Abstract
Background and Aims:
Although noninferior to stent retriever (SR) as first-line approach for endovascular treatment (EVT) of acute large vessel occlusion (LVO) stroke, little is known about the current status of direct aspiration (DA) as first-line thrombectomy in China. This analysis of a prospective, nationwide registry (ANGEL-ACT) aimed to investigate the prevalence and comparative effectiveness of DA-first thrombectomy in a real-world practice in China.
Methods:
All patients receiving thrombectomy were screened from a prospective cohort of LVO patients undergoing EVT at 111 hospitals in China between November 2017 and March 2019, and divided into two groups based upon which type of thrombectomy was attempted first (“DA-first” and “SR-first”). The following outcome measures were compared using logistic regression models with adjustment: successful recanalization after first-device alone and all procedures, use of rescue treatment, intracranial hemorrhage (ICH) within 24 h, and modified Rankin Scale (mRS) score at 90 days.
Results:
A total of 1225 patients, 102 (8.3%) in DA-first group and 1123 (91.7%) in SR-first group, were included. Patients receiving DA-first had less often successful recanalization after first-device alone [30.4 versus 66.4%; odds ratio (OR) = 0.23, 95% confidence interval (CI) = 0.15–0.37], more frequent rescue treatment (62.8 versus 27.0%; OR = 4.55, 95% CI = 2.92–7.08) and ICH (35.4 versus 22.1%; OR = 1.78, 95% CI = 1.12–2.83) than those receiving SR-first; however, no significant difference was found in successful recanalization after all procedures (84.3 versus 90.3%; p = 0.18) and 90-day mRS (median: 3 versus 3 points; p = 0.90) between both groups.
Conclusion:
This real-world registry suggested that DA-first thrombectomy for acute stroke patients lagged behind in China during the study period. Far fewer DA-first than SR-first thrombectomies were performed, and DA-first was associated with lower first-device recanalization, more frequently requiring rescue treatment, and increased ICH risk.
Clinical Trial Registration:
Keywords: stroke, thrombectomy, aspiration, stent retriever, China
Introduction
In the era of mechanical thrombectomy, there are two main modalities used to treat acute ischemic strokes with large vessel occlusion (LVO): direct aspiration (DA) and stent retriever (SR).1 The angiographic and clinical outcomes of either DA or SR as first-line thrombectomy have been reported to be similar in two recent randomized clinical trials.2,3 Thus, DA as first-line thrombectomy is recommended by the 2019 updated guidelines as noninferior to SR first-line thrombectomy for appropriate patients with acute LVO.4 DA thrombectomy techniques are being increasingly used in clinical practice in many developed countries,5–7 and based on some observational data, potential advantages of DA first-line approach might include less injury to the vessel wall, earlier puncture-to-reperfusion and a lower cost compared with SR first-line thrombectomy.3,5,6,8,9 However, the current status of DA as first-line approach in developing countries such as China are still rarely reported at present.
The current study utilized a prospective, nationwide registry to explore the prevalence and comparative effectiveness of DA-first thrombectomy in a real-world practice setting in China.
Methods
Study population
Data were derived from the ANGEL-ACT registry, a prospective nationwide registry of 1793 consecutive patients with acute LVO undergoing endovascular treatment (EVT) at 111 hospitals from 26 provinces in China between November 2017 and March 2019. Patients who met the following inclusion criteria were enrolled in the registry: (1) age ⩾18 years; (2) diagnosis of acute ischemic stroke caused by imaging-confirmed intracranial LVO, including internal carotid artery, middle cerebral artery (M1/M2), anterior cerebral artery (A1/A2), vertebral artery, basilar artery, and posterior cerebral artery (P1); (3) initiation of any type of EVT, including mechanical thrombectomy, intra-arterial thrombolysis, balloon angioplasty, stenting, and other mechanical fragmentation. The ANGEL-ACT registry aimed to reflect the current status of EVT for patients with acute LVO in real-world clinical practice in China. The protocol was approved by the ethics committees of Beijing Tiantan Hospital and each participating site. Each participant or his/her representative gave written informed consent before being enrolled in the study.
In this analysis, patients receiving thrombectomy with DA as first-line thrombectomy or SR as first-line thrombectomy were divided into two groups (“DA-first” and “SR-first”, respectively). DA thrombectomy was performed with a distal access catheter [Navien 058/072 (Medtronic), Sofia/Sofia plus (Microvention), or Catalyst 5/6 (Stryker)] or Penumbra ACE60 reperfusion catheter (Penumbra). SR thrombectomy was performed by using a Solitaire FR (Medtronic), Trevo (Stryker), Revive (Cordis) or Reco (Jiangsu Nico) device. After failure of the first-line thrombectomy approach, the choice of devices for rescue treatment was left to the discretion of the operator.
Data collection
All variables, including demographic characteristics, vascular risk factors, vital signs, stroke severity [e.g. National Institutes of Health Stroke Scale (NIHSS)], laboratory tests, neurovascular images, endovascular procedural details, peri-procedural management and complications, functional outcome [e.g. modified Rankin Scale (mRS)] and adverse events within 90 days after the procedure, were prospectively collected. Only investigators trained and qualified to use NIHSS and mRS recorded the scores.
Baseline computed tomography (CT)/magnetic resonance (MR) and CT angiography (CTA)/MR angiography (MRA), digital subtraction angiography (DSA), and post-procedural CT were evaluated by an imaging core laboratory blinded to clinical data and outcomes. Follow-up CTs were performed immediately and 24 ± 2 h after the procedure, and an additional CT was obtained at any time of neurological deterioration. All images were independently assessed by two neuroradiologists, with a third available for adjudication when needed. Core laboratory image interpretation included early ischemic changes using Alberta Stroke Program Early CT Score (ASPECTS)10 for anterior circulation strokes and posterior circulation ASPECTS (pc-ASPECTS)11 for posterior circulation strokes, occlusion site, tandem lesions, underlying intracranial atherosclerotic disease (ICAD, defined as fixed stenosis degree >70% or >50% with distal blood flow impairment or evidence of repeated re-occlusion),12 baseline and post-procedural modified Thrombolysis in Cerebral Infarction (mTICI),13 and occurrence of intracranial hemorrhage (ICH) on post-treatment imaging. For patients of whom the images could not be obtained and intra-procedural complications found on DSA (e.g. distal or new territorial embolization, arterial perforation, arterial dissection and vasospasm requiring treatment, etc.), site-reported data were used. The imaging review criteria of local investigators were the same as that of core laboratory, except that ICAD could also be determined according to previous images indicating stenotic lesion at the occlusion site.
Outcome measurement
The primary outcome was the successful recanalization (mTICI of 2b-3) of the occluded intracranial artery after first-device alone. The secondary outcomes included the complete recanalization (mTICI of 3) of the occluded intracranial artery after first-device alone, successful and complete recanalization after first-pass, number of passes, use of rescue treatment (switching to other thrombectomy devices/intra-arterial thrombolysis/balloon angioplasty/stenting), successful and complete recanalization after all procedures, procedure duration, 90-day mRS as an ordinal score, and mRS of 0–1, 0–2, 0–3 as dichotomous variables. The mRS score was assessed by trained investigators who were blinded to the baseline information via the telephone interview based on a standardized interview protocol. The safety outcomes included the distal or new territorial embolization during procedure, any ICH and symptomatic ICH within 24 h according to the Heidelberg Bleeding Classification,14 and death within 90 days.
Statistical analysis
All data were presented as the median (interquartile range [IQR]) for continuous and ordinal variables, and a number (percentage) for categorical variables. A comparison of the baseline characteristics between the two groups was performed using the Mann–Whitney U test for continuous and ordinal variables, and Pearson’s chi-square test or Fisher’s exact test for categorical variables. For comparing the outcome measures, the adjusted odds ratios (OR), common OR or β-coefficients with their 95% confidence intervals (CI) were analyzed using a binary or ordinal logistic regression model or generalized linear model, if applicable. All baseline variables with a significant difference of p < 0.05 as potential confounders were adjusted. In addition to conventional multivariable analysis, a propensity score was constructed for adjustment (the propensity score was derived using a logistic regression model that included all baseline variables for first-line treatment options). Treatment effect modification on the primary outcome was explored in the following subgroups: occlusion site (internal carotid artery versus middle cerebral artery M1 segment versus vertebra-basilar artery), tandem lesions (yes versus no), ICAD (yes versus no), stroke subtype by Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria (large artery atherosclerosis versus cardioembolism versus other or unknown etiology),15 and prior intravenous thrombolysis (yes versus no). Treatment effect size heterogeneity across the subgroups was tested by including the corresponding multiplicative interaction term into the logistic regression model with adjustment for the propensity score. All analyses were conducted with SAS software version 9.4 (SAS Institute Inc, Cary, NC). A two-sided p-value of <0.05 was considered to be statistically significant.
Results
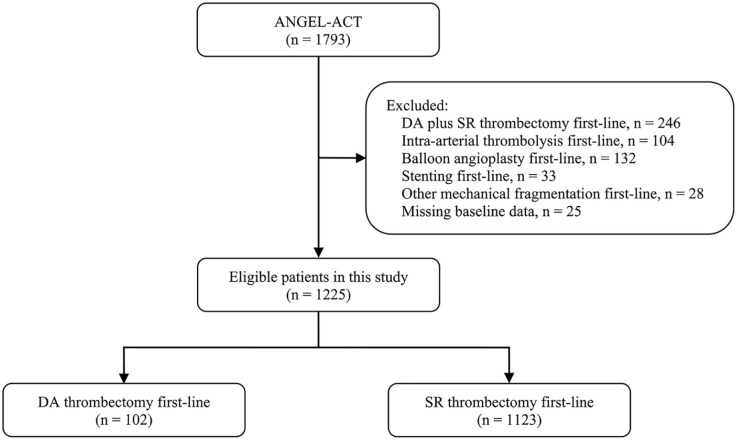
A total of 1225 eligible patients were analyzed in this study. A flow chart of the patient selection is shown in Figure 1. Among the included patients, 755 (63.3%) were male, and the median age was 65 years (IQR: 55–73). The median baseline NIHSS score and onset-to-puncture time were 17 points (IQR: 13–22) and 301 min (IQR: 212–439). Successful recanalization at the final angiogram was achieved in 1100 patients (89.8%). Symptomatic ICH within 24 h occurred in 89 (7.6%) out of 1164 patients. Of 1167 patients who attended a 90-day follow-up, the median mRS score was 3 points (IQR: 0–5), and the rate of the mRS 0–1, 0–2, 0–3, and death was 39.4%, 43.4%, 53.9%, and 16.8%, respectively.
Figure 1.
Flow chart of patient selection.
DA, direct aspiration; SR, stent retriever.
Baseline characteristics
Of the 1225 patients included, 102 (8.3%) received DA-first, and 1123 (91.7%) received SR-first. The baseline characteristics are compared in Table 1. Patients of the DA-first group had higher rates of internal carotid artery occlusion (50.0% versus 23.4%), tandem lesions (35.3% versus 14.5%), cardioembolism (48.0% versus 35.4%), and prior intravenous thrombolysis (44.1% versus 25.9%) than those of the SR-first group (all p-values < 0.05). No significant differences existed between the two groups in the remaining baseline variables.
Table 1.
Baseline characteristics of patients undergoing thrombectomy with DA first-line versus SR first-line.
| Baseline variable | DA first-line (n = 102) | SR first-line (n = 1123) | p-Value |
|---|---|---|---|
| Age, median (IQR), years | 65 (54–74) | 65 (55–73) | 0.61 |
| Male sex | 63 (61.8) | 712 (63.4) | 0.74 |
| History of hypertension | 56 (54.9) | 633 (56.4) | 0.78 |
| History of diabetes mellitus | 21 (20.6) | 194 (17.3) | 0.40 |
| History of dyslipidemia | 10 (9.8) | 98 (8.7) | 0.71 |
| History of coronary heart disease | 17 (16.7) | 175 (15.6) | 0.77 |
| History of atrial fibrillation | 39 (38.2) | 381 (33.9) | 0.38 |
| Prior stroke | 20 (19.6) | 234 (20.8) | 0.77 |
| Cigarette smoking | 0.61 | ||
| Never smoker | 64 (62.8) | 706 (62.9) | |
| Ex-smoker | 9 (8.8) | 72 (6.4) | |
| Current smoker | 29 (28.4) | 345 (30.7) | |
| Systolic blood pressure, median (IQR), mmHg | 148 (135–160) | 145 (130–161) | 0.63 |
| NIHSS score, median (IQR) | 16 (12–19) | 17 (13–22) | 0.17 |
| ASPECTS, median (IQR)a | 9 (7–10) | 9 (7–10) | 0.21 |
| Occlusion site | <0.01 | ||
| Internal carotid artery | 51 (50.0) | 263 (23.4) | |
| Middle cerebral artery (M1) | 34 (33.3) | 510 (45.4) | |
| Vertebro-basilar artery | 15 (14.7) | 224 (20.0) | |
| Other intracranial arteries | 2 (2.0) | 126 (11.2) | |
| Tandem lesions | 36 (35.3) | 163 (14.5) | <0.01 |
| Underlying ICAD | 0.12 | ||
| Yes | 21 (20.6) | 333 (29.7) | |
| No | 66 (64.7) | 666 (59.3) | |
| Undetermined | 15 (14.7) | 124 (11.0) | |
| Stroke subtype by TOAST criteria | 0.02 | ||
| Large artery atherosclerosis | 33 (32.4) | 506 (45.1) | |
| Cardioembolism | 49 (48.0) | 397 (35.4) | |
| Other or unknown etiology | 20 (19.6) | 220 (19.6) | |
| Prior use of antiplatelet agents | 16 (15.7) | 170 (15.1) | 0.88 |
| Prior use of anticoagulants | 7 (6.9) | 51 (4.5) | 0.32 |
| Prior intravenous thrombolysis | 45 (44.1) | 291 (25.9) | <0.01 |
| Intra-procedural use of heparin | 43 (42.2) | 574 (51.1) | 0.08 |
| Type of anesthesia | 0.11 | ||
| Local anesthesia only | 35 (34.3) | 477 (42.5) | |
| Local anesthesia plus sedation | 14 (13.7) | 183 (16.3) | |
| General anesthesia | 53 (52.0) | 463 (41.2) | |
| Onset-to-puncture time, median (IQR), min | 318 (210–425) | 300 (212–440) | 0.97 |
Values are numbers with percentages in parentheses, unless indicated otherwise.
ASPECTS for anterior circulation stroke; and pc-ASPECTS for posterior circulation stroke.
ASPECTS, Alberta stroke program early CT score; DA, direct aspiration; ICAD, intracranial atherosclerotic disease; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; pc-ASPECTS, posterior circulation Alberta stroke program early CT score; SR, stent retriever; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Outcome measures
A comparison of the outcome measures is presented in Table 2. Regarding the primary outcome, a multivariable analysis with adjustment for potential confounders showed that patients in the DA-first group had a lower rate of successful recanalization after first-device alone (30.4% versus 66.4%; OR = 0.23, 95% CI = 0.15–0.37; p < 0.01) compared with those in the SR-first group.
Table 2.
Outcome measures of patients undergoing thrombectomy with DA first-line versus SR first-line.
| Outcome variable | DA first-line | SR first-line | Unadjusted model |
Adjusted model 1a |
Adjusted model 2b |
|||
|---|---|---|---|---|---|---|---|---|
| Effect size (95% CI) | p-Value | Effect size (95% CI) | p-Value | Effect size (95% CI) | p-Value | |||
| Primary outcome | ||||||||
| Successful recanalization after first-device alonef | 31/102 (30.4) | 746/1123 (66.4) | 0.22 (0.14–0.34)c | <0.01 | 0.23 (0.15–0.37)c | <0.01 | 0.24 (0.15–0.37)c | <0.01 |
| Secondary outcomes | ||||||||
| Complete recanalization after first-device aloneg | 25/102 (24.5) | 582/1123 (51.8) | 0.30 (0.19–0.48)c | <0.01 | 0.33 (0.20–0.53)c | <0.01 | 0.31 (0.19–0.51)c | <0.01 |
| Successful recanalization after first-passf | 29/102 (28.4) | 404/1123 (36.0) | 0.71 (0.45–1.11)c | 0.13 | 0.82 (0.52–1.29)c | 0.39 | 0.83 (0.52–1.31)c | 0.42 |
| Complete recanalization after first-passg | 24/102 (23.5) | 325/1123 (28.9) | 0.76 (0.47–1.22)c | 0.25 | 0.84 (0.51–1.37)c | 0.48 | 0.83 (0.51–1.37)c | 0.47 |
| Number of passes, median (IQR) | 2 (1–4) | 1 (1–2) | 0.94 (0.68–1.20)d | <0.01 | 0.79 (0.53–1.05)d | <0.01 | 0.74 (0.48–1.01)d | <0.01 |
| Use of rescue treatment | 64/102 (62.8) | 303/1123 (27.0) | 4.56 (3.00–6.95)c | <0.01 | 4.55 (2.92–7.08)c | <0.01 | 4.51 (2.90–7.02)c | <0.01 |
| Switching to other thrombectomy devices | 58/102 (56.9) | 45/1123 (4.0) | 31.58 (19.30–51.67)c | <0.01 | 27.67 (16.28–47.01)c | <0.01 | 25.41 (15.18–42.53)c | <0.01 |
| Intra-arterial thrombolysis | 2/102 (2.0) | 20/1123 (1.8) | 1.10 (0.25–4.79)c | 0.90 | 0.95 (0.21–4.37)c | 0.95 | 1.15 (0.25–5.30)c | 0.86 |
| Balloon angioplasty | 12/102 (11.8) | 167/1123 (14.9) | 0.76 (0.41–1.43)c | 0.40 | 0.81 (0.42–1.55)c | 0.53 | 0.88 (0.46–1.68)c | 0.70 |
| Stenting | 11/102 (10.8) | 158/1123 (14.1) | 0.74 (0.39–1.41)c | 0.36 | 0.79 (0.40–1.54)c | 0.48 | 0.81 (0.41–1.58)c | 0.54 |
| Successful recanalization after all proceduresf | 86/102 (84.3) | 1014/1123 (90.3) | 0.58 (0.33–1.02)c | 0.06 | 0.67 (0.37–1.21)c | 0.18 | 0.74 (0.40–1.35)c | 0.33 |
| Complete recanalization after all proceduresg | 70/102 (68.6) | 777/1123 (69.2) | 0.97 (0.63–1.51)c | 0.91 | 1.07 (0.68–1.68)c | 0.77 | 1.02 (0.65–1.61)c | 0.92 |
| Procedure duration, median (IQR), min | 84 (50–150) | 80 (50–123) | 6.81 (−5.30 to 18.91)d | 0.27 | 4.39 (−8.02 to 16.81)d | 0.49 | 4.30 (−8.32 to 16.91)d | 0.50 |
| mRS at 90 d, median (IQR) | 3 (0–5) | 3 (0–5) | 0.94 (0.65–1.36)e | 0.73 | 1.03 (0.70–1.50)e | 0.90 | 0.99 (0.67–1.44)e | 0.94 |
| mRS 0–1 at 90 d | 37/95 (39.0) | 423/1072 (39.5) | 0.98 (0.64–1.51)c | 0.92 | 1.11 (0.71–1.73)c | 0.65 | 1.02 (0.66–1.59)c | 0.93 |
| mRS 0–2 at 90 d | 40/95 (42.1) | 467/1072 (43.6) | 0.94 (0.62–1.44)c | 0.78 | 1.03 (0.67–1.60)c | 0.88 | 0.96 (0.62–1.49)c | 0.86 |
| mRS 0–3 at 90 d | 46/95 (48.4) | 583/1072 (54.4) | 0.79 (0.52–1.20)c | 0.26 | 0.85 (0.55–1.31)c | 0.45 | 0.81 (0.53–1.25)c | 0.34 |
| Safety outcomes | ||||||||
| Intra-procedural embolization | 6/102 (5.9) | 61/1123 (5.4) | 1.09 (0.46–2.58)c | 0.85 | 0.79 (0.32–1.94)c | 0.61 | 0.68 (0.27–1.73)c | 0.42 |
| Any ICH within 24 h | 34/96 (35.4) | 237/1074 (22.1) | 1.94 (1.24–3.01)c | <0.01 | 1.78 (1.12–2.83)c | 0.02 | 1.69 (1.06–2.69)c | 0.03 |
| Symptomatic ICH within 24 hh | 13/96 (13.54) | 76/1068 (7.1) | 2.05 (1.09–3.84)c | 0.03 | 1.65 (0.84–3.26)c | 0.15 | 1.51 (0.77–2.96)c | 0.23 |
| Death within 90 d | 15/95 (15.8) | 181/1072 (16.9) | 0.92 (0.52–1.64)c | 0.78 | 0.82 (0.45–1.51)c | 0.53 | 0.82 (0.45–1.48)c | 0.51 |
Data are shown as the event number/total number (%), unless otherwise indicated.
Adjusted for the baseline variables with a significant difference of p < 0.05 between both groups, including occlusion site, tandem lesions, TOAST subtype, intravenous thrombolysis.
Adjusted for the propensity score.
The OR values were calculated using a binary logistic regression model.
The β-coefficients were calculated using a generalized linear model.
The common OR values were calculated using an ordinal logistic regression model and indicated the odds of improvement of 1 point on the mRS at 90 days.
Defined as mTICI of 2b-3.
Defined as mTICI of 3.
According to the Heidelberg Bleeding Classification.
CI, confidence interval; DA, direct aspiration; ICH, intracranial hemorrhage; IQR, interquartile range; mRS, modified Rankin Scale; mTICI, modified Thrombolysis in Cerebral Infarction; OR, odds ratio; SR, stent retriever; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Regarding the secondary and safety outcomes, multivariable analyses demonstrated that patients who underwent DA-first had a lower chance of complete recanalization after first-device alone (24.5% versus 51.8%; OR = 0.33, 95% CI = 0.20–0.53; p < 0.01), more thrombectomy passes (median: 2 versus 1; β = 0.79, 95% CI = 0.53–1.05; p < 0.01), higher likelihood of rescue treatment (62.8% versus 27.0%; OR = 4.55, 95% CI = 2.92–7.08; p < 0.01), switching to other thrombectomy devices (56.9% versus 4.0%; OR = 27.67, 95% CI = 16.28–47.01; p < 0.01), and any ICH within 24 h (35.4% versus 22.1%; OR = 1.78, 95% CI = 1.12–2.83; p = 0.02) than those who underwent SR-first. No significant differences were found between both groups in the remaining secondary and safety outcome measures.
As a sensitivity analysis, similar results were observed in the models with propensity score adjusted (Table 2).
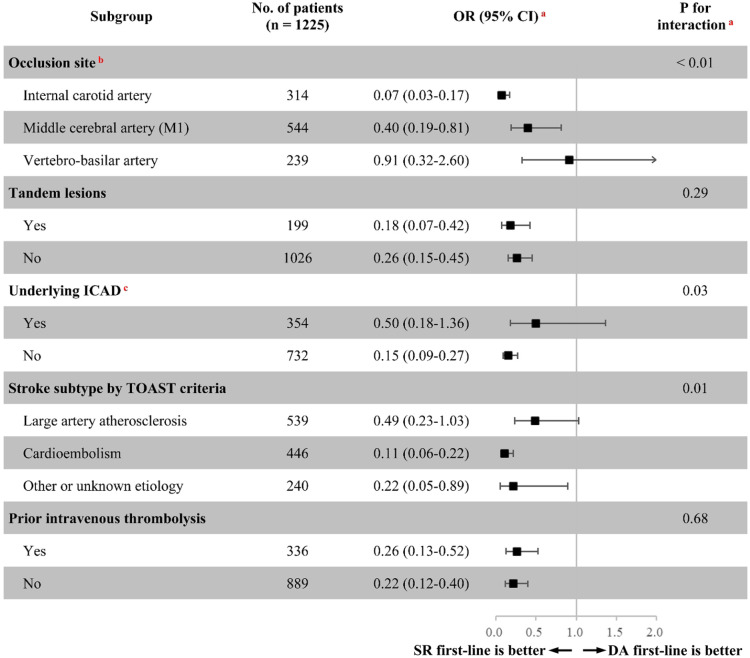
Subgroup analysis
As shown in Figure 2, exploratory subgroup analyses showed significant heterogeneity in treatment effects on the primary outcome among the studied subgroups stratified by occlusion site, underlying ICAD, and TOAST subtype (p for interactions <0.05); however, similar treatment effect sizes on the primary outcome were seen in the studied subgroups stratified by tandem lesions, and prior intravenous thrombolysis (p for interactions >0.20).
Figure 2.
Treatment effects on the primary outcome according to exploratory subgroups.
aAdjusted for the propensity score.
bA total of 128 patients with other intracranial artery occlusions were excluded.
cA total of 139 patients with undetermined ICAD were excluded.
CI, confidence interval; DA, direct aspiration; ICAD, intracranial atherosclerotic disease; OR, odds ratio; SR, stent retriever; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Discussion
The major findings of this study were (1) the number of patients undergoing DA-first thrombectomy for acute LVO in China was far less than that of SR-first approach during the study period (ratio of DA-first to SR-first was approximately 1:11); (2) DA-first thrombectomy was associated with lower recanalization after first-device alone, more thrombectomy passes and requiring rescue treatment (specifically switching to other thrombectomy devices), and increased risk of any ICH within 24 h; (3) DA-first and SR-first modalities showed no significant difference in terms of the final recanalization rate after rescue treatment and 90-day functional outcomes; and (4) significant heterogeneity in the treatment effects on the first-device successful recanalization was found in the subgroups stratified by occlusion site, ICAD, and TOAST subtype.
The development of DA-first thrombectomy in China is much behind what one might expect compared with the published literature in the Western world. Five randomized trials have established thrombectomy as the standard of care for appropriate patients with LVO, and these trials predominantly used SR devices to do a thrombectomy.16 As a result, established stroke guidelines in 2015 specifically recommend the use of SR to the exclusion of other thrombectomy techniques.17 Since then, SR first-line thrombectomy has flourished and gained popularity in China, which has in turn limited the application of DA-first thrombectomy.
One of the notable findings from our study is that the DA-first group achieved a lower recanalization without the help of rescue treatment than the SR-first group after adjustment for potential confounders and propensity scores. In the real-practice setting, a failure of the first-line modality to achieve satisfactory recanalization is not a rare instance. In such patients with LVO who were refractory to first-line modality, rescue treatment was often required. Because successful recanalization is one of the most important factors in achieving a favorable outcome,18 it seems a reasonable strategy that rescue treatment is performed after the first-line modality failure rather than aborting further treatment. Various types of rescue treatment were used in this study. Switching to other thrombectomy devices was significantly higher in DA-first, while intra-arterial thrombolysis, balloon angioplasty, and permanent stenting were comparable in both. This seems to indicate that the causes of recanalization failure might differ between DA-first and SR-first. Specifically, SR was used as a rescue treatment in more than half of DA-first cases whereas DA was needed as rescue treatment in only 4% of SR-first cases. These data suggest that the performance of a DA-first approach was not as successful as a SR-first approach in the current study population. However, in light of the literature in total, it is unlikely that a DA-first approach has inherent technical disadvantages in clot retrieval. One possible explanation could be the experience and preference of the operators. These two thrombectomy modalities are quite different in the mechanisms of action and procedural details, and the experience and preference of operators may be the major determinant in the successful angiographic outcome, rather than differences in the thrombectomy devices themselves. During the study period, DA thrombectomy was only routinely performed in a few hospitals in China, and the annual volume of DA-first in these hospitals was very small. Accordingly, the annual volume of DA-first per operator would be even smaller, so most operators would likely have limited hands-on experience with DA thrombectomy. In this analysis, the operator’s inexperience in DA may also be reflected in the fact that more thrombectomy passes and higher ICH rate were seen in the DA-first group than in the SR-first group.
The causes of recanalization failure may be various, including occlusion site, tandem lesions, pre-existing ICAD, TOAST subtype, and added value of intravenous thrombolysis.19 An exploratory subgroup analysis to investigate the difference in the causes of recanalization failure between DA-first and SR-first was completed. We found that DA-first was far less effective than SR-first in terms of first-device successful recanalization for anterior circulation LVO as compared with posterior circulation LVO, possibly due to the different anatomical characteristics (tortuous access, vessel curvature or angulation), constituent ratio of stroke causes (ICAD or embolism), and proportion of thrombus components (hard or soft thrombi, red blood cell-rich or fibrin-rich clots) between anterior and posterior circulation.20–22 We also found that the performance of DA-first was much poorer than SR-first in patients with embolic stroke, which corresponds well with previous studies.23,24 SR-first thrombectomy may be a better option for acute LVO with susceptibility vessel sign (+) or CT thrombus hyperdense (+) suggesting embolic stroke; however, our unplanned subgroup analysis was unable to draw any definitive conclusions.25,26 However, when choosing the modality of mechanical thrombectomy, we should comprehensively consider the thrombus burden, occlusion site, and anatomical access in addition to stroke etiology and thrombus component. From our point of view, DA-first thrombectomy may be suitable for the treatment of large-load soft thrombus (such as tandem lesions) and bifurcation occlusion (such as M1 bifurcation and basilar artery tip) with good access. The different causes of recanalization failure and optimizing the strategy of currently available EVT methods should be investigated in further studies.
This study has several inherent limitations that must be acknowledged. First, the results should be interpreted with the caution of selection bias caused by non-randomized treatment assignment, and measured or unmeasured variables (such as the aforementioned experience/preference of the operators) that may represent potential confounders that cannot be ruled out despite multiple adjustment models used. Second, since a relatively small number of patients were enrolled in the DA-first group, there was a possibility of a type II error in the statistical analysis. Third, as this was a retrospective analysis of a prospective registry, the number of passes determining a failure was not predefined, leading to potential heterogeneity in the use of rescue treatment between the two groups. However, the operators who participated in this study were experienced in when to perform rescue treatment. Fourth, thrombectomy for stroke is allowed in qualified tertiary and secondary hospitals in China. In the ANGEL-ACT registry, more than 90% of the patients were from tertiary hospitals, and in fact less DA first-line thrombectomy was done in secondary hospitals, so it is likely that the proportion of using DA-first could be even lower. Finally, we did not collect treatment costs, so it was not possible to compare the cost-effectiveness of the two modalities.
Conclusion
The study population from 111 hospitals across 26 (76.5%) of 34 provinces in China represents the real-world patients who received EVT between November 2017 and March 2019. Therefore, it is worthy of note that the results of this study reflect the current situation of DA as first-line thrombectomy for acute stroke patients in real clinical practice in China, suggesting that DA-first thrombectomy is less developed in China compared with the Western world. Specifically, far fewer DA-first than SR-first thrombectomies were performed during the study period, and DA-first thrombectomy was associated with lower first-device recanalization, more requiring rescue treatment, and increased risk of ICH.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_17562864211007715 for Current status of aspiration thrombectomy for acute stroke patients in China: data from ANGEL-ACT Registry by Xu Tong, Yilong Wang, Clayton T. Bauer, Baixue Jia, Xuelei Zhang, Xiaochuan Huo, Gang Luo, Anxin Wang, Ning Ma, Feng Gao, Dapeng Mo, Ligang Song, Xuan Sun, Lian Liu, Yiming Deng, Xiaoqing Li, Bo Wang, Gaoting Ma, Yongjun Wang, Zeguang Ren and Zhongrong Miao in Therapeutic Advances in Neurological Disorders
Acknowledgments
We thank all participating hospitals, relevant clinicians, statisticians, and imaging and laboratory technicians.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is supported by grants from the National Key Research and Development Program of China (2016YFC1301500, 2018YFC1312801), China Postdoctoral Science Foundation (2019M650773).
Data sharing: The data that support the findings of this study are available from the corresponding author Zhongrong Miao (zhongrongm@163.com) upon reasonable request.
ORCID iDs: Xu Tong  https://orcid.org/0000-0002-3277-6941
https://orcid.org/0000-0002-3277-6941
Dapeng Mo  https://orcid.org/0000-0002-1558-9626
https://orcid.org/0000-0002-1558-9626
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xu Tong, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Yilong Wang, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Clayton T. Bauer, Department of Neurosurgery, University of South Florida, Tampa, FL, USA
Baixue Jia, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Xuelei Zhang, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Xiaochuan Huo, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Gang Luo, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Anxin Wang, China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Ning Ma, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Feng Gao, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Dapeng Mo, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Ligang Song, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Xuan Sun, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Lian Liu, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Yiming Deng, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Xiaoqing Li, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Bo Wang, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Gaoting Ma, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Yongjun Wang, China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Zeguang Ren, Department of Neurosurgery, University of South Florida, 2 Tampa General Circle, Tampa, FL 33606, USA.
Zhongrong Miao, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, No.119 South 4th Ring West Road, Fengtai District, Beijing, 100070, China.
References
- 1. Turc G, Kim AS. First-line use of contact aspiration or stent retriever thrombectomy for large vessel occlusion stroke. Stroke 2019; 50: 2634–2636. [DOI] [PubMed] [Google Scholar]
- 2. Lapergue B, Blanc R, Gory B, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA 2017; 318: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turk AS, III, Siddiqui A, Fifi JT, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet 2019; 393: 998–1008. [DOI] [PubMed] [Google Scholar]
- 4. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 5. Bernsen M, Goldhoorn RB, van Oostenbrugge RJ, et al. Equal performance of aspiration and stent retriever thrombectomy in daily stroke treatment. J Neurointerv Surg 2019; 11: 631–636. [DOI] [PubMed] [Google Scholar]
- 6. Martini M, Mocco J, Turk A, et al. ‘Real-world’ comparison of first-line direct aspiration and stent retriever mechanical thrombectomy for the treatment of acute ischemic stroke in the anterior circulation: a multicenter international retrospective study. J Neurointerv Surg 2019; 11: 957–963. [DOI] [PubMed] [Google Scholar]
- 7. Kang DH, Kim JW, Kim BM, et al. Need for rescue treatment and its implication: stent retriever versus contact aspiration thrombectomy. J Neurointerv Surg 2019; 11: 979–983. [DOI] [PubMed] [Google Scholar]
- 8. Peschillo S, Diana F, Berge J, et al. A comparison of acute vascular damage caused by ADAPT versus a stent retriever device after thrombectomy in acute ischemic stroke: a histological and ultrastructural study in an animal model. J Neurointerv Surg 2017; 9: 743–749. [DOI] [PubMed] [Google Scholar]
- 9. Turk AS, Turner R, Spiotta A, et al. Comparison of endovascular treatment approaches for acute ischemic stroke: cost effectiveness, technical success, and clinical outcomes. J Neurointerv Surg 2015; 7: 666–670. [DOI] [PubMed] [Google Scholar]
- 10. Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta stroke programme early CT score. Lancet 2000; 355: 1670–1674. [DOI] [PubMed] [Google Scholar]
- 11. Puetz V, Sylaja PN, Coutts SB, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke 2008; 39: 2485–2490. [DOI] [PubMed] [Google Scholar]
- 12. Jia B, Feng L, Liebeskind DS, et al. Mechanical thrombectomy and rescue therapy for intracranial large artery occlusion with underlying atherosclerosis. J Neurointerv Surg 2018; 10: 746–750. [DOI] [PubMed] [Google Scholar]
- 13. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015; 46: 2981–2986. [DOI] [PubMed] [Google Scholar]
- 15. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of ORG 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 16. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 17. Powers WJ, Derdeyn CP, Biller J, et al. 2015. American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 18. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007; 38: 967–973. [DOI] [PubMed] [Google Scholar]
- 19. Kim BM. Causes and solutions of endovascular treatment failure. J Stroke 2017; 19: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banerjee G, Stone SP, Werring DJ. Posterior circulation ischaemic stroke. BMJ 2018; 361: k1185. [DOI] [PubMed] [Google Scholar]
- 21. Alonso de, Leciñana M, Kawiorski MM, Ximénez-Carrillo Á, et al. Mechanical thrombectomy for basilar artery thrombosis: a comparison of outcomes with anterior circulation occlusions. J Neurointerv Surg 2017; 9: 1173–1178. [DOI] [PubMed] [Google Scholar]
- 22. Weber R, Minnerup J, Nordmeyer H, et al. Thrombectomy in posterior circulation stroke: differences in procedures and outcome compared to anterior circulation stroke in the prospective multicentre REVASK registry. Eur J Neurol 2019; 26: 299–305. [DOI] [PubMed] [Google Scholar]
- 23. Bourcier R, Mazighi M, Labreuche J, et al. Susceptibility vessel sign in the ASTER trial: higher recanalization rate and more favourable clinical outcome after first line stent retriever compared to contact aspiration. J Stroke 2018; 20: 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ye G, Cao R, Lu J, et al. Association between thrombus density and reperfusion outcomes using different thrombectomy strategies: a single-center study and meta-analysis. Front Neurol 2019; 10: 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim SK, Yoon W, Kim TS, et al. Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient-echo MRI. AJNR Am J Neuroradiol 2015; 36: 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brinjikji W, Duffy S, Burrows A, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg 2017; 9: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_17562864211007715 for Current status of aspiration thrombectomy for acute stroke patients in China: data from ANGEL-ACT Registry by Xu Tong, Yilong Wang, Clayton T. Bauer, Baixue Jia, Xuelei Zhang, Xiaochuan Huo, Gang Luo, Anxin Wang, Ning Ma, Feng Gao, Dapeng Mo, Ligang Song, Xuan Sun, Lian Liu, Yiming Deng, Xiaoqing Li, Bo Wang, Gaoting Ma, Yongjun Wang, Zeguang Ren and Zhongrong Miao in Therapeutic Advances in Neurological Disorders