Abstract
We report 24 new records of the Brazilian cownose ray Rhinoptera brasiliensis outside its accepted geographic range. Sequencing of a 442-base pair portion of the mitochondrial NADH dehydrogenase subunit 2 gene for 282 Rhinoptera samples revealed eight records off the east coast of the USA and 16 from the eastern Gulf of Mexico. Both sexes of all life stages were documented in all seasons over multiple years in the Indian River and Lake Worth lagoons, Florida, indicating that their range extends further in the western North Atlantic than previously described.
Keywords: American cownose ray, elasmobranch, Indian River Lagoon, mitochondrial DNA, ND2, Rhinoptera bonasus
The cownose rays (Rhinopteridae) are comprised of eight morphologically similar species in the genus Rhinoptera (Last et al., 2016). Rhinopterids are circumglobally distributed, with two recognized species in the western Atlantic: the Endangered Brazilian cownose ray Rhinoptera brasiliensis Müller 1836 (Vooren & Lamónaca, 2004) and the Near Threatened American cownose ray Rhinoptera bonasus (Mitchill 1815) (Barker, 2006). Both species are benthopelagic and indigenous to tropical and temperate shallow waters along continental shelves (Last et al., 2016). Historically, tooth series counts were used to distinguish between these two species, despite ambiguity arising from overlap in series counts (Bigelow & Schroeder, 1953). Recent research documented changes in tooth morphology with age, making this characteristic unreliable (Jones et al., 2017). More reliable methods of distinguishing R. brasiliensis from R. bonasus, such as cranial anatomy and number of spiral valve lamellae, necessitate either dissection or advanced analytical techniques, such as computed tomography or magnetic resonance imaging (Jones et al., 2017).
In lieu of clear, external diagnostic characteristics to distinguish Rhinoptera spp., species identifications in the western North Atlantic have largely relied on assumed geographic ranges. This approach, however, grossly underestimated the range of R. brasiliensis in the western Atlantic. The range of R. bonasus extends from New England in the United States to northern Argentina (Last et al., 2016). Historically, R. brasiliensis was considered endemic to Brazil, restricted to the waters of southern Brazil (Barker, 2006; Bigelow & Schroeder, 1953). Recent literature extended the range of R. brasiliensis to include Central America (McEachran & Carvalho, 2002), the southern Gulf of Mexico (Palacios-Barreto et al., 2017), and the northern Gulf of Mexico (Jones et al., 2017), using a combination of morphological and molecular methods in species identifications. This range extension of R. brasiliensis substantially increased overlap in the ranges of R. brasiliensis and R. bonasus, and given the absence of clear field characteristics, the range of R. brasiliensis is still poorly understood. Given this uncertainty, existing datasets of Rhinoptera spp. in the western Atlantic may reflect a species complex rather than single species, which could lead to the use of spurious data from biological and ecological studies in their management. Here, we used genetic methods to identify Rhinoptera spp. to evaluate the extent of occurrence of R. brasiliensis beyond its currently described range in the western North Atlantic, specifically in the eastern Gulf of Mexico (hereafter eGOM) and along the east coast of the United States (hereafter Atlantic).
A total of 282 archived or opportunistically collected Rhinoptera spp. tissue samples collected between 2002 and 2018 were analysed from 12 locations in the eGOM and Atlantic, spanning from Santa Rosa Sound, Florida, to Chesapeake Bay, Virginia (Figure 1). Rhinoptera spp. were caught throughout the year, primarily by gillnet, and fin clips were collected and stored in 95% ethanol. Total genomic DNA was extracted from ~15 mg of tissue using a Qiagen DNeasy DNA extraction kit (Hilden, Germany), according to the manufacturer’s protocol, with the exception that tissue samples were digested overnight. A 442-base pair region of the mitochondrial (mtDNA) NADH dehydrogenase subunit 2 (ND2) gene was targeted using a forward primer (RhinND2F1: 5’-GAACCCYTTAATCCTCTYCATC-3’) designed by McDowell and Fisher (unpubl. data) and a reverse primer (RayND2R: 5’-GGATTGATAGTACGCCTATGG-3’) designed for this study. Polymerase chain reaction (PCR) mixtures contained 25–50 ng template DNA, 10 mM Taq buffer (Invitrogen, Carlsbad, California, USA), 1.5 mM MgCl2, 0.3 μM of each primer, 0.1 mM deoxynucleotide triphosphates (dNTP mix, Promega, Madison, Wisconsin, USA), 1 U of Taq polymerase (Invitrogen) and PCR-grade water for a final reaction volume of 25 μL. PCR cycling conditions consisted of an initial denaturation at 95°C for 5 min, then 35 cycles of 30 s denaturation at 95°C, 30 s annealing at 58°C, 30 s extension at 72°C, followed by a 5 min final extension at 72°C. Amplicons were cleaned using 3 μL of ExoSAP-IT (ThermoFisher, Waltham, Massachusetts, USA) and sequenced in the forward and reverse directions on an Applied Biosystems 3730XL DNA Analyser using a BigDye Terminator 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, California, USA) following the manufacturer’s protocol except that all sequences were run using half reactions.
FIGURE 1.
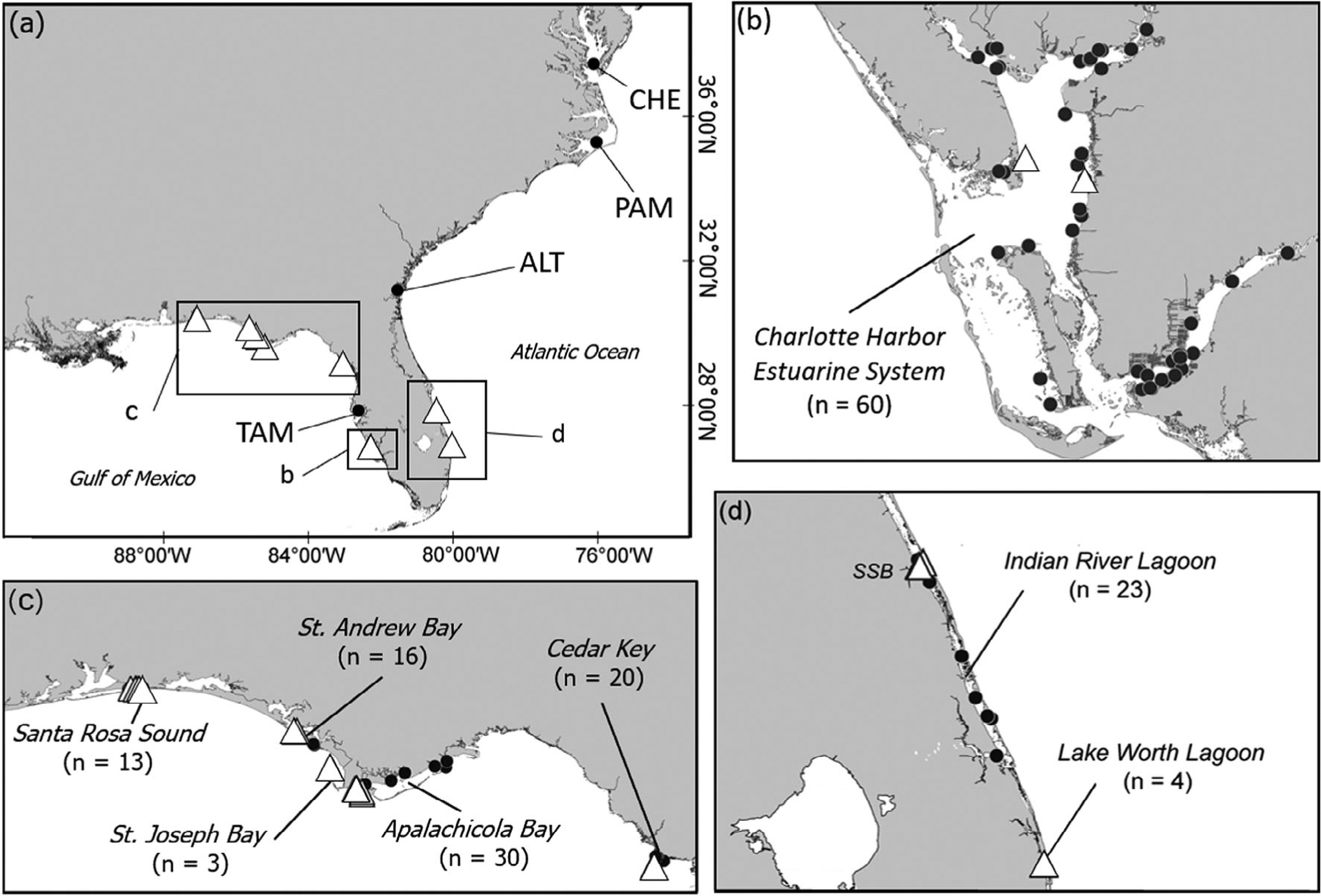
Rhinoptera spp. sampling sites in the western North Atlantic. (a) Triangles represent general locations where the Brazilian cownose ray R. brasiliensis Müller 1836 and the American cownose ray R. bonasus (Mitchill 1815) co-occurred and circles represent general locations where only R. bonasus occurred. TAM, Tampa Bay; n = 22; ALT, Altamaha River, n = 2; PAM, Pamlico Sound and its tributaries, n = 66; CHE, Chesapeake Bay, n = 23. For locations of co-occurrence, inset maps b–d show sites where each species was caught, with overall sample size for each location indicated. R. brasiliensis, triangles; R. bonasus, circles; SSB, St. Sebastian River
Consensus sequences were generated by aligning the forward and reverse sequences for each individual in CodonCode v 9.0.1 (CodonCode Corporation, Dedham, USA). Resultant haplotypes were compared to mtDNA ND2 haplotypes generated, using the methods described here, for 10 specimens each of R. brasiliensis and R. bonasus collected from Mississippi and Alabama waters (United States), which were verified using a suite of 21 external morphological measurements and genetic analysis of the cytochrome c oxidase subunit I (CO1) gene (see Jones et al., 2017). The Jukes–Cantor substitution model (Jukes & Cantor, 1969) was determined to be the nucleotide substitution model of best fit for the data in jModelTest 2 v 2.1.10 using Bayesian Information Criterion (BIC) values (Darriba et al., 2012). Phylogenetic relationships between these haplotypes were inferred using a maximum likelihood approach with 10,000 bootstrap replicates and the Jukes–Cantor substitution model in MEGA v X (Kumar et al., 2018), with the bat ray Myliobatis californica Gill 1865 (GenBank accession no. KM364985) serving as an outgroup. Relationships among haplotypes were estimated using the maximum parsimony method of Polzin and Daneshmand (2003) and visualized as a median-joining haplotype network using Network v 10.1.10 (Bandelt et al., 1999).
The 282 tissue samples revealed 14 haplotypes that formed two distinct genetic clades. Three haplotypes representing 24 individuals clustered together with the single haplotype (RBRA1) sequenced for the 10 verified R. brasiliensis (GenBank accession nos. MT410205–MT410207) and 11 haplotypes representing 258 individuals clustered with the single haplotype sequenced for the 10 verified R. bonasus (RBON1) (GenBank accession nos. MT410194–MT410204) (Supporting Information Figure S1). The sampled R. brasiliensis and R. bonasus haplotypes were differentiated by 5.43–7.24% sequence divergence, based on the number of base pair differences (Supporting Information Figure S2). In contrast, the maximum sequence differentiation between sampled haplotypes within each species was much less: 0.68% and 1.13% for R. brasiliensis and R. bonasus, respectively (Supporting Information Figure S2). The minimum sequence differentiations between M. californica and R. brasiliensis and R. bonasus were 13.80% and 12.90%, respectively.
The 24 genetically identified R. brasiliensis were collected from eight locations in the western North Atlantic; eight were from two estuaries in the Atlantic and 16 were from six estuaries in the eGOM (Figure 1). In the Atlantic, five R. brasiliensis were caught at the mouth of the St. Sebastian River in the Indian River Lagoon, Florida (hereafter IRL) and three were caught in Lake Worth Lagoon, Florida. In the eGOM, six were caught in Apalachicola Bay, five in Santa Rosa Sound, two in Charlotte Harbor and one in each of St. Andrew Bay, St. Joseph Bay and Cedar Key. Individuals included males and females, juveniles and adults, and were caught across all seasons in multiple years between 2003 and 2018 (Table 1). Evidence of reproductive habitat use in the Atlantic was identified by the presence of two particular R. brasiliensis near the mouth of the St. Sebastian River, a gravid female caught in summer (August) and a juvenile male caught in winter (January). None were identified from estuaries in the Atlantic north of the St. Sebastian River in the IRL, including the Altamaha River, Georgia (n = 2), Pamlico Sound and its tributaries, North Carolina (n = 66), and Chesapeake Bay, Virginia (n = 23) (Figure 1).
TABLE 1.
Biological details for the Brazilian cownose ray Rhinoptera brasiliensis Müller 1836 genetically identified via a 442-base pair fragment of the mitochondrial NADH dehydrogenase subunit 2 gene from sites off Florida in the eastern Gulf of Mexico (eGOM) and along the east coast of the USA (Atlantic)
| Region | Location | Date collected | Seasona | Sex | Life stageb | Disc width (cm) | Haplotype |
|---|---|---|---|---|---|---|---|
| eGOM | Santa Rosa Sound | 2013–2017 | NA | NA | NA | NA | RBRA1 |
| 2013–2017 | NA | NA | NA | NA | RBRA1 | ||
| 2013–2017 | NA | NA | NA | NA | RBRA1 | ||
| 2013–2017 | NA | NA | NA | NA | RBRA1 | ||
| 2013–2017 | NA | NA | NA | NA | RBRA1 | ||
| St. Andrew Bay | 25 October 2013 | Fall | F | Juvenile | 55.0 | RBRA1 | |
| St. Joseph Bay | 14 August 2012 | Summer | M | Adult | 90.0 | RBRA1 | |
| Apalachicola Bay | 20 June 2013 | Summer | F | Adult | 88.5 | RBRA1 | |
| 20 June 2013 | Summer | F | Adult | 71.5 | RBRA1 | ||
| 20 June 2013 | Summer | F | Juvenile | 49.0 | RBRA1 | ||
| 14 June 2012 | Summer | F | Juvenile | 48.0 | RBRA1 | ||
| 14 June 2012 | Summer | M | Juvenile | 40.0 | RBRA1 | ||
| 24 October 2013 | Fall | M | Juvenile | 49.0 | RBRA1 | ||
| Cedar Key | 6 December 2017 | Winter | F | Adult | 77.5 | RBRA3 | |
| Charlotte Harbor | 17 October 2003 | Fall | M | Juvenile | 62.5 | RBRA1 | |
| 15 December 2008 | Winter | M | Juvenile | 66.7 | RBRA1 | ||
| Atlantic | Lake Worth Lagoon | 14 February 2018 | Winter | M | Adult | 74.0 | RBRA1 |
| 14 February 2018 | Winter | F | Adult | 78.4 | RBRA1 | ||
| 14 February 2018 | Winter | F | Adult | 80.2 | RBRA1 | ||
| Indian River Lagoon | 12 January 2018 | Winter | M | Juvenile | 61.3 | RBRA1 | |
| 12 April 2017 | Spring | M | Adult | 87.0 | RBRA5 | ||
| 12 April 2017 | Spring | M | Adult | 87.5 | RBRA1 | ||
| 12 April 2017 | Spring | M | Adult | 85.1 | RBRA1 | ||
| 7 August 2017 | Summer | Fc | Adult | 93.2 | RBRA1 |
Note:Specific dates and biological details were not available (NA)
Spring, March–May; summer, June–August; fall, September–November; winter, December–February.
Life stage of R. brasiliensis was inferred from disc width at 100% maturity for both sexes (>71.2 cm disc width) of R. bonasus (Mitchill 1815) from Charlotte Harbor, Florida by Poulakis (2013).
Gravid.
The discovery of 24 R. brasiliensis based on mtDNA ND2 sequence data across sites in Florida waters further confirms its occurrence in the eGOM (see Carney et al., 2017; Jones et al., 2017; McDowell & Fisher, unpubl. data) and documents that the range extends further in the western North Atlantic than previously described, at least as far north as the St. Sebastian River in the IRL. Since mtDNA is maternally inherited, this finding assumes that these individuals are not hybrid crosses between female R. brasiliensis and male R. bonasus, which would require analysis of additional nuclear data. Hybridization has not yet been documented in cownose rays, but has been shown in freshwater rays (Potamotrygonidae, Crus et al., 2015) and fiddler rays (Rhinobatidae, Donnellan et al., 2015). While it is possible to detect patterns of introgression from mtDNA markers alone, robust evidence necessitates powerful datasets that typically use much larger DNA fragments (e.g., Rosenzweig et al., 2016).
These new records are part of a growing body of literature documenting the presence of R. brasiliensis in the western North Atlantic. Prior species accounts that used genetic identifications of R. brasiliensis in the Atlantic include four records with no life history data from Georgia in fall (October) 2007 (Quattro, unpubl. data), one of unknown size from North Carolina with an unknown capture date in 2008 (Naylor et al., 2012), and one from a deceased specimen of unknown size found on a beach in New Jersey in summer (August) 2017 (Stoeckle et al., 2020). There is one additional historic record from North Carolina, but since this identification was based on tooth series counts alone (Bigelow & Schroeder, 1953), it is not considered reliable. Despite these records, the Atlantic was not considered part of the accepted range of R. brasiliensis in subsequent accounts (e.g., Last et al., 2016), possibly because they were discounted as ‘vagrants’. The term vagrant was recently defined for elasmobranchs by Grant et al. (2019) as ‘individuals found outside of the species’ distribution, in a habitat not biologically utilized by the species’. Our work has shown the presence of both male and female R. brasiliensis of all life stages, documented across all seasons over multiple years as far north as the St. Sebastian River in the IRL in the Atlantic. This indicates that R. brasiliensis is unlikely to be a vagrant, at least in the Atlantic off Florida, and that this region is part of its range, although the northernmost extent of its range remains uncertain. Whether R. brasiliensis have long occurred in these waters but were obscured by the presence of morphologically similar R. bonasus, or this reflects a more recent range expansion, remains unknown.
Juvenile R. brasiliensis occurred near the mouth of the St. Sebastian River during winter, in St. Andrew and Apalachicola bays during summer and fall, and in Charlotte Harbor during fall and winter. The presence of juveniles in these estuaries along both coasts of Florida, as well as the presence of a gravid R. brasiliensis in the IRL during the time associated with their parturition (Rangel et al., 2017), suggests these bays may serve as nurseries. Juvenile R. bonasus are abundant in estuaries in the northern Gulf of Mexico and have been shown to tolerate low temperatures (e.g., <12°C, reported down to 8.3–9.1°C), potentially residing in estuaries over winter, and possibly until they reach maturity (Ajemian, 2011; Ajemian & Powers, 2016; Bigelow & Schroeder, 1953). Additional research is needed to better understand how and to what extent R. brasiliensis uses and relies on these estuarine habitats in the Atlantic and eGOM.
The opportunistic nature of the sampling regime in this study likely limited our understanding of the occurrence and frequency of R. brasiliensis throughout the study area. For example, for some locations four samples were collected at a single site in a single day and represented the same life stage, while elsewhere 52 samples were selected from archived samples to include all life stages, seasons and sexes over 9 years. Despite these differences, the presence of R. brasiliensis at locations outside of its accepted range, detected over multiple years, implies this species has a wider range than previously thought.
As documented here, the range of R. brasiliensis extends at least to the St. Sebastian River in the IRL in the Atlantic. More comprehensive, year-round surveys paired with genetic species identifications are still needed to clarify the northern extent of the range of R. brasiliensis in the Atlantic and seasonal patterns of occurrence in these waters. This range extension further increases range overlap between R. brasiliensis and R. bonasus, emphasizing the need to verify species identities using genetic methods prior to undertaking biological or ecological studies of either species. Furthermore, existing biological and relative abundance datasets require careful consideration as they may reflect a Rhinoptera species complex rather than single species. Future studies should use a suite of approaches, including tagging and molecular methods, to better understand and disentangle the biology and ecology of these morphologically similar, sympatric species.
Supplementary Material
ACKNOWLEDGEMENTS
This research was funded primarily by The University of Southern Mississippi and was supported by the Mississippi Institutional Development Award (IDeA) Network of Biomedical Research Excellence (INBRE), funded under IDeA number P20-GM103476 from the National Institutes of General Medical Sciences of the National Institutes of Health. Funding for sample collection from the Indian River Lagoon was provided by a Harbor Branch Oceanographic Institute Foundation Save Our Seas Specialty License Plate Program grant to MJA. Samples from the Charlotte Harbor estuarine system were collected as part of reproductive biology research on cownose rays and opportunistically during multiple projects: NA10NMF4720032, NA13NMF4720047, NA16NMF4720062. Sampling from Tampa Bay, Lake Worth Lagoon, and Cedar Key was supported by State of Florida saltwater recreational fishing licenses. Sample collection in Chesapeake Bay was funded by a California State University Program for Education & Research in Biotechnology (CSUPERB) travel grant to JDS and samples from the Pamlico Sound area were collected during sampling for projects funded through US Fish and Wildlife Service Sport Fish Restoration and Coastal Recreational Fishing License grants. Sample collection in St. Andrew Bay, St. Joseph Bay, Santa Rosa Sound, and Apalachicola Bay was accomplished by the National Marine Fisheries Service Panama City Laboratory’s GULFSPAN Survey. Funding for the GULFSPAN Survey is provided in part by the NOAA National Marine Fisheries Highly Migratory Species Division.
Thanks to Bob Fisher, George Trice Sr, George Trice Jr, Alia Court, Andrew Wooley, Rachel Scharer, Jackie DeAngelo, Amy Timmers, Charles Bangley, Christine Bedore, Jeff Eble, Michelle Edwards, Grace Roskar and Breanna DeGroot for assisting with tissue sample collection and subsampling. Thanks to Brian Kreiser for his assistance with early phylogeny construction.
Funding information
University of Southern Mississippi; Mississippi Institutional Development Award (IDeA) Network of Biomedical Research Excellence (INBRE), Grant/Award Number: P20-GM103476; National Institutes of General Medical Sciences of the National Institutes of Health; Harbor Branch Oceanographic Institute Foundation Save Our Seas Specialty License Plate Program; Charlotte Harbor Estuarine System, Grant/Award Numbers: NA10NMF4720032, NA13NMF4720047, NA16NMF4720062; State of Florida; California State University Program for Education & Research in Biotechnology (CSUPERB); US Fish and Wildlife Service Sport Fish Restoration and Coastal Recreational Fishing License; National Marine Fisheries Service Panama City Laboratory’s GULFSPAN Survey; NOAA National Marine Fisheries Highly Migratory Species Division; Univeristy of West Florida
Footnotes
ETHICS STATEMENT
Collection of tissue samples in all locations complied with animal welfare laws, guidelines and policies. Sample collection in the Indian River Lagoon, Florida was conducted under protocols approved by the Florida Atlantic University Institutional Animal Care and Use Committee (IACUC) (Animal Use Protocol #A16-16) and in accordance with federal and Florida state laws and regulations under the following permits: FWC Special Activity Licenses SAL-16-1785-SRP, SAL-17-1785-SRP, SAL-18-1785A-SRP and SAL-19-1785-SRP; Florida Department of Environmental Protection Florida Park Service Scientific Research Permits 07261610 and 07241710A; and US Fish and Wildlife Service Special Use Permits 41572-2016-04 and 41572-2017-07. Sample collections in St Andrew, St Joseph and Apalachicola bays as well as Santa Rosa Sound were permitted under Florida Fish and Wildlife Conservation Commission Special Activity Licenses SAL-11-1292-SRP, SAL-12-1292-SRP, SAL-13-1292-SRP, SAL-14-1292-SRP, SAL-15-1292-SRP and SAL-16-1292-SRP, SAL-13-1464-SR; Florida Park Service Permit number 14100111; and National Parks Service Gulf Islands Scientific Research and Collecting Permit GUIS-2013-SCI-0024. Sample collection in Chesapeake Bay, Virginia was conducted in accordance with the San Francisco State University Institutional Animal Care and Use Committee (IACUC) protocol A15-09R2. No permits were required for collection of tissue samples by researchers with Florida Fish and Wildlife Conservation Commission or the North Carolina Division of Marine Fisheries. Sample collection in Mississippi and Alabama was conducted under the University of Southern Mississippi IACUC permit numbers 11092216, 13101704, and 15101509.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- Ajemian MJ (2011). Foraging ecology of large benthic mesopredators: Effects of myliobatid rays on shellfish resources. (Doctoral thesis). University of South Alabama, Mobile, AL. [Google Scholar]
- Ajemian MJ, & Powers SP (2016). Seasonality and ontogenetic habitat partitioning of cownose rays in the northern Gulf of Mexico. Estuaries and Coasts, 39, 1234–1248. [Google Scholar]
- Bandelt HJ, Forster P, & Röhl A (1999). Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution, 16, 37–48. [DOI] [PubMed] [Google Scholar]
- Barker AS (2006). Rhinoptera bonasus. In IUCN Red List of Threatened Species 2006: e.T60128A12310195. Retrieved from: https://www.iucnredlist.org/species/60128/12310195
- Bigelow HB, & Schroeder WC (1953). Fishes of the western North Atlantic, Part 2. Sawfishes, guitarfishes, skates, rays, and chimaeroids. Memoir of the Sears Foundation for Marine Research Number 1 New Haven, CT: Yale University. [Google Scholar]
- Carney SL, McVeigh DM, Moss JB, Ferrier MD, & Morrissey JF (2017). Insights on mitochondrial genetic variation in Chesapeake Bay summer-resident cownose rays. Transactions of the American Fisheries Society, 146, 478–484. [Google Scholar]
- Crus VP, Vera M, Mendonca FF, Pardo BG, Martinez P, Oliveira C, & Foresti F (2015). First identification of interspecies hybridization in the freshwater stingrays Potamotrygon motoro and P. falkneri (Myliobatiformes, Potamotrygonidae). Conservation Genetics, 16, 241–245. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, & Posada D (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9, 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan SC, Foster R, Junge C, Huveneers C, Rogers P, Kilian A, & Bertozzi T (2015). Fiddling with the proof: the magpie fiddler ray is a colour pattern variant of the common southern fiddler ray (Rhinobatidae: Trygonorrhina). Zootaxa, 3981, 367–384. [DOI] [PubMed] [Google Scholar]
- Grant MI, Kyne PM, Simpfendorfer CA, White WT, & Chin A (2019). Categorising use patterns of non-marine environments by elasmobranchs and a review of their extinction risk. Reviews in Fish Biology and Fisheries, 29, 689–710. [Google Scholar]
- Jones CM, Hoffmayer ER, Hendon JM, Quattro JM, Lewandowski J, Roberts MA, … Márquez-Farías JF (2017). Morphological conservation of rays in the genus Rhinoptera (Elasmobranchii, Rhinopteridae) conceals the occurrence of a large batoid, Rhinoptera brasiliensis Müller, in the northern Gulf of Mexico. Zootaxa, 4286, 499–514. [Google Scholar]
- Jukes TH, & Cantor CR (1969). Evolution of protein molecules. In Munro HN (Ed.), Mammalian protein Metabolismprotein metabolism (pp. 21–132). New York, NY: Academic Press. [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, & Tamura K (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last PR, White WT, de Carvalho MR, Séret B, Stehmann MFW, & Naylor GJP (2016). Rays of the world. Clayton, VIC, Australia: CSIRO Publishing. [Google Scholar]
- McEachran JD, & de Carvalho MR (2002). Batoid fishes. In Carpenter KE (Ed.), The living marine resources of the western Central Atlantic. Volume 1: Introduction, molluscs, crustaceans, hagfishes, sharks, batoid fishes, and chimaeras. FAO species identification guide for fishery purposes (pp. 508–589). Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- Naylor GJP, Caira JN, Jensen K, Rosana KAM, White WT, & Last PR (2012). A DNA sequence-based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bulletin of the American Museum of Natural History, 367, 1–263. [Google Scholar]
- Palacios-Barreto P, Cruz VP, Foresti F, Rangel BS, Uribe-Alcocer M, & Díaz-Jaimes P (2017). Molecular evidence supporting the expansion of the geographical distribution of the Brazilian cownose ray Rhinoptera brasiliensis (Myliobatiformes: Rhinopteridae) in the western Atlantic. Zootaxa, 4341, 593–600. [DOI] [PubMed] [Google Scholar]
- Polzin T, & Daneshmand SV (2003). On Steiner trees and minimum spanning trees in hypergraphs. Operations Research Letters, 31, 12–20. [Google Scholar]
- Poulakis GR (2013). Reproductive biology of the cownose ray in the Charlotte Harbor estuarine system, Florida. Marine and Coastal Fisheries, 5, 159–173. [Google Scholar]
- Rangel BS, Cruz VP, Rodrigues A, Araujo MLG, Oliveira C, Foresti F, & Moreira RG (2017). Sympatric and syntopic occurrence of cownose rays: neonatal strategies for survival? Journal of Applied Ichthyology, 33, 542–545. [Google Scholar]
- Rosenzweig BK, Pease JB, Besansky NJ, & Hahn MW (2016). Powerful methods for detecting introgressed regions from population genomic data. Molecular Ecology, 25, 2387–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckle MY, Das Mishu M, & Charlop-Powers Z (2020). Improved environmental DNA reference library detects overlooked marine fishes in New Jersey, United States. Frontiers in Marine Science, 7, 226. [Google Scholar]
- Vooren CM, & Lamónaca AF (2004). Rhinoptera brasiliensis. In IUCN Red List of Threatened Species 2004: e.T44595A10912274. Retrieved from: https://www.iucnredlist.org/species/44595/10912274
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


