Abstract
Pharmacists are health care professionals who are actively involved in identifying and solving drug-related problems (DRPs) in neoplasm patients. However, the effectiveness of pharmaceutical services at outpatient clinic for neoplasm patients have not been reported in China. This study aims to describe and investigate the impacts of pharmacists-managed oncology outpatient clinic on ambulatory neoplasm patients. We performed a descriptive, prospective study from June 6, 2018 to June 6, 2020. Firstly, we established a pharmacists-managed oncology outpatient clinic and a Pharmacists Work System of Medication Therapy Management (MTM) software with the cooperation of oncologists, pharmacists and software engineers in 2018. Subjects were neoplasm patients who visited the pharmacists-managed outpatient clinic. The pharmacists performed a comprehensive assessment of the patient’s medication and made planned interventions based on the DRPs identified. A total of 215 eligible patients with 707 visits were enrolled and recorded in the MTM software. A total of 316 DRPs (1.47 per patient) were identified. Adverse reactions, non-adherence, untreated indication, and drug interactions were the leading DRPs. 261 (82.6%) of the identified DRPs had been confirmed as resolved and 104 (78.2%) of adverse reactions were improved following pharmacist interventions and 2 to 3 course follow-up. Of the 382 planned interventions, 345 (90.3%) were accepted by patients or physicians. This is the first pharmacists-managed oncology outpatient clinic to describe the type of DRPs in neoplasm patients and evaluate the effectiveness of pharmacist interventions in China. Pharmacist interventions were efficacious in resolving DRPs and improving adverse reactions. We confirmed that pharmacists have an important role in ambulatory neoplasm patients care.
Keywords: prospective study, pharmacists, outpatient clinic, drug-related problems, adverse reactions, neoplasm patients, medication therapy management, China
What do we already know about this topic?
Patients with cancer are at high risk of drug-related problems (DRPs) due to multi-morbidity associated polypharmacy. The effectiveness of pharmaceutical services at outpatient clinic for neoplasm patients have not been reported in China.
How does your research contribute to the field?
This is the first pharmacists-managed oncology outpatient clinic to describe the type of DRPs in neoplasm patients and evaluate the effectiveness of pharmacist interventions in China. Pharmacist interventions were efficacious in resolving DRPs and improving adverse reactions in ambulatory neoplasm patients.
What are your research’s implications toward theory, practice, or policy?
Pharmacists are health care professionals who are actively involved in identifying and solving the DRPs in neoplasm patients.
Introduction
In recent years, cancer is still rank as the leading cause of death and the single most important barrier to increasing life expectancy in the world.1 Global cancer statistics 2020 reported by the International Agency for Research on Cancer predicts that an estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths worldwide occurred in 2020. Overall, the burden of cancer incidence and mortality is rapidly growing in every country of the world, with an estimated 28.4 million new cancer cases by the year 2040.2 In terms of the absolute burden, China with a high Human Development Index (HDI) is expected to experience a greater increase in cancer incidence. A systematic analysis for the global burden of disease study shows that 8 types of cancer appeared in the 25 leading causes of death in China from 1990 to 2017.3 With the increasing number of long-term cancer survivors, there is an increasing need to provide high-quality patient health care.
Aging is an important risk factor for the development of cancer and it is common that they are also suffering from other concomitant medical conditions and organs’ dysfunction.4 Elderly neoplasm patients not only have a risk of using polypharmacy compared to their younger counterparts, the physiologic changes associated with aging also alter pharmacokinetics and pharmacodynamics of cancer therapy.5,6 What’s more, patients taking multiple medications are more likely to experience adverse drug events, overdose, drug interactions, medication non-adherence, and other drug-related problems (DRPs).7,8 Strong evidence from studies in aged neoplasm patients had shown that use of 5 or more medications was shown to almost double the risk of DRP occurrence.9,10 It is therefore important to conduct pharmaceutical services to review medications of neoplasm patients, especially for the elderly.
Pharmacists are health care professionals who are actively involved in optimizing chemotherapy protocols, adjusting drug doses, detecting and preventing adverse reactions, improving medication compliance, monitoring laboratory values, and conducting patient education.11,12 Studies demonstrated that the intervention conducted by pharmacists can greatly improve patient adherence to oral anti-neoplastic drugs, treatment outcomes, maintain or improve quality of life of patients who receiving cancer treatment, and ultimately provide a further reduction in mortality and/or health care costs.13-15 However, the effectiveness of pharmacists services at outpatient clinics for neoplasm patients have not been reported in China.
Patients with malignant tumor, especially those elderly patients with multiple comorbidities, are widely ignored in pharmaceutical care in China. A guideline on development of pharmaceutical service was issued by National Health Commission of China, which emphasizing the professional service provided by pharmacists.16 In 2018, the first pharmacists-managed oncology outpatient clinic at a tertiary teaching hospital in Shanghai was established to provide Medication Therapy Management (MTM) service and promote drug rational use. The main objectives of this study were to describe those DRPs identified and addressed by pharmacists and evaluated the impact of pharmacist interventions on the management of adverse reactions in ambulatory neoplasm patients.
Methods
Study Setting and Design
We performed a descriptive, prospective study at Shanghai Jiao Tong University affiliated Sixth People’s Hospital, Shanghai, China. This is a tertiary comprehensive teaching hospital specializing in osteosarcoma and soft tissue sarcoma. This study was approved by Ethics Committee of Shanghai Sixth People’s Hospital. Informed consent was obtained from all individual participants through signing of informed consent forms.
Prior to initiation, a collaborative agreement was reached among oncologists, pharmacists and software engineers to establish a pharmacists-managed oncology outpatient clinic and a Pharmacist Work System of MTM software in June 2018. The MTM software (intellectual property registration No.: 2018SR916520) was designed on the basis of the concept of patient-centered medication therapy management service. Through Pharmacist Work System the pharmacists can retrieve and record patient’s information and medication history from hospital information system.
Study Subjects
Consecutive patients visited the pharmacists-managed oncology outpatient clinic were enrolled from June 6, 2018 to June 6, 2020. The inclusion criteria were patients with malignant tumors regardless of demographic, tumor type or stage, and treatment characteristics. Patients were excluded if they had a severe mental disorder or had a life expectancy of fewer than 3 months. During the study period, 4 pharmacists worked as outpatient pharmacists at the outpatient clinic.
Patient Management Procedure and Pharmacist Interventions
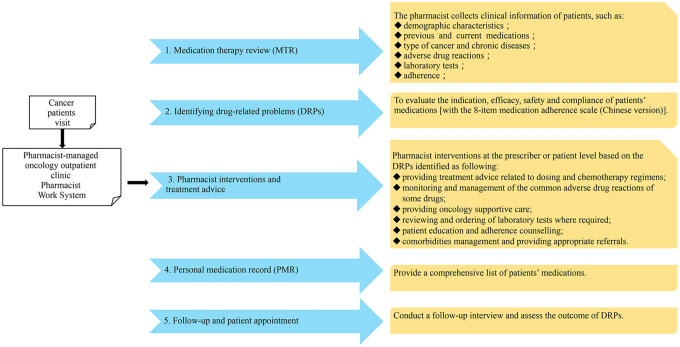
The main medication therapy management process in this study included the following 5 steps: (1) comprehensive medication therapy review (MTR). After patient’s visit, clinical diseases and medications information of patients, adverse reactions and laboratory tests would be collected and retrieved; (2) identifying drug-related problems (DRPs), to evaluate the indication, efficacy, safety and compliance of patients’ medications [with the 8-item medication adherence scale (Chinese version)]17; (3) developing an intervention plan, and providing treatment advice based on the DRPs identified; (4) making personal medication record (PMR), recording a comprehensive list of patients’ medications; and (5) follow-up (Figure 1).
Figure 1.
Patient management procedure and pharmacist interventions.
Pharmacist interventions at the prescriber level or patient level included any of the following: providing treatment advice related to dosing and chemotherapy regimens, monitoring and management of the common adverse reactions of some drugs, comorbidities management and providing appropriate referrals if required, providing oncology supportive care, reviewing and ordering of laboratory tests where required, patient education and adherence counseling. Recommendations were then communicated face-to-face or through online to the physician and the resolution of DRPs were focused on during patient follow-up. Patients will be enrolled for timely follow-up when they have DRPs such as non-adherence, new adverse reactions or with labs falling out of normal range by telephone or on-site appointment with pharmacists.
DRP Classification and Resolution
The DRPs were classified according to the DRP classification by the American Society of Health-System Pharmacists (ASHP) with slight modifications.18 In the MTM software, we classified DRPs into 9 categories including (a) drug without indication; (b) untreated indication; (c) no effect of drug treatment; (d) underdose; (e) adverse reactions; (f) overdose; (g) potential drug interactions; (h) non-adherence, and (i) other problems (such as drug use process, drug information request).
The resolution status of these DRPs were evaluated during patients’ 2 to 3 course follow-up. DRPs was considered as resolved when the pharmacists received the following confirmation that either the patient made a medication adjustment based on the pharmacist interventions (eg, discontinuing an unnecessary medication, altering the dose of a medication if it was too high or too low, switching to an alternative medication to optimize effectiveness or avoid drug interactions, initiating an indicated preventive care medication) or the problem was resolved after the pharmacist’s evaluation (eg, improvement of adverse events, achieving better medication compliance, understanding the drugs used and how to use them).
Data Collection and Analysis
The clinical data of all patients were collected from the electronic medical record of MTM information system based on pharmacist record. Data were collected on demographic characteristics (age, sex, marital status, date of hospital visiting), disease characteristics (type of cancer and chronic diseases), and treatment characteristics (chemotherapy regimens, prescription drugs, and over-the counter drugs), adverse reactions and DRPs.
All data analyses were performed using Microsoft Excel 2013 and IBM SPSS version 23.0 software. A descriptive analysis was conducted on patients’ demographics, disease characteristics, ADRs and types of DRPs. Continuous data were expressed as mean with standard deviation. Categorical variables were expressed as the number and percentages.
Results
Characteristics of Study Population
A total of 215 eligible patients with 707 visits have been recorded and managed by the pharmacist over 2 years and the average number of clinic visits per patient was 3.3 times. The patients’ characteristics are shown in Table 1. The mean age was 52.10 ± 22.11 years (range 4-88) and 81 patients (37.7%) were over 65 years. 113 (52.5%) of them were female. Osteosarcoma (27.9%), lung (18.1%), and Colorectal (13.5%) were the most common primary tumor sites among these patients. The most commonly seen comorbid conditions were hypertension (23.7%), pain (18.1%), osteoporosis (16.3%), and coronary artery disease (10.7%). 54.9% of patients had one or more comorbid disease, these patients with comorbid conditions requiring drug therapy including antihypertensive drugs, antidiabetic drugs, analgesics, bisphosphonates, vitamin D, proton pump inhibitors, hepatoprotective drugs, and other supportive agents. Seventy-four patients (34.4%) take oral medications over 5.
Table 1.
Patients’ Characteristics (n = 215).
| Characteristics | Number (%) |
|---|---|
| Gender | |
| Female | 113 (52.5) |
| Male | 102 (47.5) |
| Age, year | |
| Mean ± SD | 52.1 ± 22.11 |
| ≥65 | 81 (37.7) |
| Primary tumor site | |
| Bone | 60 (27.9) |
| Lung | 39 (18.1) |
| Colorectum | 29 (13.5) |
| Breast | 21 (9.8) |
| Soft tissue | 14 (6.5) |
| Prostate | 9 (4.2) |
| Stomach | 8 (3.7) |
| Nasopharyngeal | 6 (2.8) |
| Liver | 5 (2.3) |
| Lymphoma | 5 (2.3) |
| Ovary | 4 (1.9) |
| Uterus | 4 (1.9) |
| pancreatic | 2 (0.9) |
| Others | 9 (4.2) |
| Comorbidities | |
| None | 97 (45.1) |
| Hypertension | 51 (23.7) |
| Pain | 39 (18.1) |
| Osteoporosis | 35 (16.3) |
| Coronary artery disease | 23 (10.7) |
| Gastrointestinal disease | 15 (7.0) |
| Hypohepatia | 14 (6.5) |
| Undernutrition | 14 (6.5) |
| Diabetes | 13 (6.0) |
| Hyperlipidemia | 12 (5.6) |
| hepatitis B | 9 (4.2) |
| Renal dysfunction | 8 (3.7) |
| Venous thrombosis | 8 (3.7) |
| Pulmonary disease | 6 (2.8) |
| Prostatic hyperplasia | 4 (1.9) |
| Cerebrovascular disease | 3 (1.4) |
| Hypothyroidism | 2 (0.9) |
| Zoster | 2 (0.9) |
| Sjogren syndrome | 1 (0.5) |
| Number of oral medications used | |
| <3 | 64 (29.7) |
| 3-5 | 77 (35.9) |
| >5 | 74 (34.4) |
Others: melanoma, bladder, oviduct, kidney, thyroid, gallbladder, thymus, scrotum; SD: standard deviation.
The list of 29 different chemotherapy regimens for the treatment of 154 patients are shown in Table 2. The data showed that 45 (20.9%) patients were on IFO+MAP (ifosfamide, methotrexate, adriamycin, cisplatin) protocol, which was the most common chemotherapy regimen for the treatment of osteosarcoma. This was subsequently followed by gefitinib (5.1%, n = 11) and capecitabine (3.7%, n = 8) protocols. And during this study, a total of 55 patients were on oral chemotherapy agents.
Table 2.
Chemotherapy Regimens.
| Regimen | n (%) |
|---|---|
| IFO+MAP | 45 (20.9) |
| Gefitinib | 11 (5.1) |
| Capecitabine | 8 (3.7) |
| VAC+IE | 8 (3.7) |
| BV+PMT+CBP/DDP | 8 (3.7) |
| Aromatase inhibitor | 8 (3.7) |
| PTX+CBP/DDP | 7 (3.3) |
| GEM+TXT | 6 (2.8) |
| ADM/PLD | 6 (2.8) |
| Apatinib | 6 (2.8) |
| Bicalutamide | 4 (1.9) |
| FOLFOX | 4 (1.9) |
| XELOX | 3 (1.4) |
| FOLFIRI+BV | 3 (1.4) |
| S-1 | 3 (1.4) |
| Erlotinib | 3 (1.4) |
| Anlotinib | 3 (1.4) |
| Pazopanib | 2 (0.9) |
| Osimertinib | 2 (0.9) |
| Flutamide | 2 (0.9) |
| LHRH Analogs | 2 (0.9) |
| Etoposide | 2 (0.9) |
| Gemcitabine | 2 (0.9) |
| Paclitaxel | 1 (0.5) |
| DCT+CTX | 1 (0.5) |
| R-CHOP | 1 (0.5) |
| Imatinib | 1 (0.5) |
| Afatinib | 1 (0.5) |
| Crizotinib | 1 (0.5) |
IFO+MAP = lfosfamide + methotrexate + adriamycin + cisplatin; VAC+IE = Vincristine + Adriamycin + cyclophosphamide + lfosfamide + Etoposide; BV+PMT+CBP/DDP = Bevacizumab + pemetrexed + carboplatin/cisplatin; PTX+CBP/DDP = Paclitaxel + carboplatin/cisplatin.; GEM+TXT = Gemcitabine + docetaxel; ADM/PLD = Adriamycin/Liposome Adriamycin; FOLFOX = 5-fluorouracil + levofolinate + oxaliplatin; XELOX = Capecitabine + oxaliplatin; FOLFIRI+BV = 5-fluorouracil + levofolinate + irinotecan + bevacizumab.; S-1 = Tegafur/gimeracil/oteracil.; LHRH = Luteinizing hormone releasing hormone; DCT+CTX = Dacarbazine + cyclophosphamide; R-CHOP = Rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone.
Drug-Related Problems and Improvement of Adverse Events
During the study period, a total of 316 DRPs (1.47 per patient) were identified. The most common DRPs related to adverse reactions (42.1%) followed by non-adherence (16.1%), untreated indication (11.7%), and drug interactions (8.2%). Of these DRPs, 261 (82.6%) had been confirmed as resolved after pharmacist interventions and 2 to 3 course follow-up. More details and outcome of DRPs identified among these patients are listed in Table 3. Among these DRPs, 133 adverse reactions were diagnosed by pharmacists using the National Cancer Institute’s Common Terminology Criteria Adverse Events version 4.0 (NCI-CTCAE v4.0), including very common and specific adverse events such as myelosuppression (n = 30), constipation (n = 22), rash (n = 19), myalgia (n = 2), hyperuricemia (n = 1). Regular blood test was performed before and after chemotherapy administration at least 2 times a week for all patients. 78.2% (n = 104) of adverse reactions were improved following pharmacist interventions of medication adjustment and use of supportive drugs (Table 4). The other 29 cases of adverse reactions which did not improve were due to severity or the absence of effective supportive drugs.
Table 3.
Types of Drug-Related Problems (DRPs) Identified and Level of Intervention for each DRP.
| Drug-related problems | Identified, n (%) | Confirmed as resolved, n (%) | Pharmacist interventions | |
|---|---|---|---|---|
| Prescriber level, n | Patient level, n | |||
| Indication | ||||
| Drug without indication | 17 (5.4) | 15 (88.2) | 7 | 12 |
| Untreated indication | 37 (11.7) | 33 (89.1) | 29 | 8 |
| Efficacy | ||||
| No effect of drug treatment | 17 (5.4) | 11 (64.7) | 17 | |
| Underdose | 7 (2.2) | 7 (100) | ||
| Safety | ||||
| Adverse reactions | 133 (42.1) | 104 (78.2) | 47 | 133 |
| Overdose | 17 (5.4) | 17 (100) | 3 | 17 |
| Drug interactions | 26 (8.2) | 26 (100) | 10 | 26 |
| Compliance | ||||
| Non-adherence | 51 (16.1) | 37 (72.5) | 11 | 51 |
| Others | 11 (3.5) | 11 (100) | 2 | 9 |
| Total | 316 (100) | 261 (82.6) | 126 | 256 |
Table 4.
Improvement of Adverse Reactions due to Pharmacist Interventions.
| Adverse event | Identified, n | Improved, n (%) |
|---|---|---|
| Myelosuppression | 30 | 29 (96.7) |
| Constipation | 22 | 20 (90.9) |
| Rash | 19 | 12 (63.2) |
| Diarrhea | 11 | 6 (54.5) |
| Drug-induced liver injury | 8 | 7 (87.5) |
| Renal injury | 7 | 5 (71.4) |
| Hand-foot syndrome | 6 | 2 (33.3) |
| Hypertension | 5 | 5 (100) |
| Nausea and vomiting | 5 | 3 (60.0) |
| Fatigue | 4 | 3 (75.0) |
| Mucositis oral | 4 | 3 (75.0) |
| Peripheral neuropathy | 3 | 1 (33.3) |
| Hypothyroidism | 2 | 2 (100) |
| Myalgia | 2 | 2 (100) |
| Hypersomnia | 2 | 2 (100) |
| Albuminuria | 2 | 1 (50.0) |
| Hyperuricemia | 1 | 1 (100) |
| Total | 133 | 104 (78.2) |
Effectiveness of Pharmacist Interventions
Overall, 382 planned interventions were made and recorded by MTM pharmacists at the prescriber level (n = 126, 33.0%) and patient level (n = 256, 67.0%) based on the DRPs identified in this period (Table 3). Among the planned interventions, chemotherapy adverse event prevented and reviewing/ordering of laboratory tests for adverse reactions (n = 180, 47.1%) were the most common intervention types, and 62 (16.2%) were related to patient education and referral to the prescriber for non-adherence. In order to improve patient adherence, patients were educated on their chemotherapeutic agents, disease state, laboratory monitoring, adverse reactions management, and the importance of adherence of oral chemotherapy drugs. Among these interventions, 90.3% (n = 345) were accepted by patients or physicians. Of the 37 cases that were not accepted, 5 were due to patient rejection of regimen adjustment, for the remaining, the physician chose to increase monitoring instead of modifying the supportive medications.
Discussion
The establishment and practice of pharmacists-managed oncology outpatient clinic not only provide a novel practice setting for a pharmacist but also fill the need of patients. The majority of published studies on pharmaceutical care in ambulatory patients typically have focused on chronic diseases such as diabetes, hypertension, chronic obstructive pulmonary disease, and anticoagulation.19 This is the first study which attempted to describe the incidence and type of DRPs among neoplasm patients identified by pharmacists in outpatient clinic in mainland China. Our study showed that an average of 1.47 DRPs per patient was found among our oncology outpatient. The most common DRPs were adverse reactions, non-adherence, untreated indication and drug interactions. Among the planned interventions (n = 382), 90.3% were accepted by patients or physicians. The results were consistent with previous published studies in many aspects.
The high rate of adverse reactions can be explained by the cancer chemotherapy agents or the broader use of supportive agents (eg, antiallergics, antiemetics, analgesics, etc.). Another important reason is that the short consultation time patients had with oncologists and the priority was on cancer management-hence these common chemotherapeutic adverse events became secondary and sometimes neglected. In addition, those patients with chronic medications for coexisting conditions and altered metabolic profile incline to suffer from the adverse reactions, non-adherence, and frequency of drug interactions. What’s more, the polypharmacy, comorbidity and aging might complicate the situation and contribute to untreated indication.10,20 The high prevalence of non-adherence and drug interactions also can be attributed to the fact that patients are usually seeing multiple doctors for their various conditions. Under these circumstances, interdisciplinary and multidisciplinary collaboration is of great significance in solving the DRPs. The MTM pharmacists play an important role in counseling patients or their prescribers to empower them to manage the rational use of medicines.
In our study, nearly a quarter of adverse reactions were myelosuppression, which require reviewing laboratory tests for diagnosis. Myelosuppression is mainly due to the use of high-dose chemotherapy drugs, such as high-dose ifosfamide and methotrexate. Five leading types of cancer were related to bone, lung, colorectum, breast and soft tissue in our pharmacists-managed oncology outpatient clinic. The high incidence of bone tumors and soft tissue sarcomas may attribute to the fact that our hospital has many patients with bone tumors in the department of orthopedics. In recent years, the combination of high-dose ifosfamide, high-dose methotrexate, anthracycline, and cisplatin has been recognized as effective chemotherapy regimens for bone and soft tissue tumors.21,22 Constipation, rash and diarrhea are also common adverse reactions that require face-to-face evaluation for diagnosis and real-time treatment with medications. In particular, 78.2% of the adverse reactions were improved by the pharmacist interventions and suggested medications.
Medication Therapy Management (MTM) is defined by the collaboration of multiple national pharmaceutical organizations as a distinct service or group of services that optimize therapeutic outcomes for individual patients.23 It has been proved that MTM interventions can effectively improve clinical outcomes and save the cost, as well as improve the treatment compliance and satisfaction of patients in previous studies.24-26 Fortunately, our MTM pharmacists received training since China’s first time introducing the MTM mode from US in 2015.27 In 2018, we established a pharmacists-managed oncology outpatient clinic with a Pharmacist Work System of MTM software in Shanghai with the cooperation of oncologists, clinic pharmacists and software engineers before the implementation of MTM service. This MTM software was designed to facilitate the communication and cooperation of different medical workers, which can be viewed as an extension of hospital information system. Incorporating the MTM into the hospital medical system which would collect more medication information is one of the ongoing explorations, including previous medical history, medications, laboratory examination, and other information.
At present, 4 outpatient pharmacists collaborate with oncologists at outpatient clinic to treat neoplasm patients. To perform high-quality comprehensive medication evaluation, pharmacists should receive uniform training before providing pharmaceutical service. In addition, the pharmacists collected the medical records of each patient for 30 to 40 minutes to obtain and record more comprehensive information. However, our outpatient clinic can only serve 6 to 10 patients each time in half a day per week. In addition, as the service is new and provides a lot of support to oncologists, our pharmacy service to the outpatient clinic is free of charge.
This is the first study to identify the DRPs and investigate the pharmacist interventions among neoplasm patients in China. Our model of pharmacy service could be used as a reference to establish similar pharmaceutical services for ambulatory patients in hospitals to optimize treatment regimens in China. On the other hand, some limitations of this study have to be considered. First, it was designed to assess the frequency and type of the DRPs as well as the acceptance rate of interventions but was not designed to address the clinical outcome of these interventions or its clinical significance. Second, the number of patients was small and there was no control group.
Conclusions
This is the first pharmacists-managed oncology outpatient clinic to describe the type of DRPs in outpatients and evaluate the effectiveness of pharmacist interventions in China. The most common DRPs were adverse reactions, non-adherence, untreated indication and drug interactions in our study. Pharmacist interventions were efficacious in resolving DRPs and improving adverse reactions in ambulatory neoplasm patients. We confirmed that pharmacists have an important role in ambulatory neoplasm patients care.
Acknowledgments
The authors wish to thank the staff of the Pharmacy Department and all the patients for supporting the study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Xincai Zhao  https://orcid.org/0000-0001-7713-4339
https://orcid.org/0000-0001-7713-4339
References
- 1. Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4(11):1553-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel AL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. Published online 4 February 2021. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carmona-Bayonas A, Jimenez-Fonseca P, Castanon E, et al. Chronic opioid therapy in long-term cancer survivors. Clin Transl Oncol. 2017;19(2):236-250. [DOI] [PubMed] [Google Scholar]
- 5. Mohile SG, Magnuson A. Comprehensive geriatric assessment in oncology. Interdiscip Top Gerontol. 2013;38:85-103. [DOI] [PubMed] [Google Scholar]
- 6. Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puts MT, Costa-Lima B, Monette J, et al. Medication problems in older, newly diagnosed neoplasm patients in Canada: How common are they? A prospective pilot study. Drugs Aging. 2009;26(6):519-536. [DOI] [PubMed] [Google Scholar]
- 8. Riechelmann RP, Moreira F, Smaletz O, Saad ED. Potential for drug interactions in hospitalized neoplasm patients. Cancer Chemother Pharmacol. 2005;56(3):286-290. [DOI] [PubMed] [Google Scholar]
- 9. Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65(9):989-995. [DOI] [PubMed] [Google Scholar]
- 10. Goh I, Lai O, Chew L. Prevalence and risk of polypharmacy among elderly neoplasm patients receiving chemotherapy in ambulatory oncology setting. Curr Oncol Rep. 2018;20(5):38. [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Yang J, Yu X, Wang Z, Wang H, Liu L. Characterization of drug-related problems and associated factors in ambulatory patients in China. J Clin Pharm Ther. 2020;45(2):1058-1065. [DOI] [PubMed] [Google Scholar]
- 12. Liekweg A, Westfeld M, Jaehde U. From oncology pharmacy to pharmaceutical care: new contributions to multidisciplinary cancer care. Support Care Cancer. 2004;12:73-79. [DOI] [PubMed] [Google Scholar]
- 13. Felton MA, Londen GV, Marcum ZA. Medication adherence to oral cancer therapy: the promising role of the pharmacist. J Oncol Pharm Pract. 2016;22(2):378-381. [DOI] [PubMed] [Google Scholar]
- 14. Walter C, Mellor JD, Rice C, et al. Impact of a specialist clinical cancer pharmacist at a multidisciplinary lung cancer clinic. Asia Pac J Clin Oncol. 2016;12:e367-e374. [DOI] [PubMed] [Google Scholar]
- 15. Randolph LA, Walker CK, Nguyen AT, Zachariah SR. Impact of pharmacist interventions on cost avoidance in an ambulatory cancer center. J Oncol Pharm Pract. 2018;24(1):3-8. [DOI] [PubMed] [Google Scholar]
- 16. National Health Commission of China. Opinions on accelerating the quality development of pharmaceutical services. Published 2018. Accessed September 8, 2020. http://www.gov.cn/xinwen/2018-11/28/content_5344128.htm
- 17. Tsai YF, Huang WC, Cho SF, et al. Side effects and medication adherence of tyrosine kinase inhibitors for patients with chronic myeloid leukemia in Taiwan. Medicine. 2018;97(26):e11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Society of Health-System Pharmacists. ASHP guidelines on a standardized method for pharmaceutical care. American Society of Health-System Pharmacists. Am J Heal Pharm. 1996;53(14):1713-1716. [DOI] [PubMed] [Google Scholar]
- 19. McBane S, Trewet CB, Havican SN, et al. Tenets for developing quality measures for ambulatory clinical pharmacy services. Pharmacotherapy. 2011;31(7):723. [Google Scholar]
- 20. Cherubini A, Corsonello A, Lattanzio F. Underprescription of beneficial medicines in older people: causes, consequences and prevention. Drugs Aging. 2012;29(6):463-475. [DOI] [PubMed] [Google Scholar]
- 21. Grohar PJ, Janeway KA, Mase LD, Schiffman JD. Advances in the treatment of pediatric bone sarcomas. ASCO Educational Book. 2017;37:725-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17(10):1396-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bluml BM. Definition of medication therapy management: development of profession wide consensus. J Am Pharm Assoc. 2005;45(5):566. [DOI] [PubMed] [Google Scholar]
- 24. Ramalho de Oliveira D, Brummel AR, Miller DB. Medication therapy management: 10 years of experience in a large integrated health care system. J Manag Care Pharm. 2010;16(3):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Theising KM, Fritschle TL, Scholfield AM, Hicks EL, Schymik ML. Implementation and clinical outcomes of an employer-sponsored, pharmacist-provided medication therapy management program. Pharmacotherapy. 2016;35(11):e159-e163. [DOI] [PubMed] [Google Scholar]
- 26. Viswanathan M, Kahwati LC, Golin CE, Blalock SJ, Lohr KN. Medication therapy management interventions in outpatient settings: a systematic review and meta-analysis. JAMA intern med. 2015;175:76-87. [DOI] [PubMed] [Google Scholar]
- 27. Beijing Pharmacist Association. Introduction of CMTM training system in China. Published 2017. Accessed September 8, 2020. http://cmtm.cmtms.cn/info/cmtm.html