Abstract
Study objective:
Concurrent use of amphetamine-type stimulants among individuals with opioid use disorder can exacerbate social and medical harms, including overdose risk. The study evaluated rates of amphetamine-type stimulant use among patients with untreated opioid use disorder presenting at emergency departments in Baltimore, MD; New York, NY; Cincinnati, OH; and Seattle, WA.
Methods:
Emergency department (ED) patients with untreated opioid use disorder (N=396) and enrolled between February 2017 and January 2019 in a multisite hybrid type III implementation science study were evaluated for concurrent amphetamine-type stimulant use. Individuals with urine tests positive for methamphetamine, amphetamine, or both were compared with amphetamine-type stimulant–negative patients.
Results:
Overall, 38% of patients (150/396) were amphetamine-type stimulant positive; none reported receiving prescribed amphetamine or methamphetamine medications. Amphetamine-type stimulant–positive versus –negative patients were younger: mean age was 36 years (SD 10 years) versus 40 years (SD 12 years), 69% (104/150) versus 46% (114/246) were white, 65% (98/150) versus 54% (132/246) were unemployed, 67% (101/150) versus 49 (121/246) had unstable housing, 47% (71/150) versus 25% (61/245) reported an incarceration during 1 year before study admission, 60% (77/128) versus 45% (87/195) were hepatitis C positive, 79% (118/150) versus 47% (115/245) reported drug injection during 1 month before the study admission, and 42% (62/149) versus 29% (70/244) presented to the ED for an injury. Lower proportions of amphetamine-type stimulant–positive patients had cocaine-positive urine test results (33% [50/150] versus 52% [129/246]) and reported seeking treatment for substance use problems as a reason for their ED visit (10% [14/148] versus 19% [46/246]). All comparisons were statistically significant at P<.05 with the false discovery rate correction.
Conclusion:
Amphetamine-type stimulant use among ED patients with untreated opioid use disorder was associated with distinct sociodemographic, social, and health factors. Improved ED-based screening, intervention, and referral protocols for patients with opioid use disorder and amphetamine-type stimulant use are needed.
INTRODUCTION
Use of amphetamine-type stimulants is increasing in the United States and worldwide.1,2 They are synthetically manufactured psychostimulant substances, including pharmaceutically produced medications used for nonmedical reasons that are used orally, intranasally (insufflated), by inhaling vapors with different types of pipes, or by injection.3 In the United States, 1.9 million people aged 12 years or older used methamphetamine in 2018,1 and higher availability and prevalence of amphetamine-type stimulant use is observed in the Western and South Central regions.4 An increase in drug overdoses related to amphetamine-type stimulant use has also been observed in the United States in recent years.4 Literature suggests that amphetamine-type stimulant use among individuals with opioid use disorder may increase overall medical and social adverse effects of psychoactive substance use, interfere with treatment efforts, increase the rates of psychiatric and medical complications, and result in greater risks of infectious disease and other harms.2,5
Emergency departments (EDs) evaluate large numbers of patients presenting with a broad range of substance-use-related conditions, including opioid use disorder and amphetamine-type stimulant use, and provide treatment interventions and linkage to other components of the health care system. Screening, treatment, and referral protocols for tobacco and alcohol use problems, and more recently for opioid misuse and disorder, have been developed and successfully implemented in EDs.6 However, amphetamine-type stimulant use among ED patients with untreated opioid use disorder has not previously been systematically evaluated or reported in published literature, to our knowledge. To address this gap, data collected in the multisite National Institute on Drug Abuse Clinical Trials Network protocol 0069 (CTN-0069)7 enrolling a large cohort of ED patients with untreated opioid use disorder were evaluated to uncover potential corelates and characteristics of patients with concurrent amphetamine-type stimulant use. The aim of the current report is to inform future interventions for ED patients with untreated opioid use disorder and co-occurring amphetamine-type stimulant use.
MATERIALS AND METHODS
The detailed description of the study aims, design, methods, and participant recruitment procedures have been published elsewhere.7 Briefly, CTN-0069 screened English-speaking adults (18 years or older) for untreated opioid use disorder to enroll them in a hybrid type III implementation-effectiveness multisite clinical trial designed to determine the effectiveness of an implementation facilitation strategy to increase the rate of ED-initiated buprenorphine with referral for ongoing addiction treatment in actual ED settings.7 The CTN-0069 study research protocol was reviewed and approved by the Western Institutional Review Board. Between February 2017 and January 2019, a total of 27,748 patients presenting at 4 academic urban EDs in Baltimore, MD; New York, NY; Cincinnati, OH; and Seattle, WA, were screened. A total of 24,765 were ineligible to participate because of no opioid use in the previous 7 days, being prescribed opioid medications for pain, being currently enrolled in a formal addiction treatment, or other reasons; 219 refused to participate; and 396 were enrolled.
Patients who met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for moderate to severe untreated opioid use disorder, had a positive urine test result for opioids, and agreed to participate in the CTN-0069 study signed written informed consent and were further evaluated regarding their overall health status, current status and history of addiction treatment and other health care use, drug overdose history, and current and past substance use. Patients were enrolled by trained study research associates during 5 rotating 8-hour shifts covering all days of the week from 7 am to 11 pm during the course of their ED visit.
Urine samples of the enrolled patients were also tested for cocaine, benzodiazepine, barbiturates, tetrahydrocannabinol, ecstasy, amphetamine, and methamphetamine, using instant immunoassay strip tests; and self-reports on the use of these substances in the 7 days before study enrollment were collected with the timeline follow-back method. Urine toxicology testing for substance use and collection of additional self-reported data were not part of standard ED clinical care and were solely conducted as a part of the clinical trial research protocol.
Participants with urine samples testing positive for methamphetamine, amphetamine, or both were classified as amphetamine-type stimulant positive. Demographics and health behaviors were collected in face-to face interviews. The ED medical records of all participants were reviewed and information pertaining to the reasons for ED admission was extracted. Patients with amphetamine-type stimulant—positive and —negative results were compared with a focused exploratory research approach8 to identify key characteristics potentially associated with concurrent amphetamine-type stimulant use and factors that may exacerbate social and medical harms among ED patients with untreated opioid use disorder.
A limited set of variables informed by previous research as potentially relevant1-5,9 and available in the current data was evaluated, including sociodemographic factors, reasons for ED visit, substance use behaviors, and potential medical and social consequences of amphetamine-type stimulant use. The false discovery rate correction for multiple comparisons8 was applied to obtain the statistical significance levels of the study findings. Continuous variables were analyzed for statistical significance by the t test; the Pearson’s χ2 test was used with categoric variables.
RESULTS
Participants (N=396) had a mean age of 38.8 years (95% confidence interval 37.7 to 40.0 years), 69% (275/396) were men, 59% (235/396) were never married, 36% (142/396) had less than a high school education, 58% (230/396) were unemployed, 56% (222/396) reported current unstable housing, 33% (132/396) presented to the ED because of injury, and 16% (65/396) were identified as presenting to the ED with opioid overdose.
In the study cohort, 38% of participants (150/396) were amphetamine-type stimulant positive: 6 of 105 (6%) in Baltimore, MD; 3 of 42 (7%) in New York, NY; 39 of 121 (32%) in Cincinnati, OH; and 102 of 128 (80%) in Seattle, WA. No participant reported receiving prescribed amphetamine or methamphetamine medications. Of individuals who tested amphetamine-type stimulant positive, 74% (109/148) reported the use of methamphetamine and 36% (53/148) reported intravenous injection of methamphetamine in the 7 days before study admission (Table).
Table.
Characteristics of the study cohort
| Participant characteristics | Mean (SD) |
|---|---|
| Age, y | 38.8 (11.7) |
| % (n/N)) | |
| Men | 69 (275/396) |
| White | 55 (218/396) |
| Never married | 59 (235/396) |
| Less than high school education | 36 (142/396) |
| Unemployed | 58 (230/396) |
| With current unstable housing | 56 (222/396) |
| Reported incarceration during 1 y before study admission | 33 (132/395) |
| Have health insurance | 87 (346/396) |
| Presented in the ED because of injury | 33 (132/396) |
| Presented in the ED with opioid overdose | 16 (65/396) |
| Seeking substance use treatment during ED visit | 15 (60/394) |
| With ATS-positive urine test result | 38 (150/396) |
| ATS-positive patients who reported methamphetamine use during 7 days before study admission | 74 (109/148) |
| ATS-positive patients who reported intravenous injection of methamphetamine in the week before study admission | 36 (53/148) |
| With cocaine-positive urine test result | 45 (178/395) |
| With benzodiazepine-positive urine test result | 26 (101/396) |
| Reported injection drug use during 1 mo before study admission | 59 (233/395) |
| HCV positive | 41 (164/323) |
| HIV positive | 5 (19/349) |
ATS, Amphetamine-type stimulant.
Participants testing amphetamine-type stimulant positive compared with negative were younger: mean age was 36 years (95% confidence interval 34.7 to 38.0 years) versus 40 years (95% confidence interval 38.9 to 41.9 years), 69% (104/150) versus 46% (114/246) were white, 65% (98/150) versus 54% (132/246) were unemployed, 67% (101/150) versus 49 (121/246) had unstable housing, 47% (71/150) versus 25% (61/245) had an incarceration history during 1 year before study admission, 60% (77/128) versus 45% (87/195) were hepatitis C (HCV) positive, 79% (118/150) versus 47% (115/245) reported drug injection during 1 month before the study admission, and 42% (62/149) versus 29% (70/244) presented to the ED for an injury. Lower proportions of amphetamine-type stimulant–positive patients had a cocaine-positive urine test result (33% [50/150] versus 52% [129/246]) and reported seeking treatment for substance use problems as a reason for their ED visit (9.5% [14/148] versus 19% [46/246]) (Figure). All the above-mentioned comparisons were statistically significant at P<.05 with the false discovery rate correction.
Figure.
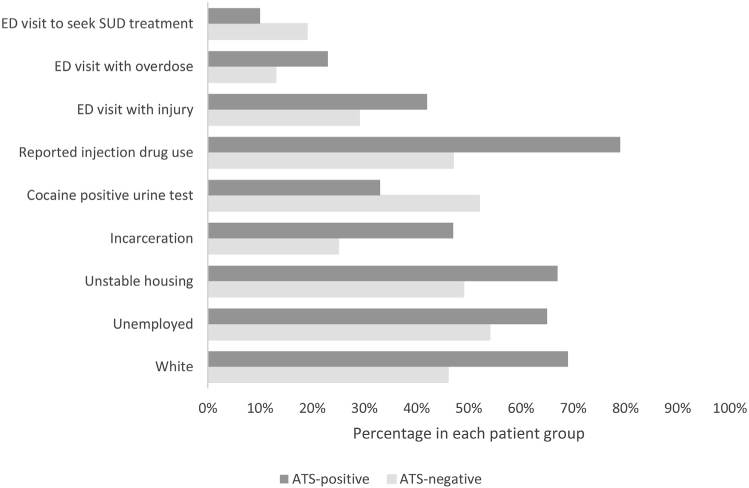
Differences between amphetamine-type stimulant-positive and -negative patients. False discovery rate–corrected significance level for ED visit with overdose was P=.06. All other false discovery rate–corrected significance levels were P<.05. SUD, Substance use disorder.
LIMITATIONS
The current findings are based on an exploratory analysis of data previously collected in a larger parent study and should be interpreted as preliminary. Substantial geographic differences in amphetamine-type stimulant availability and the prevalence of amphetamine-type stimulant use among the study sites resulted in very small numbers of amphetamine-type stimulant–positive patients in the East Coast EDs, limiting generalizability of the study findings. Small numbers of amphetamine-type stimulant–positive patients in Baltimore, MD, and New York, NY, EDs also precluded the possibility of disentangling region- or site-related patient characteristic from those related specifically to amphetamine-type stimulant use. Severity of amphetamine-type stimulant–related problems was not assessed, which limits the conclusions regarding specific intervention recommendations. Despite these limitations, the current study provides preliminary data for future epidemiologic and clinical research.
DISCUSSION
A substantial proportion of untreated opioid use disorder patients with amphetamine-type stimulant use was found in Cincinnati, OH, and Seattle, WA, with much lower rates of amphetamine-type stimulant use in in Baltimore, MD, and New York, NY. This geographic variation in the rates of amphetamine-type stimulant use found in the current study is consistent with patterns of geographic differences of amphetamine-type stimulant use prevalence reported previously.1,4
Patients with untreated opioid use disorder and amphetamine-type stimulant use in the current study represented a highly disadvantaged group at increased risk for adverse outcomes and facing challenging barriers to treatment. They were younger, had unstable housing, were mostly unemployed, and reported high rates of recent incarcerations. They also reported higher rates of injection drug use during 1 month before the study admission and had higher rates of HCV infection. Higher proportions of amphetamine-type stimulant–positive patients presented to the ED for an injury or with drug overdose (not significant after false discovery rate correction), and a lower proportion of them were seeking treatment for substance use problems. A recent study also reported that patients with co-occurring methamphetamine use and opioid use disorder were less likely to be retained in buprenorphine treatment.9 This increased risk for treatment discontinuation may be associated with unstable employment and housing and higher incarceration rates identified in the current study, as some of the amphetamine-type stimulant use correlates.
Currently, there are no standardized and broadly implemented ED-based screening, intervention, and referral protocols for patients with amphetamine-type stimulant use. Although rapid urine toxicology tests for amphetamine-type stimulant (ie, amphetamine and methamphetamine) are available, they are not routinely used, and detailed guidance on how to use and interpret such test results in the ED context is not available. Most likely, routine amphetamine-type stimulant testing would introduce additional burdens on patient evaluation in the ED and add economic costs, but they could be warranted if needed for aiding clinical decisions. However, routine collection of self-reported data on amphetamine-type stimulant use could be beneficial to ED treatment and referral for ongoing care. Identification and referral of individuals with amphetamine-type stimulant use may be challenged by the lack of efficacious and approved medications for treatment of amphetamine-type stimulant use disorder. Further rigorous research and intervention development is needed to address these challenges.
Nonetheless, the study findings suggest the need to evaluate multiple substance use among individuals presenting with either toxidrome opioid use disorder or amphetamine-type stimulant use. Despite significant barriers to providing effective interventions for concurrent opioid use disorder and amphetamine-type stimulant use, medications and behavioral or psychosocial interventions that could be potentially effective for treatment of amphetamine-type stimulant–related problems have been identified.10 Effective ED treatment approaches in the subset of individuals with opioid use disorder and concurrent amphetamine-type stimulant use will need to be augmented by additional medical or behavioral interventions and enhanced social support efforts. Providing medications for opioid use disorder with referral for ongoing treatment may not be sufficient for patients with concurrent opioid use disorder and amphetamine-type stimulant use.
Co-occurring amphetamine-type stimulant use among individuals with untreated opioid use disorder who present to the ED was associated with distinct sociodemographic and health factors. Improved ED-based identification according to self-report or urine tests and enhanced ED interventions and referral protocols for individuals with opioid use disorder and amphetamine-type stimulant use are needed to engage them in effective interventions and services.
Editor’s Capsule Summary.
What is already known on this topic
Individuals with opioid use disorder often use other agents that complicate care and recovery.
What question this study addressed
What are the characteristics of the group of emergency department (ED) patients who have untreated opioid use disorder and urine toxicology evidence of amphetamine-type co-use?
What this study adds to our knowledge
Of 396 ED patients at 4 self-selected urban EDs, 38% had concomitant amphetamine-type drugs detected. There was a complex pattern of differences between patients with and without amphetamine-type drugs in their urine.
How this is relevant to clinical practice
Many opioid use disorder patients co-use stimulants; how this affects care in the ED and afterward is unclear.
Acknowledgments
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. Funded by National Institute on Drug Abuse grants 5UG1DA015831-15 (CTN-0069) and K12 DA033312-06.
Footnotes
All authors attest to meeting the four ICMJE.org authorship criteria: (1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Trial registration number: NCT03023930
Contributor Information
Marek C. Chawarski, Department of Psychiatry, Yale School of Medicine, New Haven, CT; Department of Emergency Medicine, Yale School of Medicine, New Haven, CT.
Kathryn Hawk, Department of Emergency Medicine, Yale School of Medicine, New Haven, CT.
E. Jennifer Edelman, Department of Internal Medicine, Yale School of Medicine, New Haven, CT; Yale School of Public Health, New Haven, CT.
Patrick O’Connor, Department of Internal Medicine, Yale School of Medicine, New Haven, CT.
Patricia Owens, Department of Emergency Medicine, Yale School of Medicine, New Haven, CT.
Shara Martel, Department of Emergency Medicine, Yale School of Medicine, New Haven, CT.
Edouard Coupet, Jr, Department of Emergency Medicine, Yale School of Medicine, New Haven, CT.
Lauren Whiteside, Department of Emergency Medicine, University of Washington, Seattle, WA.
Judith I. Tsui, Department of Emergency Medicine, University of Washington, Seattle, WA.
Richard Rothman, Department of Emergency Medicine, University of Washington, Seattle, WA.
Ethan Cowan, Department of Emergency Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
Lynne Richardson, Department of Emergency Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
Michael S. Lyons, Department of Emergency Medicine, Icahn School of Medicine at Mount Sinai, New York, NY and University of Cincinnati Department of Emergency Medicine, Cincinnati, OH.
David A. Fiellin, Department of Emergency Medicine, Yale School of Medicine, New Haven, CT; Department of Internal Medicine, Yale School of Medicine, New Haven, CT; Yale School of Public Health, New Haven, CT.
Gail D’Onofrio, Department of Emergency Medicine, Yale School of Medicine, New Haven, CT; Yale School of Public Health, New Haven, CT.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration. Results From the 2018 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Center for Behavioral Health Statistics & Quality, Substance Abuse & Mental Health Services Administration; 2019. Available at: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2018R2/NSDUHDetailedTabs2018.pdf. Accessed July 21, 2020. [Google Scholar]
- 2.Ellis MS, Kasper ZA, Cicero TJ. Twin epidemics: the surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend. 2018;193:14–20. [DOI] [PubMed] [Google Scholar]
- 3.Chomchai CH, Chomchai S. Global patterns of methamphetamine use. Curr Opin Psychiatry. 2015;28:269–274. [DOI] [PubMed] [Google Scholar]
- 4.Artigiani EE, Hsu MH, McCandlish D, et al. Methamphetamine: A Regional Drug Crisis. College Park, MD: National Drug Early Warning System; 2018. [Google Scholar]
- 5.Pilowsky DJ, Wu LT, Burchett B, et al. Co-occurring amphetamine use and associated medical and psychiatric comorbidity among opioid-dependent adults: results from the Clinical Trials Network. Subst Abuse Rehabil. 2011;2:133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein SL, D’Onofrio G. Screening, treatment initiation, and referral for substance use disorders. Addict Sci Clin Pract. 2017;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Onofrio G, Edelman EJ, Hawk KF, et al. Implementation facilitation to promote emergency department–initiated buprenorphine for opioid use disorder: protocol for a hybrid type III effectiveness-implementation study (Project ED HEALTH). Implement Sci. 2019;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J,Zhang S. Multiple comparison procedures. JAMA. 2014;312:543–544. [DOI] [PubMed] [Google Scholar]
- 9.Tsui JI, Mayfield J,Speaker EC,et al. Association between methamphetamine use and retention among patients with opioid use disorderstreated with buprenorphine. J Subst Abuse Treat. 2020;109:80–85. [DOI] [PubMed] [Google Scholar]
- 10.UNODC. Treatment of stimulant use disorders: current practices and promising perspectives. Discussion paper. Available at: https://www.unodc.org/documents/drug-prevention-and-treatment/Treatment_of_PSUD_for_website_24.05.19.pdf. Accessed July 21, 2020. [Google Scholar]


