Abstract
Background:
To date, few data have been reported on clinical outcomes following interventions in elderly populations with acute basilar artery occlusion. Using data from the Endovascular Treatment for Acute Basilar Artery Occlusion Study (BASILAR), we evaluated the efficacy and safety of intervention and determined predictors of outcomes among elderly patients in China.
Methods:
Patients from January 2014 to May 2019 were dichotomized into elderly (75 years or older) and nonelderly patients (under 75 years). Pearson’s Chi-square test and multivariate logistic regression were performed to assess 90-day favorable functional outcome (defined as a modified Rankin scale score of 0–3), mortality and symptomatic intracranial hemorrhage between intervention and conservative cohorts in elderly patients.
Results:
Among the 829 patients in the BASILAR, 182 patients aged 75 years or older were analyzed. These patients were divided into intervention (127 patients) and conservative (55 patients) cohorts. Compared with the conservative cohort, the intervention cohort presented more frequently with a favorable functional outcome (28.3% versus 12.7%; p = 0.023) and with a decreased mortality (54.3% versus 76.4%; p = 0.005). There was no difference in symptomatic intracranial hemorrhage (4.7% versus 0, p = 0.235). Multivariate analysis indicated that intervention was associated with favorable functional outcome (adjusted odds ratio, 0.262; 95% confidence interval, 0.088–0.778, p = 0.016) and lower mortality (adjusted odds ratio, 0.257; 95% confidence interval, 0.109–0.606, p = 0.002). In the intervention cohort, initial National Institutes of Health Stroke Scale (NIHSS) score and occlusion site were associated with functional outcome, and initial NIHSS score and recanalization were associated with mortality.
Conclusions:
Although the overall outcome following intervention was worse with age, intervention was more effective and safer than conservative treatment for elderly Chinese patients with basilar artery occlusion. Predictors of desirable outcome in elderly patients undergoing intervention included lower initial NIHSS score, occlusion site and successful recanalization.
Clinical Trial Registration-URL: http://www.chictr.org. Unique identifier: ChiCTR-1800014759
Keywords: basilar artery occlusion, elderly, endovascular treatment, intervention, stroke
Introduction
To date, patients with basilar artery occlusion (BAO) would have had more severe outcomes if left untreated. This devastating disease presents with high mortality, causing up to 68% of victims to have poor outcomes.1 Previous studies have shown that acute BAO was usually associated with a high rate of morbidity and mortality without active treatment.2 The benefit of intervention has yet to be demonstrated, as recent independent randomized controlled trials focusing on management of acute BAO have failed to statistically demonstrate the superiority of intervention.3,4 However, intervention treatment has been increasingly applied as a common strategy for patients with BAO in daily clinical practice. In the Basilar Artery International Cooperation Study (BASICS) from 2009, as many as 329 (55.6%) of the 592 patients were treated with intra-arterial therapy.1 In the nationwide, multicenter, prospective registry study, termed BASILAR (the Endovascular Treatment for Acute Basilar Artery Occlusion Study) from 2020, this number was 647 (78%).5 These results demonstrated that for patients with acute BAO, the devastating outcome limited the acceptance of conservative, and treating physicians tended to apply more aggressive endovascular interventions in many cases in expectation of lowering disability and mortality rates. Although interventional approaches have been used to treat BAO with a tendency toward a benefit over the last few years, limited data on the efficacy and safety of intervention were available in the elderly population.
Patient demographics were characterized by a marked aging due to the increase in life expectancy and improvements in medical care. The ever-growing elderly population was much more vulnerable to acute cerebral ischemia than the younger population, leading to a higher incidence of cerebral ischemia in this group.6 In addition, this patient population became disabled after stroke more often than the younger population.7 Elderly patients will soon represent the majority of BAO victims, resulting in a substantial impact on health services in the near future.8 While interventional therapy for elderly patients with acute BAO was currently not considered the standard of care, it was offered at many comprehensive stroke centers or tertiary hospitals in China. It was believed that there was clinical equipoise and that further study was warranted.
Here, we analyzed data from the BASILAR to assess the efficacy and safety of intervention for patients ⩾75 years of age and to determine the factors affecting the clinical outcome.
Methods
Patients selection
Patients from the BASILAR were included. The investigator-initiated multicenter perspective BASILAR registry aimed to assess the efficacy and safety of intervention for patients with acute BAO. Consecutive patients from 47 senior stroke sites across 15 provinces in China were included from January 2014 to May 2019. Patients at each stroke site were required to be included to avoid selection bias. Eligible patients were aged 18 years or older, presented with acute, symptomatic angiographically confirmed BAO within 24 h of estimated occlusion time, and underwent intravenous thrombolysis if in the therapeutic time window. The choice of treatment was at the discretion of the treating physician at the investigation site.
Ethical approval for the study was obtained by the local institutional review board of each site. Written informed consent was obtained from the patients or, if unable to provide consent, their legal surrogates, as required by the authorities’ guidelines. BASILAR was registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn, ChiCTR1800014759).
Parameter definitions
The BASILAR cohort was dichotomized into elderly and nonelderly groups. Elderly patients were defined as those 75 years or older and nonelderly patients were defined as those younger than 75 years. Patients in both age groups were divided into intervention cohorts (standard medication plus mechanical thrombectomy, thromboaspiration, balloon angioplasty, stenting, or a combination of these approaches) and conservative cohorts (standard medication alone, including antiplatelet or anticoagulation therapy, intravenous thrombolysis, or a combination of these therapies). The specific method of treatment was left to the discretion of the local interventionist. The presumed causative mechanism of stroke was assessed based on the Trial of ORG10172 in Acute Stroke Treatment (TOAST) classification.9
The ischemic changes were quantified by the posterior circulation Alberta Stroke Program Early Computed Tomography Score (pc-ASPECTS, range 0–10, with scores ⩾8 correlating with favorable outcome).10 The collateral circulation status was assessed by the posterior circulation collateral score (PC-CS) based on the presence of potential collateral pathways on computed tomography angiography.11 The technical outcome of interventional treatment was assessed by the modified Thrombolysis in Cerebral Infarction score.12 Successful vessel recanalization was defined as a modified Thrombolysis in Cerebral Infarction score of 2b or 3 at the end of the procedure as confirmed by the imaging core laboratory according to individual angiographic data.
The efficacy outcome was the proportion of patients with favorable functional outcome defined by the follow-up modified Rankin scale (mRS) score at 90 days. The mRS is a seven-level categorical scale that measures functional outcomes with scores ranging from 0 (no symptoms) to 6 (death). Given the documented dismal natural history of BAO,13 favorable functional outcome was defined as an mRS of 3 or less, and poor functional outcome defined as an mRS of 4 or more. We also investigated whether the results changed if favorable functional outcome was defined as an mRS of 0–2 and 0–1.
The safety outcomes were the incidence of death within 90 days after symptoms onset and the symptomatic intracranial hemorrhage as diagnosed within 48 h after intervention treatment. Symptomatic intracranial hemorrhage, defined according to the Heidelberg Bleeding Classification, was detected by brain imaging associated with any of the following items: an increase by ⩾4 points of the total National Institutes of Health Stroke Scale (NIHSS), an increase by ⩾2 points of a NIHSS subcategory as a relevant change in neurological status, the absence of alternative explanation for deterioration or leading to intubation/hemicraniectomy/external ventricular draining placement or other major medical/surgical intervention.14
Statistical analysis
Data were presented as medians and interquartile ranges (IQRs) unless indicated otherwise. Univariate analysis was first performed to compare the baseline demographic and clinical characteristics of elderly patients between the intervention and conservative cohorts, using the Mann–Whitney U test for numerical variables and Pearson’s Chi-square test or Fisher’s exact test for categorical variables as appropriate. The t test was used for variables that were normally distributed (Kolmogorov–Smirnov test: p > 0.05), such as the initial systolic and diastolic blood pressure for the elderly patients. Binary outcomes were analyzed with logistic regression to estimate the treatment effect and to determine predictors of functional outcome and mortality. The adjusted and unadjusted odds ratios (ORs) were reported with 95% confidence intervals (CIs) to indicate statistical precision. To generate the benefit curves related to the treatment modalities, outcome-specific predicted probabilities for continuous age values were computed by setting other variables in the model to their mean values.
In this study, the missing values of key variables were excluded from analysis, and thus there was no need for imputation. The significance level was set at p < 0.05 and all p-values were two-sided. Statistical analysis was performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA) and STATA version 15.2 (Stata Corp LLC, TX, USA). Percentage bar plots were drawn in Excel 2020 software (Microsoft). Distribution surfaces representing changes in predicted outcome probabilities were generated using SigmaPlot 12.5, with models assessed using the R2 correlation metric.
Results
Baseline characteristics of the BASILAR full cohort
There were 829 acute BAO patients from 47 stroke sites in China enrolled in the BASILAR registry.5 Of these, 647 were <75 years and 182 were ⩾75 years of age. The median (IQR) age of the full cohort was 65 (57–74) years.
Outcome with intervention versus conservative in the elderly
There were 182 patients aged 75 years or older [58.8% male; median (IQR) age, 78 (76–81) years; age range, 75–91 years]. When dichotomizing the elderly patients, 127 of 182 (69.8%) were intervention-treated cohort [median (IQR) age, 78 (76–81) years] and 55 of 182 (30.2%) were conservative-treated cohort [median (IQR) age, 79 (77–81) years]. The baseline characteristics of the elderly patients and univariate analysis are presented in Table 1.
Table 1.
Baseline characteristics of patients ⩾75 years with acute BAO.
| Characteristics | All patients | Intervention | Conservative | p-value |
|---|---|---|---|---|
| (n = 182) | (n = 127) | (n = 55) | ||
| Age, median (IQR), years | 78 (76–81) | 78 (76–81) | 79 (77–81) | 0.363 |
| Sex, male, n (%) | 107 (58.8) | 79 (62.2) | 28 (50.9) | 0.155 |
| NIHSS baseline score, median (IQR) | 28 (16–34) | 28 (17–34) | 22 (14–31) | 0.025 |
| pc-ASPECTS baseline, median (IQR)a | 8 (6–9) | 8 (7–9) | 7 (6–9) | 0.062 |
| PC-CS score, median (IQR) | 4 (3–6) | 4 (4–6) | 4 (2–6) | 0.196 |
| SBP, mean (SD), mm Hg | 156 (27.4) | 152 (26.0) | 164 (28.9) | 0.008 |
| DBP, mean (SD), mm Hg | 85 (15.8) | 84 (16.0) | 89 (15.1) | 0.062 |
| Medical history, n (%) | ||||
| Hypertension | 142 (78.0) | 102 (80.3) | 40 (72.7) | 0.256 |
| Hyperlipidemia | 34 (18.7) | 21 (16.5) | 13 (23.6) | 0.259 |
| Diabetes mellitus | 36 (19.8) | 24 (18.9) | 12 (21.8) | 0.650 |
| Atrial fibrillation | 68 (37.4) | 56 (44.1) | 12 (21.8) | 0.004 |
| Cerebral infarction | 48 (26.4) | 32 (25.2) | 16 (29.1) | 0.584 |
| Intracerebral hemorrhage | 6 (3.3) | 3 (2.4) | 3 (5.5) | 0.283 |
| Coronary artery disease | 39 (21.4) | 30 (23.6) | 9 (16.4) | 0.273 |
| Heart failure | 14 (7.7) | 9 (7.1) | 5 (9.1) | 0.870 |
| Chronic bronchitis | 3 (1.6) | 2 (1.6) | 1 (1.8) | 1.000 |
| Current or ex-smoker | 34 (18.7) | 28 (22.0) | 6 (10.9) | 0.077 |
| Cause of stroke, n (%) | 0.001 | |||
| Large artery atherosclerosis | 86 (47.3) | 56 (44.1) | 30 (54.5) | |
| Cardioembolism | 80 (44.0) | 65 (51.2) | 15 (27.3) | |
| Other causes | 16 (8.8) | 6 (4.7) | 10 (18.2) | |
| Occlusion sites, n (%) | 0.032 | |||
| Distal basilar artery | 84 (46.2) | 65 (51.2) | 19 (34.5) | |
| Middle basilar artery | 61 (33.5) | 34 (26.8) | 27 (49.1) | |
| Proximal basilar artery | 18 (9.9) | 13 (10.2) | 5 (9.1) | |
| Vertebral artery-V4 | 19 (10.4) | 15 (11.8) | 4 (7.3) | |
| Intravenous thrombolysis, n (%) | 35 (19.2) | 22 (17.3) | 13 (23.6) | 0.321 |
| OTT, median (IQR), min | 239 (121–362) | 261 (151–365) | 208 (89–329) | 0.046 |
Data were missing for one patient in the conservative cohort.
BAO, basilar artery occlusion; DBP, diastolic blood pressure; IQR, interquartile rage; NIHSS, National Institutes of Health Stroke Scale; OTT, onset-treatment time; pc-ASPECTS, posterior circulation Alberta Stroke Program Early Computed Tomography Score; PC-CS score, posterior circulation collateral system score; SBP, systolic blood pressure.
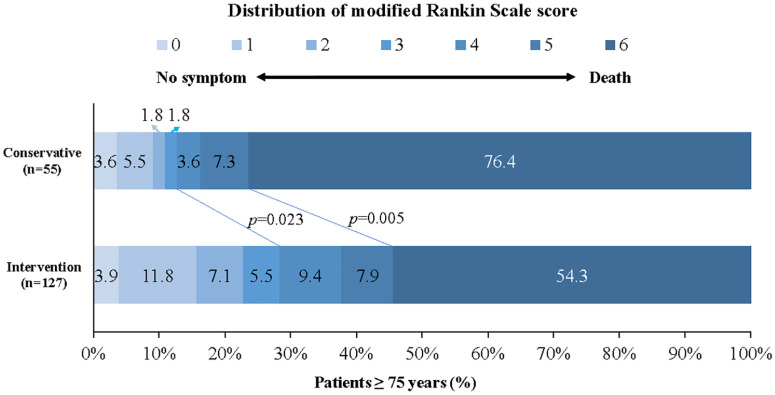
In the univariate analysis, compared with the conservative cohort, the intervention cohort presented a higher rate of favorable functional outcome (28.3% versus 12.7%; p = 0.023) and a decreased mortality (54.3% versus 76.4%; p = 0.005) (Figure 1). No difference was observed for functional outcome when it was defined by different mRS scores (mRS 0–2: 22.8% versus 10.9%, p = 0.061; mRS 0–1: 15.7% versus 9.1%, p = 0.231).
Figure 1.
Distribution of modified Rankin scale score at 90 days in patients ⩾75 years.
Shown was the distribution of the modified Rankin scale scores at 90 days in patients aged 75 years and older. The distribution showed that compared with conservative treatment, intervention treatment was associated with a higher rate of favorable functional outcome and lower mortality among patients ⩾75 years.
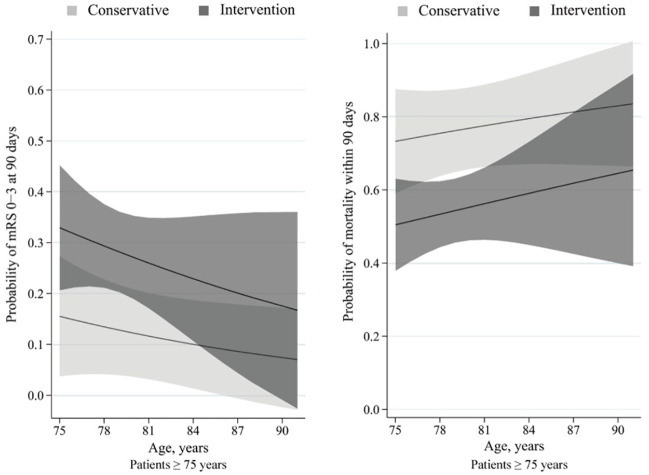
The probability of favorable functional outcome decreased with age, and the probability of mortality increased with age. The between-line difference in the probability of both outcomes was approximately 20% between the intervention and conservative cohorts (Figure 2). No difference was detected in the proportion of symptomatic intracranial hemorrhage (4.7% versus 0, p = 0.235) between the intervention and conservative cohorts, respectively.
Figure 2.
Predicted probability of clinical outcome by age in patients ⩾75 years.
Curves showed that among patients aged 75 years or older, the intervention cohort presented with a higher predicted probability of favorable functional outcome and a lower predicted probability of mortality than the conservative cohort.
The solid line indicates its predicted probability of outcomes and grey shading indicates its 95% confidence interval.
Adjusting for age, initial NIHSS, PC-CS, occlusion site, onset to treatment time, intravenous thrombolysis and type of treatment, the multivariate analysis confirmed that initial NIHSS (OR, 1.106; 95% CI, 1.055–1.160; p < 0.001), occlusion site and intervention treatment (OR, 0.262; 95% CI, 0.088–0.788; p = 0.016) were associated with functional outcome, and that initial NIHSS (OR, 1.082; 95% CI, 1.040–1.126; p < 0.001) and intervention treatment (OR, 0.257; 95% CI, 0.109–0.606; p = 0.002) were associated with mortality (Table 2).
Table 2.
Multivariate analysis: predictors of outcome in patients ⩾75 years.
| Variables | Unadjusted OR (95% CI) | p-value | Adjusted ORa (95% CI) | p-value |
|---|---|---|---|---|
| Functional outcome | ||||
| Initial NIHSS score | 1.067 (1.03–1.106) | <0.001 | 1.106 (1.055–1.160) | <0.001 |
| PC-CS score | 0.787 (0.649–0.954) | 0.015 | 0.908 (0.709–1.163) | 0.446 |
| Occlusion sites | ||||
| Distal basilar arteryb | 0.039 | 0.005 | ||
| Middle basilar artery | 2.889 (1.246–6.695) | 0.013 | 4.997 (1.792–13.931) | 0.002 |
| Proximal basilar artery | 1.750 (0.527–5.812) | 0.361 | 2.290 (0.582–9.004) | 0.236 |
| Vertebral artery-V4 | 4.250 (0.917–19.702) | 0.064 | 8.020 (1.498–42.924) | 0.015 |
| Intervention | 0.369 (0.153–0.890) | 0.027 | 0.262 (0.088–0.778) | 0.016 |
| Mortality | ||||
| Initial NIHSS score | 1.063 (1.030–1.098) | <0.001 | 1.082 (1.040–1.126) | <0.001 |
| PC-CS score | 0.742 (0.622–0.885) | 0.001 | 0.817 (0.663–1.008) | 0.059 |
| Intervention | 0.368 (0.180–0.751) | 0.006 | 0.257 (0.109–0.606) | 0.002 |
Adjusted estimates of effect were calculated using multiple regression taking the following variables into account: age, initial NIHSS, PC-CS score, occlusion site, onset to treatment time, intravenous thrombolysis and type of treatment.
Distal basilar artery was taken as reference.
CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; PC-CS score, posterior circulation collateral system score.
Predictors of outcome with intervention in the elderly
To identify the predictors of outcome following intervention, the 127 elderly patients with intervention were dichotomized by functional outcome (favorable versus poor) and mortality (alive versus deceased) to determine the variables to be adjusted. The adjusted variables were calculated using multiple logistics regressions, taking the following into account: age, initial NIHSS, initial PC-CS, occlusion site, puncture to recanalization time and recanalization. Analysis demonstrated initial NIHSS (OR, 1.142; 95% CI, 1.070–1.219; p < 0.001) and occlusion site as predictors of functional outcome. And initial NIHSS (OR, 1.121; 95% CI, 1.059–1.186; p < 0.001) and recanalization (OR, 0.230; 95% CI, 0.064–0.826; p = 0.025) were predictors of mortality (Table 3). The predicted probability of outcome with age and initial NIHSS were shown via distribution surfaces (Figure I in the Supplementary Material).
Table 3.
Multivariate analysis: predictors of outcome following intervention in patients ⩾75 years.
| Variables | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Functional outcome | ||||
| Age | 1.017 (0.907–1.140) | 0.779 | 0.933 (0.808–1.079) | 0.351 |
| Initial NIHSS score | 1.080 (1.034, 1.129) | 0.001 | 1.142 (1.070–1.219) | <0.001 |
| Occlusion sites | ||||
| Distal basilar arterya | 0.034 | 0.014 | ||
| Middle basilar artery | 3.867 (1.325–11.284) | 0.013 | 8.101 (1.942–33.797) | 0.004 |
| Proximal basilar artery | 2.222 (0.558–8.854) | 0.258 | 2.711 (0.521–14.110) | 0.236 |
| Vertebral artery-V4 | 4.333 (0.902–20.813) | 0.067 | 7.681 (1.311–45.001) | 0.024 |
| Mortality | ||||
| Age | 1.014 (0.915–1.124) | 0.787 | 0.928 (0.815–1.056) | 0.256 |
| Initial NIHSS score | 1.092 (1.046–1.141) | <0.001 | 1.121 (1.059–1.186) | <0.001 |
| Recanalization | 0.267 (0.092–0.774) | 0.015 | 0.230 (0.064–0.829) | 0.025 |
Distal basilar artery was taken as reference.
CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio.
Outcome of intervention in the elderly versus nonelderly patients
Among all patients treated with intervention in the BASILAR, 520 of 647 (80.4%) were nonelderly (median, 62; IQR, 54–67) and 127 of 647 (19.6%) were elderly patients (median, 78; IQR, 76–81). The characteristics of patients with intervention and univariate analysis are presented in Table I in the Supplementary Material.
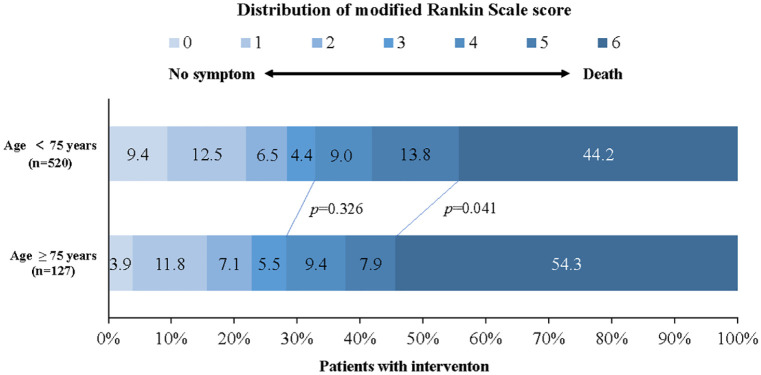
In the univariate analysis, the elderly patients with intervention showed a higher mortality (54.3% versus 44.2%; p = 0.041; Figure 3) and comparable rates of favorable functional outcome (28.3% versus 32.9%; p = 0.326) and symptomatic intracranial hemorrhage (4.7% versus 7.5%; p = 0.270) compared with those nonelderly. The probability of favorable functional outcome decreased and that of mortality increased from a young age to the old (Figure 4). An age greater than 75 years remained a predictor of mortality (OR, 1.623; 95% CI, 1.022–2.578; p = 0.040) when adjusting for dichotomized age, initial NIHSS, PC-CS, hypertension, diabetes mellitus, occlusion site, puncture to recanalization time and recanalization on the multivariate analysis (Table 4).
Figure 3.
Distribution of modified Rankin scale score at 90 days in patients with intervention.
Shown was the distribution of the modified Rankin scale scores at 90 days in all patients with intervention. The distribution showed that compared with younger patients, the older patients were associated with a higher rate of mortality among all the intervention-treated patients. The overall prognosis following intervention tended to be poor with age.
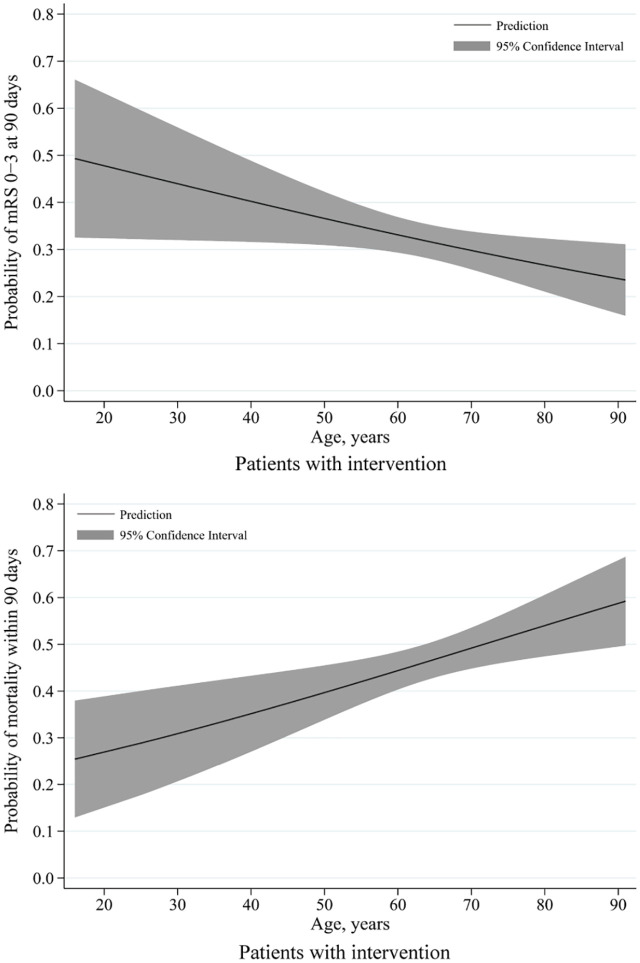
Figure 4.
Predicted probability of clinical outcome by age in patients with intervention.
Of all patients with intervention, the curves showed that decreases in predicted probabilities of favorable functional outcome and increases in predicted probabilities of mortality with age.
The solid line indicates the predicted probability of outcomes and grey shading indicates the 95% confidence interval.
Table 4.
Multivariate analysis: predictors of outcome following intervention.
| Variables | Functional outcome | Mortality | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
| Dichotomized age | 1.465 (0.881–2.436) | 0.141 | 1.623 (1.022–2.578) | 0.040 |
| Initial NIHSS score | 1.102 (1.077–1.127) | <0.001 | 1.087 (1.063–1.112) | <0.001 |
| PC-CS score | 0.834 (0.746–0.934) | 0.002 | 0.887 (0.797–0.986) | 0.026 |
| Hypertension | 0.674 (0.432–1.052) | 0.082 | 1.183 (0.781–1.790) | 0.428 |
| Diabetes mellitus | 2.122 (1.274–3.536) | 0.004 | 1.973 (1.270–3.064) | 0.002 |
| Occlusion sites | ||||
| Distal basilar arterya | 0.016 | 0.986 | ||
| Middle basilar artery | 2.257 (1.356–3.757) | 0.002 | 1.009 (0.628–1.620) | 0.972 |
| Proximal basilar artery | 1.454 (0.795–2.660) | 0.224 | 0.909 (0.514–1.608) | 0.744 |
| Vertebral artery-V4 | 1.791 (0.987–3.251) | 0.055 | 0.991 (0.573–1.713) | 0.973 |
| PTR | 1.009 (1.005–1.012) | <0.001 | 1.007 (1.004–1.010) | <0.001 |
| Recanalization | 0.194 (0.097–0.386) | <0.001 | 0.150 (0.088–0.256) | <0.001 |
Distal basilar artery was taken as reference.
CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; PC-CS score, posterior circulation collateral system score; PTR, puncture to recanalization.
Discussion
This multicenter study represented the real-world experience of patients 75 years or older with acute BAO treated with intervention using modern devices and advanced technologies. The major finding of this study was a strong correlation between intervention and favorable outcome among elderly patients with a threefold increase in the likelihood of achieving a 90-day favorable functional outcome and nearly four times as high mortality rate in conservative-treated patients. Along with prior data and case series of BAO, our results provided extensive evidence for further implementation of intervention in the posterior circulation stroke population.
Published literature has reported outcomes following thrombectomy in elderly patients with anterior circulation stroke compared with conservative counterparts. Among the randomized thrombectomy trials of anterior circulation occlusion, the overall outcomes following intervention were significantly more beneficial than with compared with conservative treatment in the elderly subgroups of the ESCAPE trial, DAWN trial (both divided into older versus younger than 80 years) and SWIFT-PRIME trial (divided into older versus younger than 70 years).7 Relatively few studies have reported on elderly patients with BAO. Some studies have reported poor outcome rates ranging from 54% to 95% for conservatively treated BAO patients,2 and have examined the effect of intervention in patients with acute BAO.15–19 However, few studies have solely focused on elderly stroke population. Our results clearly showed the superiority of intervention with special reference to patients aged 75 years or older. Thus, our study, specifically highlighting the aging population, may threw some light on the treatment of elderly patients with acute BAO.
In our study, there was a higher rate of favorable functional outcome (adjusted OR, 0.262; 95% CI, 0.088–0.788) and decreased mortality (adjusted OR, 0.257; 95% CI, 0.109–0.606) in the interventional cohort than in the conservative cohort. Several of the following observations might contribute to this result. First, the study benefited from the use of widespread, advanced techniques and newer-generation devices, the application of neuroimaging and rigorous patient selection. This led to a high rate of successful recanalization in patients undergone intervention, reaching up to 81.9% in our study. In a series of 214 patients, Bouslama et al.16 demonstrated a strong correlation between recanalization and outcome in patients with vertebrobasilar occlusion stroke treated with intervention with a 10-fold increase in the likelihood of achieving good functional outcomes if successful recanalization is attained and twice as high mortality rates in nonrecanalized patients. Additionally, the considerate healthcare provided, proper postprocedural care and timely rehabilitation training services also contributed to the functional outcomes.20
Second, we observed a higher proportion of embolic rather than atherosclerotic occlusion (51.2% versus 44.1%) in the intervention cohort. This was mainly due to the aging population and the increasing prevalence and incidence of atrial fibrillation with age among elderly patients, which were in accordance with the real-world clinical practice in China.21 Elderly patients with an embolic cause of BAO had a relatively higher rate of good functional outcome than those with an atherosclerotic cause.19 This is an important point, as the difference in clot morphology and the pathologic mechanism of stroke (i.e. intracranial atherosclerosis) may exert a variable influence on management and outcomes. Of the elderly patients undergone intervention in our study, the rate of favorable functional outcome was 33.8% among patients with an embolic cause versus 21.4% among those with an atherosclerotic cause, but this difference was not statistically significant (p = 0.130). Mortality was also not significantly different between groups (p = 0.976). This might be explained by the sampling variability caused by the small number of patients.
Third, the time from onset to treatment, an important prognostic factor for the elderly patients in our study, tended to be shorter than that in patients ⩾75 years of age with symptom onset within 24 h in the BASICS registry.22 We compared the number and proportion of patients during five time periods stratified by time to treatment between the elderly patients in the BASICS and BASILAR registries: <3 h: 50 (34.7%) versus 91 (50.0%); 4–6 h: 50 (34.7%) versus 59 (32.4%); 7–9 h: 17 (11.8%) versus 16 (8.8%); 10–12 h: 13 (9.0%) versus 9 (4.9%); 13–24 h: 14 (9.7%) versus 7 (3.8%). And the difference was significant (p = 0.022). Time to treatment has been widely described as having a major impact on clinical outcome. As indicated previously, a shorter time to treatment was associated with improved odds for a better clinical outcome.23,24 Our positive result might result from improvement in emergency triage systems, more timely recognition and earlier administration of medical treatment.24
Prior studies of the posterior territories also examined predictors of elderly patients with intervention. Rentzos et al.25 demonstrated in a single series of 110 patients with underwent endovascular stroke treatment in the posterior circulation that a significant association was seen between favorable outcome and younger age (p = 0.023). Rangaraju et al.26 evaluated 376 of 619 participants in the BASICS registry and showed that age was a predictor for poor outcome. An analysis of 212 BAO patients reported by Kang et al.18 showed that younger age was significantly associated with a favorable shift in the overall distribution of 90-day mRS. In contrast, Mokin and colleagues showed that there were 12 (12%) patients >80 years in the cohort, and only 5 (42%) had an mRS of 0–2 at 3 months versus 30 (34%) in patients younger than 80.15 The authors found that age >80 years was not a predictor for clinical outcome. Bouslama et al.16 also found that age was not associated with good outcome. These results were partially influenced by their one-arm, single-center, study design with a small sample size, the use of earlier-generation retrieval devices and unsophisticated interventionists in some institutions. Only a minority of patients included were of elderly age. Therefore, the results above should be cautiously interpreted. Our study aimed to examine risk factors among intervention-treated patients ⩾75 years to identify predictors of the prognosis of acute BAO. In our multivariate analysis, factors that may influence functional outcome included initial NIHSS score and occlusion site; initial NIHSS and successful recanalization were found to be predictors of mortality in the elderly population. Age, analyzed as a continuous variable, however, showed no correlation with favorable clinical outcome.
The strengths of this study were the comparator arm of the elderly BAO patients who received standard medical therapy alone and the large sample size (up to 182) from a representative database. The use of newer-generation devices and advanced interventional technologies made the results more applicable to current institutions and populations. The study limitations included the observational nature of our BASILAR registry study, which had the inherent weakness of the nonrandomized clinical trial as exemplified by a selection bias in the treatment of the patients. Among the elderly population with cardioembolic stroke or distal basilar occlusion which were likely to recanalize, the proportion of patients undergone intervention was significantly higher than the proportion of those undergone conservative treatment. This bias can be avoided by developing associated randomized controlled trials. Second, the study was unable to define an upper age limit above which intervention treatment was no longer effective because of the lack of cases. In addition, confounding factors were not fully controlled for between the two cohorts, which may have potentially influenced our results.
Conclusions
While the elderly population presented with a high overall mortality, we concluded that they were more likely to achieve a favorable outcome after intervention than after conservative treatment. A lower initial NIHSS, occlusion site and successful recanalization were associated with desirable outcomes in elderly patients undergone intervention. For elderly patients with BAO, potential intervention should not be ruled out simply because they are 75 years of age or older.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_17562864211000453 for Endovascular intervention for basilar artery occlusion in the elderly by Weidong Luo, Wenguo Huang, Min Zhang, Xing Liu, Zhangbao Guo, Peiyang Zhou, Li Wang, Xinmin Fu, Shiquan Yang, Shuai Zhang, Zhiming Zhou, Min Zhang, Junjie Yuan, Shuai Liu, Jiaxing Song, Zhongming Qiu, Hongfei Sang, Fengli Li, Wenjie Zi, Deping Wu, Wenhua Liu and Qingwu Yang in Therapeutic Advances in Neurological Disorders
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (nos. 81901236 and 82071323), Chongqing Natural Science Foundation (no. cstc2020jcyj-msxmX0926), and Clinical Medical Research Talent Training Program of Army Medical University (nos. 2019XLC2008 and 2019XLC3016).
ORCID iD: Weidong Luo  https://orcid.org/0000-0001-5282-3090
https://orcid.org/0000-0001-5282-3090
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Weidong Luo, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Wenguo Huang, Department of Neurology, Maoming Traditional Chinese Medicine Hospital, Maoming, China.
Min Zhang, Department of Neurology, Maoming Traditional Chinese Medicine Hospital, Maoming, China.
Xing Liu, Department of Medicine, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Zhangbao Guo, Department of Neurology, Wuhan No. 1 Hospital, Wuhan, China.
Peiyang Zhou, Department of Neurology, The First People’s Hospital of Xiangyang, Xiangyang, China.
Li Wang, Department of Neurology, The Third People’s Hospital of Zigong, Zigong, China.
Xinmin Fu, Department of Neurology, Xuzhou Central Hospital, Xuzhou, China.
Shiquan Yang, Department of Neurology, The 902th Hospital of The Chinese People’s Liberation Army, Bengbu, China.
Shuai Zhang, Department of Neurology, The Affiliated Hospital of Yangzhou University, Yangzhou, China.
Zhiming Zhou, Department of Neurology, Yijishan Hospital of Wannan Medical College, Wuhu, China.
Min Zhang, Department of Neurology, Jiangmen Central Hospital, Jiangmen, China.
Junjie Yuan, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Shuai Liu, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Jiaxing Song, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Zhongming Qiu, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Hongfei Sang, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Fengli Li, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Wenjie Zi, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Deping Wu, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Wenhua Liu, Department of Neurology, Wuhan No. 1 Hospital, No. 215 Zhongshan Avenue, Qiaokou District, Wuhan 430000, China.
Qingwu Yang, Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), No. 183 Xinqiao Main Street, Shapingba District, Chongqing, 400037, China.
References
- 1. Schonewille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol 2009; 8: 724–730. [DOI] [PubMed] [Google Scholar]
- 2. Mortimer AM, Bradley M, Renowden SA. Endovascular therapy for acute basilar artery occlusion: a review of the literature. J Neurointerv Surg 2012; 4: 266–273. [DOI] [PubMed] [Google Scholar]
- 3. Liu X, Dai Q, Ye R, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol 2020; 19: 115–122. [DOI] [PubMed] [Google Scholar]
- 4. Forman R. ESO-WSO large clinical trials webinar: BASICS, https://journals.heart.org/bloggingstroke/2020/05/14/eso-wso-large-clinical-trials-webinar-basics/ (2020, accessed 3 September 2020).
- 5. Writing Group for the BASILAR Group, Zi W, Qiu Z, et al. Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol 2020; 77: 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019; 18: 394–405. [DOI] [PubMed] [Google Scholar]
- 7. Jayaraman MV, McTaggart RA. Endovascular treatment of anterior circulation large vessel occlusion in the elderly. Front Neurol 2017; 8: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Z, Jiang Y, Li H, et al. China’s response to the rising stroke burden. BMJ 2019; 364: l879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 10. Puetz V, Sylaja PN, Coutts SB, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke 2008; 39: 2485–2490. [DOI] [PubMed] [Google Scholar]
- 11. van der Hoeven EJ, McVerry F, Vos JA, et al. Collateral flow predicts outcome after basilar artery occlusion: the posterior circulation collateral score. Int J Stroke 2016; 11: 768–775. [DOI] [PubMed] [Google Scholar]
- 12. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schonewille WJ, Wijman CAC, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS) a prospective registry study. Lancet Neurol 2009; 8: 724–730. [DOI] [PubMed] [Google Scholar]
- 14. von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015; 46: 2981–2986. [DOI] [PubMed] [Google Scholar]
- 15. Mokin M, Sonig A, Sivakanthan S, et al. Clinical and procedural predictors of outcomes from the endovascular treatment of posterior circulation strokes. Stroke 2016; 47: 782–788. [DOI] [PubMed] [Google Scholar]
- 16. Bouslama M, Haussen DC, Aghaebrahim A, et al. Predictors of good outcome after endovascular therapy for vertebrobasilar occlusion stroke. Stroke 2017; 48: 3252–3257. [DOI] [PubMed] [Google Scholar]
- 17. Uno J, Kameda K, Otsuji R, et al. Mechanical thrombectomy for acute basilar artery occlusion in early therapeutic time window. Cerebrovasc Dis 2017; 44: 217–224. [DOI] [PubMed] [Google Scholar]
- 18. Kang DH, Jung C, Yoon W, et al. Endovascular thrombectomy for acute basilar artery occlusion: a multicenter retrospective observational study. J Am Heart Assoc 2018; 7: e009419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baik SH, Park HJ, Kim JH, et al. Mechanical thrombectomy in subtypes of basilar artery occlusion: relationship to recanalization rate and clinical outcome. Radiology 2019; 291: 730–737. [DOI] [PubMed] [Google Scholar]
- 20. Balami JS, White PM, McMeekin PJ, et al. Complications of endovascular treatment for acute ischemic stroke: prevention and management. Int J Stroke 2018; 13: 348–361. [DOI] [PubMed] [Google Scholar]
- 21. Chao TF, Liu CJ, Lin YJ, et al. Oral anticoagulation in very elderly patients with atrial fibrillation: a nationwide cohort study. Circulation 2018; 138: 37–47. [DOI] [PubMed] [Google Scholar]
- 22. Vergouwen MD, Compter A, Tanne D, et al. Outcomes of basilar artery occlusion in patients aged 75 years or older in the Basilar Artery International Cooperation Study. J Neurol 2012; 259: 2341–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA 2015; 313: 1451–1462. [DOI] [PubMed] [Google Scholar]
- 24. Zweifler RM. Initial assessment and triage of the stroke patient. Prog Cardiovasc Dis 2017; 59: 527–533. [DOI] [PubMed] [Google Scholar]
- 25. Rentzos A, Karlsson JE, Lundqvist C, et al. Endovascular treatment of acute ischemic stroke in the posterior circulation. Interv Neuroradiol 2018; 24: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rangaraju S, Jovin TG, Frankel M, et al. Neurologic examination at 24 to 48 hours predicts functional outcomes in basilar artery occlusion stroke. Stroke 2016; 47: 2534–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_17562864211000453 for Endovascular intervention for basilar artery occlusion in the elderly by Weidong Luo, Wenguo Huang, Min Zhang, Xing Liu, Zhangbao Guo, Peiyang Zhou, Li Wang, Xinmin Fu, Shiquan Yang, Shuai Zhang, Zhiming Zhou, Min Zhang, Junjie Yuan, Shuai Liu, Jiaxing Song, Zhongming Qiu, Hongfei Sang, Fengli Li, Wenjie Zi, Deping Wu, Wenhua Liu and Qingwu Yang in Therapeutic Advances in Neurological Disorders