Abstract
Objectives
The Medicines and Healthcare Products Regulatory Agency in the United Kingdom (UK) formally reclassified sildenafil citrate 50 mg tablets as a pharmacy medicine (sildenafil‐P) in 2017 for adult men with erectile dysfunction (ED). A 1‐year prospective real‐world observational study was conducted to track men's health behaviour, particularly their healthcare resource utilisation (HCRU) and quality of life (QoL) before and after the availability of sildenafil‐P.
Methods
Adult men with ED aged ≥18 years provided data at baseline (prior to launch of sildenafil‐P) and every 3 months after the launch. Demographics, health characteristics, treatments at baseline and HCRU, including number of pharmacist and physician/nurse practitioner visits over time are reported. QoL‐related outcomes were assessed via the Self‐Esteem and Relationship Questionnaire (SEAR), 2‐Item Patient Health Questionnaire and ratings of sexual satisfaction. Generalised linear models were used to assess the association of sildenafil‐P use with total physician/nurse practitioner and pharmacist visits and QoL‐related outcomes at 12 months.
Results
Overall, 1162 men completed the survey at all 5 time points. The mean ± SD age was 59.02 ± 12.06 years; 55.42% reported having a moderate‐to‐severe ED. Hypertension (37.52%) and hypercholesterolaemia (31.50%) were the most common risk factors for ED. At baseline, 62.99% were not using any ED treatment. After adjusting for baseline visits/other covariates, mean physician/nurse practitioner (3.68 vs 2.87; P = .003) and pharmacist visits for any reason (2.10 vs 1.34; P < .001) at 12 months were significantly higher among sildenafil‐P users than those who never used sildenafil‐P. Sildenafil‐P users also had significantly higher SEAR total and domain (sexual relationship and self‐esteem) scores at 12 months.
Conclusion
Following the reclassification to a pharmacy medicine in the UK, sildenafil‐P was associated with a higher number of physician/nurse practitioner and pharmacist visits for any reason. Sildenafil‐P use was also associated with better QoL, although group differences were small in magnitude.
Whats known
Erectile dysfunction (ED) is a result of underlying disorders and affects patients’ quality of life. However, men delay seeking treatment due to unwillingness to discuss ED with their physicians. In 2017, sildenafil citrate 50 mg was reclassified to a pharmacy medicine in the UK, which is expected to increase access to quality and legitimate care and healthcare visits.
Whats new
In the UK, the reclassifying of branded sildenafil to a pharmacy medicine was associated with a higher healthcare resource utilisation (number of physician/nurse practitioner and pharmacist visits for any reason) and better ED‐specific quality of life in the 12 months following the switch. The observed greater engagement with the healthcare system could facilitate early diagnosis and management of both ED and underlying comorbidities.
1. INTRODUCTION
Erectile dysfunction (ED) is defined as the persistent inability to achieve and/or maintain penile erection sufficient for performing sexual intercourse. 1 According to the multinational Men's Attitudes to Life Events and Sexuality (MALES) study, the overall self‐reported prevalence of ED among men aged 20–‐75 years was estimated at 16%. 2 A recent retrospective cross‐sectional study of men aged ≥18 years from eight countries reported an overall self‐reported ED prevalence of 40.5%, with a higher estimated prevalence of 42.6% in the United Kingdom (UK). 3 The prevalence and severity of ED increase with age, and prior research has consistently demonstrated that ED is normally the result of underlying cardiovascular or endocrine disease or depression. 4
ED adversely affects a patient's quality of life (QoL) and is associated with physical and psychological conditions, including depressive symptoms and anxiety regarding sexual performance. 5 , 6 Additionally, ED is associated with higher work productivity loss and activity impairment. 7 While ED is both underdiagnosed and undertreated, 8 there is evidence that higher uptake of effective ED treatments could improve well‐being and, given appropriate management arrangements, facilitate the earlier control of underlying disorders.
Phosphodiesterase type 5 inhibitors (PDE5Is), the first‐line therapy for ED, have been the main ED treatment since the launch of branded sildenafil in 1998. 9 , 10 , 11 PDE5Is have improved the treatment of ED, as well as the awareness of ED among the general public and clinicians. 9 , 10 Treatment with PDE5Is correlates with improved QoL, sexual satisfaction, adherence to treatment for comorbid health conditions, and emotional well‐being in men with ED. 12 , 13 , 14 Additionally, cost‐effectiveness research has shown that sildenafil use was associated with fewer costly hospitalisations/complications through improved adherence to other treatments for comorbidities. 13
Awareness of PDE5Is was shown to shorten the delay of time between the onset of ED symptoms and seeking treatment for ED. 15 Furthermore, availability and awareness of a new pharmacological option in the treatment of ED were associated with a change of behaviour among general practitioners and men with ED. 16 The number of men presenting with ED symptoms at general practitioner and specialist consultations, as well as sildenafil prescription rates, increased from 1997 to 2000; however, the percentage of men with ED who used treatment was generally low during this time period, ranging from 15% to 40%. 17 , 18 , 19 , 20 , 21 Additionally, new prescriptions of sildenafil by specialists have remained steady since 1999, with primary care physicians being the leading prescribers of sildenafil. 22 In a cross‐sectional study, only 5.6% of men were identified with an ED diagnosis or prescription for a PDE5I. 8 Furthermore, many men delay seeking treatment for over 2 years, on average, due to an unwillingness to discuss ED with their doctor. 23
In November 2017, the UK Medication and Healthcare Products Regulatory Agency approved the reclassifying of branded sildenafil 50 mg tablets to a pharmacy medicine (P). 24 This change to pharmacy sildenafil (sildenafil‐P) availability is expected to increase utilisation of PDE5Is and healthcare visits, based on a projection model. 25 Pharmacy availability will facilitate broader access to safe, effective treatment for ED. Accordingly, the role of community pharmacists will be integral in facilitating both treatment and preventative care for men with ED. 26 More active involvement by community pharmacists can help men to make more informed self‐care decisions regarding ED treatment.
As sildenafil‐P has been made available in the market only recently, 27 there were no previous studies that have evaluated the relationship between the use of sildenafil‐P and men's degree of engagement with the healthcare system and different aspects of their QoL. The objective of this study was to provide a real‐world assessment of the impact of sildenafil‐P availability on real‐world healthcare resource utilisation (HCRU) and QoL among men with ED in the UK.
2. METHODS
2.1. Study sample and design
This longitudinal real‐world observational study prospectively collected data from adult men (aged ≥18 years) in the UK general population, who could read and write in English and were willing to provide informed consent, using a self‐reported internet survey. Participants were recruited through the opt‐in online panel of Lightspeed Research (LSR), in which panelists choose to participate in surveys. LSR panels were formed in such a way as to represent the demographic characteristics of the adult general population in the UK (ie respondents are recruited from diverse online sources, such as partner panels, opt‐in emails, etc). The panel was regularly maintained by LSR, with panelists’ demographic information updated routinely to ensure appropriate sample selection. Men who completed the survey were compensated with reward points offered by the panel of which they were a member.
Data were collected from 30 March 2018 to 9 April 2019. At baseline, the study recruited 12,506 men with and without ED from the UK general population. The recruitment process was designed to ensure roughly similar proportions of men from the following age groups: 18‐39, 40‐49, 50‐64 and ≥65 years. During the baseline assessment, participants were screened for the presence of self‐reported ED via a single validated question from the Massachusetts Male Aging Study. 28 Participants who had ED in the baseline survey (pre‐launch of sildenafil‐P), according to their responses to the aforementioned screening item, were re‐invited to complete 3‐, 6‐, 9‐ and 12‐month follow‐up surveys. A brief “pulse” survey each month in between the follow‐up assessment points was conducted to maintain participant engagement with the study and collect additional details on treatment use. The study protocol was reviewed and granted exemption by the Sterling Institutional Review Board (Atlanta, GA, USA); participants provided their informed consent electronically.
2.2. Study variables
2.2.1. Sociodemographics, health characteristics and ED treatment use
Sociodemographic variables assessed included age, education, employment status, geographic region, income, race and marital status. Health characteristics variables assessed included body mass index (BMI), comorbidities and other risk factors (eg diabetes, depression, etc), smoking status, alcohol consumption, exercise, and overall level of life stress, as well as ED severity (assessed using the validated Erection Hardness Score 29 ) and other men's health conditions experienced (eg low testosterone, benign prostatic hyperplasia, etc). Comorbidity burden was measured via the Charlson Comorbidity Index (CCI). 30 The CCI is a validated measure that assigns weights to 12 different medical conditions (eg mild liver disease, metastatic solid tumour) and sums these weights to generate a total index score; scores can range from 0 to 24, with higher scores signifying greater comorbidity burden, Current prescription and non‐pharmacological (eg alternative medicine, herbal supplements) ED treatments and current use of medications for comorbid health conditions (eg nitrates, statins, alpha‐blockers) were also assessed.
2.2.2. HCRU outcomes
The number of physician/nurse practitioner visits for any reason was assessed by a single item: “In the past 3 months, how many times have you visited the doctor for any reason related to your own health?” The item instructions informed participants that the term “doctor” referred to general practitioners, specialists or nurse practitioners. The number of visits for cardiovascular reasons was assessed by two items: “Of the [fill in the number] visits to the doctor that you had in the past 3 months, how many times did you see each type of healthcare provider [cardiologist]?” and “Of the [fill in the number] visits to the doctor that you had in the past 3 months, what was the reason for each visit [heart disease check‐up/discussion]?” The number of pharmacist visits for any reason was assessed by a single item: “In the past 3 months, how many times have you visited the pharmacy and spoke to the pharmacist for any reason?” The number of pharmacist visits for sexual functioning discussions was assessed by a single item: “Of the [fill in the number] of pharmacist visits you had in the past 3 months, at how many did you discuss sexual function issues?”
2.2.3. QoL outcomes
ED‐specific QoL was assessed using the Self‐Esteem and Relationship Questionnaire (SEAR). 31 The SEAR is a 14‐item validated measure focusing on the impact of ED on psychosocial functioning and well‐being. For the SEAR, a total score is computed, as well as scores for each of four domains: sexual relationship, confidence, self‐esteem and overall relationship. Scores on this measure range from 0 to 100, with higher scores indicating better ED‐specific QoL. Positive depression screen was measured using the 2‐Item Patient Health Questionnaire (PHQ‐2). The PHQ‐2 has been validated as an initial screening tool for major depressive disorder. Scores on the PHQ‐2 can range from 0 to 6, with scores ≥ 3 indicating a positive screen for major depressive disorder. 32 In the current study, PHQ‐2 scores were dichotomised (ie positive depression screen: yes vs no). Sexual satisfaction was assessed using responses to a single item: “Which of the following best describes how satisfied you are with the frequency of sexual intercourse you currently engage in?” (1 = extremely dissatisfied to 5 = extremely satisfied).
2.3. Statistical analyses
Descriptive analysis included means and standard deviations (SDs) for continuous or discrete variables and frequencies and percentages for categorical variables. Descriptive analysis was conducted for all variables at baseline, as well as for the HCRU and QoL outcome variables at each time point. Multivariable analyses for all outcomes, except positive depression screen, used linear regression models, controlling for HCRU visits and QoL outcomes at baseline, as well as other relevant covariates, to estimate the association of sildenafil‐P use with HCRU and QoL outcomes at 12 months. Analysis for each outcome only included participants with complete data for the particular outcome. HCRU outcome variables were first transformed by taking the square root to approximate a normal distribution. After running the linear regression models, adjusted means were then back‐transformed to facilitate interpretation. For positive depression screen (yes vs no), logistic regression models were used to estimate the adjusted odds associated with sildenafil‐P use. P < .05 (two‐tailed) were considered statistically significant. Initial analysis was performed at each of the follow‐up time points (data not shown), with results reported for comparisons at 12 months.
For all multivariable models, covariates included sociodemographics, health characteristics and ED treatment variables, as well as HCRU visits and QoL outcomes at baseline, identified via backward stepwise elimination. The collective set of possible covariates were input as the first step of the process. In successive regression models, predictor variables were subsequently eliminated from the next model iteration based on results of the F‐test; predictors were retained in the next model iteration if P < .100, two‐sided. The process ended when no more predictor variables met the threshold for elimination.
Following the backward stepwise elimination process, the final list of baseline covariates included in the analysis for each HCRU outcome variable were as follows: (aa) CCI score, medication use for comorbid health conditions, cardiovascular disease (CVD), overall level of life stress and ED treatment use (physician/nurse practitioner visits for any reason), (b) depression (cardiologist visits), (c) smoking and ED treatment use (CVD check‐up visits), (d) CCI score, medication use for comorbid health conditions, depression, age and ED treatment use (pharmacist visits for any reason) and (e) household income (pharmacist visits for sexual functioning discussions). In addition to these baseline covariates, each linear regression model also controlled for the number of visits reported at baseline. For QoL outcomes, the final list of covariates were as follows: (a) CVD diagnosis, depression, ED severity, overall life stress and household income (SEAR total and domain scores), (b) age, ED severity, overall level of life stress, depression and household income (sexual satisfaction), and (c) age, overall level of life stress, CVD diagnosis, obesity, ED severity, CCI score, household income and medication use for comorbid health conditions (positive depression screen). Each linear regression model also controlled for these QoL outcomes at baseline.
3. RESULTS
Of the subset of 5185 men with ED who were surveyed at baseline, a total of 1162 completed the survey at all four follow‐up time points (3, 6, 9 and 12 months post‐launch of sildenafil‐P), thus comprising the final study sample for analysis. Overall, 234 men with ED in the final study sample reported using sildenafil‐P at ≥1 time point post‐launch, and 928 men never used sildenafil‐P at any time point during the study (hereafter referred to as never users).
3.1. Descriptive analysis
3.1.1. Sociodemographics, health characteristics and ED treatment
Men with ED had a mean ± SD age of 59.02 ± 12.06 years; sildenafil‐P users tended to be younger than never users (54.87 ± 13.21 vs 60.06 ± 11.53 years) (Table 1). Nearly two‐thirds of sildenafil‐P users (63.68%) were currently employed at baseline (full‐time, part‐time or self‐employed), whereas the majority (57.87%) of never users were not currently employed. Sildenafil‐P users and never users were generally similar on the other sociodemographic characteristics assessed (Table 1).
TABLE 1.
Baseline demographic characteristics by sildenafil‐P use
| Variable | Sildenafil‐P use | |
|---|---|---|
| Never users (n = 928) | Sildenafil‐P users (n = 234) | |
| n (%) | n (%) | |
| Age (mean ± SD) | 60.06 ± 11.53 | 54.87 ± 13.21 |
| Age category | ||
| 18‐39 | 60 (6.47) | 28 (11.97) |
| 40‐49 | 111 (11.96) | 60 (25.64) |
| 50‐64 | 344 (37.07) | 80 (34.19) |
| ≥65 | 413 (44.50) | 66 (28.21) |
| Education | ||
| No college degree | 483 (52.05) | 121 (51.71) |
| College degree | 444 (47.84) | 113 (48.29) |
| Prefer not to answer | 1 (0.11) | 0 (0.00) |
| Employment | ||
| Unemployed | 537 (57.87) | 85 (36.32) |
| Employed | 391 (42.13) | 149 (63.68) |
| Prefer not to answer | 0 (0.00) | 0 (0.00) |
| Household income | ||
| <£50k | 704 (75.86) | 176 (75.21) |
| ≥£50k | 184 (19.83) | 51 (21.79) |
| Prefer not to answer | 40 (4.31) | 7 (2.99) |
| Race | ||
| White | 891 (96.01) | 221 (94.44) |
| Non‐White | 34 (3.66) | 12 (5.13) |
| Prefer not to answer | 3 (0.32) | 1 (0.43) |
| Marital status | ||
| Married/domesticpartner | 663 (71.44) | 153 (65.38) |
| Not married | 265 (28.56) | 81 (34.62) |
| Region | ||
| Scotland | 92 (9.91) | 17 (7.26) |
| Wales | 50 (5.39) | 19 (8.12) |
| Northern Ireland | 16 (1.72) | 6 (2.56) |
| England | 768 (82.76) | 192 (82.05) |
| Other UK region | 2 (0.22) | 0 (0.00) |
Abbreviations: sildenafil‐P, pharmacy medicine sildenafil; SD, standard deviation; UK, United Kingdom.
Sildenafil‐P users and never users were likewise similar in terms of most baseline health characteristics (Table 2). The majority (55.42%) reported having a moderate or severe ED in the past month. Almost three‐quarters had either overweight (sildenafil‐P users: 42.33% vs never users: 47.92%) or obese BMI (sildenafil‐P users: 24.19% vs never users: 27.27%). Hypertension (37.52%) and hypercholesterolaemia (31.50%) were the most commonly reported ED risk factors, although these conditions tended to be reported less often by sildenafil‐P users than never users (hypertension: 26.50% vs 40.30%; hypercholesterolaemia: 25.64% vs 32.97%). Sildenafil‐P users (vs never users) more frequently reported smoking (41.89% vs 21.65%) and drinking alcohol (80.35% vs 74.89%) ≥1 day per week. Additionally, sildenafil‐P users more often reported exercising ≥1 day in the past week (83.34% vs 73.93%), relative to never users (Table 2).
TABLE 2.
Baseline health characteristics by sildenafil‐P use
| Variable | Sildenafil‐P use | |
|---|---|---|
| Never users (n = 928) | Sildenafil‐P users (n = 234) | |
| n (%) | n (%) | |
| BMI (mean ± SD) | 28.16 ± 5.43 | 27.45 ± 4.75 |
| BMI category | ||
| Underweight (<18.5 kg/m2) | 5 (0.56) | 3 (1.40) |
| Normal weight (18.5 to <25.0 kg/m2) | 216 (24.24) | 69 (32.09) |
| Overweight (25.0 to <30.0 kg/m2) | 427 (47.92) | 91 (42.33) |
| Obese (≥30.0 kg/m2) | 243 (27.27) | 52 (24.19) |
| CCI (mean ± SD) | 0.42 ± 1.10 | 0.47 ± 1.55 |
| CCI category | ||
| 0 | 736 (79.31) | 190 (81.20) |
| 1 | 92 (9.91) | 18 (7.69) |
| ≥2 | 100 (10.78) | 26 (11.11) |
| Diabetes | 163 (17.56) | 33 (14.10) |
| Depression | 143 (15.41) | 51 (21.79) |
| CVD | 77 (8.30) | 16 (6.84) |
| Hypertension | 374 (40.30) | 62 (26.50) |
| Hypercholesterolemia | 306 (32.97) | 60 (25.64) |
| Number of days smoked in past week | ||
| Did not smoke in past week | 410 (44.18) | 69 (29.49) |
| Smoked on 1‐7 days in past week | 201 (21.65) | 98 (41.89) |
| Have never smoked | 317 (34.16) | 67 (28.63) |
| Number of days consumed alcohol in past week | ||
| Did not consume alcohol in past week | 211 (22.74) | 33 (14.10) |
| Consumed alcohol on 1‐7 days in past week | 695 (74.89) | 188 (80.35) |
| Have never drank alcohol | 22 (2.37) | 13 (5.56) |
| Number of days exercised in past week | ||
| Did not exercise in past week | 242 (26.08) | 39 (16.67) |
| Exercised on 1‐2 days in past week | 265 (28.56) | 72 (30.77) |
| Exercised on 3‐7 days in past week | 421 (45.37) | 123 (52.57) |
| Overall level of life stress | ||
| Extremely stressful | 31 (3.34) | 8 (3.42) |
| Very stressful | 76 (8.19) | 30 (12.82) |
| Moderately stressful | 243 (26.19) | 73 (31.20) |
| Somewhat stressful | 269 (28.99) | 68 (29.06) |
| Not at all stressful | 309 (33.30) | 55 (23.50) |
| Erection problem | ||
| None | 0 (0.00) | 0 (0.00) |
| Minimal/mild | 402 (43.32) | 116 (49.57) |
| Moderate | 326 (35.13) | 75 (32.05) |
| Severe | 200 (21.55) | 43 (18.38) |
| EHS | ||
| Penis does not enlarge | 100 (10.78) | 20 (8.55) |
| Penis is larger, but not hard | 153 (16.49) | 31 (13.25) |
| Penis is hard, but not hard enough for penetration | 227 (24.46) | 56 (23.93) |
| Penis is hard enough for penetration, but not completely hard | 355 (38.25) | 107 (45.73) |
| Penis is completely hard and fully rigid | 93 (10.02) | 20 (8.55) |
| Low testosterone | 48 (5.17) | 18 (7.69) |
| BPH | 97 (10.45) | 19 (8.12) |
| Premature ejaculation | 88 (9.48) | 31 (13.25) |
| Decreased libido | 277 (29.85) | 65 (27.78) |
| Inability/difficulty achieving orgasm | 141 (15.19) | 38 (16.24) |
| Peyronie's disease | 16 (1.72) | 12 (5.13) |
CVD includes self‐reported diagnosis of heart attack, congestive heart failure and/or cardiovascular disease.
Abbreviations: BMI, body mass index; BPH, benign prostatic hyperplasia; CCI, Charlson Comorbidity Index; CVD, cardiovascular disease; EHS, Erection Hardness Score; sildenafil‐P, pharmacy medicine sildenafil; SD, standard deviation.
Approximately one in five men (19.28%) reported currently using any prescription medication for ED at baseline, with this being more frequently reported by sildenafil‐P users than never users (36.32% vs 14.98%) (Table 3). The most commonly used prescription branded medications for ED were sildenafil (41.52%), followed by tadalafil (23.66%). Branded prescription sildenafil was more often used by sildenafil‐P users than never users (57.65% vs 31.65%), whereas similar percentages of sildenafil‐P users (22.35%) and never users (24.46%) reported taking prescription branded tadalafil. The most frequently reported non‐pharmacological ED treatment was lifestyle changes (9.47%), with this being reported by a greater percentage of sildenafil‐P users than never users (17.52% vs 7.44%). Overall, nearly two‐thirds of men (62.99%) had never tried anything to get and/or maintain an erection at baseline, but this was only the case for a minority of sildenafil‐P users (39.32% vs 68.97%) (Table 3). Overall, 52.99% and 25.54% of sildenafil‐P users and never users, respectively, reported using a prescription medication for ED at ≥1 time point post‐launch of sildenafil‐P.
TABLE 3.
Baseline ED treatment by sildenafil‐P use
| Variable | Sildenafil‐P use | |
|---|---|---|
| Never users (n = 928) | Sildenafil‐P users (n = 234) | |
| n (%) | n (%) | |
| Any prescription medication | 139 (14.98) | 85 (36.32) |
| Branded prescription tadalafil | 34 (24.46) | 19 (22.35) |
| Branded prescription vardenafil | 3 (2.16) | 5 (5.88) |
| Branded prescription sildenafil | 44 (31.65) | 49 (57.65) |
| Branded prescription avanafil | 1 (0.72) | 1 (1.18) |
| Generic prescription sildenafil | 64 (46.04) | 24 (28.24) |
| Generic prescription tadalafil | 10 (7.19) | 4 (4.71) |
| Other prescription medication | 2 (1.44) | 0 (0.00) |
| Injectables | 6 (0.65) | 5 (2.14) |
| Alternative medicine | 3 (0.32) | 3 (1.28) |
| Lifestyle changes | 69 (7.44) | 41 (17.52) |
| Penile implant | 3 (0.32) | 4 (1.71) |
| Penile vacuum or pump | 16 (1.72) | 9 (3.85) |
| Herbal treatments or supplements | 27 (2.91) | 15 (6.41) |
| Testosterone therapy | 5 (0.54) | 9 (3.85) |
| Other treatment | 53 (5.71) | 14 (5.98) |
| Never tried anything for ED | 640 (68.97) | 92 (39.32) |
| Medication use for comorbid health conditions | 577 (62.20) | 107 (45.70) |
ED, erectile dysfunction; sildenafil‐P, pharmacy medicine sildenafil.
3.1.2. HCRU outcomes
Across all HCRU outcomes among the overall sample of men with ED, the number of visits increased from baseline, relative to the total number of visits in the aggregate 12‐month post‐launch period (Figure 1). Specifically, the mean ± SD number of visits to physicians/nurse practitioners for any reason (4.53 ± 5.45 vs 2.81 ± 3.44, respectively), cardiologist visits (0.27 ± 1.02 vs 0.06 ± 0.34, respectively) and CVD check‐up visits (1.01 ± 2.47 vs 0.29 ± 1.05, respectively) in the aggregate 12‐month period were higher than baseline. The mean ± SD total number of visits to pharmacists for any reason (2.83 ± 4.69 vs 1.37 ± 3.94, respectively) and visits to pharmacists for sexual functioning discussions (0.92 ± 1.84 vs 0.14 ± 1.08, respectively) in the aggregate 12‐month period were likewise higher, relative to baseline. Apart from CVD check‐up visits, sildenafil‐P users had a higher mean total number of visits in the aggregate 12‐month period following the baseline survey than never users for all HCRU outcomes assessed (Figure 1).
FIGURE 1.
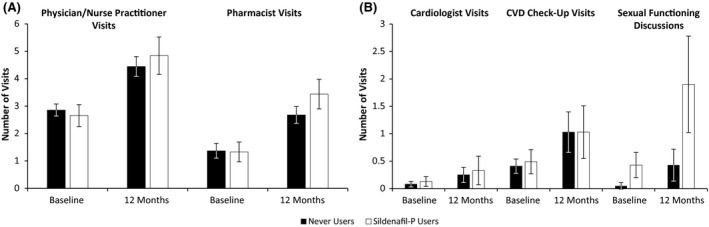
HCRU outcomes at baseline and aggregate 12‐month period by sildenafil‐P use. Total visits for any reason: physician/nurse practitioner visits and pharmacist visits. Total visits for cardiologists, CVD check‐ups and sexual functioning discussions. Baseline and total visits over 12 months variables reflect a 12‐month recall period. For physician/nurse practitioner and pharmacist visits for any reason, n = 1,162 participants provided data for all timepoints. For cardiologist and CVD check‐up visits, n = 282 participants provided data for all time points. For pharmacist visits for sexual functioning discussions, n = 107 participants provided data for all timepoints. Error bars depict 95% confidence intervals. CVD, cardiovascular disease; sildenafil‐P, pharmacy medicine sildenafil.
The percentages of respondents who reported ≥1 visit to physicians/nurse practitioners across the aggregate 12‐month follow‐up period for check‐ups or discussions about diabetes (22.22% vs 19.61%), hypertension (27.78% vs 25.22%) or high cholesterol (21.37% vs 18.00%) were similar, albeit slightly higher, among sildenafil‐P users than never users. However, the percentage of sildenafil‐P users who reported ≥1 visit for mental health check‐ups or discussions was over two times greater than the percentage of never users (25.21% vs 11.75%). Approximately three times more sildenafil‐P users than never users had ≥1 visit to physicians/nurse practitioners across the aggregate 12‐month follow‐up period for discussions about sexual functioning issues, either when the respondent raised this topic with his physician/nurse practitioner (32.91% vs 11.31%) or the physician/nurse practitioner raised this topic with the respondent (17.95% vs 5.50%).
3.1.3. QoL outcomes
In the total sample, SEAR scores assessed across the post‐launch follow‐up period remained similar to those assessed during the pre‐launch baseline period. The SEAR total scores were similar between sildenafil‐P users and never users at both time points (baseline, 56.00 ± 21.32 vs 55.09 ± 23.52; 12 months, 55.12 ± 20.61 vs 53.88 ± 24.50). SEAR sexual relationship scores at both time points were slightly higher in sildenafil‐P users, compared with never users (baseline, 53.86 ± 23.10 vs 49.91 ± 26.97; 12 months, 52.58 ± 22.51 vs 48.84 ± 27.54). SEAR confidence scores were slightly lower in sildenafil‐P users, compared with never users (baseline, 58.85 ± 22.57 vs 62.00 ± 23.84; 12 months, 58.51 ± 21.30 vs 60.60 ± 25.38). Similar trends were observed for scores on the self‐esteem (baseline, 57.22 ± 24.74 vs 59.45 ± 26.96; 12 months, 57.10 ± 22.62 vs 58.51 ± 27.18) and overall relationship (baseline, 62.10 ± 25.82 vs 67.09 ± 27.50; 12 months, 61.35 ± 25.50 vs 64.79 ± 29.70) SEAR domains. Approximately 17% of men with ED screened positive for depression on the PHQ‐2 at baseline and 12‐month follow‐up. A higher percentage of sildenafil‐P users screened positive, compared with never users, across both time points (baseline, 21.79% vs 15.41%; 12 months, 22.22% vs 15.20%). Mean sexual satisfaction ratings remained similar at 12‐month follow‐up, relative to the baseline assessment, and were comparable between sildenafil‐P users and never users (baseline, 3.35 ± 1.25 vs 2.99 ± 1.26; 12 months, 3.15 ± 1.23 vs 2.91 ± 1.28).
3.2. Multivariable analysis
3.2.1. HCRU outcomes
Multivariable analyses revealed that, after adjusting for baseline visits and other covariates, sildenafil‐P users reported significantly more visits to physicians/nurse practitioners for any reason (3.68 vs 2.87, P = .003) and to pharmacists for any reason (2.10 vs 1.34, P < .001) in the total 12‐month post‐launch period (Table 4). While it did not reach the prespecified threshold for statistical significance, sildenafil‐P users reported marginally more pharmacist visits for sexual functioning discussions than never users (0.71 vs 0.14, P = .070). Sildenafil‐P users and never users did not differ on any of the other HCRU outcomes assessed (Table 4).
TABLE 4.
Association of sildenafil‐P use with HCRU outcomes at 12 months, adjusted for baseline visits and covariates
| Outcome | Adjusted mean ± SE | 95% LCL | 95% UCL | P value |
|---|---|---|---|---|
| Physician/nurse practitioner visits for any reason a | ||||
| Sildenafil‐P users (n = 234) | 3.68 ± 0.07 | 3.19 | 4.20 | .003 |
| Never users (n = 928) | 2.87 ± 0.03 | 2.65 | 3.09 | |
| Cardiologist visits b | ||||
| Sildenafil‐P users (n = 67) | 0.04 ± 0.12 | 0.00 | 0.19 | .824 |
| Never users (n = 201) | 0.03 ± 0.07 | 0.00 | 0.09 | |
| CVD check‐up visits c | ||||
| Sildenafil‐P users (n = 67) | 0.33 ± 0.13 | 0.11 | 0.68 | .642 |
| Never users (n = 201) | 0.26 ± 0.07 | 0.14 | 0.42 | |
| Pharmacist visits for any reason d | ||||
| Sildenafil‐P users (n = 234) | 2.10 ± 0.07 | 1.73 | 2.50 | <.001 |
| Never users (n = 928) | 1.34 ± 0.03 | 1.20 | 1.50 | |
| Pharmacist visits for sexual functioning discussions e | ||||
| Sildenafil‐P users (n = 29) | 0.71 ± 0.20 | 0.20 | 1.52 | .070 |
| Never users (n = 54) | 0.14 ± 0.14 | 0.01 | 0.43 | |
Annual visits were calculated by summing the number of visits reported at 3‐, 6‐, 9‐ and 12‐month follow‐up.
Abbreviations: CCI, Charlson Comorbidity Index; CVD, cardiovascular disease; ED, erectile dysfunction; HCRU, healthcare resource use; LCL, lower confidence limit; sildenafil‐P, pharmacy medicine sildenafil; SE, standard error; UCL, upper confidence limit.
Model controlled for the following baseline covariates: physician/nurse practitioner visits for any reason, CCI score, medication use for comorbid health conditions, CVD, overall life stress and ED treatment.
Model controlled for the following baseline covariates: cardiologist visits and depression.
Model controlled for the following baseline covariates: CVD check‐up visits, smoking and ED treatment.
Model controlled for the following baseline covariates: pharmacist visits for any reason, CCI score, medication use for comorbid health conditions, depression, age and ED treatment.
Model controlled for the following baseline covariates: pharmacist visits for sexual functioning discussions and household income.
3.2.2. QoL outcomes
After adjusting for the baseline covariates, sildenafil‐P users had statistically significantly higher SEAR total score, sexual relationship score and self‐esteem score, compared with never users (55.64 vs 54.80, 51.34 vs 50.00, and 59.67 vs 59.28, respectively; for all, P < .001; Table 5). However, the SEAR overall relationship score was significantly lower for sildenafil‐P users than never users (63.86 vs 65.34; P < .001). Differences in the adjusted means between sildenafil‐P users and never users did not reach the minimal clinically important difference of 10 points on the SEAR. 33 Although sildenafil‐P users were 1.26 times more likely than never users to screen positive for depression on the PHQ‐2 at the 12‐month follow‐up, this association was not statistically significant (P = .287). Sildenafil‐P users and never users also reported similar ratings of sexual satisfaction after adjusting for baseline covariates (Table 5).
TABLE 5.
Association of sildenafil‐P use with QoL outcomes at 12 months, adjusted for baseline scores and covariates
| Outcome | Adjusted mean ± SE | 95% LCL | 95% UCL | P value |
|---|---|---|---|---|
| SEAR total score a | ||||
| Sildenafil‐P users (n = 172) | 55.64 ± 0.08 | 55.49 | 55.79 | <.001 |
| Never users (n = 603) | 54.80 ± 0.04 | 54.72 | 54.88 | |
| SEAR sexual relationship score a | ||||
| Sildenafil‐P users (n = 172) | 51.34 ± 0.08 | 51.19 | 51.49 | <.001 |
| Never users (n = 603) | 50.00 ± 0.04 | 49.92 | 50.08 | |
| SEAR confidence score a | ||||
| Sildenafil‐P users (n = 172) | 61.15 ± 0.08 | 61.00 | 61.30 | .158 |
| Never users (n = 603) | 61.27 ± 0.04 | 61.19 | 61.35 | |
| SEAR self‐esteem score a | ||||
| Sildenafil‐P users (n = 172) | 59.67 ± 0.08 | 59.52 | 59.82 | <.001 |
| Never users (n = 603) | 59.28 ± 0.04 | 59.20 | 59.36 | |
| SEAR overall relationship score a | ||||
| Sildenafil‐P users (n = 172) | 63.86 ± 0.08 | 63.71 | 64.01 | <.001 |
| Never users (n = 603) | 65.34 ± 0.04 | 65.26 | 65.42 | |
| Satisfaction with sexual intercourse frequency b | ||||
| Sildenafil‐P users (n = 222) | 3.03 ± 0.07 | 2.90 | 3.17 | .408 |
| Never users (n = 841) | 2.97 ± 0.03 | 2.90 | 3.04 | |
| Outcome | Adjusted OR ± SE | 95% LCL | 95% UCL | P value |
|---|---|---|---|---|
| Positive depression screen (PHQ‐2) c | ||||
| Sildenafil‐P users (n = 227) | 1.26 ± 0.21 | 0.83 | 1.91 | .287 |
| Never users (n = 888) | ||||
Satisfaction with sexual intercourse frequency was rated from 1 = extremely dissatisfied to 5 = Extremely satisfied.
CCI, Charlson Comorbidity Index; CVD, cardiovascular disease; ED, erectile dysfunction; LCL, lower confidence limit; OR, odds ratio; PHQ‐2, 2‐item Patient Health Questionnaire; sildenafil‐P, pharmacy medicine sildenafil; SE, standard error; SEAR, Self‐Esteem and Relationship Questionnaire; UCL, upper confidence limit.
Model controlled for the following baseline covariates: SEAR score, CVD, depression, ED severity, overall life stress and household income.
Model controlled for the following baseline covariates: satisfaction with sexual intercourse frequency, age, ED severity, overall level of life stress, depression and household income.
Model controlled for the following baseline covariates: positive depression screen, age, overall level of life stress, CVD, obesity, ED severity, CCI score, household income and medication use for comorbid health conditions.
4. DISCUSSION
To determine the suitability of sildenafil‐P for men with ED, a pharmacist must first ask them a series of general questions about cardiovascular health, concomitant medication use and co‐occurring health conditions. Pharmacists are to advise men buying sildenafil‐P to follow‐up with their doctor within 6 months or as soon as possible for those considered to be at lower or higher cardiovascular risk, respectively, to ensure that any underlying health conditions are investigated. 34 Results of this 1‐year, real‐world prospective study demonstrated that the total number of visits to physicians/nurse practitioners for any reason in the total 12‐month post‐launch period was significantly higher among sildenafil‐P users than never users. This suggests that men are increasingly following the recommendations of pharmacists to follow‐up with their doctors. These consultations are needed for treating ED, but, of great importance, they are essential for the secondary prevention of CVD, diabetes and depression, which have a considerable economic impact. 35
We additionally found that, for the aggregate 12‐month follow‐up period, sildenafil‐P users (vs never users) had a significantly greater number of visits to pharmacists for any reason. These results suggest that reclassifying to a pharmacy medicine may have increased treatment utilisation among sildenafil‐P users. We also observed that sildenafil‐P users (vs never users) reported a marginally greater total number of sexual function discussions. These results are encouraging, since research has reported there are several barriers, namely cultural/societal beliefs, embarrassment, lack of training around sexual health issues among healthcare providers, or a poor‐quality relationship with their healthcare provider, that preclude men, especially in older age groups, from discussing or seeking treatment for sexual health problems. 36 Moreover, the burden on physicians can be reduced, as consultations can instead be managed by pharmacists, which is also more economical and less time‐consuming for patients. 37 , 38
Overall, nearly two‐thirds of men with ED in the current study were not using any treatment at all, which suggests that better services for this group could, for a significant number of its members and their partners, improve well‐being and, in the longer term, enhance the prevention and treatment of life‐threatening illnesses. Pharmacy medication availability has been associated with improvements in HCRU and cost‐effectiveness in other therapeutic areas in treating non‐communicable diseases, such as in pain management and CVD risk reduction. 39 , 40 Future research is needed to determine whether the availability of sildenafil‐P has a similar impact on costs.
Results also revealed that a greater percentage of sildenafil‐P users than never users reported smoking in the past week. This finding has important public health implications, as it is well established that smoking elevates CVD risk. Thus, pharmacists could also play a vital role in providing smoking cessation counselling and behavioural support for these men as part of a comprehensive CVD risk reduction strategy. Such an approach likewise aligns with the aim of the UK National Health Service to prioritise CVD treatment and risk reduction as a key strategy to prevent premature mortality over the next decade. 41 Whether increased contact with healthcare providers is associated with subsequent improvements in lifestyle‐related ED risk factors is an important topic that warrants future research.
After adjusting for covariates, satisfaction with sexual intercourse frequency and positive depression screen at 12‐month follow‐up did not differ between sildenafil‐P users and never users, although results suggested that sildenafil‐P users have better ED‐specific QoL than never users on most SEAR metrics. Yet, these observed group differences, while statistically significant, were small in magnitude.
In summary, results from the current study show that sildenafil‐P users have significantly greater engagement with the healthcare system, which can facilitate early diagnosis and management of both ED and underlying comorbidities and CVD risk factors. The early and effective management of long‐term diseases is associated with better outcomes, including enhanced QoL. In light of the reclassifying of sildenafil 50 mg tablets to a pharmacy medicine, community pharmacists can play a pivotal role in both treating ED and guiding appropriate self‐care decisions to mitigate the health and lifestyle risk factors that cause ED. 26 Administering ED treatment can be integrated in a complementary manner with other services pharmacists provide, such as blood pressure checks and smoking cessation consultations. Hence, pharmacists can help to treat ED, as well as to address its underlying causes via diagnostic assessment and lifestyle‐related support services. Future directions for research should include an investigation of whether the broader availability of sildenafil‐P has increased overall ED treatment uptake in the UK. Likewise, it will critical to determine whether the reclassifying of sildenafil to a pharmacy medicine has resulted in a greater proportion of UK men with ED who are being treated for its underlying causes.
4.1. Limitations
The results of the current study must be interpreted considering inherent limitations. In particular, the results of this study might be subject to recall bias, particularly at baseline assessment, which had a 12‐month recall period. Furthermore, small sample sizes may have limited statistical power to detect statistically significant differences between sildenafil‐P users and never users on pharmacist visits for sexual functioning discussions, thereby increasing the likelihood of Type 2 error on this specific outcome. The sample included in the study may be affected by selection bias, and it is possible the study underrepresents those without internet access, which consists of 10% of the UK adult population, 42 as well as men with severe comorbidities or disabilities; given the small sample of sildenafil‐P users, men who are more affluent and proactive about their health may have been overrepresented among this group. However, CVD risk factors commonly cited by men with ED in this study, such as hypertension and hypercholesterolemia, are consistent with prior research, 1 , 2 , 43 suggesting the study sample is, at least to some extent, representative of the general ED population.
All data were self‐reported, and responses regarding diagnoses and treatment could not be independently confirmed, although ED status was determined using a validated self‐report measure. Nevertheless, the use of an anonymous online self‐report survey can facilitate more candid responses to questions about highly sensitive topics, such as ED. 44
Results for QoL outcomes reflect conservative estimates because the sildenafil‐P users included in this study could have used this treatment at any point (or multiple points) over the 12‐month follow‐up period. Future research could include a longer follow‐up period and standardise the commencement and duration of treatment use across all participants to more clearly discern the effect of sildenafil‐P use on QoL outcomes.
5. CONCLUSIONS
This is the first real‐world study that assessed the impact of reclassifying sildenafil 50 mg tablets to a pharmacy medicine on HCRU and QoL. Findings showed that the use of sildenafil‐P is associated with better ED‐specific QoL in the 12 months following the reclassifying of sildenafil to a pharmacy medicine. However, observed group differences were small in magnitude. Notably, sildenafil‐P use was associated with a higher number of healthcare provider (physicians/nurse practitioners) and pharmacist visits, which has important implications for improving public health. Specifically, greater engagement with the healthcare system over time can increase the likelihood of diagnosis and treatment of both ED and underlying long‐term diseases. Furthermore, results underscore the necessity for more frequent and active involvement of community pharmacists in preventative care among men with ED. Future research should determine whether healthcare providers report greater uptake of ED medication and treatment for the underlying causes of ED.
CONFLICT OF INTEREST
LJL and BE are employees of Pfizer Inc. JZL, KHZ and SSD are employees of Viatris. The views expressed are their own and do not necessarily represent those of their employers. TAM and DT have no conflicts of interest to declare. MCM, VWL and MJ are employees of Kantar, which received funding from Pfizer for conducting and reporting on this study.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the design of the study, interpretation of results, drafting and review of the manuscript and publication decisions. MCM, VWL and MJ also contributed to the data collection and data analysis.
Funding information
This study was sponsored by Pfizer. Pfizer provided funding to Kantar for carrying out this study and funding to Sudha Korwar and Ramu Periyasamy at Indegene Pvt Ltd. for assistance with literature review and medical writing. The following authors (LJL, BE) are employees of Pfizer, and (JZL, KHZ and SSD) are employees of Viatris.
ACKNOWLEDGEMENTS
The authors thank Sudha Korwar, PhD and Ramu Periyasamy, PhD, Indegene Pvt Ltd. for assistance with literature review and writing, which was funded by Pfizer. Data sharing: The study data will be provided for non‐commercial use upon request.
Lee LJ, Maguire TA, Maculaitis MC, et al. Increasing access to erectile dysfunction treatment via pharmacies to improve healthcare provider visits and quality of life: Results from a prospective real‐world observational study in the United Kingdom. Int J Clin Pract.2021;75:e13849. 10.1111/ijcp.13849
REFERENCES
- 1. McCabe MP, Sharlip ID, Atalla E, et al. Definitions of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation on Sexual Medicine 2015. J Sex Med. 2016;13:135‐143. 10.1016/j.jsxm.2015.12.019 [DOI] [PubMed] [Google Scholar]
- 2. Rosen RC, Fisher WA, Eardley I, Niederberger C, Nadel A, Sand M. The multinational Men’s Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004;20:607‐617. 10.1185/030079904125003467 [DOI] [PubMed] [Google Scholar]
- 3. Goldstein I, Goren A, Li VW, Tang WY, Hassan TA. Epidemiology update of erectile dysfunction in eight countries with high burden. Sex Med Rev. 2020;8:48‐58. 10.1016/j.sxmr.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 4. Hackett G, Kirby M, Wylie K, et al. British society for sexual medicine guidelines on the management of erectile dysfunction in men—2017. J Sex Med. 2018;15:430‐457. 10.1016/j.jsxm.2018.01.023 [DOI] [PubMed] [Google Scholar]
- 5. Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nature Reviews Disease Primers. 2016;2(1):16003. 10.1038/nrdp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaman Huri H, Mat Sanusi N, Razack AHA, Mark R. Association of psychological factors, patients’ knowledge, and management among patients with erectile dysfunction. Patient Prefer Adherence. 2016;10:807‐823. 10.2147/PPA.S99544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldstein I, Goren A, Li VW, Maculaitis MC, Tang WY, Hassan TA. The association of erectile dysfunction with productivity and absenteeism in eight countries globally. Int J Clin Pract. 2019;73: 10.1111/ijcp.13384 [DOI] [PubMed] [Google Scholar]
- 8. Mulhall JP, Luo X, Zou KH, Stecher V, Galaznik A. Relationship between age and erectile dysfunction diagnosis or treatment using real‐world observational data in the USA. Int J Clin Pract. 2016;70:1012‐1018. 10.1111/ijcp.12908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen L, Staubli SEL, Schneider MP, et al. Phosphodiesterase 5 inhibitors for the treatment of erectile dysfunction: a trade‐off network meta‐analysis. Eur Urol. 2015;68:674‐680. 10.1016/j.eururo.2015.03.031 [DOI] [PubMed] [Google Scholar]
- 10. Hatzimouratidis K, Salonia A, Adaikan G, et al. Pharmacotherapy for erectile dysfunction: recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015). J Sex Med. 2016;13:465‐488. 10.1016/j.jsxm.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 11. US Food and Drug Administration . Drug approval package. Viagra (sildenafil citrate). https://www.accessdata.fda.gov/drugsatfda_docs/NDA/98/viagra/viagra_toc.cfm. Accessed December 12, 2019.
- 12. Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Ranasinghe P, Levy JC. Erectile dysfunction is a strong predictor of poor quality of life in men with Type 2 diabetes mellitus. Diabet Med. 2014;31:699‐706. 10.1111/dme.12412 [DOI] [PubMed] [Google Scholar]
- 13. Martin AL, Huelin R, Wilson D, Foster TS, Mould JF. A systematic review assessing the economic impact of sildenafil citrate (Viagra®) in the treatment of erectile dysfunction. J Sex Med. 2013;10:1389‐1400. 10.1111/jsm.12068 [DOI] [PubMed] [Google Scholar]
- 14. Tang W‐H, Zhuang X‐J, Ma L‐L, et al. Effect of sildenafil on erectile dysfunction and improvement in the quality of sexual life in China: a multi‐center study. Int J Clin Exp Med. 2015;8:11539‐11543. [PMC free article] [PubMed] [Google Scholar]
- 15. Salonia A, Ferrari M, Saccà A, et al. Delay in seeking medical help in patients with new‐onset erectile dysfunction remained high over and despite the PDE5 era—an ecological study. J Sex Med. 2012;9:3239‐3246. 10.1111/j.1743-6109.2012.02953.x [DOI] [PubMed] [Google Scholar]
- 16. Schouten BWV, Bohnen AM, Groeneveld FPMJ, Dohle GR, Thomas S, Ruud Bosch JLH. Erectile dysfunction in the community: trends over time in incidence, prevalence, GP consultation and medication use—the krimpen study: Trends in ED. J Sex Med. 2010;7(7):2547–2553. 10.1111/j.1743-6109.2010.01849.x [DOI] [PubMed] [Google Scholar]
- 17. Anastasiadis AG, Ghafar MA, Shabsigh R, Burchardt M. Economic aspects of medical erectile dysfunction therapies. Expert Opin Pharmacother. 2002;3:257‐263. 10.1517/14656566.3.3.257 [DOI] [PubMed] [Google Scholar]
- 18. Costa P, Avances C, Wagner L. Erectile dysfunction: knowledge, wishes and attitudes. Results of a French study of 5.099 men aged 17 to 70. Prog Urol. 2003;13:85‐91. [PubMed] [Google Scholar]
- 19. Heruti RJ, Yossef M, Shochat T. Screening for erectile dysfunction as part of periodic examination programs—concept and implementation. Int J Impot Res. 2004;16:341‐345. 10.1038/sj.ijir.3901173 [DOI] [PubMed] [Google Scholar]
- 20. Shabsigh R, Stone B. Understanding the needs and objectives of erectile dysfunction patients. World J Urol. 2006;24:618‐622. 10.1007/s00345-006-0128-5 [DOI] [PubMed] [Google Scholar]
- 21. Wilson ECF, McKeen ES, Scuffham PA, Brown MCJ, Wylie K, Hackett G. The cost to the United Kingdom National Health Service of managing erectile dysfunction: the impact of sildenafil and prescribing restrictions. Pharmacoeconomics. 2002;20:879‐889. 10.2165/00019053-200220130-00002 [DOI] [PubMed] [Google Scholar]
- 22. Shabsigh R. Socioeconomic considerations in erectile dysfunction treatment. Urol Clin North Am. 2001;28:417‐422. 10.1016/S0094-0143(05)70149-X [DOI] [PubMed] [Google Scholar]
- 23. Gulpinar O, Haliloglu AH, Abdulmajed MI, Bogga MS, Yaman O. Help‐seeking interval in erectile dysfunction: analysis of attitudes, beliefs, and factors affecting treatment‐seeking interval in Turkish men with previously untreated erectile dysfunction. J Androl. 2012;33:624‐628. 10.2164/jandrol.111.013946 [DOI] [PubMed] [Google Scholar]
- 24. Medicines and Healthcare Products Regulatory Agency . MHRA reclassifies Viagra Connect tablets to a pharmacy medicine. November 2017. https://www.gov.uk/government/news/mhra‐reclassifies‐viagra‐connect‐tablets‐to‐a‐pharmacy‐medicine. Accessed December 11, 2019.
- 25. Stomberg C, Philipson T, Albaugh M, Sood N. Utilization effects of Rx‐OTC switches and implications for future switches. Health (Irvine Calif). 2013;05:1667‐1680. 10.4236/health.2013.510225 [DOI] [Google Scholar]
- 26. Taylor DG, Giuliano F, Hackett G, et al. The pharmacist’s role in improving the treatment of erectile dysfunction and its underlying causes. Res Soc Adm Pharm. 2019;15:591‐599. 10.1016/j.sapharm.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 27. Johnsen M. Viagra Connect available OTC in the UK. 2018. https://drugstorenews.com/otc/viagra‐connect‐available‐otc‐u‐k. Accessed July, 2020.
- 28. O’Donnell AB, Araujo AB, Goldstein I, McKinlay JB. The validity of a single‐question self‐report of erectile dysfunction. J Gen Intern Med. 2005;20:515‐519. 10.1111/j.1525-1497.2005.0076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mulhall JP, Goldstein I, Bushmakin AG, Cappelleri JC, Hvidsten K. Validation of the erection hardness score. J Sex Med. 2007;4:1626‐1634. 10.1111/j.1743-6109.2007.00600.x [DOI] [PubMed] [Google Scholar]
- 30. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676‐682. 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 31. Cappelleri JC, Althof SE, Siegel RL, Shpilsky A, Bell SS, Duttagupta S. Development and validation of the Self‐Esteem And Relationship (SEAR) questionnaire in erectile dysfunction. Int J Impot Res. 2004;16:30‐38. 10.1038/sj.ijir.3901095 [DOI] [PubMed] [Google Scholar]
- 32. Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire‐2: validity of a two‐item depression screener. Med Care. 2003;41:1284‐1292. 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- 33. Cappelleri JC, Althof SE, O’Leary MP, et al. Clinically meaningful improvement on the Self‐Esteem And Relationship questionnaire in men with erectile dysfunction. Qual Life Res. 2007;16:1203‐1210. 10.1007/s11136-007-9232-2 [DOI] [PubMed] [Google Scholar]
- 34. Viagra Connect . Summary of product characteristics. November 2017. Pfizer Consumer Healthcare Ltd, UK. https://www.Medicines.Org.Uk/Emc/Product/8725/Smpc. Accessed January 30, 2020.
- 35. Pastuszak AW, Hyman DA, Yadav N, et al. Erectile dysfunction as a marker for cardiovascular disease diagnosis and intervention: a cost analysis. J Sex Med. 2015;12:975‐984. 10.1111/jsm.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ezhova I, Savidge L, Bonnett C, Cassidy J, Okwuokei A, Dickinson T. Barriers to older adults seeking sexual health advice and treatment: a scoping review. Int J Nurs Stud. 2020;107:103566. 10.1016/j.ijnurstu.2020.103566 [DOI] [PubMed] [Google Scholar]
- 37. Bell J, Dziekan G, Pollack C, Mahachai V. Self‐care in the twenty first century: a vital role for the pharmacist. Adv Ther. 2016;33:1691‐1703. 10.1007/s12325-016-0395-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fielding S, Porteous T, Ferguson J, et al. Estimating the burden of minor ailment consultations in general practices and emergency departments through retrospective review of routine data in North East Scotland. Fam Pract. 2015;32:165‐172. 10.1093/fampra/cmv003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee L, DiBonaventura M, Kudel I, Schepart A, Kellstein D. The incremental effect of over‐the‐counter medications on work productivity and healthcare resource utilization among adults with pain in the United States. Value Health. 2016;19:A250. [Google Scholar]
- 40. Stomberg C, Albaugh M, Shiffman S, Sood N. A cost‐effectiveness analysis of over‐the‐counter statins. Am J Manag Care. 2016;22:e294‐e303. [PubMed] [Google Scholar]
- 41. UK National Health Service . Cardiovascular disease (CVD): our ambition for CVD prevention. https://www.england.nhs.uk/ourwork/clinical‐policy/cvd/. Accessed July 8, 2020.
- 42. UK Office for National Statistics . Exploring the UK’s digital divide. March 4, 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/householdcharacteristics/homeinternetandsocialmediausage/articles/exploringtheuksdigitaldivide/2019‐03‐04#toc. Accessed May 15, 2020.
- 43. Skeldon SC, Detsky AS, Goldenberg SL, Law MR. Erectile dysfunction and undiagnosed diabetes, hypertension, and hypercholesterolemia. Ann Fam Med. 2015;13:331‐335. 10.1370/afm.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fischbacher C, Chappel D, Edwards R, Summerton N. Health surveys via the Internet: Quick and dirty or rapid and robust? J R Soc Med. 2000;93:356‐359. 10.1177/014107680009300705 [DOI] [PMC free article] [PubMed] [Google Scholar]


