Abstract
Gastrointestinal helminth parasites are a concern for the poultry industry worldwide as they can affect the health, welfare, and production performance. A systematic review of the prevalence over time in different countries may improve our understanding of gastrointestinal helminthiasis in chickens and subsequently lead to improved poultry health. The aim of this systematic review and meta-analysis was to provide an overview of the published information regarding the epidemiology and the diagnostic approaches of chicken helminth infection. Six databases were searched for studies, and a total of 2,985 articles published between 1942 and 2019 were identified and subsequently screened for eligibility using title or abstract and full text assessment, resulting in 191 publications to be used in the study. Postmortem diagnostics (73.8%) and the flotation technique (28.8%) were commonly used to detect helminth infections with a pooled prevalence of 79.4% ranging from 4 to 100%. More than 30 helminth species in chicken populations were identified including Ascaridia galli (35.9%), Heterakis gallinarum (28.5%), Capillaria spp. (5.90%), and Raillietina spp. (19.0%) being the most prevalent. The reported prevalence of helminth infection decreased over time in developing countries while it increased in the developed world. Chicken kept in backyard and free-range systems had a markedly higher pooled prevalence of helminth infection (82.6 and 84.8%, respectively) than those housed in cage production systems (63.6%). This may indicate the need for more rigorous control and prevention measures in free-range and backyard production systems using regular deworming coupled with access to early and accurate diagnosis allowing for early intervention.
Key words: cestode, chicken, epidemiology, nematode, parasite
Introduction
The increasing demand for poultry products for human consumption has resulted in substantial growth of extensively and intensively housed poultry over the last few decades (Permin and Hansen, 1998; Ola-Fadunsin et al., 2019). As a result, poultry production is making a significant and increasing contribution to the national economy of most countries (Dube et al., 2010; Adang et al., 2014; Ferdushy et al., 2016). However, the production performance of poultry can be significantly reduced because of various intestinal helminth parasites (Permin et al., 1997; Ruff, 1999; Ogbaje et al., 2012; Adang et al., 2014; Bachaya et al., 2015; Van et al., 2019).
Gastrointestinal helminthiasis is caused by roundworms (nematodes), tapeworms (cestodes), and flukes (trematodes) (Ruff, 1999; Macklin, 2013; Ola-Fadunsin et al., 2019). With respect to the health impact of the infection, the abundance of pathogenic species, and the economic importance, nematodes are the most important intestinal worms in the poultry industry (Ruff, 1999; Macklin, 2013; Bachaya et al., 2015; Ola-Fadunsin et al., 2019). Impacts associated with nematode infections include reduced health, welfare, and production performance due to reduced feed conversion ratio, reduced growth rates or weight loss, reduced egg production and egg quality, intestinal damage, and in severe cases, death (Ramadan and Znada, 1991; Das et al., 2010, 2012; Dube et al., 2010; Sreedevi et al., 2016; Rufai and Jato, 2017). Nematode infections can have direct adverse effects on the host, inducing the breakdown of the gastrointestinal barrier, but indirect damage can also occur via increased susceptibility to secondary infectious diseases (Dahl et al., 2002; Eigaard et al., 2006; Permin et al., 2006; Dube et al., 2010; Sharma et al., 2019) and reduced host immune response (Nnadi and George, 2010; Hørning et al., 2003; Pleidrup et al., 2014; Dalgaarda et al., 2015).
A prevalence rate of as high as 100% has been reported in chickens housed in backyard (Rabbi et al., 2006) and free-range systems (Sherwin et al., 2013). The prevalence of helminth infections can be influenced by many factors such as the climatic conditions and agro-ecological zones, the accumulation of infective stages of larvae or eggs in the environment, the presence of intermediate hosts, and the individual susceptibility of the final host (Magwisha et al., 2002). Temperature and humidity can be considered as determinants for the occurrence and the level of helminth infection by influencing transmission through survival in the environment and developmental success of the infective stage (Permin et al., 1997; Magwisha et al., 2002; Dube et al., 2010; Naphade and Chaudhari, 2013; Ola-Fadunsin et al., 2019). Most poultry nematodes have a direct lifecycle, and the fecal-oral route is the main route of infection contributing to the higher susceptibility of poultry in free-range and floor poultry production systems due to being in close contact to their excreta and soil (Permin et al., 1999; Jansson et al., 2010; Kaufmann et al., 2011b; Wongrak et al., 2014, 2015). As such, the ongoing global growth in poultry production coupled with the trend to move from caged to more extensive housing conditions (free-range, barn) will favor the parasite infection. Therefore, the objective of this review was to provide compiled information on the epidemiology of chicken helminthiasis, the change in housing systems over time, and the diagnostic methods used to obtain information on prevalence.
Materials and methods
Data Collection Strategy
A preliminary search of key databases (PubMed, Embase, ProQuest, Web of Science, Google Scholar, and Scopus) was conducted to ensure the availability of sufficient and relevant published articles, to validate the rationality for this review objectives, and to identify and refine key search terms. Based on these findings, the following combination of terms was used to search in the PubMed, Embase, ProQuest, Web of Science, and Scopus databases for the main study: (Prevalen∗ OR Epidemiolog∗ OR Magnitude OR Occurrence) AND (Helminth∗ OR Gastrointestinal Worm∗ OR Gastrointestinal Nematode∗ OR Cestode∗) AND (Poultry OR Chicken∗ OR Domestic fowl OR Hen∗) by using title or abstract and years from 1942 to 2019 in the search engines. When searching the database of Google scholar “advanced search”, the following search for any of the following words or phrases being present anywhere in the articles was conducted: prevalence helminth chicken; epidemiology helminth poultry; prevalence gastrointestinal nematodes or cestodes; occurrence intestinal helminth hens or domestic fowl; and at least one of the following words anywhere in the article or in the title of an article: prevalence helminth nematode cestodes chicken. All searched articles from each database were imported into Endnote X9 (Clarivate Analytics, Philadelphia, PA, USA) to identify and delete duplicate articles.
Article Selection
Eligibility criteria were then applied to screen articles. These criteria were selected in line with the guidelines by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (Moher et al., 2009). The following inclusion criteria were applied: 1) providing prevalence data on helminths in avian species, 2) only relating to chicken or domestic fowl populations, 3) English language only, 4) published studies and original articles, and 5) reports on natural (not experimental) infection. Exclusion criteria were 1) unrelated articles; 2) repeated publications using the same data; 3) unpublished articles, proceeding papers, conference, books, case reports, review articles, systematic reviews, and articles without full text available; and 4) experimental infection.
Data Extraction
Data were extracted manually and entered into a Microsoft Excel sheet (2016, Microsoft Corporation, Redmond, WA, USA). The extracted information included study details (such as authors, years, study regions, breed or strain, age, sex, production system, study design, sampling type, and sample size), diagnostic method, and helminth species and prevalence.
Quality Assessment
Quality assessment was carried out by 2 independent appraisers based on the Joanna Briggs Institute critical appraisal tool for systematic reviews (Munn et al., 2015) using 9 selection criteria and 4 rating variables (“yes”, “no”, “unclear”, and “not applicable”) for each criteria. Calculating the overall score was conducted by adding the values for all 9 selection criteria where “yes” was valued as one while “no”, “unclear”, and “not applicable” were valued as zero. The published articles were then categorized as poor, fair, and good studies using the same approach described by Tawfik et al. (2019) where a score mark was given for each article grouped as 1 to 3, 4 to 6, and 7 to 9 representing poor, fair, and good quality research, respectively.
Data Synthesis
The production systems of domestic chicken were categorised as village or backyard, free-range or organic, deep litter or barn, and enriched cage or conventional cage production systems. Village or backyard chickens were defined as any domestic chicken kept extensively in the village as rural or scavenging in a backyard or traditional production system being allowed to roam freely at least during daylight hours. Free-range was considered to be a commercial husbandry farming system where chicken had access to a range area. Deep litter and barn production system studies included chicken reared on the ground with indoor housing, whereas caged housing referred to chicken confined in single cage or group cages raised off the ground. The sample size varied significantly across studies and was mainly based on individual birds. Postmortem examination was defined as an inspection and dissection of the intestine of dead chickens to determine the presence of parasite worms and magnitude of infection. Excreta examination was defined as a microscopic examination of the presence of parasite eggs in the excreta of chickens. The reported individual or flock prevalence of helminth infection was categorized as low (<11%), moderate (12–30%), high (31–75%), or very high (76–100%). The median prevalence and interquartile range of helminth prevalence were calculated for each category of continents, production systems, and helminth species.
Statistical Analysis
All statistical meta-analyses were performed using RevMan Review manager 5.4 (Cochran collaborations, 2020) while regression analyses were performed using JMP14 (SAS Institute Inc., Cary, NC, USA). Owing to the anticipated heterogeneity of the prevalence studies, random-effect meta-analysis was performed based on inverse variance model using the effect size of the total sample size, number of positive samples, and standard error. Pooled estimated prevalence presented at 95% confidence interval (Der Simonian and Laird, 1986; Field, 2001). The prevalence data (proportions) were transformed into logits (log odds) and analyzed using logistic regression as described previously (Sutton et al., 1999; Bland and Altman, 2000; Sanchez et al., 2007; Barendregt et al., 2013). In brief, prevalence proportion odds ratio (POR) = (P) / (1 – P). Hence, logit (P) or log (POR) = 1n (P/ (1 – P)), where P is the proportion of prevalence value. Standard error (SE) of the log odds ratio (logits) was calculated as the square root of variance (Var) log odds ratio: SE (ln (POR) = (Var (1n (POR))) 1/2. Var(ln(POR) was computed as /the sum of reciprocal of the number of positive cases (N+ event) and negative cases (N- event) for prevalence data. . Therefore, SE of is equivalent to , where P is proportion of prevalence value and N is total number of cases (sample size). The 95% CI of logit proportion was defined as logit (P) ± 1.96 × SE of logit (P). Thus, 95% (lower, upper limit). The transformed results of the prevalence estimate for the meta-analysis were back-transformed to obtain informative pooled prevalence and confidence intervals by using the following inverse logits formula as described in the study by Roalfe et al. (2008).
Heterogeneity between studies was tested by the Tau-squared, I-squared, Cochran's Q test, or Chi-squared (P > 0.05) tests. The values of 25, 50, and 75% for I2 statistics testing the degree of heterogeneity was considered as low, moderate, and high, respectively (Higgins and Thompson, 2002). Subgroup meta-analysis was performed to identify potential heterogeneity of prevalence estimates across study continents, years, and production systems and chicken types. Linear trends of helminth prevalence over time were investigated using linear regression analysis. A P value < 0.05 was considered statistically significant for all analyses.
Results
Descriptive Outcome of Systematic Studies
Description of the Data set
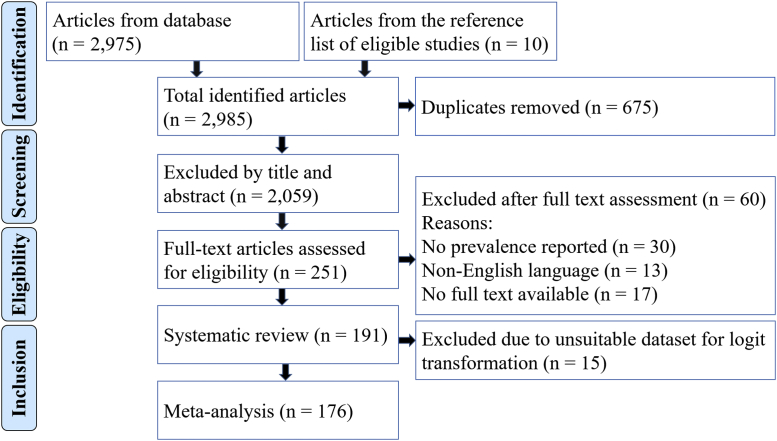
From a total of 2985 published studies identified in the 6 search databases and the reference list of eligible studies, 191 published articles were retained after the screening process (Figure 1). Applying the Joanna Briggs Institute critical appraisal checklist, 30 of the 191 articles were deemed to be poor while the remaining 107 and 54 articles were classified as fair and good quality, respectively.
Figure 1.
Adapted PRISMA article selection process. The figure provides details of the selection of publications used for the systematic review and the meta-analysis.
Data Investigation
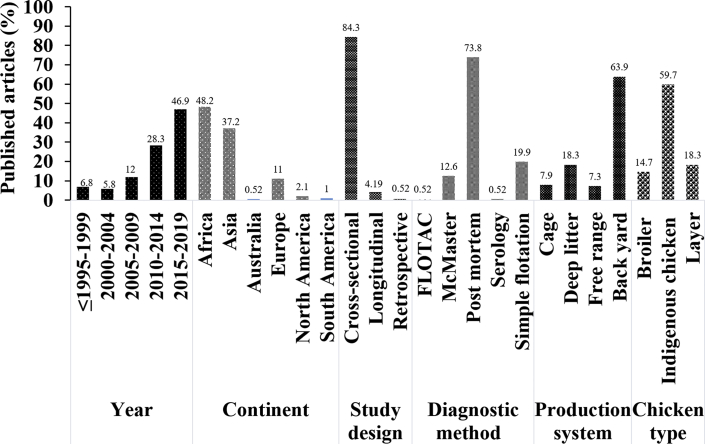
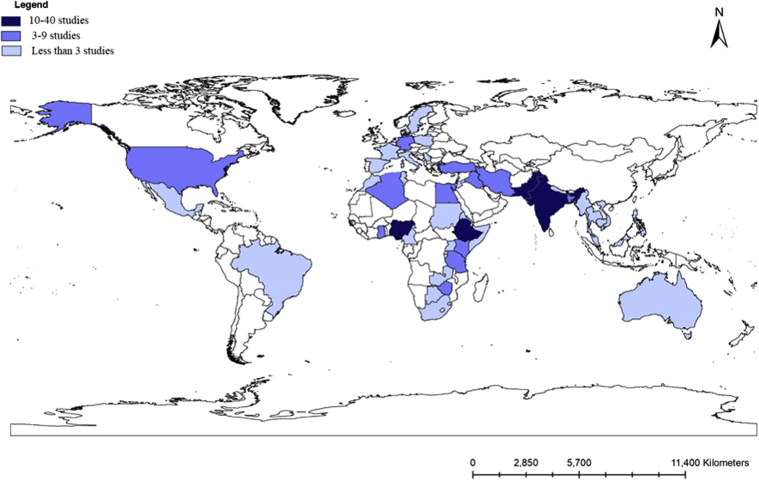
A total of 191 studies published between 1942 and 2019 were selected for systematic review. The 191 included studies were conducted in 50 different countries, and approximately 66307 samples with the mean ± SD sample size of 347.2 ± 474.9 were analyzed. The current review showed that the number of published studies has increased over time and varied across regions and production systems. Number of studies per continent, year, study design, diagnostic methods, and production systems are shown in Figure 2. Most studies (178; 93.2%) were conducted during the last 2 decades, whereas the smallest number of studies were conducted before 2000. More than 86% of studies were conducted from developing countries of which 39 of 191 (20%) and 23 of 191 (12%) studies were conducted in Nigeria and India, respectively. The details of the individual countries' prevalence study distribution are shown in Figure 3. Diagnosis was based on postmortem examination in 141 studies (73.8%), whereas 55 (28.8%) used the egg flotation techniques (simple flotation, McMaster; or FLOTAC). The vast majority of studies were conducted on backyard or village chicken production systems followed by barn or deep litter, cage, and free-range or organic production systems, respectively (Figure 2). Furthermore, most studies were conducted on indigenous chicken breeds while a smaller number of studies were conducted on commercial layer and broiler chicken strains (Figure 2). In addition, 45 and 31 studies were conducted on female and male chickens, respectively, while the remaining studies did not specify. Overall, the reported helminth prevalence varied from 4 to100%.
Figure 2.
Distribution of the 191 research articles by year, region, study design, diagnostic methods, production system, and chicken type.
Figure 3.
Individual countries where the prevalence studies were conducted.
Prevalence Report by Helminth Species
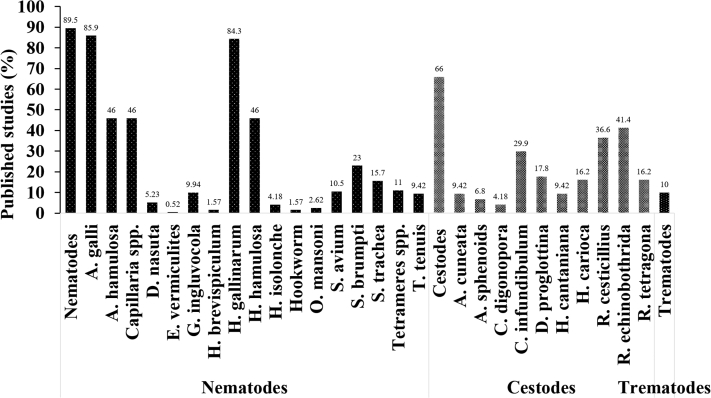
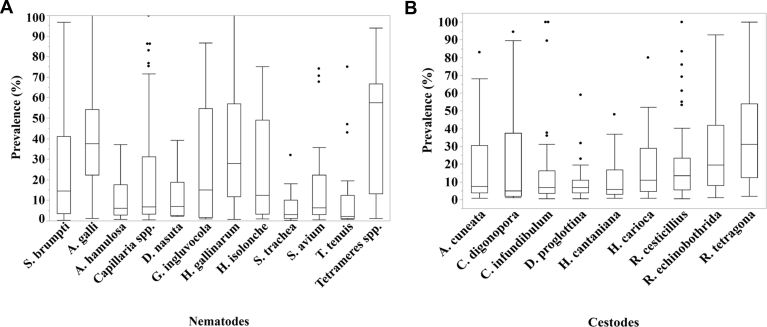
More than 30 different helminth parasite species were identified in total, of which Ascaridia galli (n = 164), Heterakis gallinarum (n = 161), Capillaria spp. (n = 88), Raillietina tetragona (n = 83), Raillietina echinobothrida (n = 79), and Raillietina cesticillus (n = 70) were the most studied parasite species, respectively. In general, nematodes were the most studied helminth parasite (90%), followed by cestodes (66%) and trematodes (10%; Figure 4). The reported prevalence of helminth species varied among species and ranged from 30 to 100% (Figures 5A and 5B).
Figure 4.
Distribution of number of published studies by nematodes, cestodes, and trematodes species.
Figure 5.
(A) Prevalence distribution by nematodes and (B) cestodes parasites. The box plots include the minimum and maximum prevalence, median prevalence (Q2), lower quartile (Q1), upper quartile (Q3), and outliers (indicated by dots).
Spacio-temporal Distribution of Helminth Prevalence and its Trend Over Time
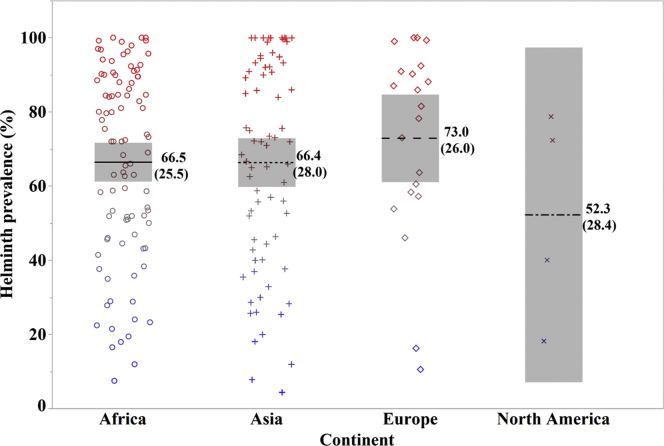
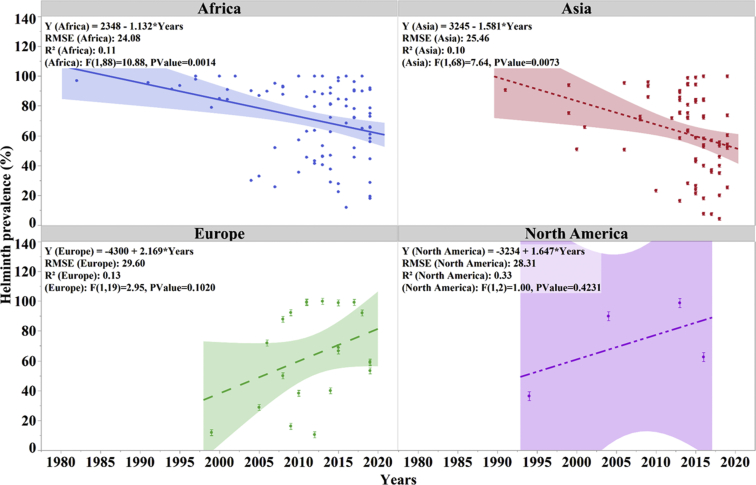
The overall helminth prevalence reported across regions ranged between 4 and 100%. The details of reported helminth prevalence by regions are shown in Figure 6. Most studies that reported high prevalence range (31–100%) were conducted in Africa (n = 83), Asia (n = 59), Europe (n = 16), and North America (n = 4). Among 191 studies, 14 studies reported a prevalence of 100% including Africa (11), Asia (2), and Europe (1). The prevalence range in Africa, Asia, and Europe was 11.9 to 100%, 4 to 100%, and 10.5 to 100%, respectively. Regression analysis indicated a decreasing trend in reported helminth prevalence in Africa and Asia, whereas an increase helminth prevalence in Europe and North America has been seen recently (Figure 7). However, the relatively low R2 value reflects considerable variation of the reported prevalence.
Figure 6.
Descriptive presentation of the reported helminth prevalence by region. Different colored symbols represent reported prevalence value for each prevalence study and show the pattern of prevalence distribution across each region. The dot or solid lines indicate the Mean ± SD.
Figure 7.
Helminth prevalence reported by year across regions. Each symbol represents one research study. The trend lines were created based on the individual reported values (dots).
Reported Prevalence by Production System
Description of production systems and chicken type across regions and the prevalence range are presentenced in Table 1. A total of 186 studies provided information about their production system. Most studies that were conducted in backyard or village chickens were from Africa and Asia, whereas all free-range or organic systems were conducted in Europe. The prevalence was expressed in terms of range, mean ± SD, and quartile range. The prevalence range across production system was 0 to 100%. Most studies (80%) reported a prevalence of 31 to 100%, of which 46.6% of studies reported a prevalence of 76 to 100%, and 66.6% of studies were conducted on backyard production with average prevalence of 73.6%. The 14 studies that reported a 100% prevalence were conducted in backyard or village chickens (n = 13) and the free range or organic (n = 1) production system. Furthermore, 35 and 28 studies were conducted on layer and broiler chickens with a prevalence range of 4 to 100% and 0 to 99%, respectively (Table 1). Only 76 studies revealed the sex of their study population: 45 studies were conducted on female chickens while 31 studies were conducted on male chickens. The helminth prevalence for males ranged from 11 to 92% with a median of 53%, whereas the prevalence of female birds had a wider range (11–100%) and higher median (68%).
Table 1.
Description of production systems, chicken types, diagnostic methods, number of studies across continents and their corresponding helminth prevalence rates.
| Study characteristics | Number of studies |
Sample size |
Prevalence distribution |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Africa | Asia | Europe | North America | Mean ± SD | Range | Prevalence range (%) | Mean ± SD | Quartile (1st, 2nd, 3rd) | ||
| Production system | Backyard | 71 | 47 | 4 | 0 | 268 ± 368.8 | 75-3,773 | 4.39-100 | 73.6 ± 24.0 | 58.7, 81.5, 92.5 |
| Free range | 0 | 0 | 14 | 0 | 447 ± 295.8 | 60-907 | 24.0-100 | 78.4 ± 20.8 | 58.3, 81.5, 99.2 | |
| Deep litter | 19 | 9 | 5 | 2 | 457 ± 802.5 | 45-3,100 | 1.0-98.9 | 43.4 ± 25.9 | 25.4, 36.6, 58.7 | |
| Cage | 5 | 7 | 3 | 0 | 401 ± 976.9 | 58-500 | 0-80.0 | 20.8 ± 25.9 | 3.0, 11.0, 31.7 | |
| Chicken type | Broiler | 11 | 9 | 5 | 3 | 509 ± 832.1 | 90-3,542 | 0-98.9 | 39.1 ± 32.7 | 12.1, 33.2, 63.5 |
| Layer | 11 | 9 | 15 | 0 | 347 ± 375.7 | 65-1,996 | 4.0-100 | 54.5 ± 30.5 | 26.5, 57.7, 75.4 | |
| Indigenous chicken | 70 | 43 | 1 | 0 | 228 ± 186.2 | 75-889 | 4.39-100 | 72.7 ± 24.8 | 58.3, 81.0, 92.2 | |
| Diagnostic method | Postmortem examination | 69 | 56 | 14 | 2 | 294 ± 347.1 | 55-3,100 | 4.0-100 | 70.6 ± 26.3 | 52.4, 75.6, 92.5 |
| Simple flotation technique | 20 | 8 | 1 | 1 | 424 ± 625.8 | 50-3,773 | 7.43-100 | 56.2 ± 24.7 | 40.3, 58.3, 76.7 | |
| McMaster technique | 6 | 7 | 6 | 1 | 515 ± 527.2 | 70-1,996 | 7.8-99.2 | 56.5 ± 26.2 | 38.3, 56, 72.3 | |
Meta-analysis Results of Published Studies
A total of 176 studies were included for meta-analyses, with 15 studies excluded because of unsuitable data set for logit transformation. The result of meta-analysis is summarized in Table 2. The overall pooled helminth prevalence estimate was 79.4%, and heterogeneity between studies was significantly high (P < 0.00001). Therefore, sub-group meta-analysis was conducted using continents, years, production systems, and chicken type. Heterogeneity between subgroup studies ranged from 84 to 97%.
Table 2.
Subgroup meta-analysis for pooled prevalence of helminth infection and test of heterogeneity across study period, continents, production systems, and chicken type.
| Logit transformed |
Logit back transformed |
Tests of heterogeneity |
||||||
|---|---|---|---|---|---|---|---|---|
| Study characteristics | Pooled proportion (%) | 95% CI | Pooled prevalence (%) | 95% CI | Cochran's (Q)/(chi2) test | I-squared (I2) | DF | P value |
| Year | ||||||||
| <1995-1999 | 2.22 | 1.44-3.43 | 90.2 | 27.9-100 | 358.1 | 97% | 11 | <0.00001 |
| 2000-2004 | 1.63 | 1.14-2.34 | 83.6 | 25.7-99.9 | 34.9 | 80% | 7 | <0.0001 |
| 2005-2009 | 1.66 | 1.28-2.15 | 84.0 | 27.0-99.8 | 243.1 | 91% | 22 | <0.00001 |
| 2010-2014 | 1.39 | 1.22-1.59 | 80.1 | 26.9-98.9 | 475.7 | 89% | 50 | <0.00001 |
| 2015-1019 | 1.15 | 1.04-1.27 | 76.0 | 29.1-97.4 | 1,059 | 92% | 80 | <0.00001 |
| Continent | ||||||||
| Africa | 1.49 | 1.33-1.66 | 81.6 | 24.7-99.1 | 926.9 | 91% | 82 | <0.00001 |
| Australasia | 1.23 | 1.10-1.38 | 77.4 | 28.4-98.1 | 921.3 | 92% | 68 | <0.00001 |
| Europe | 1.28 | 1.02-1.61 | 78.9 | 29.9-99.1 | 235.9 | 92% | 19 | <0.00001 |
| North America | 1.55 | 0.89-2.71 | 82.5 | 21.7-99.9 | 33.0 | 91% | 3 | <0.00001 |
| Production system | ||||||||
| Backyard | 1.56 | 1.41-1.72 | 82.6 | 23.1-99.3 | 1,059 | 90% | 107 | <0.00001 |
| Free range | 1.72 | 1.36-2.17 | 84.8 | 22.6-99.8 | 76.9 | 84% | 12 | <0.00001 |
| Deep litter | 0.91 | 0.8-1.02 | 71.3 | 34.1-94.8 | 287.3 | 88% | 34 | <0.00001 |
| Cage | 0.56 | 0.38-0.83 | 63.6 | 42.9-91.7 | 62.4 | 84% | 10 | <0.00001 |
| Chicken type | ||||||||
| Layer | 1.04 | 0.87-1.24 | 73.9 | 33.9-96.9 | 413.9 | 92% | 32 | <0.00001 |
| Broiler | 0.77 | 0.66-0.9 | 68.4 | 35.5-93.3 | 186.6 | 89% | 21 | <0.00001 |
| Indigenous | 1.55 | 1.4-1.71 | 82.5 | 23.2-99.3 | 864.6 | 88% | 102 | <0.00001 |
| Overall effect estimates | 1.35 | 1.26-1.46 | 79.4 | 24.6-98.5 | 2,258.2 | 92% | 175 | <0.00001 |
Abbreviation: DF, degree of freedom.
Discussion
This review provides evidence on the prevalence of helminth parasites in chickens stratified by region, production type, helminth species, and diagnostic methods using 191 published studies across the globe from 1942 to 2019. The number of publications on chicken helminth infections increased markedly in the last 2 decades. Most of the studies were conducted in Africa (n = 92) and Asia (n = 72), followed by Europe (n = 21). The pooled prevalence of helminth infection was 79.4%, ranging across studies from 4 to 100%. The prevalence in Africa and Asia was decreasing significantly overtime, while the prevalence in Europe and North America was increasing trend. The vast majority of the studies (73.8%) used postmortem examination to detect presence of worms, whereas a considerable number of studies were based on egg flotation techniques. A. galli and H. gallinarum were the most commonly reported helminth parasites followed by Capillaria and Raillietina spp.
The number of publications on helminth prevalence increased over time. The number of publications on helminth prevalence increased exponentially in the last 2 decades indicating that 1) attention has been given to chicken health by researchers, stakeholders, producers, governmental, and non-governmental institutions at regional, national, and global levels; and 2) scientific evidence and awareness about the impact of helminth infection on chicken profitability and productivity is more frequently communicated. One possible reason might be that chicken health receives more attention because of the increased demand and volume of production as well as a relative shift in egg production towards free-range in some regions. In addition, the economic impact of helminths on chicken production might have gained larger awareness, and welfare concerns result in higher demands on chicken health. Consequently, veterinary interest has expanded which not only might have supported diagnostic procedures but also initiate research interest on chicken health.
Most prevalence studies were conducted in Africa (n = 92), followed by Asia (n = 72), and Europe (n = 21). One reason for the high interest on helminths in Africa and Asia may be the fact that approximately 80% of meat and eggs produced in these continents are from backyard chickens and small-scale production system (Pym et al., 2006; Dube et al., 2010) where not only the uncontrolled exposure to the soil but also the warm and partially humid tropical climate provide ideal conditions for the presence of insect vectors and the helminths to develop. In contrast, Europe and North America housed their chicken traditionally in barns or cages with only recent shifts to free-range, also reflected in the recent increased number of publications in these countries.
Overall pooled prevalence of helminth infection in chicken was 79.4%, ranging across studies from 4 to 100%. Reasons for this variation might be the large variety of production systems, management procedures investigated, the broad range of the agro-ecological zones being investigated, climatic or environmental conditions, seasonal dynamics, the number and availability of intermediate hosts involved, the diagnostic and sampling methods used, and various host factors including susceptibility to genetic resistance of the host. While evidence is provided that a high prevalence (>76%) of helminths can be obtained in every continent, most of the high prevalence studies were conducted in Africa (n = 83) with the overall pooled prevalence of 81.6%. The possible reasons for these may be associated with a relatively high proportion of studies being conducted in backyard production systems (Abebe et al., 1997; Nnadi and George, 2010; Abdullah and Mohammed, 2013). In addition to this, the tropical climatic condition is suitable or favorable for the propagation and development of the infective stage of helminth infection (Abebe et al., 1997; Slimane, 2016). Adequate moisture and temperature of the environment in the tropics are indeed the most determinant factors for the development of the infective stage of helminth parasite and that may influence the epidemiology of parasite infection (Wuthijaree et al., 2019). Although a high pooled helminth prevalence was reported in developing countries associated with the backyard production system and environmental factors, the prevalence was decreasing overtime in these countries most likely due to increasing access to anthelmintic treatment over the last few decades. However, the current review showed that a high prevalence (78.9%) was also reported across European countries with increasing prevalence overtime. This is likely linked with the re-emerging of helminth infection due to shifting production systems into a free-range and organic production system (Jansson et al., 2010; Thapa et al., 2015). Moreover, anthelmintic application has been prohibited in the organic production systems in Europe, further promoting helminths as a re-emerging disease (Kaufmann et al., 2011a, 2011b; Wongrak et al., 2015).
Most studies were conducted on backyard, and free-range systems reported a relatively high prevalence rate. This is reflected in the fact that 14 backyard and free-range studies reported a 100% prevalence. These findings agreed with the report of Permin and Hansen (1998) and Nnadi and Georg (2010). Moreover, the overall pooled prevalence in backyard and free-range production systems was 82.6 and 84.8%, respectively. These results are not surprising, and the most likely explanation would be due to scavenging activities and roaming habit of free-range and backyard chicken where the direct contact with excreta and soil and the noncommercial approach of owners would most likely include poor management including a lack of anthelminthic drug application (Abdelqader et al., 2008; Abdullah and Mohammed, 2013). Furthermore, the free-range and backyard scavenging production system can play an important role in shedding helminth infections by contaminating the environment with parasite eggs and larvae (Mwale and Masika, 2011; Wamboi et al., 2020). In contrast, birds housed in caged systems off the ground are largely separated from their excreta, disrupting the direct life cycle of the parasite which is reflected in the significantly lower average prevalence than backyard and free range (Permin and Hansen, 1998; Permin et al., 1999). However, some caged flocks still experienced a prevalence of up to 80%, which is somewhat surprising, and the authors suggested poor sanitation including cross-contamination of feed with excreta and heavy accumulation of excreta and dust that may have facilitated the fast development and propagation of embryonated eggs and possibly insect vectors (Ponnudurai and Chellappa, 2001). Another possible reason may be that the breeder pullets have been reared on the floor and therefore became infected in the rearing facility before being caged. In addition, caged birds are more commonly laying hens, and the longer life span of these hens (usually at least 70–80 wk, sometimes multiple laying cycles) allows for a greater manifestation of helminth infection than broiler production where the life span may be as short as 32 days (McDougald, 2020).
Detection of the presence of helminth parasite in chicken population was mostly carried out using either postmortem examination of chicken's gastrointestinal tract or excretal examination. Vast majority of the studies (73.8%) used postmortem examination, and some egg floatation techniques (simple flotation, McMaster technique, and FLOTAC) were used in studies subject to this review to detect presence of worms in chickens. Our systematic review indicated that postmortem examination, known for its accurate and reliable diagnostic approach for helminth species identification, was a widely used diagnostic method (Macklin, 2013) but may not be the most economically viable method for routine diagnosis when sacrificing birds only for this purpose. However, equipment such as a microscope and glass slides are required to use alternative, less invasive techniques such as the flotation technique based on excreta egg count analysis (Das et al., 2017). In addition, owing to the intermittent egg shedding nature of the nematodes, it allows only for reliable egg detection if samples were collected over multiple days (Das et al., 2011, 2017). With the McMaster techniques being known for its uncomplicated and cheap use, it was unsurprisingly the most commonly used technique (12.6%), whereas the relatively new FLOTAC method with improved sensitivity and precision was only used once due probably to the expense of equipment and time (Cringoli et al., 2010; Das et al., 2020).
Nematodes were the most studied helminth parasite (90%), followed by cestodes (66%) and trematodes (10%). The most commonly reported and prevalent nematodes species were A. galli (35.7%), H. gallinarum (29.5%), and capillaria species (5.90%) with an overall prevalence ranging from 0.30 to 100%. A. galli was the most prevalent helminth in all production systems, and coinfections with H. gallinarum were common, which is not surprising given the fact that both parasites have the same life cycle and require same environmental conditions (Das et al., 2017). Cestodes were detected less often, with the highest prevalence among cestodes species being that of Raillietina species, including R. tetragona (30.6%), R. echinobothrida (20.0%), and R. cesticillius (13.0%), once again being highest in backyard and free-range chicken most likely due to the reasons discussed previously. In addition, cestodes require an intermediate host such as houseflies or beetles for their transmission, and their overall lower prevalence may have been linked to less opportunities of transmission (Dube et al., 2010; AbdelAziz, 2016). Likewise, trematodes require 2 to 4 intermediate hosts to complete their life cycle, and the eggs hatch only in water (Permin and Hansen, 1998; Abdelqader et al., 2008; Afolabi et al., 2016; Mcdougald, 2020). As a result, basic biosecurity that prevents chicken to access lake or water bodies might have been sufficient to control the transmission of these trematodes.
Heterogeneity test indicated that substantial heterogeneity was found between prevalence studies. Source of heterogeneity between prevalence studies may arise due to methodological and clinical variation which contribute for the presence of statistical heterogeneity (variation of effect size between studies) (Higgins and Thompson, 2002). Barendregt et al. (2013) argued that the main concern for meta-analysis is heterogeneity; however, the aim of disease burden studies, such as prevalence study, is to obtain best prevalence estimate based on the available data. Thus, a reported pooled prevalence estimate is considered to be valid as a measure of helminth prevalence. The potential source of variation between studies in the current meta-analysis may be due to variation in study design, sampling methods, level of exposure to parasite, physiological and genetic status of animal, sample size, study location, environmental condition, and diagnostic methods.
Conclusions
Helminths are globally common and highly prevalent in chicken with significant variation across the regions and production systems. The prevalence is decreasing overtime in developing countries because of increasing recognition and regular deworming over the last few decades. However, a higher helminth infection prevalence has been recently seen in certain regions where a continuous shifting from cages toward the extensive systems (free-range, organic, backyard) is happening. The rapid growth of commercial free-range production has changed the dynamic of helminth transmission; hence, epidemiological evidences on the existence, spreading, and the underlying factors are needed to understand transmission models and institute control strategies. Therefore, increased awareness among those producers combined with access to accurate and early diagnosis would be crucial for early intervention.
Acknowledgments
A.S. was supported by a University of New England international postgraduate research award scholarship.
Disclosures
The authors declare no conflict of interest.
References
- Abdel Aziz A.R. Prevalence of gastrointestinal helminthes of Gallus gallus domesticus (Linnaeus, 1758) in free-range system at Upper Egypt. World J. Clin. Pharmacol. Microbiol. Toxicol. 2016;2:13–18. [Google Scholar]
- Abdullah S.H., Mohammed A.A. Ecto and Endo parasites prevalence in domestic chickens in Sulaimani region. Iraqi J. Vet. Med. 2013;37:149–155. [Google Scholar]
- Abdelqader A., Gauly M., Wollny C.B.A., Abo-Shehada M.N. Prevalence and burden of gastrointestinal helminthes among local chickens, in northern Jordan. Prev. Vet. Med. 2008;85:17–22. doi: 10.1016/j.prevetmed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Abebe W., Asfaw T., Genete B., Kassa B., Dorchies P. Comparative studies of external parasites and gastro-intestinal helminths of chickens kept under different management system in and around Addis Ababa (Ethiopia) Revue de Medecine Veterinaire. 1997;148:497–500. [Google Scholar]
- Adang K., Asher R., Abba R. Gastrointestinal helminths of domestic chickens Gallus gallus domestica and ducks Anas platyrhynchos slaughtered at Gombe main market, Gombe state, Nigeria. Asian J. Poult. Sci. 2014;8:32–40. [Google Scholar]
- Afolabi O.J., Simon-Oke I.A., Olasunkanmi A.O. Intestinal parasites of domestic chicken (Gallus gallus domesticus) in Akure, Nigeria. J. Biomed. 2016;1:1–4. [Google Scholar]
- Bachaya H.A., Raza M.A., Anjum M.A., Khan I.A., Aziz A., Manzoor Z., Munawar S.H. Prevalence of Ascaridia galli in white leghorn layers and Fayoumi-Rhode Island Red crossbred flock at government poultry farm Dina, Punjab, Pakistan. Trop. Biomed. 2015;32:11–16. [PubMed] [Google Scholar]
- Bland J.M., Altman D.G. The odds ratio. Br. J. Med. 2000;320:1468. doi: 10.1136/bmj.320.7247.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendregt J.J., Doi S.A., Lee Y.Y., Norman R.E., Vos T. Meta-analysis of prevalence. J. Epidemiol. Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- Cringoli G., Rinaldi L., Maurelli M.P., Utzinger J. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010;5:503–515. doi: 10.1038/nprot.2009.235. [DOI] [PubMed] [Google Scholar]
- Eigaard N.M., Schou T.W., Permin A., Christensen J.P., Ekstrøm C.T., Ambrosini F., Cianci D., Bisgaard M. Infection and excretion of Salmonella enteritidis in two different chicken lines with concurrent Ascaridia galli infection. Avian Pathol. 2006;35:487–493. doi: 10.1080/03079450601071696. [DOI] [PubMed] [Google Scholar]
- Dahl C., Permin A., Christensen J.P., Bisgaard M., Muhairwa A.P., Petersen K.D., Poulsen J.D., Jensen A.L. The effect of concurrent infections with Pasteurella multocida and Ascaridia galli on free range chickens. Vet. Microbiol. 2002;86:313–324. doi: 10.1016/s0378-1135(02)00015-9. [DOI] [PubMed] [Google Scholar]
- Dalgaard T.S., Skovgaard K., Norup L.R., Pleidrup J., Permin A., Schou T.W., Vadekær D.F., Jungersen G., Juul-Madsen H.R. Immune gene expression in the spleen of chickens experimentally infected with Ascaridia galli. Vet. Immunol. Immunopathol. 2015;164:79–86. doi: 10.1016/j.vetimm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Daş G., Abel H., Humburg J., Schwarz A., Rautenschlein S., Breves G., Gauly M. The effects of dietary non-starch polysaccharides on Ascaridia galli infection in grower layers. Parasitology. 2012;139:110–119. doi: 10.1017/S0031182011001636. [DOI] [PubMed] [Google Scholar]
- Das G., Hennies M., Sohnrey B., Rahimian S., Wongrak K., Stehr M., Gauly M. A comprehensive evaluation of an ELISA for the diagnosis of the two most common ascarids in chickens using plasma or egg yolks. Parasite and Vectors. 2017;10:187. doi: 10.1186/s13071-017-2121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daş G., Klauser S., Stehr M., Tuchscherer A., Metges C.C. Accuracy and precision of McMaster and Mini-FLOTAC egg counting techniques using egg-spiked faeces of chickens and two different flotation fluids. Vet. Parasitol. 2020;283:109158. doi: 10.1016/j.vetpar.2020.109158. [DOI] [PubMed] [Google Scholar]
- Daş G., Kaufmann F., Abel H., Gauly M. Effect of extra dietary lysine in Ascaridia galli-infected grower layers. Vet. Parasitol. 2010;170:238–243. doi: 10.1016/j.vetpar.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Das G., Savas T., Kaufmann F., Idris A., Abel H., Gauly M. Precision, repeatability and representative ability of faecal egg counts in Heterakis gallinarum infected chickens. Vet. Parasitol. 2011;183:87–94. doi: 10.1016/j.vetpar.2011.07.005. [DOI] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials. Controlled Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dube S., Zindi P., Dube J., Mbanga C. A study of scavenging poultry gastrointestinal and ecto-parasites in rural areas of Matebeleland Province, Zimbabwe. Int. J. Poult. Sci. 2010;9:911–915. [Google Scholar]
- Ferdushy T., Hasan M.T., Kadir A.G. Cross sectional epidemiological investigation on the prevalence of gastrointestinal helminths in free range chickens in Narsingdi district, Bangladesh. J. Parasitic Dis. 2016;40:818–822. doi: 10.1007/s12639-014-0585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A.P. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed-and random-effects methods. Psychol. Methods. 2001;6:161–180. doi: 10.1037/1082-989x.6.2.161. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hørning G., Rasmussen S., Permin A., Bisgaard M. Investigations on the influence of helminth parasites on vaccination of chickens against Newcastle disease virus under village conditions. Trop. Anim. Health Prod. 2003;35:415–424. doi: 10.1023/a:1025863412078. [DOI] [PubMed] [Google Scholar]
- Jansson D.S., Nyman A., Vagsholm I., Christensson D., Goransson M., Fossum O., Hoglund J. Ascarid infections in laying hens kept in different housing systems. Avian Pathol. 2010;39:525–532. doi: 10.1080/03079457.2010.527923. [DOI] [PubMed] [Google Scholar]
- Kaufmann F., Daş G., Sohnrey B., Gauly M. Helminth infections in laying hens kept in organic free range systems in Germany. Livestock Sci. 2011;141:182–187. [Google Scholar]
- Kaufmann F., Daş G., Preisinger R., Schmutz M., König S., Gauly M. Genetic resistance to natural helminth infections in two chicken layer lines. Vet. Parasitol. 2011;176:250–257. doi: 10.1016/j.vetpar.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Macklin K. Merck Sharp and Dohme Corp., a subsidiary of Merck and Co., Inc.; Kenilworth, NJ: 2013. Overview of Helminthiasis in Poultry. [Google Scholar]
- Magwisha H.B., Kassuku A.A., Kyvsgaard N.C., Permin A. A comparison of the prevalence and burdens of helminth infections in growers and adult free-range chickens. Trop. Anim. Health Prod. 2002;34:205–214. doi: 10.1023/a:1015278524559. [DOI] [PubMed] [Google Scholar]
- McDougald L.R. Diseases of Poultry. 13th ed. Wiley-Blackwell, New York City, USA; 2020. Internal parasites; pp. 1157–1191. [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int. J. Evidence-Based Healthcare. 2015;13:147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- Mwale M., Masika P.J. Point prevalence study of gastro-intestinal parasites in village chickens of Centane district, South Africa. Afr. J. Agric. Res. 2011;6:2033–2038. [Google Scholar]
- Naphade S., Chaudhari K. Studies on the seasonal prevalence of parasitic helminths in Gavran (desi) chickens from Marathwada region of Maharashtra. Int. J. Fauna Biol. Stud. 2013;1:4–7. [Google Scholar]
- Nnadi P.A., George S.O. A cross-sectional survey on parasites of chickens in selected villages in the subhumid zones of South-Eastern Nigeria. J. Parasitol. Res. 2010;2010:1–6. doi: 10.1155/2010/141824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbaje C.I., Agbo E.O., Ajanusi O.J. Prevalence of Ascaridia galli, Heterakis gallinarum and tapeworm infections in birds slaughtered in Makurdi township. Int. J. Poult. Sci. 2012;11:103–107. [Google Scholar]
- Ola-Fadunsin S.D., Uwabujo P.I., Sanda I.M., Ganiyu I.A., Hussain K., Rabiu M., Elelu N., Alayande M.O. Gastrointestinal helminths of intensively managed poultry in Kwara Central, Kwara State, Nigeria: its diversity, prevalence, intensity, and risk factors. Vet. World. 2019;12:389–396. doi: 10.14202/vetworld.2019.389-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permin A., Bisgaard M., Frandsen F., Pearman M., Kold J., Nansen P. Prevalence of gastrointestinal helminths in different poultry production systems. Br. Poult. Sci. 1999;40:439–443. doi: 10.1080/00071669987179. [DOI] [PubMed] [Google Scholar]
- Permin A., Christensen J.P., Bisgaard M. Consequences of concurrent Ascaridia galli and Escherichia coliinfections in chickens. Acta Veterinaria Scand. 2006;47:43–54. doi: 10.1186/1751-0147-47-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permin A., Hansen J.W. FAO Animal Health Manual 4; Roma, Italy: 1998. Epidemiology, diagnosis and control of poultry parasites. 1–160. [Google Scholar]
- Permin A., Magwisha H., Kassuku A.A., Nansen P., Bisgaard M., Frandsen F., Gibbons L. A cross-sectional study of helminths in rural scavenging poultry in Tanzania in relation to season and climate. J. Helminthol. 1997;71:233–240. doi: 10.1017/s0022149x00015972. [DOI] [PubMed] [Google Scholar]
- Pleidrup J., Dalgaard T.S., Norup L.R., Permin A., Schou T.W., Skovgaard K., Vadekaer D.F., Jungersen G., Sorensen P., Juul-Madsen H.R. Ascaridia galli infection influences the development of both humoral and cell-mediated immunity after Newcastle Disease vaccination in chickens. Vaccine. 2014;32:383–392. doi: 10.1016/j.vaccine.2013.11.034. [DOI] [PubMed] [Google Scholar]
- Ponnudurai G., Chellappa D.J. Prevalence of helminth parasites of chicken in different systems of management. J. Vet. Parasitol. 2001;15:73–74. [Google Scholar]
- Pym R., Guerne Bleich E., Hoffmann I. The relative contribution of indigenous chicken breeds to poultry meat and egg production and consumption in the developing countries of Africa and Asia. Proc. XII Eur. Poult. Conf. 2006:10–14. [Google Scholar]
- Rabbi A.K., Islam A., Majumder S., Anisuzzaman A., Rahman M.H. Gastrointestinal helminths infection in different types of poultry. Bangladesh J. Vet. Med. 2006;4:13–18. [Google Scholar]
- Ramadan H.H., Znada A.N.Y. Some pathological and biochemical studies on experimental ascaridiasis in chickens. Food/Nahrung. 1991;35:71–84. doi: 10.1002/food.19910350120. [DOI] [PubMed] [Google Scholar]
- Roalfe A.K., Holder R.L., Wilson S. Standardisation of rates using logistic regression: a comparison with the direct method. Biomed. Cent. Health Serv. Res. 2008;8:275. doi: 10.1186/1472-6963-8-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufai M., Jato A. Assessing the prevalence of gastrointestinal tract parasites of poultry and their environmental risk factors in poultry in Iwo, Osun state Nigeria. Ife J. Sci. 2017;19:7–13. [Google Scholar]
- Ruff M.D. Important parasites in poultry production systems. Vet. Parasitol. 1999;84:337–347. doi: 10.1016/s0304-4017(99)00076-x. [DOI] [PubMed] [Google Scholar]
- Sanchez J., Dohoo I.R., Christensen J., Rajic A. Factors influencing the prevalence of Salmonella spp. in swine farms: a meta-analysis approach. Prev. Vet. Med. 2007;81:148–177. doi: 10.1016/j.prevetmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Sharma N., Hunt P.W., Hine B.C., Ruhnke I. The impacts of Ascaridia galli on performance, health, and immune responses of laying hens: new insights into an old problem. Poult. Sci. 2019;98:6517–6526. doi: 10.3382/ps/pez422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin C.M., Nasr M.A.F., Gale E., Petek M., Stafford K., Turp M., Coles G.C. Prevalence of nematode infection and faecal egg counts in free-range laying hens: relations to housing and husbandry. Br. Poult. Sci. 2013;54:12–23. doi: 10.1080/00071668.2012.757577. [DOI] [PubMed] [Google Scholar]
- Slimane B.B. Prevalence of the gastro-intestinal parasites of domestic chicken Gallus domesticus Linnaeus, 1758 in Tunisia according to the agro-ecological zones. J. Parasitic Dis. 2016;40:774–778. doi: 10.1007/s12639-014-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedevi C., Jyothisree C., Devi V.R., Annapurna P., Jeyabal L. Seasonal prevalence of gastrointestinal parasites in desi fowl (Gallus gallus domesticus) in and around Gannavaram, Andhra Pradesh. J. Parasitic Dis. 2016;40:656–661. doi: 10.1007/s12639-014-0553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A.J., Abrams K.R., Jones D.R., Sheldon T.A., Song F. Systematic reviews of trials and other studies. Health Technol. Assess. 1999;2:53–60. [PubMed] [Google Scholar]
- Tawfik G.M., Dila K.A.S., Mohamed M.Y.F., Tam D.N.H., Kien N.D., Ahmed A.M., Huy N.T. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop. Med. Health. 2019;47:46. doi: 10.1186/s41182-019-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa S., Hinrichsen L.K., Brenninkmeyer C., Gunnarsson S., Heerkens J.L., Verwer C., Niebuhr K., Willett A., Grilli G., Thamsborg S.M. Prevalence and magnitude of helminth infections in organic laying hens (Gallus gallus domesticus) across Europe. Vet. Parasitol. 2015;214:118–124. doi: 10.1016/j.vetpar.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Van N.T., Cuong N.V., Yen N.T., Nhi N.T., Kiet B.T., Hoang N.V., Hien V.B., Thwaites G., Carrique-Mas J.J., Ribas A. Characterisation of gastrointestinal helminths and their impact in commercial small-scale chicken flocks in the Mekong Delta of Vietnam. Trop. Anim. Health Prod. 2019;52:53–62. doi: 10.1007/s11250-019-01982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamboi P., Waruiru R.M., Mbuthia P.G., Nguhiu J.M., Bebora L.C. Haemato-biochemical changes and prevalence of parasitic infections of indigenous chicken sold in markets of Kiambu County, Kenya. Int. J. Vet. Sci. Med. 2020;8:18–25. doi: 10.1080/23144599.2019.1708577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongrak K., Das G., Moors E., Sohnrey B., Gauly M. Establishment of gastro-intestinal helminth infections in free-range chickens: a longitudinal on farm study. Berliner und Münchener tierärztliche Wochenschrift. 2014;127:314–321. [PubMed] [Google Scholar]
- Wongrak K., Daş G., von Borstel U.K., Gauly M. Genetic variation for worm burdens in laying hens naturally infected with gastro-intestinal nematodes. Br. Poult. Sci. 2015;56:15–21. doi: 10.1080/00071668.2014.981147. [DOI] [PubMed] [Google Scholar]
- Wuthijaree K., Lambertz C., Vearasilp T., Anusatsananun V., Gauly M. Prevalence of gastrointestinal helminths in Thai indigenous chickens raised under backyard conditions in Northern Thailand. J. Appl. Poult. Res. 2019;28:221–229. [Google Scholar]