Abstract
One of the rare causes of diffuse T-wave inversion (TWI) in electrocardiogram (ECG) is memory T-waves. This should be considered among the differentials of diffuse TWI in ECG of patients presenting to the emergency department (ED), especially when they have previous episodes of ventricular tachycardia (VT) or pacemaker implantation or Wolff-Parkinson-White syndrome. These TWIs are benign and do not require any treatment. However, it is of paramount importance for the emergency physician to differentiate it from ischemia-related T-wave changes. In the following case series, we report three cases of memory T-waves. Two of the cases had TWI in leads II, III, aVF, and V3 to V6 following reversion of VT. The other patient, with a VVI (Left ventricle paced, Left ventricle sensed, Inhibition to sensing) pacemaker, had memory T-waves in the ECG taken during normal sinus rhythm. In all the three patients, we considered memory T-waves to be the possible cause of TWI. The electrocardiographic diagnostic criteria for memory T-waves are positive T in lead aVL and positive/isoelectric T in the lead I; and precordial TWI >inferior TWI. These criteria are 92% sensitive and 100% specific. In the following case series, we also provide an algorithmic approach for patients with suspected memory T-waves in their 12-lead ECG when they present to the ED.
Keywords: Chatterjee phenomenon, memory T-waves, postpacing T-wave inversion, posttachycardia T-wave inversion, T-wave inversion
INTRODUCTION
The causes of T-wave inversion (TWI) in 12-lead electrocardiogram (ECG) include ischemia, left ventricular hypertrophy, hypokalemia, raised intracranial pressure, and hypertrophic obstructive cardiomyopathy. One of the rare causes of TWI is memory T-waves, which is also called the Chatterjee phenomenon. Memory T-waves are observed in an ECG after an event that causes abnormal electrical activation patterns such as ventricular tachycardia (VT), bundle branch block, Wolff-Parkinson-White (WPW) syndrome, and postpacing. We report a series of three patients, in which memory T-waves were observed.
CASE REPORT
Case 1
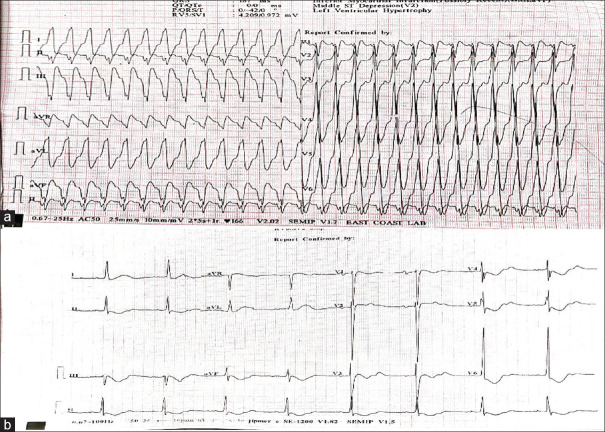
A 48-year-old male patient presented to the emergency department (ED) with complaints of palpitations and chest pain for 1 h duration. The patient was put on cardiac monitoring, which showed a regular wide complex tachycardia. The patient was hemodynamically unstable with a heart rate of 180/min, blood pressure of 60 mm of Hg systolic, and saturation of 98% in room air. The patient was planned for synchronized electrical cardioversion. A 12-lead ECG was also obtained, which confirmed the diagnosis of regular wide complex tachycardia [Figure 1a]. The patient was given procedural sedation, and synchronized cardioversion was done with 100 joules. After successful cardioversion, a 12-lead ECG was taken, which showed a heart rate of 50 beats/min: regular sinus rhythm and TWI in leads II, III, aVF, and V3 to V6 [Figure 1b]. Serum troponin levels were normal, and echocardiography showed no regional wall motion abnormality. The patient’s serum electrolyte levels were also normal. These T-wave changes were consistent with memory T-waves. The patient was followed up till discharge (9 days), and these TWIs were persistent until discharge.
Figure 1.
(a) 12-lead electrocardiogram of the patient showing regular wide complex tachycardia. (b) Electrocardiogram of the same patient postcardioversion showing positive T-wave in leads I and aVL and negative T-wave in leads II, III, aVF, and V3 to V6 with precordial T-wave inversion more than T-wave inversion in inferior leads suggestive of memory T-waves
Case 2
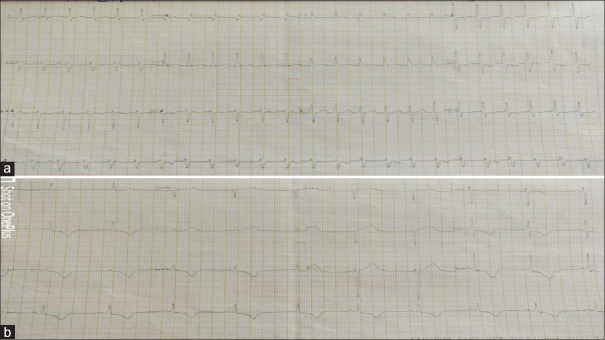
A 36-year-old male patient presented to ED with complaints of palpitations and syncope. He is a known case of recurrent fascicular VT. The patient was put on cardiac monitoring, which showed a regular wide complex tachycardia. The patient was hemodynamically stable with a heart rate of 150 beats/min, blood pressure of 110/70 mm of Hg, and a saturation of 99% in room air. A 12-lead ECG was obtained, which showed a heart rate of 150 beats/min with a regular wide complex tachycardia. The ECG also showed the right bundle branch block morphology with a left axis deviation, which suggested a fascicular VT [Figure 2a]. Two doses of adenosine (6 and 12 mg) were given, which had no effect on the rhythm. The rhythm was reverted with 12.5 mg of diltiazem. After successful chemical cardioversion, a 12-lead ECG was taken, which showed a rate of 65 beats/min with normal sinus rhythm. The ECG also showed TWIs in leads II, III, aVF, and V3 to V6, with positive T-waves in leads I and aVL [Figure 2b]. These T-wave changes were also consistent with memory T-waves. Serum troponin levels were normal, and echocardiography showed no regional wall motion abnormality. The patient’s serum electrolyte levels were also normal. These T-wave changes were consistent with memory T-waves. The patient was followed up till discharge (3 days), and these TWIs were persistent till discharge.
Figure 2.
(a) 12-lead electrocardiogram of the patient showing regular intermediate QRS complex tachycardia with right bundle branch morphology with left axis deviation and AV dissociation suggestive of fascicular ventricular tachycardia. (b) Electrocardiogram of the same patient postcardioversion showing positive T-wave in leads I and aVL and negative T-wave in leads II, III, aVF, and V4 to V6 with precordial T-wave inversion more than T-wave inversion in inferior leads suggestive of memory T-waves
Case 3
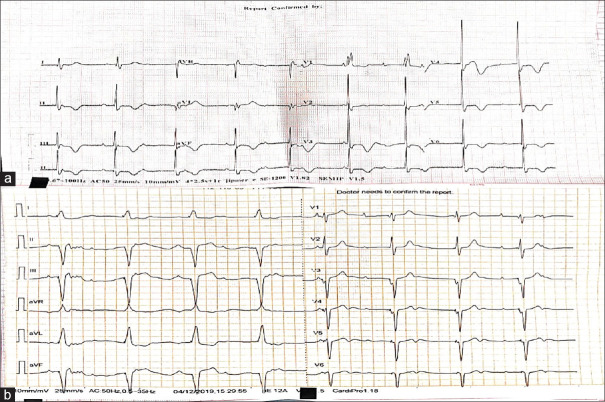
A 65-year-old female patient presented to ED with complaints of atypical chest pain for 6 h duration. The patient had a pacemaker implanted in the past for sick sinus syndrome. It was a VVI pacemaker which was kept at a minimal rate of 50 beats/min to prolong the battery life [Figure 3b]. The patient was hemodynamically stable with a pulse rate of 53 beats/min, blood pressure of 110/70 mm of Hg, and saturation of 100% in room air. A 12-lead ECG was taken which showed a heart rate of 53 beats/min, normal sinus rhythm with no pacemaker spikes suggestive of appropriate sensing, and diffuse TWI in leads II, III, aVF, and V1 to V6 with positive T-waves in leads I and avL [Figure 3a]. Serum troponin levels were normal, and echocardiography showed no regional wall motion abnormality. These T-wave changes were also consistent with memory T-waves. The patient was followed up. On his next visit a month later to cardiology outpatient department, 12-lead ECG was taken, which also showed memory T-waves.
Figure 3.
(a) 12-lead electrocardiogram of the patient with a pacemaker in situ showing positive T-wave in leads I and aVL and negative T-wave in leads II, III, aVF, and V4 to V6 with precordial T-wave inversion more than T-wave inversion in inferior leads suggestive of memory T-waves. (b) 12-lead electrocardiogram of the same patient showing regular wide complex rhythm at a rate of 50 beats/min with pacer spikes suggestive of pacemaker rhythm. (Note: The lower rate of pacemaker is set at 50 beats/min to prolong the life of pacemaker)
DISCUSSION
Memory T-waves are TWIs occurring in ECG of patients in their normal sinus rhythm when they had abnormal ventricular activation transiently due to various causes such as right ventricular pacing, VT, intermittent left bundle branch block (LBBB), and intermittent WPW syndrome.
Chatterjee et al., in 1969, observed abnormal ventricular repolarization after ventricular pacing. This abnormal ventricular depolarization produces TWIs in the 12-lead surface ECG after the pacing stimulus is withdrawn. Chatterjee et al. observed massive TWIs and ST depressions in unpaced (sensed ECG) 12-lead ECG of 29 patients who had recent ventricular pacemaker implantation.[1]
Rosenbaum et al. in 1982 were the first to coin the term cardiac memory, and they postulated that the electrotonic modulation of ventricular repolarization is the basic electrophysiological mechanism of cardiac memory and may result in normal and abnormal T-waves. They also formulated the three important principles of cardiac memory. These principles include:
The direction of the T-waves in sinus rhythm follows (remembers) the direction of the QRS complex during the past episode of abnormal activation
The amplitude of memory T-waves depends on the duration of abnormal conduction and is directly proportional to one another
Repeat episodes of abnormal activation after complete normalization of T-waves result in more rapid and prominent accumulation of T-wave changes (hence the term memory).[2]
Molecular mechanisms of cardiac memory
Although molecular mechanisms of cardiac memory have been extensively studied, the exact mechanism causing cardiac memory is still unclear. However in most of the studies, the common mechanism observed for the electrophysiological basis of cardiac memory is enhanced regional repolarization gradient.[3]
It is also observed that whatever may be the source of altered activation, it causes two distinct pathophysiological mechanisms depending on the site of the myocardium with respect to the activation region. The region of the myocardium, which is very close to the site of altered activation, is called “Early activated region” and the region of the myocardium, which is far away from the site of altered activation, is called “Late activated region.”
The potential mechanism causing cardiac memory is different in these two regions. In the early activated region, the potential mechanisms involved are electrotonic modulation and angiotensin II-mediated signaling. These mechanisms produce an attenuation of epicardial phase I notch because of reduced expression of Ito channel, Ica/Ikr channel, and connexin 43. In the late activated region, the potential mechanism involved in the mechanical strain. This results in prolonged action potential duration.
Because of the epicardial phase I notching in the early activated region and the increased action potential duration in the late activated region, there is a development of enhanced regional repolarization gradient, which produces cardiac memory.
Triggers of memory T-waves
Memory T-waves are triggered by any conditions which produce wide QRS transiently. These conditions include
Distinguishing memory T-waves from ischemic T-wave changes
The important clinical implication of memory T-waves in the ED is to differentiate memory T-waves from ischemic T-wave changes. This diagnostic dilemma is a very common occurrence in the ED as the triggers of memory T-waves such as VT, LBBB may be due to ischemia. Hence, it is very important for an emergency physician to differentiate memory T-waves from ischemic T-wave changes.
Shvilkin et al. proposed the electrocardiographic criteria to differentiate memory T-waves from ischemic T-wave changes. The combination of:
Positive T in lead aVL and positive/isoelectric T in the lead I; and
Precordial TWI > inferior TWI produces a unique pacing-induced cardiac memory signature that was 92% sensitive and 100% specific in differentiating pacing-induced TWI from ischemia.[9]
Although this study has certain limitations such as small sample size and included only patients with only postpacing triggers, these simple ECG criteria can be applied in the day-to-day practice.
Nakagawa et al. proposed ECG criteria to differentiate memory T-waves from ischemia related T-wave changes in idiopathic left VT:
Positive T in aVL
Negative or isoelectric T in II, and
Negative T in V4–6 or
QTc <430 ms
These ECG criteria were 100% sensitive and 96% specific in differentiating the cardiac memory group from the ACS group.[10] They proposed these criteria based on the study done on 16 patients with idiopathic left VT with TWIs postcardioversion.
Although both the studies looked at the ECG criteria for differentiating memory T-waves from ischemia-related T-waves, the sample of patients they studied was different. However, the ECG criteria they proposed were similar except that in the Nakagawa study, they looked at QTc interval.
Based on these studies and our personal experience, we propose an algorithm to differentiate memory T-waves from ischemia related T-wave changes [Figure 4].
Figure 4.
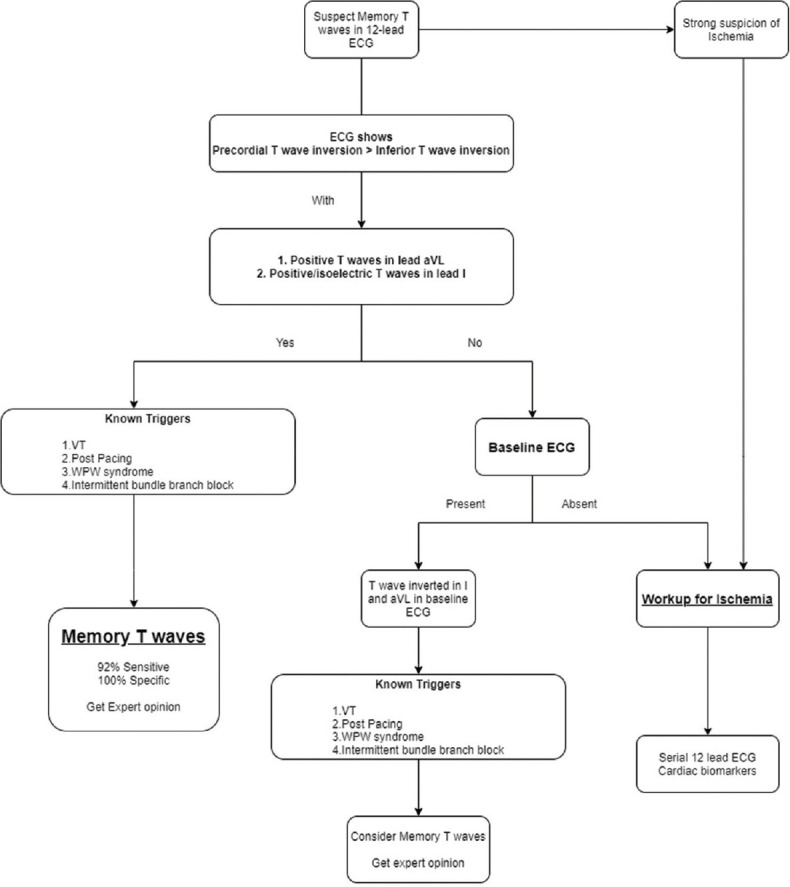
Algorithm for differentiating memory T-waves from ischemia related T-wave changes
CONCLUSION
It is important to differentiate memory T-waves from ischemia related T-waves in the ED. By following this simple algorithm in patients with suspected memory T-waves, we can differentiate memory T-waves from ischemia related T-wave changes. However when in doubt, always consider TWI to be due to ischemia until proven otherwise.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chatterjee K, Harris A, Davies G, Leatham A. Electrocardiographic changes subsequent to artificial ventricular depolarization. Br Heart J. 1969;31:770–9. doi: 10.1136/hrt.31.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbaum MB, Blanco HH, Elizari MV, Lázzari JO, Davidenko JM. Electrotonic modulation of the T wave and cardiac memory. Am J Cardiol. 1982;50:213–22. doi: 10.1016/0002-9149(82)90169-2. [DOI] [PubMed] [Google Scholar]
- 3.Jeyaraj D, Ashwath M, Rosenbaum DS. Pathophysiology and clinical implications of cardiac memory. Pacing Clin Electrophysiol. 2010;33:346–52. doi: 10.1111/j.1540-8159.2009.02630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sucu MM, Davutoglu V. Cardiac memory T-wave changes after ventricular tachycardia in pregnancy. Am J Emerg Med. 2008;26:968:e1–3. doi: 10.1016/j.ajem.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Waks JW, Steinhaus DA, Shvilkin A, Kramer DB. Post-pacemaker T-wave Inversions: Cardiac memory. Am J Med. 2016;129:170–2. doi: 10.1016/j.amjmed.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subham G, Rhee Edward K, Avari Jennifer N, Woodard Pamela Yoram R. Cardiac memory in patients with Wolff-Parkinson-White Syndrome. Circulation. 2008;118:907–15. doi: 10.1161/CIRCULATIONAHA.108.781658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seibolt L, Maestas C, Lazkani M, Fatima U, Loli A, Chesser M. Rate-related left bundle branch block and cardiac memory in a patient with bradycardia: Case report and literature review. Clin Cardiol. 2018;41:1097–102. doi: 10.1002/clc.22997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manne JR. The heart remembers: anterior T wave inversions in a patient with intermittent left bundle branch block. [Last acessed on 2020 Apr 27];Int J Clin Cardiol. 2017 4:105. Available from: https://clinmedjournalsorg/articles/ijcc/international-journal-of-clinical-cardiology-ijcc-4-105phpjid=ijcc . [Google Scholar]
- 9.Shvilkin A, Huang HD, Josephson ME. Cardiac memory: Diagnostic tool in the making. Circ Arrhythm Electrophysiol. 2015;8:475–82. doi: 10.1161/CIRCEP.115.002778. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa T, Yagi T, Ishida A, Mibiki Y, Yamashina Y, Sato H, et al. Differences between cardiac memory T wave changes after idiopathic left ventricular tachycardia and ischemic T wave inversion induced by acute coronary syndrome. J Electrocardiol. 2016;49:596–602. doi: 10.1016/j.jelectrocard.2016.04.001. [DOI] [PubMed] [Google Scholar]