kit‐positive interstitial cells of Cajal are thought to be causes of impaired inhibitory innervation of the efferent loop of the rectoanal reflex. 15 Prospective studies are needed to demonstrate whether the finding of an absent RAIR in a child with IASA is one that is permanent or that can resolve with time. Still, the association between IASA and a more severe phenotype raises the question of whether children with IASA warrant more aggressive medical treatment and closer follow‐up—and whether IASA could one day be used as a prognostic factor. Children with an earlier onset of constipation and who have colonic redundancy or dilation may therefore also warrant prompt anorectal manometry to not only evaluate for HD but also for IASA, particularly if future studies better delineate whether children with IASA are more likely to respond to specific interventions (targeting outlet dysfunction) than children with FC. We believe that the absence of a RAIR in a child with FC, while still incompletely understood, is meaningful and worthy of further investigation.
It is interesting that two patients were found to have an absent RAIR while awake but had a RAIR when evaluated under anesthesia. Both patients were described as severely distressed during their awake studies, a factor which interfered with the study protocol and prevented inflation to higher rectal balloon volumes. It is possible that these two patients obscured the RAIR by increasing their external anal sphincter pressure, although we feel it is more likely the balloon inflations during ARM were insufficient as in both of these patients the RAIR was only elicited when asleep at higher balloon volumes compared with the volumes used during their awake studies.
Strengths of our study include the large number of reviewed ARM tests by at least two physicians and the extended chart review of the IASA patients, including results of other relevant tests. Limitations of our study include the retrospective study design and the relatively short follow‐up of IASA patients. We also have to be aware of the possible limitations of ARM, especially with more IASA patients having abnormal contrast enemas, it is possible that in children with a dilated rectum the balloon inflations were insufficient to trigger a RAIR. 30
In conclusion, we demonstrate that the absent RAIR is not so rare in children and confirm that it is not always absent due to the diagnosis of HD. Patients diagnosed with IASA may represent a more severe patient population compared to patients with FC, but have a later onset of symptoms compared to patients with HD. Patients with IASA may require different treatment strategies for their constipation. Future research should focus on prospectively evaluating outcomes of children with IASA, including whether the RAIR remains absent in these patients after appropriate treatment of constipation. In order to adequately study this patient population, we suggest that experts in the field develop consensus on a standardized way of diagnosing children with IASA.
CONFLICT OF INTERESTS
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
DFB, MM, CDL, and PLL involved in design of the work. DFB, MM, and PLL contributed to acquisition and analysis of data. DFB contributed to analysis of data. DFB, MAB, NB, KHV, DY, CDL, and PLL contributed to interpretation of data. DFB and PLL drafted the initial manuscript. MM, MAB, NB, KHV, DY, and CDL critically revised the manuscript for important intellectual content. All authors made substantial contributions to the work, approved the final version of the manuscript as submitted, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGMENTS
Preliminary findings were presented during poster presentation sessions at the 2019 NASPGHAN/APGNN/CPNP Annual Meeting in Chicago, IL, USA, October 17‐19, 2019, and at the 2020 Digestive Disease Week in Chicago, IL, USA, May 2‐5, 2020.
Funding informationDesiree F. Baaleman received financial support from the Prins Bernhard Cultuurfonds with support from the Jadefonds, and the VSBfonds to conduct this research.
REFERENCES
- 1. Rodriguez L, Sood M, Di Lorenzo C, Saps M. An ANMS‐NASPGHAN consensus document on anorectal and colonic manometry in children. Neurogastroenterol Motil. 2017;29(1):8. [DOI] [PubMed] [Google Scholar]
- 2. Sangwan YP, Solla JA. Internal anal sphincter: advances and insights. Dis Colon Rectum. 1998;41(10):1297–1311. [DOI] [PubMed] [Google Scholar]
- 3. Kenny SE, Tam PKH, Garcia‐Barcelo M. Hirschsprung's disease. Semin Pediatr Surg. 2010;19(3):194–200. [DOI] [PubMed] [Google Scholar]
- 4. de Lorijn F, Boeckxstaens GE, Benninga MA. Symptomatology, pathophysiology, diagnostic work‐up, and treatment of Hirschsprung disease in infancy and childhood. Curr Gastroenterol Rep. 2007;9(3):245–253. [DOI] [PubMed] [Google Scholar]
- 5. Collins L, Collis B, Trajanovska M, et al. Quality of life outcomes in children with Hirschsprung disease. J Pediatr Surg. 2017;52(12):2006–2010. [DOI] [PubMed] [Google Scholar]
- 6. de Lorijn F, Reitsma JB, Voskuijl WP, et al. Diagnosis of Hirschsprung's disease: a prospective, comparative accuracy study of common tests. J Pediatr. 2005;146(6):787–792. [DOI] [PubMed] [Google Scholar]
- 7. de Lorijn F, Kremer LC, Reitsma JB, Benninga MA. Diagnostic tests in Hirschsprung disease: a systematic review. J Pediatr Gastroenterol Nutr. 2006;42(5):496–505. [DOI] [PubMed] [Google Scholar]
- 8. Meinds RJ, Trzpis M, Broens PMA. Anorectal Manometry May Reduce the Number of Rectal Suction Biopsy Procedures Needed to Diagnose Hirschsprung Disease. J Pediatr Gastroenterol Nutr. 2018;67(3):322–327. [DOI] [PubMed] [Google Scholar]
- 9. Puri P, Gosemann JH. Variants of Hirschsprung disease. Semin Pediatr Surg. 2012;21(4):310–318. [DOI] [PubMed] [Google Scholar]
- 10. Howard ER, Nixon HH. Internal anal sphincter. Observations on development and mechanism of inhibitory responses in premature infants and children with Hirschprung's disease. Arch Dis Child. 1968;43(231):569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lorijn FD, Omari TI, Kok JH, Taminiau JAJM, Benninga MA. Maturation of the rectoanal inhibitory reflex in very premature infants. The Journal of Pediatrics. 2003;143(5):630–633. [DOI] [PubMed] [Google Scholar]
- 12. Neilson IR, Yazbeck S. Ultrashort Hirschsprung's disease: Myth or reality. J Pediatr Surg. 1990;25(11):1135–1138. [DOI] [PubMed] [Google Scholar]
- 13. Ciamarra P, Nurko S, Barksdale E, Fishman S, Di Lorenzo C. Internal Anal sphincter achalasia in children: clinical characteristics and treatment with clostridium botulinum Toxin. J Pediatr Gastroenterol Nutr. 2003;37(3):315–319. [DOI] [PubMed] [Google Scholar]
- 14. Caluwé DD, Yoneda A, Akl U, Puri P. Internal anal sphincter achalasia: Outcome after internal sphincter myectomy. J Pediatr Surg. 2001;36(5):736–738. [DOI] [PubMed] [Google Scholar]
- 15. Doodnath R, Puri P. Internal anal sphincter achalasia. Semin Pediatr Surg. 2009;18(4):246–248. [DOI] [PubMed] [Google Scholar]
- 16. Friedmacher F, Puri P. Comparison of posterior internal anal sphincter myectomy and intrasphincteric botulinum toxin injection for treatment of internal anal sphincter achalasia: a meta‐analysis. Pediatr Surg Int. 2012;28(8):765–771. [DOI] [PubMed] [Google Scholar]
- 17. Youn JK, Han J‐W, Oh C, Kim S‐Y, Jung S‐E, Kim H‐Y. Botulinum toxin injection for internal anal sphincter achalasia after pull‐through surgery in Hirschsprung disease. Medicine (Baltimore). 2019;98(45):e17855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Lorenzo C, Hillemeier C, Hyman P, et al. Manometry studies in children: minimum standards for procedures. Neurogastroenterol Motil. 2002;14(4):411–420. [DOI] [PubMed] [Google Scholar]
- 19. Faure C, Thapar N, Di Lorenzo C. Pediatric Neurogastroenterology: Gastrointestinal Motility and Functional Disorders in Children. Springer; 2016. 10.1007/978-3-319-43268-7 [DOI] [Google Scholar]
- 20. Keef KD, Cobine CA. Control of motility in the internal anal sphincter. J Neurogastroenterol Motil. 2019;25(2):189–204. 10.5056/jnm18172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Lorenzo C. Childhood constipation: finally some hard data about hard stools!. J Pediatr. 2000;136(1):4–7. [DOI] [PubMed] [Google Scholar]
- 22. Dehghani SM, Kulouee N, Honar N, Imanieh MH, Haghighat M, Javaherizadeh H. Clinical manifestations among children with chronic functional constipation. Middle East J Dig Dis. 2015;7(1):31–35. [PMC free article] [PubMed] [Google Scholar]
- 23. Loening‐Baucke V. Constipation in early childhood: patient characteristics, treatment, and longterm follow up. Gut. 1993;34(10):1400–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Engelenburg‐van Lonkhuyzen ML, Bols EMJ, Benninga MA, Verwijs WA, de Bie RA. Bladder and bowel dysfunctions in 1748 children referred to pelvic physiotherapy: clinical characteristics and locomotor problems in primary, secondary, and tertiary healthcare settings. Eur J Pediatr. 2017;176(2):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Irani K, Rodriguez L, Doody DP, Goldstein AM. Botulinum toxin for the treatment of chronic constipation in children with internal anal sphincter dysfunction. Pediatr Surg Int. 2008;24(7):779–783. [DOI] [PubMed] [Google Scholar]
- 26. Zar‐Kessler C, Kuo B, Belkind‐Gerson J. Botulinum toxin injection for childhood constipation is safe and can be effective regardless of anal sphincter dynamics. J Pediatr Surg. 2018;53(4):693–697. [DOI] [PubMed] [Google Scholar]
- 27. Halleran DR, Lu PL, Ahmad H, et al. Anal sphincter botulinum toxin injection in children with functional anorectal and colonic disorders: A large institutional study and review of the literature focusing on complications. J Pediatr Surg. 2019;54(11):2305–2310. [DOI] [PubMed] [Google Scholar]
- 28. Keshtgar AS, Ward HC, Clayden GS. Diagnosis and management of children with intractable constipation. Semin Pediatr Surg. 2004;13(4):300–309. [DOI] [PubMed] [Google Scholar]
- 29. Azpiroz F, Enck P, Whitehead WE. Anorectal functional testing: review of collective experience11Participants: L. M. A. Akkermanns, P. Arhan, F. Azpiroz, E. Corazziari, G. Coremans, M. Dapoigny, M. Delvaux, P. Denis, G. Devroede, P. Enck, B. Flourie, M. Hemond, V. Loening‐Baucke, S. Nordgren, M. M. Schuster, G. Tougas, A. Watier, A. Wald, W. E. Whitehead, and P. Whorwell. The American Journal of Gastroenterology. 2002;97(2):232–240. [DOI] [PubMed] [Google Scholar]
- 30. Thapar N, Borrelli O. Anorectal manometry for the diagnosis of hirschsprung disease: new heights for the balloon or just hot air? J Pediatr Gastroenterol Nutr. 2018;67(3):311–312. [DOI] [PubMed] [Google Scholar]


