Abstract
Background
Blood-brain barrier (BBB) breakdown, as an early biomarker for vascular mild cognitive impairment (vMCI), has only been validated by a few studies. The aim of this study was to investigate whether compromised BBB integrity is involved in vMCI patients, and detect the relationship between BBB breakdown and cognitive function. BBB leakage in vMCI was explored, and the relationship between BBB leakage and cognitive function was discussed in this study.
Methods
This is a cross-sectional study involving 26 vMCI patients and 21 sex- and age-matched healthy controls. Dynamic contrast-enhanced-magnetic resonance imaging was performed for all participants, to determine BBB leakage. Leakage volume, leakage rate, and fractional blood plasma volume (Vp) in the grey and white matter were evaluated. Neuropsychological tests were used to determine cognitive function. Leakage rate, leakage volume, and Vp in different brain locations, including deep grey matter, cortical grey matter, white matter hyperintensity, and normal-appearing white matter were compared between the two groups.
Results
Multivariable linear regression analyses revealed that in all regions of interest, the leakage rate was significantly higher in vMCI patients relative to controls. Leakage volume in normal-appearing white matter and white matter hyperintensity were significantly higher, while Vp in normal-appearing white matter, deep grey matter, and cortical grey matter were significantly lower in vMCI patients. Moreover, Montreal Cognitive Assessment scores decreased with the increase of leakage rate in white matter hyperintensity.
Conclusion
Increased BBB permeability was detected in vMCI patients and was related to cognitive decline, which suggested that BBB breakdown might be involved in cognitive dysfunction pathogenesis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-021-02189-6.
Keywords: Blood-brain barrier, Vascular mild cognitive impairment, Dynamic contrast-enhanced-magnetic resonance imaging, Cognitive function
Background
Vascular cognitive impairment (VCI) includes subjective cognitive decline, vascular mild cognitive impairment (vMCI), and dementia [1]. Vascular dementia is the second most common subtype of dementia, and comprises about 14.5% of the total number of dementias [2]. VMCI is considered as an early stage of VCI, and it is most prevalent in elderly Chinese [3]. Its pathophysiology remains unclear, but various pathophysiological processes may affect its progress, including blood-brain barrier (BBB) defects, inflammation altered vascular reactivity, hypoperfusion, and innate immune processes [4–6].
It had been studied that BBB breakdown occurs in the early stage of cognitive dysfunction [7]. Neuropathological, cerebrospinal fluid (CSF) biomarker, and non-quantitative neuroimaging studies have revealed the increase of BBB permeability in cognitively declining patients [8–10]. Some studies used dynamic contrast-enhanced-magnetic resonance imaging (DCE-MRI) to quantitatively evaluate BBB permeability in cerebral small vessel disease(cSVD) and stroke [7, 11, 12], showing that subtle BBB leakage was detected. Although increased BBB leakage in white matter was found in patients with VCI using DCE-MRI [13], there were scarce studies focusing on vMCI patients, and understanding of the relationship between cognitive function and BBB leakage was deficient. The aim of this study was to investigate whether BBB permeability change in different brain regions in vMCI patients. In addition, the matter whether the cognitive function was independently associated with BBB leakage change was tentatively examined.
Methods
Study participants
This is a cross-sectional study. Participants were recruited through Beijing Chao-Yang Hospital, Capital Medical University from May 2016 to June 2019. Twenty-six vMCI patients were enrolled, along with 21 sex- and age-matched healthy controls. All participants were subjected to neuropsychological evaluation and DCE-MRI to assess BBB permeability. Participants were recruited into the vMCI group if they met the following criteria: (1) complaints of cognitive function, activities of daily living may be normal or mildly impaired, (2) objective cognitive impairment in at least one cognitive domain, (3) MRI findings suggest that cognitive impairment is associated with vascular diseases [14, 15]. The MRI findings include:(1) > two lacunar infarcts outside the brainstem, (2) Single lacunes(> 5 mm)placed strategically in the striatum or the thalamus, (3)confluent deep WMH (Fazekas score = 2 or 3) or irregular periventricular WMH extending into deep white matter (Fazekas score = 3) combined with or without lacunes or microbleeds [14].Sex- and age-matched controls were recruited which presented for physical examination at the medical examination centre of Beijing Chao-Yang Hospital. Exclusion criteria included psychiatric disorders, alcohol/drug abuse, epilepsy, brain tumor, brain trauma, vascular malformation, cardiac and systemic disease, neurodegenerative disease, Parkinson disease, intracranial infection, and contraindications to MRI or intravenous gadolinium.
Ethics
Ethical clearance for this study was given by the institutional ethics committee of Beijing Chao-Yang Hospital, Capital Medical University. All participants provided signed informed consent before participating in the study.
Neuropsychological evaluation
All participants underwent a face-to-face neuropsychological test by two trained interviewers within 3 days before or after MRI examination. We calculated cognitive function using Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Digit Span Test (DST, including forward and backward), Trail-Making Test (TMT, including Part A and Part B), Symbol Digital Modalities Test (SDMT), Stroop color word Test (SCWT, including Part A, B and C), Clock Drawing Test (CDT, 4-point scale), and verbal fluency test (VFT). Overall cognitive function was assessed by MMSE and MoCA. Other cognitive domains included memory (delayed racall test in MoCA, DST), information processing speed (TMT-A), attention (SDMT), executive function (TMT B-A, SCWT C-B), spatial function (CDT), and language function (VFT) [14, 16].
Imaging protocol and analysis
MRI examinations were executed using a Siemens 3 T MR system(Siemens, Prisma, Munich, Germany), with a 64-channel array head coil. Structural MRI was first performed(See Supplementary Table 1, Additional file 1). DCE-MRI was then performed. The T1 DCE protocol was a fast sequence (repetition time/echo time [TR]/[TE]5.08/1.8 ms, flip angle 15°, slice number = 20 per volume, voxel size 1.2 × 1.2 × 3 mm3, field of view [FOV]23 × 23 cm2), this sequence included 60 volumes of continuous acquisition. Gadolinium (1.0 mmol/ml, at an injection volume of 0.1 mmol/kg body weight and an injection speed of 2.5 ml/s) was injected using a high-pressure injector after the acquisition of 4 volumes T1WI, flushing with 20 mL saline. Before dynamic imaging, baseline T1 mapping was performed using two flip angles (3°, 15°). T1 mapping [17] was done to convert the contrast-enhanced signal strength into concentration in the tissue.
DCE-MRI post-processing was carried out using Nordic ICE (Nordic Neuro Lab). Vascular input function was gained from the superior sagittal sinus [18]. Patlak model was applied to assess the subtle BBB leakage [19]. This approach obtained 3 parameters: area under the leakage curve (AUC), BBB leakage rate (Ktrans), and Vp (Fig. 1). Regions of interest (ROIs) were manually outlined according to Axial T2 FLAIR sequence. ROIs included 4 areas: deep grey matter (DGM), cortical grey matter (CGM), white matter hyperintensity (WMH), and normal-appearing white matter (NAWM) (Fig. 2). The ROIs of control subjects were similar in size and location to those of patients. ROIs were 5 mm2 in area. Medial temporal lobe atrophy (MTA) scale was used to measure the hippocampal atrophy on coronal T1-weighted MRI [20].
Fig. 1.
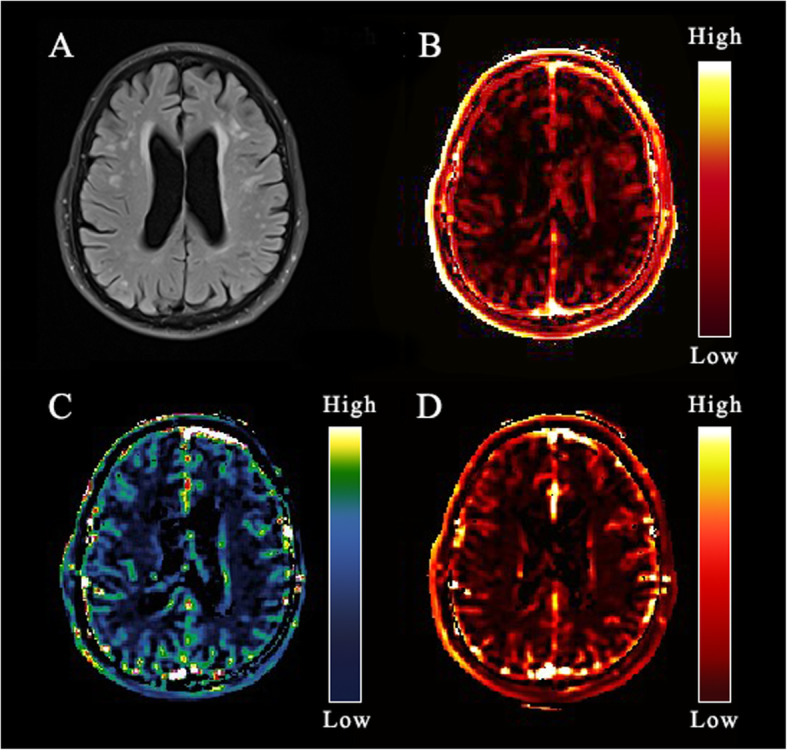
An example map. a Axial fluid-attenuated inversion recovery (FLAIR) image of a79-year-old man; b BBB leak (Ktrans) map; c Area under the leakage curve (AUC) map; d Fractional blood plasma volume (Vp) map
Fig. 2.
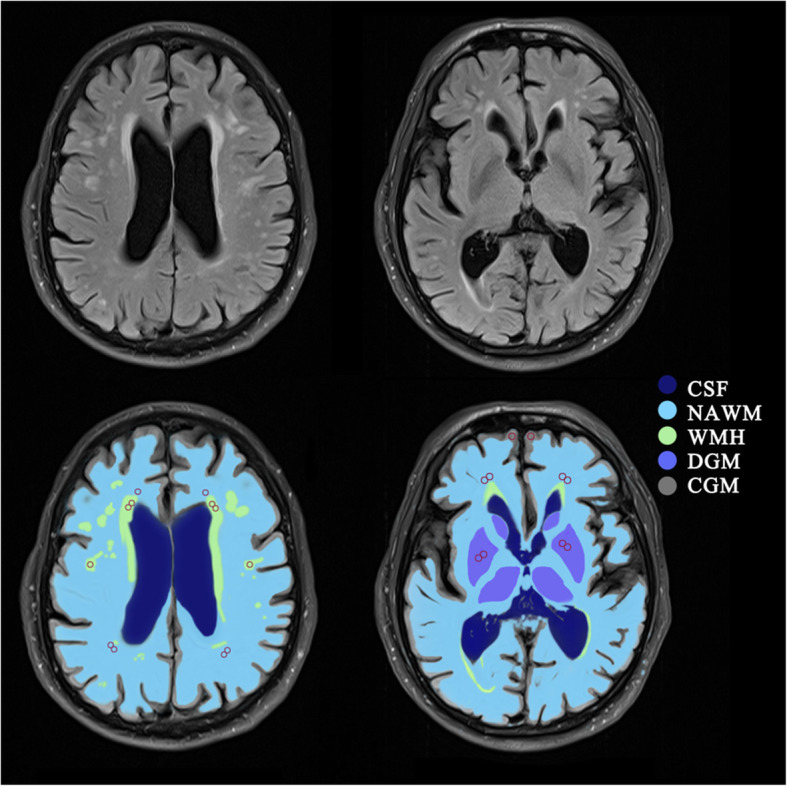
Regions of interest (ROIs). Example of the template for sampling ROIs (red circles) in normal-appearing white matter (NAWM), white matter hyperintensities (WMH), cortical gray matter (CGM), and deep gray matter (DGM). CSF, cerebrospinal fluid
Statistical analysis
Independent sample t-test, Fisher’s exact test, Mann-Whitney U test, and χ2 test were used to compare the characteristics of participants and cognitive function in the vMCI vs control group. BBB leakage and Vp of all ROIs between the two groups were compared using univariable linear regression analysis, multivariable linear regression analysis was performed to correct sex, age, and vascular risk factors. The relationship between cognitive function and BBB leakage was assessed using Spearman correlation analysis. Next, associations of BBB leakage with cognitive function were investigated using univariable linear regression analyses; then, multivariable linear regression analyses were performed to correct vascular risk factors, sex, age, and education years. P < 0.05 indicated statistical significance.
Results
Participants characteristics
Fifty subjects participated in this study, of which, three were excluded due to MRI contraindication, image artifacts, or brain tumor. Of the remaining 47 participants, 26 had vMCI, and 21 were sex- and age-matched controls. Analysis of baseline characteristics revealed significant differences in hypertension between the two groups (P < 0.001; Table 1).
Table 1.
Clinical characteristics of participants at baseline
| vMCI group N = 26 | Control N = 21 |
P | |
|---|---|---|---|
| Male, N (%) | 12(46.2%) | 8(38.1%) | 0.583 |
| Age, years | 71.04 ± 8.99 | 66.67 ± 9.19 | 0.108 |
| Education, years | 11.58 ± 3.18 | 11.62 ± 3. 49 | 0.966 |
| Vascular risk factors | |||
| BMI, kg/m2 | 26.02 ± 4.21 | 25.72 ± 2.42 | 0.774 |
| Hypertension, N(%) | 26(100.0%) | 11(52.4%) | <0.001 |
| Current Smoking, N(%) | 4(15.4%) | 3(14.3%) | 0.623 |
| T2DM, N(%) | 4(15.4%) | 3(14.3%) | 0.623 |
| Hyperlipidemia, N(%) | 20(76.9%) | 11(52.4%) | 0.081 |
| MTA scale | |||
| Right(median (range)) | 0(0,1) | 0(0,1) | 0.085 |
| Left(median (range)) | 0(0,1) | 0(0,1) | 0.085 |
| MoCA | 23.04 ± 2.32 | 28.00 ± 1.22 | <0.001 |
| MMSE | 26.31 ± 1.12 | 29.24 ± 0.70 | <0.001 |
Data are presented as mean ± SD, or counts (%)
Abbreviations: N number of persons, vMCI vascular mild cognitive impairment, BMI body mass index, T2DM type 2 diabetes mellitus, MTA medial temporal lobe atrophy, MoCA Montreal Cognitive Assessment, MMSE mini-mental state examination
Cognitive function
Analysis of cognitive functions revealed significant differences in MoCA, MMSE, information processing speed, attention executive function, spatial function, and language function between the two groups. The specific data are shown in Table 2.
Table 2.
Cognitive function in different domains of participants
| vMCI group N = 26 |
Control N = 21 |
P | |
|---|---|---|---|
| Overall cognitive function | |||
| MMSE | 26.31 ± 1.12 | 29.24 ± 0.70 | <0.001 |
| MoCA | 23.04 ± 2.32 | 28.00 ± 1.22 | <0.001 |
| Memory | |||
| Delayed recall | 4.62 ± 0.50 | 4.86 ± 0.36 | 0.059 |
| DST-forward | 7.85 ± 0.78 | 8.33 ± 0.97 | 0.063 |
| DST-backward | 3.77 ± 0.65 | 4.10 ± 0.70 | 0.106 |
| Information processing speed | |||
| TMT-A | 59.23 ± 10.88 | 39.81 ± 9.11 | <0.001 |
| Attention | |||
| SDMT | 31.81 ± 4.45 | 39.57 ± 6.52 | <0.001 |
| Executive function | |||
| TMT B-A | 85.96 ± 31.97 | 56.71 ± 19.54 | 0.001 |
| SCWT C-B | 68.31 ± 24.81 | 49.81 ± 21.02 | 0.009 |
| Spatial function | |||
| Clock Drawing Test | 3.31 ± 0.55 | 3.81 ± 0.40 | 0.001 |
| Language function | |||
| VFT | 42.38 ± 10.09 | 51.43 ± 7.21 | 0.001 |
Data are presented as mean ± SD, or counts (%)
Abbreviations: N number of persons, vMCI mild vascular cognitive impairment, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, DST digit span test, SDMT symbol digital modalities test, SCWT stroop color word test, CDT clock drawing test, VFT verbal fluency test
Correlation between BBB leakage and vMCI
Quantitative analysis and comparison results between the two groups are shown in Table 3. Univariable and multivariable (corrected for vascular risk factors, sex, and age) linear regression analyses showed that participants in vMCI group had higher BBB leakage rate in all ROIs and higher BBB leakage volume in NAWM and WMH (Table 3).
Table 3.
Leakage rate, area under the leakage curve, and fractional blood plasma volume in patients with vascular mild cognitive impairment (vMCI) and controls
| vMCI group | Control | Univariable a | Multivariable b | |||
|---|---|---|---|---|---|---|
| β P | β P | |||||
| NAWM | ||||||
| Ktrans (10− 4 min− 1) | 0.31 ± 0.14 | 0.20 ± 0.17 | − 0.109 | 0.016 | − 0.109 | 0.016 |
| AUC | 4.41 ± 1.04 | 3.52 ± 1.20 | −0.886 | 0.008 | − 0.888 | 0.006 |
| Vp (10–2) | 4.76 ± 1.97 | 7.51 ± 2.33 | 2.751 | <0.001 | 2.751 | <0.001 |
| WMH | ||||||
| Ktrans (10−4 min−1) | 0.56 ± 0.24 | 0.29 ± 0.18 | −0.275 | <0.001 | −0.275 | <0.001 |
| AUC | 6.02 ± 1.77 | 4.13 ± 1.25 | −1.896 | <0.001 | −1.896 | <0.001 |
| Vp (10−2) | 8.43 ± 4.39 | 10.49 ± 5.04 | 2.058 | 0.129 | 0.218 | 0.115 |
| CGM | ||||||
| Ktrans (10−4 min−1) | 1.57 ± 0.70 | 0.93 ± 0.56 | −0.642 | 0.001 | −0.642 | 0.001 |
| AUC | 17.90 ± 4.37 | 16.10 ± 5.76 | −1.798 | 0.217 | 0.453 | 0.800 |
| Vp (10−2) | 20.92 ± 7.97 | 30.12 ± 9.73 | 9.192 | 0.001 | 9.170 | <0.001 |
| DGM | ||||||
| Ktrans (10−4 min−1) | 0.99 ± 0.50 | 0.54 ± 0.35 | −0.442 | 0.001 | −0.442 | 0.001 |
| AUC | 11.44 ± 2.11 | 10.24 ± 3.43 | −1.200 | 0.123 | 0.122 | 0.429 |
| Vp (10−2) | 15.36 ± 5.51 | 19.79 ± 7.10 | 4.434 | 0.017 | 4.445 | 0.009 |
Data are presented as mean ± SD
Abbreviations: NAWM normal-appearing white matter, WMH white matter hyperintensities, CGM cortex grey matter, DGM deep grey matter, Ktrans leakage rate, AUC area under the leakage curve, Vp fractional blood plasma volume
Correlation between fractional blood plasma volume and vMCI
Univariable and multivariable (corrected for vascular risk factors, sex, and age) linear regression analyses found that the vMCI group had lower Vp in DGM, CGM, and NAWM (Table 3).
Correlation between BBB leakage and cognitive function
Spearman correlation analysis showed that BBB leakage rate and leakage volume was negatively correlated with MoCA in all ROIs. Multivariable linear regression analysis revealed that the MoCA scores decreased with the increase of leakage rate in WMH to correct sex, age, vascular risk factors, and education years (Table 4).
Table 4.
Association of leakage rate, area under the leakage curve, and fractional blood plasma volume with MoCA
| Spearman correlation | Univariable a | Multivariable b | ||||
|---|---|---|---|---|---|---|
| r | P | β | P | β | P | |
| NAWM | ||||||
| Ktrans (10−4 min−1) | −0.384 | 0.008 | −6.260 | 0.026 | −4.718 | 0.080 |
| AUC | −0.316 | 0.030 | −0.719 | 0.063 | −0.246 | 0.590 |
| Vp (10–2) | 0.425 | 0.003 | 0.463 | 0.009 | 0.439 | 0.011 |
| WMH | ||||||
| Ktrans (10−4 min−1) | −0.540 | <0.001 | −6.205 | <0.001 | −4.363 | 0.010 |
| AUC | −0.551 | <0.001 | −0.780 | 0.002 | −0.511 | 0.063 |
| Vp (10–2) | 0.122 | 0.412 | 0.048 | 0.631 | −0.003 | 0.977 |
| CGM | ||||||
| Ktrans (10−4 min−1) | −0.425 | 0.003 | −1.214 | 0.061 | −0.571 | 0.358 |
| AUC | −0.264 | 0.073 | −0.140 | 0.115 | 0.011 | 0.913 |
| Vp (10–2) | 0.443 | 0.002 | 0.129 | 0.003 | 0.132 | 0.004 |
| DGM | ||||||
| Ktrans (10−4 min−1) | −0.437 | 0.002 | −2.322 | 0.011 | −1.346 | 0.132 |
| AUC | −0.288 | 0.049 | −0.317 | 0.054 | 0.006 | 0.971 |
| Vp (10–2) | 0.267 | 0.070 | 0.085 | 0.237 | 0.056 | 0.449 |
Abbreviations: MoCA Montreal Cognitive Assessment, NAWM normal-appearing white matter, WMH white matter hyperintensities, CGM cortex grey matter, DGM deep grey matter, Ktrans leakage rate, AUC area under the leakage curve, Vp fractional blood plasma volume
aUnivariable linear regression analysis with MoCA as dependent variable, and Ktrans, AUC, and Vp respectively, as independent variable
bMultivariable linear regression analysis with MoCA as dependent variable, and Ktrans, AUC, Vp, age, sex, vascular risk factors, and education years as independent variables
β, Unstandardized regression coefficient
Discussion
In this study, it was found that BBB leakage rate rose in WMH, NAWM, CGM, and DGM in vMCI patients compared with the controls. Additionally, leakage volume in vMCI patients also increased in NAWM and WMH relative to controls. MoCA was negatively correlated with the leakage rate in WMH after correcting sex, age, vascular risk factors, and education years. These results indicated that BBB leakage was elevated widespread in brain parenchyma in vMCI patients and was independently related to cognitive decline.
DCE-MRI, as the most reliable noninvasive quantitative neuroimaging method to quantify BBB leakage in vivo [21],has been used to show BBB breakdown in mild cognitive impairment patients in some studies, but only a few studies focused simply on vascular factor. One study paid attention to the white matter area and found evaluated BBB permeability in white matter in VCI using DCE-MRI [13], but failed to quantify BBB permeability in grey matter which was also involved in VCI [22, 23]. A recent study found the increase of BBB leakage volume in CGM, WMH, and NAWM in vMCI and recent small subcortical infarct patients. In contrast, no differences were found in leakage rate [12], which was inconsistent with the results of this study. The disparity may be caused by different participant selection standard that only patients with vMCI diagnosed according to both cognitive function and MRI findings were included, as well as different image acquisition and post-processing methods.
It was found that BBB leakage rate increased widespread in the brain in vMCI patients and cognitive function decreased with the increase of BBB permeability, which coincided with previous research hypotheses, and may be caused by the neurovascular unit (NVU) disruption [9, 24]. Endothelial cells are the most important NVU component, and their dysfunction usually occurs first [25]. Tight junctions (TJs) are located between endothelial cells, and their disruption allows paracellular diffusion of water-soluble molecules from the blood into brain [26], upsetting brain environment homeostasis and impairing BBB function in vMCI patients [27, 28]. These changes are widely distributed in the brain tissue, rather than limited. A recent study found that BBB damage was an early initiating factor of cognitive dysfunction, which was independent of Aβ and tau protein [7]. Alterations in cerebral microvascular structure and neurovascular coupling dysfunction can lead to micro-vascular thrombosis and BBB dysfunction [29, 30]. This process may trigger a series of biochemical events, including microglial and astroglial activation, deposition of blood-borne proteins in the brain, release of pro-inflammatory neurotoxic cytokines, edema, and hemorrhage, eventually leading to neuronal degeneration and cognitive decline [31–33].
VMCI patients had impaired cognitive domains in information processing speed, attention executive function, spatial function, and language function rather than memory. This is in agreement with previous study [34]. And BBB dysfunctions was only correlated with MOCA rather than MMSE. A previous study exhibited that the MoCA was more sensitive than MMSE in the assessment of vMCI, and it had preferential sensitivity to early screening of VCI and evaluation of global cognitive function [35]. In addition, the MOCA only decreased with the increase of leakage rate in WMH, which was consistent with the result of a previous study [12]. A possible reason was that BBB breakdown may result in deposition of harmful substances. It has been found that WMH can mediates iron deposition, thus leading to cognitive impairment [36].
The study also revealed that vMCI patients displayed lower fractional blood plasma volume in CGM, DGM, and NAWM compared with control participants, indicating cerebral vascular hypoperfusion throughout the brain (including white and grey matter) in vMCI patients, which was consistent with the result of a previous study using DCE-MRI in cSVD [11]. It may be caused by structural abnormalities of blood vessels, including narrowing of the lumen and thickening of the wall due to hyaline arteriolosclerosis [37]. Moreover, reduced vascular density and cerebral vascular reactivity would also lead to hypoperfusion [38, 39]. Microvascular endothelial cells can release vasoactive substances to modulate vessel tone and regulate other functional non-vascular cells [40], while pericytes degeneration changes energy matrix transfer to neurons, reducing of cerebral blood flow (CBF) response in brain activation [41]. Changes of these cerebral vessels and local inflammation induced by ischemia may aggravate white matter damage and cognitive dysfunction [38, 42].
However, WMH was not involved, which was consistent with the results of previous studies that CBF was not associated with total WMH volume at baseline [43]. As speculated, the compensatory effect of capillaries may lead to increased local perfusion in WMH. A longitudinal study found that white matter area with low CBF progressed to new WMH during follow-up [44], suggesting that white matter hypoperfusion may contribute to disease progression.
In addition, vMCI is different from the non-amnestic mild cognitive impairment(MCI). MCI is divided into amnestic MCI and non-amnestic MCI with the subtypes of single or multiple domain impaired classification. Both multidomain non-amnestic MCI and multidomain amnestic MCI may be vMCI which is identified based on the evidence of presumed vascular etiology(See Supplementary Figure 1, Additional file 2).
Although this study was limited by its small sample size, significant differences were revealed, indicating the sensibility of the method. It was also limited by the lack of neuropathological biomarkers for indicating increased BBB permeability. However, the results may be confounded by the differences in transport patterns between gadolinium contrast agents and neurobiological markers. Furthermore, the scan time was a bit short, but motion artifacts were reduced, while image signal-to-noise ratio was improved for the application of the 64-channel coil, and significant differences were obtained after multiple measurements, indicating that the method was feasible. Moreover, it is unsure whether the control group will have cognitive impairment in the future, and the control group will be follow up in further study. Finally, no causal relationship between BBB damage and cognitive function was established in this study, which should be further investigated through longitudinal studies.
Conclusions
The current study found that BBB leakage was higher in both grey matter and white matter in vMCI patients, and cognitive function decreased with the increase of BBB leakage in WMH, which indicated that generalized BBB disruption in vMCI patients could contribute to the pathophysiological mechanisms of impaired cognitive function.
Supplementary Information
Additional file 1: Supplementary Table 1. Structural MRI scanning parameters.
Additional file 2: Supplementary Figure 1. How to distinguish different subtypes of MCI and how they can be paired with possible etiologies. This is referenced from Petersen RC: Mild Cognitive Impairment. Continuum (Minneap Minn) 2016, 22(2 Dementia):404–418.
Acknowledgements
Not applicable.
Abbreviations
- AUC
Area under the leakage curve
- BBB
Blood-brain barrier
- CGM
Cortical gray matter
- CSF
Cerebrospinal fluid
- cSVD
Cerebral small vessel disease
- CBF
Cerebral blood flow
- CDT
Clock drawing test
- DCE-MRI
Dynamic contrast-enhanced-magnetic resonance imaging
- DGM
Deep gray matter
- DST
Digit span test
- FOV
Field of view
- MCI
Mild cognitive impairment
- MoCA
Montreal Cognitive Assessment
- MTA
Medial temporal lobe atrophy
- NAWM
Normal-appearing white matter
- NVU
Neurovascular unit
- ROIs
Regions of interest
- SDMT
Symbol digital modalities test
- SCWT
Stroop color word test
- TMT
Trail-making test
- TR
Repetition time
- TE
Echo time
- TJs
Tight junctions
- vMCI
Vascular mild cognitive impairment
- Vp
Fractional blood plasma volume
- VCI
Vascular cognitive impairment
- VFT
Verbal fluency test
- WMH
White matter hyperintensities
Authors’ contributions
Study design: ML and TJ. Data collection and analysis: ML, YL, and LZ. The drafting of the manuscript: ML and YL. Manuscript revision: ML, YL, LZ, WH, and TJ. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All data derived from this study are available upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethical clearance for this study was given by the institutional ethics committee of Beijing Chao-Yang Hospital, Capital Medical University. All participants provided signed informed consent before participating in the study. WH have ruled that all patients and participants have been deemed capable of ethically and medically consenting for their participation in the research presented in this manuscript.
Consent for publication
Not applicable.
Competing interests
All authors state that there is no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Man Li, Email: pkumanli@163.com.
Yue Li, Email: 9119yueyue@sina.com.
Long Zuo, Email: zuolonglotus@163.com.
Wenli Hu, Email: wenlihu3366@126.com.
Tao Jiang, Email: jiangtao@bjcyh.com.
References
- 1.van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, Scheltens P. Vascular cognitive impairment. Nat Rev Dis Primers. 2018;4(1):18003. doi: 10.1038/nrdp.2018.3. [DOI] [PubMed] [Google Scholar]
- 2.Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011-2013. Alzheimer’s Dement. 2017;13(1):28–37. doi: 10.1016/j.jalz.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia J, Zhou A, Wei C, Jia X, Wang F, Li F, Wu X, Mok V, Gauthier S, Tang M, Chu L, Zhou Y, Zhou C, Cui Y, Wang Q, Wang W, Yin P, Hu N, Zuo X, Song H, Qin W, Wu L, Li D, Jia L, Song J, Han Y, Xing Y, Yang P, Li Y, Qiao Y, Tang Y, Lv J, Dong X. The prevalence of mild cognitive impairment and its etiological subtypes in elderly Chinese. Alzheimer’s Demen. 2014;10(4):439–447. doi: 10.1016/j.jalz.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg GA, Bjerke M, Wallin A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke. 2014;45(5):1531–1538. doi: 10.1161/STROKEAHA.113.004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wharton SB, Simpson JE, Brayne C, Ince PG. Age-associated white matter lesions: the MRC cognitive function and ageing study. Brain Pathol. 2015;25(1):35–43. doi: 10.1111/bpa.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joutel A, Chabriat H. Pathogenesis of white matter changes in cerebral small vessel diseases: beyond vessel-intrinsic mechanisms. Clin Sci. 2017;131(8):635–651. doi: 10.1042/CS20160380. [DOI] [PubMed] [Google Scholar]
- 7.Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourassa P, Tremblay C, Schneider JA, Bennett DA, Calon F. Beta-amyloid pathology in human brain microvessel extracts from the parietal cortex: relation with cerebral amyloid angiopathy and Alzheimer's disease. Acta Neuropathol. 2019;137(5):801–823. doi: 10.1007/s00401-019-01967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowman GL, Dayon L, Kirkland R, Wojcik J, Peyratout G, Severin IC, Henry H, Oikonomidi A, Migliavacca E, Bacher M, Popp J. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimer’s Demen. 2018;14(12):1640–1650. doi: 10.1016/j.jalz.2018.06.2857. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Golob EJ, Su MY. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J Magnet Resonan Imaging. 2006;24(3):695–700. doi: 10.1002/jmri.20669. [DOI] [PubMed] [Google Scholar]
- 11.Wong SM, Jansen JFA, Zhang CE, Hoff EI, Staals J, van Oostenbrugge RJ, Backes WH. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology. 2019;92(15):e1669–e1677. doi: 10.1212/WNL.0000000000007263. [DOI] [PubMed] [Google Scholar]
- 12.Zhang CE, Wong SM, van de Haar HJ, Staals J, Jansen JF, Jeukens CR, Hofman PA, van Oostenbrugge RJ, Backes WH. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88(5):426–432. doi: 10.1212/WNL.0000000000003556. [DOI] [PubMed] [Google Scholar]
- 13.Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, Grossetete M, Shah NJ, Wills J, Qualls C, Rosenberg GA. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42(8):2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sachdev P, Kalaria R, O'Brien J, Skoog I, Alladi S, Black SE, Blacker D, Blazer DG, Chen C, Chui H, Ganguli M, Jellinger K, Jeste DV, Pasquier F, Paulsen J, Prins N, Rockwood K, Roman G, Scheltens P, Internationlal Society for Vascular Behavioral and Cognitive Disorders Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28(3):206–218. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skrobot OA, Black SE, Chen C, DeCarli C, Erkinjuntti T, Ford GA, Kalaria RN, O'Brien J, Pantoni L, Pasquier F, Roman GC, Wallin A, Sachdev P, Skoog I, VICCCS group. Taragano FE, Kril J, Cavalieri M, Jellinger KA, Kovacs GG, Engelborghs S, Lafosse C, Bertolucci PH, Brucki S, Caramelli P, de Toledo Ferraz Alves TC, Bocti C, Fulop T, Hogan DB, Hsiung GR, Kirk A, Leach L, Robillard A, Sahlas DJ, Guo Q, Tian J, Hokkanen L, Jokinen H, Benisty S, Deramecourt V, Hauw J, Lenoir H, Tsatali M, Tsolaki M, Sundar U, Coen RF, Korczyn AD, Altieri M, Baldereschi M, Caltagirone C, Caravaglios G, di Carlo A, di Piero V, Gainotti G, Galluzzi S, Logroscino G, Mecocci P, Moretti DV, Padovani A, Fukui T, Ihara M, Mizuno T, Kim SY, Akinyemi R, Baiyewu O, Ogunniyi A, Szczudlik A, Bastos-Leite AJ, Firmino H, Massano J, Verdelho A, Kruglov LS, Ikram MK, Kandiah N, Arana E, Barroso-Ribal J, Calatayud T, Cruz-Jentoft AJ, López-Pousa S, Martinez-Lage P, Mataro M, Börjesson-Hanson A, Englund E, Laukka EJ, Qiu C, Viitanen M, Biessels GJ, de Leeuw FE, den Heijer T, Exalto LG, Kappelle LJ, Prins ND, Richard E, Schmand B, van den Berg E, van der Flier WM, Bilgic B, Allan LM, Archer J, Attems J, Bayer A, Blackburn D, Brayne C, Bullock R, Connelly PJ, Farrant A, Fish M, Harkness K, Ince PG, Langhorne P, Mann J, Matthews FE, Mayer P, Pendlebury ST, Perneczky R, Peters R, Smithard D, Stephan BC, Swartz JE, Todd S, Werring DJ, Wijayasiri SN, Wilcock G, Zamboni G, Au R, Borson S, Bozoki A, Browndyke JN, Corrada MM, Crane PK, Diniz BS, Etcher L, Fillit H, Greenberg SM, Grinberg LT, Hurt SW, Lamar M, Mielke M, Ott BR, Perry G, Powers WJ, Ramos-Estebanez C, Reed B, Roberts RO, Romero JR, Saykin AJ, Seshadri S, Silbert L, Stern Y, Zarow C, Ben-Shlomo Y, Passmore AP, Love S, Kehoe PG. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the vascular impairment of cognition classification consensus study. Alzheimer’s Dement. 2018;14(3):280–292. doi: 10.1016/j.jalz.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Moleiro C, Madureira S, Verdelho A, Ferro JM, Poggesi A, Chabriat H, Erkinjuntti T, Fazekas F, Hennerici M, O'Brien J, Pantoni L, Salvadori E, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D, LADIS Study Confirmatory factor analysis of the neuropsychological assessment battery of the LADIS study: a longitudinal analysis. J Clin Exp Neuropsychol. 2013;35(3):269–278. doi: 10.1080/13803395.2013.770822. [DOI] [PubMed] [Google Scholar]
- 17.Larsson HB, Courivaud F, Rostrup E, Hansen AE. Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magn Reson Med. 2009;62(5):1270–1281. doi: 10.1002/mrm.22136. [DOI] [PubMed] [Google Scholar]
- 18.Lavini C, Verhoeff JJ. Reproducibility of the gadolinium concentration measurements and of the fitting parameters of the vascular input function in the superior sagittal sinus in a patient population. Magn Reson Imaging. 2010;28(10):1420–1430. doi: 10.1016/j.mri.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Heye AK, Culling RD, Valdes Hernandez Mdel C, Thrippleton MJ, Wardlaw JM. Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. NeuroImage Clin. 2014;6:262–274. doi: 10.1016/j.nicl.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheltens P, Launer LJ, Barkhof F, Weinstein HC, van Gool WA. Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol. 1995;242(9):557–560. doi: 10.1007/BF00868807. [DOI] [PubMed] [Google Scholar]
- 21.Smith EE, Beaudin AE. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol. 2018;31(1):36–43. doi: 10.1097/WCO.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 22.Mufson EJ, Binder L, Counts SE, DeKosky ST, de Toledo-Morrell L, Ginsberg SD, Ikonomovic MD, Perez SE, Scheff SW. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol. 2012;123(1):13–30. doi: 10.1007/s00401-011-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smallwood A, Oulhaj A, Joachim C, Christie S, Sloan C, Smith AD, Esiri M. Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the Oxford project to investigate memory and ageing (OPTIMA) cohort. Neuropathol Appl Neurobiol. 2012;38(4):337–343. doi: 10.1111/j.1365-2990.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 24.Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, Schmidt MHH. Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol. 2016;131(3):347–363. doi: 10.1007/s00401-015-1524-y. [DOI] [PubMed] [Google Scholar]
- 25.Rajani RM, Quick S, Ruigrok SR, Graham D, Harris SE, Verhaaren BFJ, Fornage M, Seshadri S, Atanur SS, Dominiczak AF, Smith C, Wardlaw JM, Williams A. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci Transl Med. 2018;10(448):eaam9507. 10.1126/scitranslmed.aam9507. [DOI] [PubMed]
- 26.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 27.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimer’s Dement. 2015;11(6):710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett C, Samikkannu M, Mohammed F, Dietrich WD, Rajguru SM, Prasad A. Blood brain barrier (BBB)-disruption in intracortical silicon microelectrode implants. Biomaterials. 2018;164:1–10. doi: 10.1016/j.biomaterials.2018.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv J, Hu W, Yang Z, Li T, Jiang S, Ma Z, Chen F, Yang Y. Focusing on claudin-5: a promising candidate in the regulation of BBB to treat ischemic stroke. Prog Neurobiol. 2018;161:79–96. doi: 10.1016/j.pneurobio.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, Barnes SR, Daianu M, Ramanathan A, Go A, Lawson EJ, Wang Y, Mack WJ, Thompson PM, Schneider JA, Varkey J, Langen R, Mullins E, Jacobs RE, Zlokovic BV. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med. 2018;24(3):326–337. doi: 10.1038/nm.4482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Nordlund A, Rolstad S, Klang O, Lind K, Hansen S, Wallin A. Cognitive profiles of mild cognitive impairment with and without vascular disease. Neuropsychology. 2007;21(6):706–712. doi: 10.1037/0894-4105.21.6.706. [DOI] [PubMed] [Google Scholar]
- 35.Koski L. Validity and applications of the Montreal cognitive assessment for the assessment of vascular cognitive impairment. Cerebrovasc Dis. 2013;36(1):6–18. doi: 10.1159/000352051. [DOI] [PubMed] [Google Scholar]
- 36.Valdes Hernandez M, Allerhand M, Glatz A, Clayson L, Munoz Maniega S, Gow A, Royle N, Bastin M, Starr J, Deary I, et al. Do white matter hyperintensities mediate the association between brain iron deposition and cognitive abilities in older people? Eur J Neurol. 2016;23(7):1202–1209. doi: 10.1111/ene.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koizumi K, Hattori Y, Ahn SJ, Buendia I, Ciacciarelli A, Uekawa K, Wang G, Hiller A, Zhao L, Voss HU, Paul SM, Schaffer C, Park L, Iadecola C. Apoepsilon4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat Commun. 2018;9(1):3816. doi: 10.1038/s41467-018-06301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marstrand JR, Garde E, Rostrup E, Ring P, Rosenbaum S, Mortensen EL, Larsson HB. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke. 2002;33(4):972–976. doi: 10.1161/01.STR.0000012808.81667.4B. [DOI] [PubMed] [Google Scholar]
- 40.Hu X, De Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res. 2017;120(3):449–471. doi: 10.1161/CIRCRESAHA.116.308427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagata K, Yamazaki T, Takano D, Maeda T, Fujimaki Y, Nakase T, Sato Y. Cerebral circulation in aging. Ageing Res Rev. 2016;30:49–60. doi: 10.1016/j.arr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62(2):127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 43.ten Dam VH, van den Heuvel DM, de Craen AJ, Bollen EL, Murray HM, Westendorp RG, Blauw GJ, van Buchem MA. Decline in total cerebral blood flow is linked with increase in periventricular but not deep white matter hyperintensities. Radiology. 2007;243(1):198–203. doi: 10.1148/radiol.2431052111. [DOI] [PubMed] [Google Scholar]
- 44.Bernbaum M, Menon BK, Fick G, Smith EE, Goyal M, Frayne R, Coutts SB. Reduced blood flow in normal white matter predicts development of leukoaraiosis. J Cereb Blood Flow Metab. 2015;35(10):1610–1615. doi: 10.1038/jcbfm.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Structural MRI scanning parameters.
Additional file 2: Supplementary Figure 1. How to distinguish different subtypes of MCI and how they can be paired with possible etiologies. This is referenced from Petersen RC: Mild Cognitive Impairment. Continuum (Minneap Minn) 2016, 22(2 Dementia):404–418.
Data Availability Statement
All data derived from this study are available upon reasonable request.


