Abstract
The underlying molecular mechanism of lipid metabolism in peripheral blood lymphocytes from chicken infected with reticuloendotheliosis virus (REV) remains poorly understood. Therefore, this scientific question was explored in vitro and in vivo. The results indicated that triglyceride content was significantly reduced, but the free fatty acid content and carnitine palmitoyltransferase-1 activity were significantly increased in blood lymphocytes after REV infection. By RNA sequencing, 97 known differentially expressed genes (DEG) related to lipid metabolism or glycometabolism were screened via Gene Ontology term analysis. On the basis of these 97 DEG, enriched pathways, including the peroxisome proliferators-activated receptor (PPAR) signaling pathway, were identified. Among these 97 DEG, some representative genes were related to lipolysis and fatty acid utilization (PPARG, LPL, PLIN2, ACOX1, ACSL1, FABP3, and FABP4). However, other genes related to lipid biosynthesis (ACSL3, ACSL6, DGAT2, LPIN1, and LPIN2) were downregulated. The quantitative polymerase chain reaction results confirmed the accuracy of the RNA sequencing data, and the in vivo outcome supports theses in vitro results. Our findings revealed that REV regulates fatty acid and lipid metabolism in peripheral blood lymphocytes from chicken. After the lymphocytes were infected with REV, the exogenous fatty acids were preferentially used; genes involved in fatty acid utilization were upregulated via the PPAR pathway, whereas genes involved in lipid and fatty acid biosynthesis were downregulated.
Key words: lipid and fatty acid metabolism, gene expression, chicken, lymphocyte, REV
Introduction
Reticuloendotheliosis is an immunosuppressive disease in chicken caused by lymphocytes or reticuloendothelial cells infected with the reticuloendotheliosis virus (REV) (Witter et al., 1979), which seriously affects chicken production. REV infection inhibits the proliferation and differentiation of T cells (Hrdlicková et al., 1994; Kim et al., 2003) and causes acute reticular cell or chronic lymphoid and other tissue tumors.
Immune cells require a large amount of energy to maintain function, and fatty acid metabolism is very important for T cells. Tumor cells mainly need glucose via glycolysis (Warburg, 1956). Large consumption of glucose induces tumor progression by limiting the metabolism of T cells and inhibiting their mammalian target of rapamycin activity, glycolytic ability, and production of interferons. Fatty acids are also a major component of cell membranes, which are necessary for cell proliferation (Currie et al., 2013). Fatty acid synthesis provides the cell membrane and other key lipid cell structures needed for T cell proliferation. The intermediate products of the glycolytic pathway and tricarboxylic acid cycle metabolism are raw materials for T cells to synthesize fatty acids; thus, inhibition of glycolysis can lead to the weakening of T cell antitumor ability (Chang et al., 2015; Gupta et al., 2017).
Abnormal proliferation and metabolism are common characteristics of all malignant tumor cells (Hanahan and Weinberg, 2011). Fatty acid metabolism plays an important role in tumor development. The glycolytic pathway provides considerably low energy supply; thus, tumor cells also increase energy supply via fat metabolism. During abnormal cell proliferation, the formation of cell membrane and signaling molecules requires greater involvement of fatty acid metabolism and lipid droplet metabolism (Santos and Schulze, 2012). Fatty acid synthesis and fatty acid oxidation may constitute the metabolic cycle of fatty acid. Fatty acid oxidation provides raw material acetyl CoA for the synthesis of fatty acid in the cytoplasm, suggesting that fatty acid synthesis in tumor cells and fatty acid oxidation are interdependent and mutually causal (Caro et al., 2012).
Fatty acids required for tumor growth and proliferation are mainly derived from de novo synthesis, while normal cells tend to absorb exogenous fatty acids owing to inhibition of DE novo synthesis (Medes et al., 1953; Santos and Schulze, 2012; Veigel et al., 2015). The fatty acid synthesis pathway is the intracellular lipid synthesis necessary for cell growth and proliferation. Fatty acids can also be condensed with glycolytic glycerol to produce various combinations of triacylglycerol and phospholipids, which are key components of many cellular structures (Tannahill et al., 2013). Selective inhibition of fatty acid synthase, acetyl-CoA carboxylase, and acyl-CoA synthetase long-chain family member 5 (ACSL5) genes, which are responsible for the synthesis of endogenous fatty acids, may induce tumor cell apoptosis and significantly inhibit tumor recurrence and metastasis (Mashima et al., 2009; Murata et al., 2010; Guseva et al., 2011; Jump et al., 2011; Lee et al., 2013; Agostini et al., 2014; Sounni et al., 2014) to effectively control the occurrence and development of cancer. Inhibition of either key enzymes in fatty acid synthesis or fatty acid oxidation metabolism can inhibit tumor cell growth in vitro and in vivo (Menendez and Lupu, 2007; Liang and Mulholland, 2014).
In the present study, we explored the effect of REV infection on peripheral blood lymphocytes of chicken. However, the underlying molecular mechanism of lipid metabolism in peripheral blood lymphocytes from chicken infected with REV is poorly understood. Based on the previous data obtained via RNA sequencing (RNA-seq), the differential expression profile of genes related to fatty acid and lipid metabolism was analyzed in chicken lymphocytes infected with REV in vitro and in controls. The aim was to explore the change of regulation on lipid metabolism in peripheral blood lymphocytes with REV infection and provide an insight into the pathogenesis of REV on chicken blood lymphocytes.
Materials and methods
Animals
Experimental SPF Rugao pure line chickens aged 1 d were purchased from the Poultry Institute, Chinese Academy of Agricultural Sciences (Yangzhou, Jiangsu, China). Birds were raised in an environmentally controlled room. Feed and water were provided ad libitum during the experiment. Diets were formulated in accordance with existing recommendations (Nutrient Requirements of Yellow-feathered Broilers, NY/T 33-2004, China). All experimental procedures were performed in accordance with the Administration Act on the Use and Care of Experimental Animals in Jiangsu Province (#115th Jiangsu Province Government Notice in 2008). All animal experimental operations were approved and guided by the Animal Care and Use Committee of Yangzhou University.
REV Infection Model of Chicken Blood Lymphocytes In Vitro
First, lymphocytes were derived from chicken blood according to the protocol of the chicken blood lymphocyte separation medium kit. Cells (5 × 105/ml) were incubated with RPMI-1640 medium containing 10% fetal bovine serum in 10-cm dishes for 24 h and then infected with reticuloendotheliosis virus strain HA1101 (REV, GenBank accession number: KF305089.1) with 105 TCID50/0.1 ml. Cells were harvested after infection for 36 h for use in subsequent experiments.
REV Infection Model of Chicken and Sample Collection
For preparing the REV infection model in vivo of chicken, the aforementioned REV strain was used to infect chickens (5 × 105 TCID50/0.1 mL) by intramuscular injection. Uninfected control birds received the same volume of RPMI-1640 medium. Birds were raised in an environmentally controlled room. Feed and water were provided ad libitum during the experiment. Two weeks after REV infection, each group of 10 REV-infected chickens and control chickens with similar weights were selected. Blood was collected from the wing vein to derive blood lymphocytes for the subsequent detection by the quantitative polymerase chain reaction (qPCR).
Analysis of Differentially Expressed Genes Related to Lipid Metabolism Between Blood Lymphocytes Infected With REV In Vitro and Controls
RNA-seq data coming from our previous research (Bi et al., 2018) were used in this study. Blood lymphocytes with or without REV infection were prepared for RNA-seq, and genes were considered as differentially expressed genes (DEG) only when the fold-change in abundance for all comparisons exceeded 2.0, with a P value < 0.05. According to the results of gene ontology (GO), enrichment analysis was performed based on the DEG using the GOEAST software toolkit, and the related DEG on lipid metabolism were screened. Based on the DEG related to lipid metabolism, the enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were identified by hypergeometric testing using the R package (P < 0.01, FDR-adjusted) to explore the regulation of REV on lipid metabolism in blood lymphocytes of chicken. Pathways with fewer than 3 known genes were discarded.
Quantitative Polymerase Chain Reaction
qPCR Was performed to detect the expression levels of DEG related to lipid and fatty acid metabolism. The primers used are listed in Table 1. β-actin was used as a housekeeping gene. Complementary DNA (cDNA) was obtained using a RevertAid First Strand cDNA Synthesis Kit (TAKARA, Dalian, China). Each 20 μL of the PCR mixture contained 10 μL of the 2 × iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), 50 ng of cDNA, and 0.5 μL (10 mm) of each primer. The mixtures were incubated in an ABI7500 Real-Time PCR Detection System (Applied Biosystems, Carlsbad, CA). A melting curve was constructed to verify that only a single PCR product was amplified. Samples were assayed in triplicate with standard deviations of the threshold cycle values not exceeding 0.5 on a within-run basis (Liu et al., 2015). Moreover, correlation of gene expression between 2 methods was analyzed.
Table 1.
The specific primers for qPCR in this study.
| Gene | Sequence | Product size (bp) | Accession no. |
|---|---|---|---|
| β-actin | F:5′-GAGAAATTGTGCGTGACATCA-3′ | 152 | NM_205518 |
| R:5′-CCTGAACCTCTCATTGCCA-3′ | |||
| ACSL1 | F:5′-CAACAGGGAGACCAAAGGGT-3′ | 245 | NM_001012578 |
| R:5′-TGCCTGACATCCACAAGTTCA-3′ | |||
| ACSL3 | F:5′-GGGTTAGCAGTGTTGGGTCA-3′ | 141 | XM_015277032 |
| R:5′-TGCACCTCCTAGGGTAGCAT-3′ | |||
| ACSL6 | F:5′-TGAGAAGGAGCTGACGGAGA-3′ | 162 | XM_015294077 |
| R:5′-TTGGGGTTTCCTGTAGTGCC-3′ | |||
| ACOX1 | F:5′-GGAGATCGAGGCCTTAGTGA-3′ | 187 | NM_001006205 |
| R:5′-CCGTCCACGATGAACAAAGC-3′ | |||
| DGAT2 | F:5′-GGCTACGTTGGCTGGTAACT-3′ | 196 | NM_026384 |
| R:5′-CTTCAGGGTGACTGCGTTCT-3′ | |||
| FABP3 | F:5′- CTCCTCCTGGCTGTTCCGATG -3′ | 148 | AY648562 |
| R:5′- GGGTAATTGGTTGGCTGGCTC -3′ | |||
| FABP4 | F:5′-GGGGTTTGCTACCAGGAAGATG-3′ | 276 | NM_204290 |
| R:5′-CATTCCACCAGCAGGTTCCC-3′ | |||
| LPIN1 | F:5′-CTTGGTGCTGATGGCGTCTA-3′ | 138 | XM_015276089 |
| R:5′-AGAATGAGTGGCAGACCGTG-3′ | |||
| LPIN2 | F:5′-TCAGCCCCTTAGCCCTTACT-3′ | 121 | NM_001006386 |
| R:5′-GAATCCTCCCCACGTCCATT-3′ | |||
| LPL | F:5′-AGGAGAAGAGGCAGCAATA-3′ | 222 | AB016987 |
| R:5′-AAAGCCAGCAGCAGATAAG-3′ | |||
| PLIN2 | F:5′-GCTGGAGCCAAGGATTCTGT-3′ | 142 | NM_007408 |
| R:5′-TCATGAACTGCACCATCCCC-3′ | |||
| PPARG | F:5′-TAAAGTCCTTCCCGCTGACCAAA-3′ | 230 | NM_001001460 |
| R:5′-AAATTCTGTAATCTCCTGCACTGCCTC-3′ | |||
| FOXO1 | F:5′-GATCCGGGTACCATGGGC-3′ | 162 | NM_204328 |
| R:5′-GCCCCATGCAGGCTCC-3′ | |||
| INSR | F:5′-AACGAGCTGTGCTACCTGTC-3′ | 178 | XM_001233398 |
| R:5′-AGCGCTCGATGAAGATACCG-3′ | |||
| IRS2 | F:5′-TCCTGGAGGCTATGAAGGCT-3′ | 167 | XM_015277882 |
| R:5′-CAGGTTGACCAGGTGGTGG-3′ | |||
| IRS4 | F:5′-AAGCTTAACTCGGAGGTGCC-3′ | 153 | XM_003641084 |
| R:5′-GGCCACCACAGAATCATCCA-3′ | |||
| PYGB | F:5′-GAAGGTGGACTGGGACAAGG-3′ | 260 | NM_001031034 |
| R:5′-GGTGTGCCATGTTGATACGC-3′ | |||
| PYGL | F:5′-TTCCTCGACTCGATGGCAAC-3′ | 195 | NM_204392 |
| R:5′-CTCCACACGGCCATAGAAGT-3′ |
Determination of Biochemical Indicators
Blood lymphocytes infected with REV in vitro and in the controls were used, respectively. The lipid in cells was determined by ether extraction with the physiological saline as the solvent as previous reported (Hung et al., 2017). Triglyceride (TG) and free fatty acid (FFA) contents in lymphocytes were measured using the commercial kit (Nanjing Jian Cheng Bio-engineering Institute, Nanjing, China). Mitochondria in the lymphocytes infected with REV in vitro and in the controls were first isolated from the cells as described in previous reports, with modifications (Morash et al., 2008); the cells were immediately placed in mitochondrial isolation buffer on ice, washed, homogenized, and then spun; the supernatant was discarded; and the mitochondrial pellet was resuspended in an appropriate volume. The carnitine palmitoyltransferase-1 (CPT-1) activity was determined by using the previous method (Bieber and Fiol, 1986): The samples were homogenized in 20 volumes of an enzyme extraction buffer (20 mM HEPES, 1 mM EDTA, and 0.1% Triton at pH 7.4) using a glass-on-glass homogenizer, and enzyme assays were performed on this crude homogenate. The process was based on the measurement of the initial CoA-SH formation by 5,50-dithio-bis-(2-nitrobenzoic acid) reaction from palmitoyl-CoA with L-carnitine at 412 nm.
Statistical Analyses
Statistical differences between groups were evaluated using the Student's t-test. P < 0.05 (∗) or P < 0.01 (∗∗) was considered significant. Data were presented as the mean ± SEM. Data analyses were performed using the statistical R package and SPSS (ver. 1.70; SPSS, Inc., Chicago, IL).
Results
Changes in TG, FFA Contents, and CPT-1 Activity
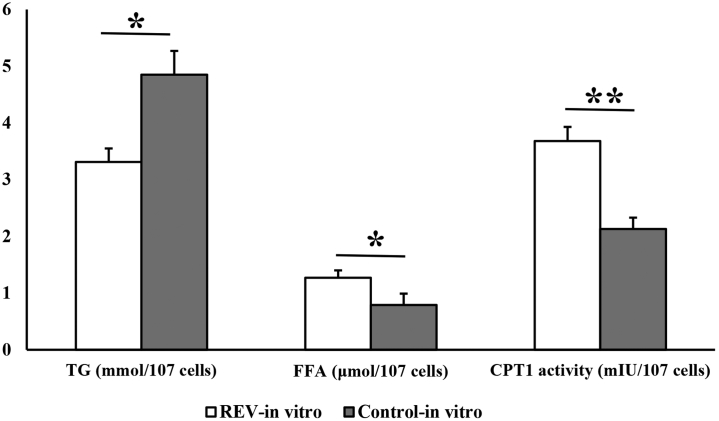
As shown in Figure 1, the TG content is significantly (P < 0.05) lower, but the FFA content and CPT-1 activity are significantly (P < 0.05 or P < 0.01) higher in the REV-infected blood lymphocytes.
Figure 1.
Changes in triglyceride (TG) content, free fatty acid (FFA) content, and carnitine palmitoyltransterase-1 (CPT-1) activity in the REV-infected blood lymphocytes. Data are presented as means ± standard error of the means (SEM); n = 6. ∗P < 0.05, ∗∗P < 0.01.
Go and KEGG Analysis Based on DEG in the Lymphocytes Infected With REV In Vitro and in the Control Cells
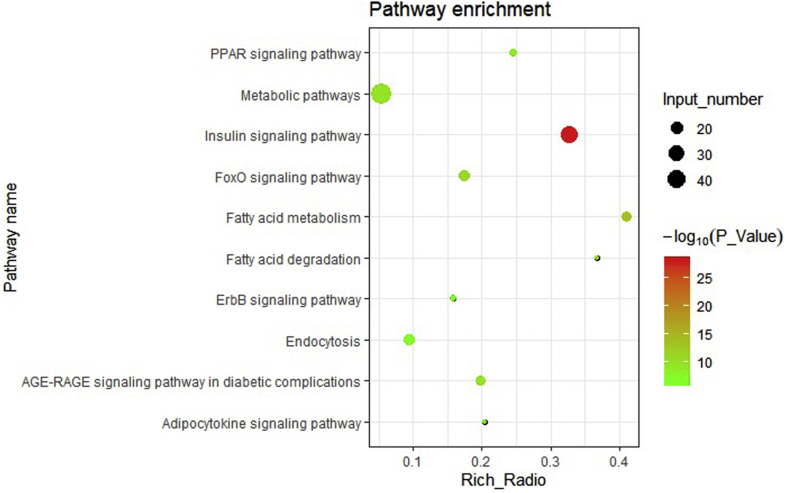
The RNA-seq results obtained using peripheral blood lymphocytes infected with REV and the control indicated that 2,977 known DEG (infected vs. control) with consistent fold changes ≥2.0 were detected (Supplementary Table 1). GO analysis based on 2,977 DEG showed that the enriched GO terms (P < 0.05) were screened, including the phospholipid biosynthesis, negative regulation of the insulin receptor signaling pathway, glycolytic process, gluconeogenesis, response to fatty acid, and so on. In accordance with the GO term analysis, KEGG analysis, and known information on gene function, 55 known DEG related to lipid metabolism (Supplementary Table 2) and 42 known DEG related to glycometabolism (Supplementary Table 3) were screened. After KEGG pathway analysis, 50 enriched pathways (Supplementary Table 4) identified based on 97 DEG related to lipid metabolism or glycometabolism (including insulin, PPAR, fatty acid metabolism, and the fatty acid degradation signaling pathway) were enriched. This occurrence affected lipid metabolism, one of the top 10 pathways (Figure 2).
Figure 2.
Top 10 pathways from the enriched pathways based on the 97 differentially expressed genes (DEG) related to lipid metabolism or glycometabolism.
DEG Related to Lipid Metabolism or Glycometabolism in the Lymphocytes Infected With REV In Vitro and in the Controls
The 55 known DEG related to lipid metabolism are mainly involved in transcriptional regulation (CEBPB, CEBPG, and PPARG), lipidolysis (DGKI, DGKK, LPL, and PLIN2), fatty acid transport (FABP3 and FABP4), acyl–coA synthetases (ACSBG2, ACSF2, ACSF3, ACSL1, ACSL3, and ACSL6), acetyl–coA transferases (ACAA2), acyl–coA oxidases (ACOX1), acyl–coA dehydrogenases (ACADL and ACADS), elongation of very-long-chain fatty acids (ELOVL1 and ELOVL7), and lipid synthesis (DGAT2, LPIN1, and LPIN2), among others. Moreover, some known DEG related to cholesterol metabolism were found in 55 known DEG related to lipid metabolism, mainly involved in cholesterol biosynthesis (VLDLR), cholesterol homeostasis (ABCA3 and LDLRAP1), and cholesterol catabolism (CYP7B1 and HMGB2).
Among the 55 known DEG related to lipid metabolism, the expression levels of ACADS, ACSBG2, ACSF2, ACSF3, ACSL1, ACOX1, DGKI, ELOVL7, FABP3, FABP4, LPL, PLIN2, and PPARG were upregulated; however, the expression levels of ACAA2, ACADL, ACSL3, ACSL6, CEBPB, CEBPG, DGAT2, DGKK, ELOVL1, LPIN1, and LPIN2 were downregulated in the lymphocytes infected with REV in vitro relative to those in the control cells. Similarly, the expression levels of ABCA3, CYP7B1, and VLDLR were upregulated, and the expression levels of HMGB2 and LDLRAP1 were downregulated.
Moreover, 42 known DEG related to glycometabolism were screened. Some genes (such as INSR, IRS2, IRS4, PYGL, PYGB, FOXO1, and FLOT2) were enriched in the insulin signaling pathway. Among them, INSR, IRS2, IRS4, PYGL, PYGB, and FOXO1 exhibited reduced expression levels for REV infection (Supplementary Table 3).
Validating the Expression Level of DEG Related to Lipid Metabolism
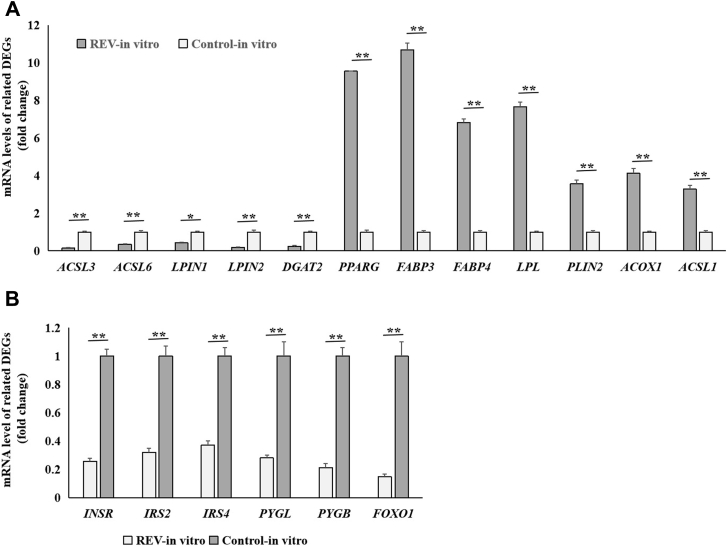
To ensure the effectiveness of the results by RNA-seq, the accuracy of data on the related genes was analyzed in this study. Using the lymphocytes infected with REV in vitro and in the controls, the expression levels of 12 representative genes (ACOX1, ACSL1, ACSL3, ACSL6, DGAT2, LPIN1, LPIN2, PPARG, LPL, PLIN2, FABP3, and FABP4) related to lipid metabolism were verified by qPCR. The results showed that the mRNA levels of ACOX1, ACSL1, PPARG, LPL, PLIN2, FABP3, and FABP4 were significantly (P < 0.05 or P < 0.01) upregulated, whereas those of ACSL3, ACSL6, DGAT2, LPIN1, and LPIN2 were significantly (P < 0.05 or P < 0.01) downregulated in the lymphocytes infected with REV (Figure 3A). In addition, the results obtained by qPCR showed that the mRNA levels of INSR, IRS2, IRS4, PYGL, PYGB, and FOXO1, which are associated with glycometabolism were significantly (P < 0.01) downregulated with REV infection (Figure 3B).
Figure 3.
mRNA Expression levels of 18 representative genes by determined by quantitative PCR (qPCR) in the lymphocytes infected with reticuloendotheliosis virus (REV) in vitro and in the control cells. (A) Twelve genes related to lipid metabolism; (B) mRNA levels of 6 genes related to glycometabolism. Data are means ± SEM; n = 6. ∗P < 0.05, ∗∗P < 0.01.
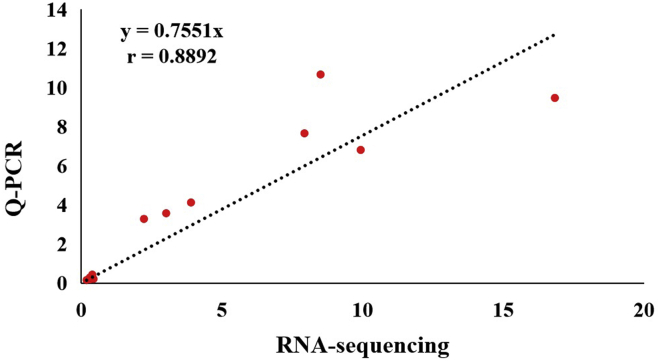
Correlation analysis of gene expression levels in vitro by RNA-seq and qPCR was performed based on the selected 12 representative DEG from 55 known DEG related to lipid metabolism and 6 representative DEG related to glycometabolism from 42 known DEG. We further selected 18 DEG with moderate fold changes to analyze the correlation between the expression data obtained by RNA-seq and qPCR. Figure 4 shows that the fold changes of these 18 genes by RNA-seq and qPCR are highly correlated (r = 0.8892, P < 0.01).
Figure 4.
Correlation analysis of gene expression levels determined by RNA-seq and qPCR. Based on 13 DEG related to lipid metabolism or glycometabolism, the analysis indicated a strong correlation on the fold changes of DEG's expression levels quantified by RNA-seq and qPCR. The correlation coefficient (r) was 0.8892 (P < 0.01).
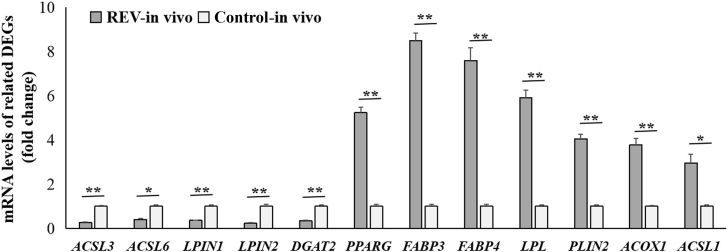
Furthermore, the blood lymphocytes were directly isolated from chickens with or without REV infection in vivo for 2 wks, and 12 genes related to lipid metabolism were detected by qPCR. The results revealed the fold-change consistency of these gene expression levels in vivo and in vitro. The expression levels of ACSL1, ACOX1, PPARG, LPL, PLIN2, FABP3, and FABP4 mRNA levels were significantly (P < 0.05 or P < 0.01) upregulated, and the expression levels of ACSL3, ACSL6, DGAT2, LPIN1, and LPIN2 were significantly (P < 0.05 or P < 0.01) upregulated in lymphocytes with REV infection (Figure 5).
Figure 5.
mRNA Levels of 12 representative genes related to lipid metabolism in the lymphocytes from chickens with or without REV infection for 2 wks by using qPCR. Data are presented as means ± SEM; n = 10. ∗P < 0.05, ∗∗P < 0.01.
Discussion
Reticuloendotheliosis for REV infection would cause huge economic losses of poultry production, accompanied by inhibiting the T cells' proliferation and inducing the tumorigenesis (Witter et al., 1979; Hrdlicková et al., 1994; Kim et al., 2003). Lymphocytes or reticuloendothelial cells were the target of REV; the immune response mechanism on REV in vitro in peripheral blood lymphocytes of chicken had been explored in our previous study (Bi et al., 2018). Considering the importance of fatty acid metabolism and abundant glucose consumption in tumor development and immunosuppression (Hapala et al., 2011; Currie et al., 2013; Gupta et al., 2017), the gene expression profile on lipid metabolism in chicken peripheral blood lymphocytes with REV infection and controls was explored to reveal the change of molecular regulation on lipid metabolism after REV infected in vitro, which will help to understand the immune mechanism of lymphocytes on response REV infection.
First, we focused on the identification of genes on lipid metabolism or glycometabolism. After GO term analysis, some related GO terms (phospholipid biosynthesis, negative regulation of the insulin receptor signaling pathway, glycolysis, gluconeogenesis, response to fatty acid, and so on) were enriched, suggesting that lipid metabolism or glycometabolism was associated with the effect of REV on blood lymphocytes. Based on the results of GO terms, we screened 97 known DEG related to lipid metabolism (55 genes) or glycometabolism (42 genes), which are mainly involved in the insulin, PPAR, fatty acid metabolism, and fatty acid degradation signaling pathways.
Among 55 known DEG related to lipid metabolism, DGAT2, LPIN1, and LPIN2 have been identified as important regulatory factors in lipid deposition (Chen et al., 2015; Hung et al., 2017). ACSL3 and ACSL6 can catalyze the production of acyl–coA synthetases (Van Horn et al., 2005; Valente et al., 2019). Thus, the downregulation of ACSL3, ACSL6, DGAT2, LPIN1, and LPIN2 expression levels was consistent with the decrease in TG content in the lymphocytes infected with REV in vitro relative to that in the control cells. Thus, it was considered that REV infection would inhibit the lipid synthesis in blood lymphocytes of chicken.
The fatty acids within the cell had 2 sources of extracellular fatty acid absorption and intracellular fatty acid biosynthesis. Fatty acids required for tumor growth and proliferation are mainly derived from de novo synthesis, but normal cells tend to absorb exogenous fatty acids owing to the inhibition of de novo synthesis (Medes et al., 1953; Santos and Schulze, 2012; Veigel et al., 2015). As previously reported, LPL and PLIN2 can release fatty acids from extracellular lipids (Bildirici et al., 2018; Wu et al., 2020), FABP3 and FABP4 are transported to the intracellular fatty acids (Andersen et al., 2019), and ACOX1 and ACSL1 are positive for fatty acid β-oxidation (Zhao et al., 2016; Li et al., 2019). As the known transcription factor (Oh et al., 2019), PPARG can regulate the expressions of ACOX1, ACSL1, LPL, PLIN, FABP3, FABP4, and so on (Jahansouz et al., 2017; Yan et al., 2017; Smith et al., 2018). Moreover, the acyl-coA synthetases ACSBG2, ACSF2, and ACSF3 can regulate the biosynthesis of endogenous fatty acid (Yamasaki et al., 2005; Ellis et al., 2010; Lopes-Marques et al., 2013). Considering the expression changes of these genes and the increases in the FFA content and CPT-1 activity in the lymphocytes infected with REV in vitro relative to those in the control cells, it was suggested that extracellular lipolysis could be enhanced for exogenous fatty acids in the lymphocytes infected with REV. This view of fatty acid metabolism in the blood lymphocytes with REV infection is the same as that in normal cells and tissues but different from that in tumor cells and tissues as previously reported (Medes et al., 1953; Hapala et al., 2011; Santos and Schulze, 2012; Veigel et al., 2015).
Meanwhile, 42 known DEG related to glycometabolism were also screened. Some of these genes (such as INSR, IRS2, IRS4, PYGL, PYGB, and FOXO1) enriched the insulin signaling pathway, and their expression levels were lower in the lymphocytes with REV infection than in the control cells. In accordance with the functional information of these genes or the insulin pathway (O-Sullivan et al., 2015), glycometabolism might be inhibited in the lymphocytes infected with REV in vitro rather than in the control cells, which was consistent with the view on the aforementioned enhancement of fatty acid synthesis.
In the present study, the expression levels of 18 representative genes related to lipid metabolism or glycometabolism were verified again by qPCR. The consistency of data between qPCR and RNA-seq was supported by a high positive correlation, indicating the validity and authenticity of the data by RNA-seq. By using the lymphocytes infected with REV in vivo, further verification was performed to evaluate the gene expression levels; 12 genes related to lipid metabolism were detected by qPCR. The results showed the consistency in fold change of these gene expression levels in vivo and in vitro. However, the proposed approach only used RNA-seq to elucidate the regulation of lipid and fatty acid metabolism in blood lymphocytes from chickens infected with REV, and a complete understanding would require further studies.
In conclusion, we presented the regulation of fatty acid and lipid metabolism in peripheral blood lymphocytes from chicken by using REV. After the lymphocytes were infected with REV, the exogenous fatty acids were preferentially used; genes involved in fatty acid utilization were upregulated via the PPAR pathway, whereas the genes involved in lipid and fatty acid biosynthesis were downregulated. This novel suggestion of REV regulating lipid and fatty acid metabolism in blood lymphocytes was different from the metabolism in virus-induced tumor tissues and should guide further investigation into the pathogenesis of REV infection in chickens.
Acknowledgments
The authors would like to thank Professor Kun Qian (Key Laboratory of Jiangsu Preventive Veterinary Medicine, Yangzhou University, Yangzhou, China) and Zhizhong Cui (Shandong Agriculture University, TaiAn, China) for providing the REV strain HA1101.
The research was funded by grants from the National Natural Science foundation of China (31802057), the open funds of the Ministry of Education Key Lab for Avian Preventive Medicine (NO.YF201902), the second Batch of Special Grant from China Postdoctoral Science Foundation (2020TQ0252), Jiangsu Province postdoctoral research funding project in 2020 (2020Z213), the Open Project Program of Joint International Research Laboratory of Agriculture and Agri-Product Safety, the Ministry of Education of China, Yangzhou University (JILAR-KF202017).
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2021.101081.
Disclosures
All authors have read and approved this version of the article, and no conflict of interest exists in the submission of this manuscript. Finally, this article is our original unpublished work and has not been submitted to any other journal for reviews.
Supplementary data
The list of all 2977 DEG.
The list of all 2977 DEG related to lipid metabolism.
The list of 47 DEG related to glycometabolism.
The list of enriched pathways based on the 102 DEG related to lipid metabolism or glycometabolism.
References
- Agostini M., Almeida L.Y., Bastos D.C., Ortega R.M., Moreira F.S., Seguin F., Zecchin K.G., Raposo H.F., Oliveira H.C.F., Amoedo N.D. The fatty acid synthase inhibitor orlistat reduces the growth and metastasis of orthotopic tongue oral squamous cell carcinomas. Mol. Cancer Ther. 2014;13:585–595. doi: 10.1158/1535-7163.MCT-12-1136. [DOI] [PubMed] [Google Scholar]
- Andersen E., Ingerslev L.R., Fabre O., Donkin I., AltNta A., Versteyhe S., Bisgaard T., Kristiansen V.B., Simar D., Barrès R. Preadipocytes from obese humans with type 2 diabetes are epigenetically reprogrammed at genes controlling adipose tissue function. Int. J. Obes. 2019;43:306–318. doi: 10.1038/s41366-018-0031-3. [DOI] [PubMed] [Google Scholar]
- Bi Y., Lu X., Qiu L., Wang S., Liu X., Zhang Y., Yang C., Yang Z., Qi X., Chang G. Reticuloendotheliosis virus inhibits the immune response acting on lymphocytes from peripheral blood of chicken. Front. Physiol. 2018;9:4. doi: 10.3389/fphys.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber L.L., Fiol C. Purification and assay of carnitine acyltransferases. Methods Enzymol. 1986;123:276–284. doi: 10.1016/s0076-6879(86)23031-1. [DOI] [PubMed] [Google Scholar]
- Bildirici I., Schaiff W.T., Chen B., Morizane M., Oh S.Y., O’Brien M., Sonnenberg Hirche C., Chu T., Barak Y., Nelson D.M. PLIN2 is essential for trophoblastic lipid droplet accumulation and cell survival during hypoxia. Endocrinology. 2018;159:3937–3949. doi: 10.1210/en.2018-00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro P., Kishan A.U., Norberg E., Stanley I.A., Chapuy B., Ficarro S.B., Polak K., Tondera D., Gounarides J., Hong Y. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.H., Qiu J., O’Sullivan D., Buck M., Noguchi T., Curtis J., Chen Q., Gindin M., Gubin M., Van Windt G.J.W. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Rui B.B., Tang L.Y., Hu C.M. Lipin family proteins - key regulators in lipid metabolism. Ann. Nutr. Metab. 2015;66:10–18. doi: 10.1159/000368661. [DOI] [PubMed] [Google Scholar]
- Currie E., Schulze A., Zechner R., Walther T.C., Farese R.V. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J.M., Frahm J.L., Li L.O., Coleman R.A. Acyl-coenzyme A synthetases in metabolic control. Curr. Opin. Lipidol. 2010;21:212–217. doi: 10.1097/mol.0b013e32833884bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Roy A., Dwarakanath B.S. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68. doi: 10.3389/fonc.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseva N.V., Rokhlin O.W., Glover R.A., Cohen M.B. TOFA (5-tetradecyl-oxy-2-furoic acid) reduces fatty acid synthesis, inhibits expression of AR, neuropilin-1 and Mcl-1 and kills prostate cancer cells independent of p53 status. Cancer Biol Ther. 2011;12:80–85. doi: 10.4161/cbt.12.1.15721. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hapala I., Marza E., Ferreira T. Is fat so bad? Modulation of endoplasmic reticulum stress by lipid droplet formation. Biol. Cell. 2011;103:271–285. doi: 10.1042/BC20100144. [DOI] [PubMed] [Google Scholar]
- Horn C.G.V., Caviglia J.M., Lei O.L., Wang S., Coleman R.A. Characterization of Recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: identification of a novel variant of Isoform 6. Biochemistry. 2005;44:1635–1642. doi: 10.1021/bi047721l. [DOI] [PubMed] [Google Scholar]
- Hrdlicková R., Nehyba J., Humphries E.H. v-rel Induces expression of three avian immunoregulatory surface receptors more efficiently than c-rel. J. Virol. 1994;68:308–319. doi: 10.1128/jvi.68.1.308-319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y.H., Carreiro A.L., Buhman K.K. Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat. Bba-mol. Cell. Biol. L. 2017;1862:600–614. doi: 10.1016/j.bbalip.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahansouz C., Xu H., Hertzel A.V., Kizy S.S., Steen K.A., Foncea R., Serrot F.J., Kvalheim N., Luthra G., Ewing K. Partitioning of adipose lipid metabolism by altered expression and function of PPAR isoforms after bariatric surgery. Int. J. Obes. 2017;42:139–146. doi: 10.1038/ijo.2017.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump D.B., Torres-Gonzalez M., Olson L.K. Soraphen A, An inhibitor of acetyl CoA carboxylase activity, interferes with fatty acid elongation. Biochem. Pharmacol. 2011;81:649–660. doi: 10.1016/j.bcp.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Brown T.P., Pantinjackwood M.J. Effects of cyclosporin A treatment on the pathogenesis of avian leukosis virus subgroup J infection in broiler chickens with Marek's disease virus exposure. J. Vet. Sci. 2003;4:245–255. [PubMed] [Google Scholar]
- Lee J.S., Sul J.Y., Jun P., Lee B., Sun M., Sung C.E. Fatty acid synthase inhibition by amentoflavone suppresses HER2/neu (erbB2) oncogene in SKBR3 human breast cancer cells. Phytother. Res. 2013;27:713–720. doi: 10.1002/ptr.4778. [DOI] [PubMed] [Google Scholar]
- Li G., Fu S., Chen Y., Jin W., Kang X. MicroRNA-15a regulates the differentiation of intramuscular preadipocytes by targeting ACAA1, ACOX1 and SCP2 in chickens. Int. J. Mol. Sci. 2019;20:4063. doi: 10.3390/ijms20164063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M., Mulholland D.J. Lipogenic metabolism: a viable target for prostate cancer treatment? Asian J. Androl. 2014;16:661–663. doi: 10.4103/1008-682X.132947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liu L., Zhang M., Yang N., Li X. Messenger RNA expression of chicken CLOCK gene in the response to Campylobacter jejuni inoculation. Poult. Sci. 2015;94:2124–2130. doi: 10.3382/ps/pev203. [DOI] [PubMed] [Google Scholar]
- Lopes-Marques M., Cunha I., Reis-Henriques M.A., Santos M.M., Castro L.F. Diversity and history of the long-chain acyl-CoA synthetase (Acsl) gene family in vertebrates. BMC Evol. Biol. 2013;13:271. doi: 10.1186/1471-2148-13-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima T., Sato S., Sugimoto Y., Tsuruo T., Seimiya H. Promotion of glioma cell survival by acyl-CoA synthetase 5 under extracellular acidosis conditions. Oncogene. 2009;28:9–19. doi: 10.1038/onc.2008.355. [DOI] [PubMed] [Google Scholar]
- Medes G., Thomas A., Weinhouse S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953;13:27–29. [PubMed] [Google Scholar]
- Menendez J.A., Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- Morash A.J., Kajimura M., Mcclelland G.B. Intertissue regulation of carnitine palmitoyltransferase I (CPTI): mitochondrial membrane properties and gene expression in rainbow trout (Oncorhynchus mykiss) Biochim. Biophys. Acta. 2008;1778:1382–1389. doi: 10.1016/j.bbamem.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Murata S., Yanagisawa K., Fukunaga K., Oda T., Kobayashi A., Sasaki R., Ohkohchi N. Fatty acid synthase inhibitor cerulenin suppresses liver metastasis of colon cancer in mice. Cancer Sci. 2010;101:1861–1865. doi: 10.1111/j.1349-7006.2010.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O-Sullivan I., Zhang W., Wasserman D.H., Liew C.W., Liu J., Paik J., DePinho R.A., Stolz D.B., Kahn C.R., Schwartz M.W. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat. Commun. 2015;6:7079. doi: 10.1038/ncomms8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.H., Karadeniz F., Lee J.I., Seo Y., Chang-Suk K. Artemisia princeps inhibits adipogenic differentiation of 3T3-L1 pre-adipocytes via downregulation of PPARγ and MAPK pathways. Preven Nutri. Food Sci. 2019;24:299–307. doi: 10.3746/pnf.2019.24.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C.R., Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- Smith A., Yu X., Yin L. Diazinon exposure activated transcriptional factors CCAAT-enhancer-binding proteins α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) and induced adipogenesis in 3T3-L1 preadipocytes. Pestic. Biochem. Physiol. 2018;150:48–58. doi: 10.1016/j.pestbp.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounni N.E., Cimino J., Blacher S., Primac I., Truong A., Mazzucchelli G., Paye A., Calligaris D., Debois D., De Tullio P. Blocking lipid synthesis overcomes iumor regrowth and metastasis after antiangiogenic therapy withdrawal. Cell Metabol. 2014;20:280–294. doi: 10.1016/j.cmet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente R.S., Almeida T.G.d., Alves M.F., Camargo J.d., Sudano M.J. Modulation of long-chain Acyl-CoA synthetase on the development, lipid deposit and cryosurvival of in vitro produced bovine embryos. Plos One. 2019;14:e0220731. doi: 10.1371/journal.pone.0220731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veigel D., Wagner R., Stübiger G. Fatty acid synthase is a metabolic marker of cell proliferation rather than malignancy in ovarian cancer and its precursor cells. Int. J. Cancer. 2015;136:2078–2090. doi: 10.1002/ijc.29261. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Witter R.L., Lee L.F., Bacon L.D., Smith E.J. Depression of vaccinal immunity to Marek's disease by infection with reticuloendotheliosis virus. Infect. Immun. 1979;26:90–98. doi: 10.1128/iai.26.1.90-98.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Ma H., Wang L., Song X., Zhang J., Liu W., Ge Y., Sun Y., Yu X., Wang Z., Wang J., Zhang Y., Li C., Li N., Gao L., Liang X., Yue X., Ma C. Tumor suppressor ZHX2 inhibits NAFLD-HCC progression via blocking LPL-mediated lipid uptake. Cell Death Differ. 2020;27:1693–1708. doi: 10.1038/s41418-019-0453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M., Hasegawa S., Suzuki H., Hidai K., Saitoh Y., Fukui T. Acetoacetyl-CoA synthetase gene is abundant in rat adipose, and related with fatty acid synthesis in mature adipocytes. Biochem. Biophys. Res. Commun. 2005;335:215–219. doi: 10.1016/j.bbrc.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Yan G., Li X., Peng Y., Long B., Fan Q., Wang Z., Shi M., Xie C., Zhao L., Yan X. The fatty acid β-oxidation pathway is activated by leucine deprivation in HepG2 cells: a comparative proteomics study. Sci. Rep. 2017;7:1914. doi: 10.1038/s41598-017-02131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.D., Zan L.S., Li A.N., Cheng G., Li S.J., Zhang Y.R., Wang X.Y., Zhang Y.Y. Characterization of the promoter region of the bovine long-chain acyl-CoA synthetase 1 gene: Roles of E2F1, Sp1, KLF15, and E2F4. Sci. Rep. 2016;6:19661. doi: 10.1038/srep19661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The list of all 2977 DEG.
The list of all 2977 DEG related to lipid metabolism.
The list of 47 DEG related to glycometabolism.
The list of enriched pathways based on the 102 DEG related to lipid metabolism or glycometabolism.