Abstract
Background:
Recent federal policy changes attempt to expand veterans’ access to providers outside the Department of Veterans Affairs (VA). Receipt of prescription medications across unconnected systems of care may increase the risk of unsafe prescribing – particularly in persons with dementia.
Objective:
To investigate the risk of dual healthcare system use on potentially unsafe medication (PUM) prescribing.
Design:
Retrospective cohort study.
Setting:
National VA outpatient care users in 2010, continuously enrolled in Medicare from 2007–2010. Dual system use and baseline risk factors in 2009 were used to predict PUM exposure in 2010.
Participants:
75 829 veterans with a dementia diagnosis prior to 2010; 80% VA only and 20% dual VA/Part D users.
Measurements:
Inverse propensity weighting was used to estimate the effect of dual system use versus VA only use for prescriptions on indicators of PUM prescribing: any prescription for a Healthcare Effectiveness Data and Information Set high risk medication in the elderly (PUM-HEDIS); any daily exposure to prescriptions with a cumulative Anticholinergic Cognitive Burden score of ≥ 3 (PUM-ACB); any antipsychotic prescription (PUM-Antipsychotic); and any PUM exposure (ANY-PUM). We also examined annual number of days of each PUM exposure.
Results:
Being a dual user more than doubled the odds of ANY-PUM exposure (59% versus 6139%, Odds Ratio [OR] 2.2; 95% CI: 2.2 – 2.3), PUM-HEDIS (OR 2.4; 95% CI: 2.2 – 2.8), and PUM-ACB (OR 2.1; 95% CI: 2.0 – 2.2). The odds of PUM-antipsychotic were also greater in dual users (OR 1.5; 95% CI: 1.4–1.6). Dual users had an adjusted average of 44.1 additional days of ANY-PUM (95% CI: 37.2 – 45.0 days).
Limitations:
Observational study design, sample of veteran outpatient-users.
Conclusion:
Rates of potentially unsafe prescribing are significantly higher among dual-using veterans with dementia compared to VA-only prescription users.
Keywords: Dual-system use, Medicare, Veterans Affairs, Dementia, Medication Safety, Quality of Care
INTRODUCTION
Dementia is a growing public health priority affecting all health care systems in the United States (1). As a large national health care organization, the U.S. Department of Veterans Affairs (VA) cares for one of the country’s largest cohorts of persons with dementia (2). Because of the aging veteran population and range of dementia risk factors among veterans (e.g., traumatic brain injury, depression, post-traumatic stress disorder), the prevalence of dementia in veterans is expected to double by 2030. Consequently, improving dementia care quality is among VA’s top strategic planning priorities and a national priority area in Healthy People 2020 (2, 3).
Dementia care is challenging for health care systems (4). The average dementia patient has four comorbidities, and receives care from five different providers annually (5). Medication management is particularly challenging, as the average patient takes five different medications and 16% take nine or more (6). Although multiple medications may be indicated, using a greater number of medications (7) and prescribers (8) are major risk factors for potentially unsafe prescribing. Thus, provision of highly-coordinated care is fundamental to prescribing safety in dementia patients.
Recent federal policy changes aimed at expanding access to care may have unintended consequences that thwart VA’s efforts to enhance care-coordination. In 2006, the introduction of the Medicare Part D prescription drug program expanded veterans’ access to medications through non-VA health care systems, where eligibility for Part D is independent of VA benefits. In 2014, the Patient Protection and Affordable Care Act offered expanded access to health care benefits including medication coverage through Medicaid and other payers. Most recently, the Veterans Access, Choice, and Accountability Act (Choice Act) expanded access to non-VA care to veterans unable to schedule an appointment within 30 days and those living >40 miles from the nearest VA facility. Although beneficial in some respects, policies that expand veterans’ access to non-VA care may have the unintended consequence of negatively impacting quality of care (9–11), including safe and effective prescribing (12).
The effect of dual VA and non-VA use on prescribing quality and safety has not previously been studied. However, prior research has shown dual VA and non-VA use to be associated with duplication and overuse of other health services (9) and worse health outcomes (11). Dual use of VA and non-VA prescribing may be particularly hazardous for veterans with dementia who have complex medication needs coupled with impaired cognitive and functional abilities, and in turn may be more vulnerable to increased care fragmentation.
This study aimed to examine the prevalence and effect of dual use of VA and Medicare Part D prescription medications on prescribing safety among a national cohort of >75,000 veteran outpatients with dementia who were dually-eligible for VA and Part D prescription benefits. This research may assist in guiding national dementia care policies and calls for improving coordination across federal systems of care (12–14).
METHODS
Data sources
We linked national patient-level data from 2007–2010 from the VA and Centers for Medicare & Medicaid Services (CMS). VA data comprised the medical SAS data sets for patient demographics and diagnosis codes/dates for all inpatient and outpatient visits, and VA Pharmacy Benefits Management data for dispensed outpatient medications. The six CMS data files included 1) the Medicare Provider Analysis and Review (MedPAR) file summarizing stays in inpatient hospitals and skilled nursing facilities; 2) Minimum Dataset (MDS) file containing information on stays in Medicare/Medicaid-certified long-term care facilities ; 3) outpatient facility file containing claims submitted by institutional outpatient providers (e.g., hospital outpatient departments, outpatient rehabilitation facilities, renal dialysis facilities); 4) carrier file containing claims from physicians and other non-institutional providers and free-standing facilities (e.g., independent clinical laboratories, free-standing ambulatory surgical centers); 5) enrollment files; and 6) Part D event files containing information on prescription drugs dispensed through Part D.
Sample
We identified all veterans with a diagnosis of Alzheimer’s disease or related dementia that were ≥68 years old on January 1, 2010 (n=508,998) (15). Veterans less than 68 years old were excluded because of lack of eligibility for Medicare during the look-back period of 2007–2009. A three year look-back period has been shown to maximize the sensitivity of identifying persons with dementia using claims data (sensitivity = 0.80) (16). To identify active VA users, we excluded veterans with no VA outpatient encounter in 2010, including those with only VA emergency department encounters (n=324,690). We further excluded veterans not continuously enrolled in Medicare Parts A and B during 2007–2010 (n=22,295) due to lack of availability of medical claims, and those with Medicare managed care or employer-sponsored creditable drug coverage (n=47,905) during 2010 for whom non-VA medication use could not be assessed. Veterans with no prescriptions through VA or Part D in 2010 (n=10,593) and veterans receiving all prescriptions through Medicare Part D in 2010 (n=2,791) were also excluded. A small number of veterans living in Puerto Rico (n=1,045) were excluded because there is no comparable zip code or county-level data. Finally, we excluded veterans who spent ≥31 days in a hospital or nursing facility in 2010 (n=23,850) . The final sample consisted of 75,829 veterans.
Measures
Dependent variables: Potentially Unsafe Medications for Older Adults with Dementia
We constructed three indicators of potentially unsafe medication (PUM) exposure in 2010: exposure to any drug to be avoided in older adults (PUM-HEDIS); any exposure to a score of ≥ 3 on the anticholinergic cognitive burden scale (PUM-ACB); and any prescription for an antipsychotic medication (PUM-Antipsychotics). For each PUM type, we also calculated the number of days of exposure. PUM-HEDIS was based on the 2010 Healthcare Effectiveness Data and Information Set (HEDIS) list of potentially harmful drugs in the elderly (17). PUM-ACB was assessed using the Anticholinergic Cognitive Burden (ACB) scale, an expert-based, validated index that classifies each medication by severity of anticholinergic effect on cognition (0=none; 1= mild; 2=moderate; 3=severe) (18). Total ACB for each day in 2010 was calculated for each patient by summing ACB scores across their medications for that day. Higher ACB scores are associated with greater anticholinergic load, with scores of ≥ 3 indicating clinically-relevant burden (19). Antipsychotics are associated with increased mortality in dementia patients and now carry a boxed warning issued by the FDA (20, 21). PUM-Antipsychotic exposure was defined as the receipt of at least one prescription for an atypical or conventional antipsychotic in 2010 (22). Finally, we created a variable, Any-PUM, indicating any PUM-HEDIS, PUM-ACB, or PUM-Antipsychotic medication exposure as well as the number of Any-PUM exposure-days. Any-PUM exposure-days is the sum of PUM-HEDIS days, PUM-ACB days, and PUM-Antipsychotic days in 2010. A day where a patient was exposed to all 3 PUMs would count as 3 exposure days. Therefore, it was theoretically possible for the Any-PUM-exposure days to exceed 365 days
Independent variable: VA-Medicare Drug Benefit User Group
We defined VA-Medicare drug benefit user group as a dichotomous variable: VA-only users obtained all 2010 prescriptions through VA; and dual VA/Part D users obtained at least one prescription from each source.
Covariates
Covariates were included if they were expected to affect likelihood of dual use or risk of PUM exposure. Age, sex, race, U.S. Census region, county-level rurality (23), distance to nearest VA medical center, VA enrollment priority status, and use of VA home-based primary care in 2009 were captured from VA data. Missing VA race/ethnicity values were filled in using race/ethnicity from Medicare files. Clinical factors were derived using 2007–2009 utilization data for all inpatient, outpatient and emergency room visits in both VA and non-VA settings, specifically using the VA Medical SAS files for all inpatient and outpatient visits and Medicare MedPAR, outpatient, and carrier claims. These factors included individual and total count of Elixhauser comorbidities in 2008–2009 (24), type of dementia (Alzheimer’s only, vascular dementia only, other dementing disorders only, or multiple dementia types coded in 2007–2009), memantine use in 2009 as a proxy for moderate/severe dementia, and counts of emergency department and hospitalizations in 2009. Number of days alive in 2010 was captured using the date of death in the Medicare enrollment file. Veterans Integrated Service Networks (VISN) dummy variables were included to account for VISN-level fixed effects.
Statistical Analysis
The primary hypothesis was that dual users would be at greater risk for each of the four PUM exposures compared to VA-only users. To address potential for selection bias, we used augmented inverse probability weighting (AIPW) propensity score methods to estimate the average effect of drug benefit user group and PUM exposure using the teffects aipw command in STATA, version 13 (StataCorp). AIPW combines two models: inverse probability weighting in the drug benefit user group selection propensity model (model 1) with regression adjustment in the outcomes model (PUM exposure) (25). By combining these two approaches, AIPW is referred to as “doubly robust estimation” because only 1 of the 2 models need be correctly specified to obtain an unbiased estimator. Specifically, logistic regression was used in model 1 to estimate the probability of belonging to either user group, and weighted logistic regression or weighted linear regression were used to model PUM exposure and days of PUM exposure, respectively (25). All covariates described above were included in both the drug benefit user group selection model and the PUM exposure model. To account for the highly skewed nature of the days of PUM exposure variables, we estimated standard errors and 95% confidence intervals using a bias-corrected bootstrap approach (26). With <2% missing data across all variables, hotdeck imputation was used to impute a single complete dataset (27).
Sensitivity Analyses
We conducted a number of sensitivity analyses. First, we examined the level of residual confounding necessary to produce the odds ratio observed in our study for Any-PUM (28). Second, we examined an alternative definition of dual use: proportion of total prescriptions (VA + Medicare) received from VA. Because we hypothesized a curvilinear relationship, we included a quadratic term in these models. Third, we analyzed for potential confounding by VA service network. (VISN) by conducting all analyses stratified by VISN. After stratification, results were pooled. We conducted two additional sensitivity analyses not presented in the appendix (results available upon request). We tested for undue influence of unusual cases in the AIPW analysis by excluding the lowest and highest 2.5% of propensity scores (29). Finally, we ran a model that included veterans who spent ≥31 days in a hospital or nursing facility in 2010.
RESULTS
Study Sample
The sample included 75,829 older VA outpatients with dementia dually enrolled in VA and fee-for-service Medicare with at least one prescription from VA or Part D, in 2010. The mean age was 82 years, 98% were male, and 88% were non-Hispanic white. Nearly 20% (19.7%) were dual VA/Part D Users, while 80% were VA-only users.
Balancing Covariates Via Inverse Propensity Score Weighting
Compared to VA-only users, dual users had the following characteristics (Table 1): lived in the Northeast, lived farther away from nearest VA, no service connected disability, more comorbidities, memantine use, and multiple hospitalizations in 2009. After AIPW adjustment, no covariates had a standardized difference greater than 10.0 percent, indicating sufficient balance between dual users and VA-only users. This is reflected in comparison between the unadjusted and AIPW-adjusted differences across covariates.
Table 1:
Characteristics of Older Veterans with Dementia Using VA Outpatient Services in 2010 by Source of Prescription Medications (N = 75,829)
| Unadjusted |
AIPW-Adjusted |
AIPW-Adjusted |
|||
|---|---|---|---|---|---|
| Sample Characteristics |
Dual VA/Part D use (n=14,941) |
VA-only use (n=60,888) |
Dual VA/Part D use |
VA-only use |
Standardized Difference |
| Age in years, % | |||||
| 68–74 | 12 | 13 | 13 | 13 | -0.9 |
| 75–79 | 23 | 22 | 22 | 22 | -1.0 |
| 80–84 | 32 | 29 | 30 | 30 | 0.2 |
| 85+ | 33 | 35 | 35 | 35 | 1.4 |
| Sex | |||||
| Female | 2 | 2 | 2 | 2 | |
| Male | 98 | 98 | 98 | 98 | −0.3 |
| Race/Ethnicity, % | |||||
| Hispanic | 2 | 3 | 3 | 3 | 0.6 |
| White, non-Hispanic | 91 | 88 | 88 | 89 | −0.4 |
| Black, non-Hispanic | 5 | 8 | 7 | 7 | 0.2 |
| Other race, non-Hispanic | 2 | 1 | 1 | 1 | −0.2 |
| Region of United States, % | |||||
| Northeast | 20 | 15 | 16 | 16 | 0.3 |
| Midwest | 22 | 26 | 26 | 25 | 1.0 |
| South | 45 | 45 | 44 | 45 | −0.4 |
| West | 13 | 14 | 14 | 14 | −0.9 |
| County Rurality, % | |||||
| Large metro. | 39 | 38 | 38 | 38 | −1.2 |
| Small metro. | 35 | 37 | 36 | 36 | 0.5 |
| Micropolitan | 14 | 14 | 14 | 14 | 0.7 |
| Non-core rural | 12 | 11 | 12 | 12 | 0.2 |
| Distance to nearest VA, miles | 18 miles | 17 miles | 17 miles | 17 miles | 0.5 |
| VA enrollment priority status, % | |||||
| Highly disabled | 18 | 28 | 26 | 25 | 0.5 |
| Low/Moderately disabled | 13 | 15 | 14 | 15 | −0.5 |
| Low income | 27 | 23 | 24 | 24 | 0.3 |
| No service-connected disability | 42 | 35 | 36 | 36 | −0.3 |
| Number of Comorbidities, % | |||||
| 0 | 1 | 2 | 2 | 2 | −0.1 |
| 1–2 | 17 | 22 | 21 | 21 | −0.5 |
| 3–4 | 30 | 32 | 32 | 32 | 0.2 |
| 5+ | 52 | 44 | 45 | 45 | 0.3 |
| Comorbid conditions | |||||
| Congestive heart failure | 26 | 21 | 22 | 22 | 0.2 |
| Valvular disease | 19 | 16 | 16 | 16 | 0.2 |
| Pulmonary circulation disorder | 5 | 4 | 4 | 4 | 0.4 |
| Peripheral vascular disorder | 31 | 25 | 26 | 26 | 0.3 |
| Hypertension | 79 | 78 | 78 | 78 | 0.2 |
| Paralysis | 5 | 5 | 5 | 5 | 0.4 |
| Neurological disorder | 50 | 43 | 44 | 44 | 0.4 |
| Chronic pulmonary disease | 33 | 28 | 29 | 29 | 0.0 |
| Diabetes, uncomplicated | 28 | 26 | 27 | 27 | −0.3 |
| Diabetes, complicated | 18 | 15 | 15 | 15 | 0.0 |
| Hypothyroidism | 20 | 17 | 17 | 17 | 0.2 |
| Renal failure | 20 | 18 | 19 | 19 | 0.6 |
| Tumor – without metastasis | 22 | 20 | 20 | 20 | −0.3 |
| Rheumatoid arthritis | 4 | 4 | 4 | 4 | −0.4 |
| Coagulopathy | 8 | 6 | 6 | 6 | 0.2 |
| Obesity | 7 | 6 | 6 | 6 | 0.1 |
| Weight loss | 8 | 8 | 8 | 8 | 0.9 |
| Fluid and Electrolyte disorder | 24 | 21 | 21 | 21 | 0.5 |
| Blood loss anemia | 2 | 2 | 2 | 2 | 0.6 |
| Deficiency anemia | 38 | 32 | 33 | 33 | 0.4 |
| Alcohol abuse | 3 | 3 | 3 | 3 | 0.5 |
| Psychoses | 16 | 15 | 15 | 15 | −0.2 |
| Depression | 26 | 24 | 24 | 24 | 0.1 |
| Epilepsy | 4 | 3 | 3 | 3 | −0.2 |
| Syncope | 10 | 8 | 9 | 9 | 0.1 |
| Memantine user in 2009 | |||||
| No | 76 | 87 | 85 | 85 | |
| Yes | 24 | 13 | 15 | 15 | −0.1 |
| In Home-Based Primary Care | |||||
| No | 97 | 93 | 94 | 94 | |
| Yes | 4 | 7 | 6 | 6 | 1.2 |
| Dementia type, % | |||||
| Alzheimer’s disease only | 8 | 9 | 8 | 8 | −0.3 |
| Vascular dementia only | 11 | 14 | 13 | 13 | −0.4 |
| Other dementing disorder only | 33 | 40 | 39 | 39 | −0.9 |
| Multiple dementia diagnoses | 48 | 38 | 40 | 39 | 1.3 |
| Hospitalizations in 2009, % | |||||
| 0 | 62 | 66 | 65 | 66 | −0.9 |
| 1 | 21 | 20 | 20 | 20 | 0.6 |
| 2+ | 17 | 14 | 15 | 14 | 0.5 |
| Emergency Dept. Visits in 2009, % | |||||
| 0 | 60 | 61 | 60 | 61 | −0.3 |
| 1 | 23 | 23 | 23 | 23 | −0.1 |
| 2+ | 17 | 17 | 17 | 17 | 0.5 |
| Days alive in 2010, days | 352 days | 349 days | 350 days | 350 days | 0.4 |
| Veteran Integrated Service Network | |||||
| 1 | 5 | 6 | 6 | 5 | 0.6 |
| 2 | 2 | 1 | 2 | 2 | −0.2 |
| 3 | 3 | 6 | 4 | 4 | −0.2 |
| 4 | 5 | 7 | 6 | 6 | 0.0 |
| 5 | 3 | 2 | 3 | 3 | −0.2 |
| 6 | 5 | 5 | 5 | 5 | −0.3 |
| 7 | 7 | 7 | 7 | 7 | −0.3 |
| 8 | 10 | 11 | 9 | 9 | −0.4 |
| 9 | 5 | 5 | 5 | 5 | −0.1 |
| 10 | 4 | 3 | 4 | 3 | 0.4 |
| 11 | 5 | 5 | 5 | 5 | 0.4 |
| 12 | 5 | 5 | 5 | 5 | 0.8 |
| 15 | 5 | 5 | 5 | 5 | −0.3 |
| 16 | 9 | 9 | 10 | 9 | 0.7 |
| 17 | 4 | 4 | 4 | 4 | −0.3 |
| 18 | 4 | 3 | 4 | 4 | −0.6 |
| 19 | 3 | 2 | 2 | 2 | −0.2 |
| 20 | 3 | 3 | 3 | 3 | −0.5 |
| 21 | 3 | 3 | 3 | 3 | −0.7 |
| 22 | 3 | 3 | 3 | 3 | −0.4 |
| 23 | 6 | 5 | 6 | 6 | 0.9 |
Unadjusted Results
Overall, 44% of VA outpatient users with dementia were exposed to at least one type of PUM. Compared to VA-only users, the prevalence of Any-PUM exposure among dual users was 19.8 percentage points higher (95% CI: 19.0 – 20.7), and an additional 44.8 Any-PUM days of exposure (95% CI: 40.2 – 48.4). A similar pattern emerged for the specific PUM measures. Compared to VA-only users, dual users were more likely to be exposed to PUM-HEDIS (10.7 percentage points; 95% CI: 10.0 – 11.4), PUM-ACB (18.4 percentage points; 95% CI: 17.5 – 19.3), and PUM-Antipsychotic (5.2 percentage points; 95% CI: 4.6 – 5.8). Dual users had an additional 6.4 days of PUM-HEDIS exposure (95% CI: 5.2 – 7.6), 30.1 days of PUM-ACB (95% CI: 27.5 – 32.6), and 8.2 days of PUM-Antipsychotic exposure (95% CI: 6.3 – 9.8).
AIPW-PS Adjusted Results
Dual versus VA-only Users.
In AIPW-adjusted analyses (Table 3), being a dual user more than doubled the odds of any type of PUM exposure (odds ratio [OR], 2.2; 95% CI, 2.2 to 2.3). The adjusted percentage point difference was 19.1 (95% CI: 18.1 – 20.7). Dual users had an adjusted average of 44.1 additional PUM-days of exposure (95% CI, 37.2 to 45.0). Dual users also more than doubled the odds of PUM-HEDIS exposure (OR, 2.4; 95% CI, 2.2 to 2.8). The adjusted percentage point difference was 10.9 (95% CI: 10.2 – 11.7). Dual users had an adjusted average of 6.6 additional days of PUM-HEDIS exposure (95% CI, 5.4–7.9). Odds of PUM-ACB exposure were 2.1 times higher for dual users compared to VA-only users (OR, 2.1; 95% CI, 2.0 to 2.2), with an adjusted percentage point difference of 17.6 (95% CI: 16.7 – 18.6). Dual users had an adjusted average of 27.6 additional days of PUM-ACB exposure (95% CI, 25.1 to 30.1). Odds of PUM-Antipsychotic exposure were 1.5 times higher for dual users compared to VA-only users (OR, 1.5; 95% CI, 1.4 to 1.6), with an adjusted percentage point difference of 4.6 (95% CI, 3.9–5.3). Dual users had an adjusted average of 6.8 additional days of PUM-Antipsychotic exposure (95% CI, 5.1–8.4).
Table 3:
Adjusted Use of Potentially Unsafe Medication Among Older Veterans with Dementia Using VA Outpatient Services in 2010 by Source of Prescription Medications (N = 75,829)
| Dual Use versus VA-only
Use |
||
|---|---|---|
| Drug System User Group | Unadjusted | AIPW-PS Adjusted |
|
Potentially Unsafe Medication
Exposure (PUM-HEDIS, PUM-ACB, or PUM-Antipsychotic) |
||
| Any PUM exposure | ||
| Difference in percentages, (95% CI) | +19.8 (19.0 – 20.7) | +19.1 (18.1 – 20.7) |
| OR (95% CI) | 2.2 (2.2 – 2.3) | 2.2 (2.1 – 2.3) |
| Number of days (95% CI) a | 44.8 days (40.9 – 48.6) | 44.1 days (37.2 – 45.0) |
| PUM-HEDIS | ||
| Any HEDIS drug | ||
| Difference in percentages, (95% CI) | +10.7 (10.0 – 11.4) | +10.9 (10.2 – 11.7) |
| OR (95% CI) | 2.4 (2.3 – 2.5) | 2.4 (2.2 – 2.8) |
| Number of days (95% CI) a | +6.5 (5.4 – 7.6) | +6.6 (5.4 – 7.9) |
| PUM-Anticholinergic Cognitive Burden (ACB) | ||
| Any day with ACB score ≥ 3, % | ||
| Difference in percentages, (95% CI) | +18.5 (17.5 – 19.3) | +17.6 (16.7 – 18.6) |
| OR (95% CI) | 2.1 (2.1 – 2.2) | 2.1 (2.0 – 2.2) |
| Number of days (95% CI) a | +30.4 (28.1 – 32.7) | +27.6 (25.1 – 30.1) |
| PUM-Antipsychotics | ||
| Any Antipsychotic drug | ||
| Difference in percentages, (95% CI) | +5.2 (4.6 – 5.8) | +4.6 (3.9 – 5.3) |
| OR (95% CI) | 1.5 (1.5 – 1.6) | 1.5 (1.4 – 1.6) |
| Number of days (95% CI) a | +8.2 (6.5 – 9.7) | +6.8 (5.1 – 8.4) |
Bias-corrected bootstrapped 95% Confidence Intervals
Abbreviations: VA, Veterans Affairs; AIPW-PS, Augmented Inverse Propensity Weighted – Propensity Score; PUM, Potentially Unsafe Medication; HEDIS, Healthcare Effectiveness Data and Information Set potentially harmful medications in the elderly; ACB, Anticholinergic Cognitive Burden.
All results were highly robust across the described sensitivity analyses.
Sensitivity Analysis Results: Unobserved Confounder Assessment.
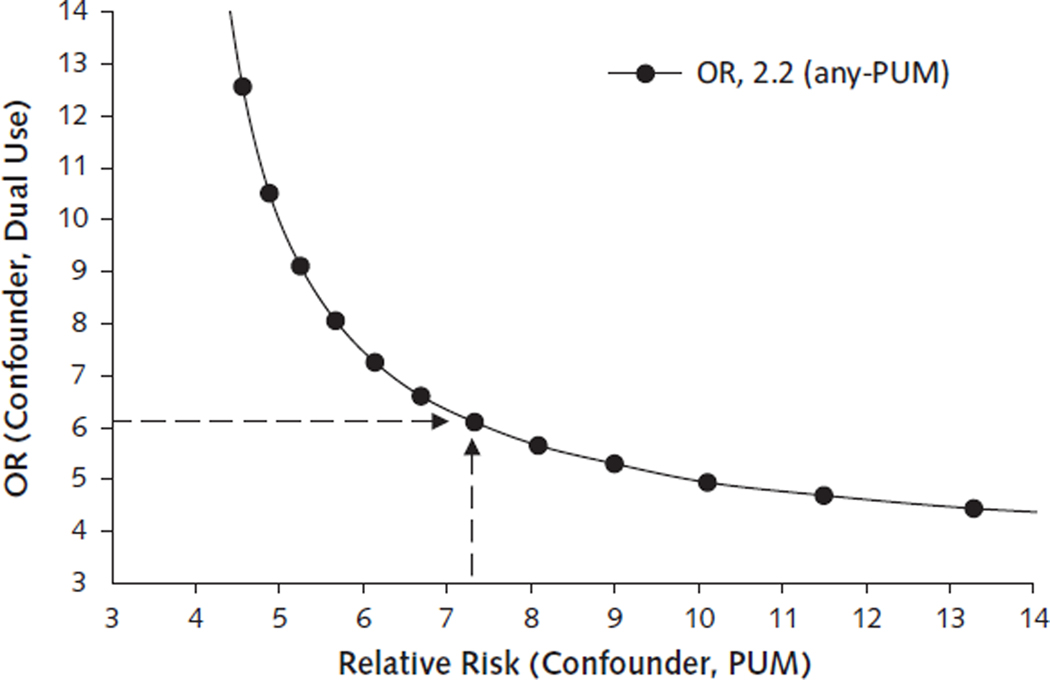
We focused sensitivity analyses on Any-PUM (Appendix Figure 1). For the dual use versus VA-only finding, a relative risk ratio of ˃7.0 between an unobserved confounder and Any-PUM (Relative Risk Ratio of Confounder and PUM exposure; labeled RRC-D) would be needed to nullify the observed OR of 2.2. Even if an unobserved cofounder met the RRC-D threshold, an odds ratio of 6.0 or greater between the confounder and dual use (Odds Ratio of Confounder and Drug Benefit Group; labeled as ORC-E) would be needed to nullify the observed result.
Appendix Figure 1.
Rule-out sensitivity analysis for any-PUM exposure.
Area above the curve represents values of the levels of confounding necessary to produce the observed OR (2.2). Area below the curve represents levels of confounding that would not be sufficient on their own, after adjustment for observed confounders, to produce the observed ORs. For example, the dashed lines indicate that an unobserved confounder would need to have a relative risk of greater than 7.0 with PUM exposure and an OR of 6.0 or greater with dual use of the VA and Medicare Part D to nullify the observed adjusted OR of 2.2. ACB = Anticholinergic Cognitive Burden; any-PUM = includes exposure to PUM-HEDIS, PUM-ACB, and PUM-antipsychotic; HEDIS = Healthcare Effectiveness Data and Information Set; OR = odds ratio; PUM = potentially unsafe medication; PUM-ACB = daily exposure to drugs that have a cumulative ACB scale score of ≥3; PUM-antipsychotic = any prescription for antipsychotic medication; PUM-HEDIS = exposure to any high-risk drug based on the 2010 HEDIS list of potentially harmful drugs in older adults; VA = U.S. Department of Veterans Affairs.
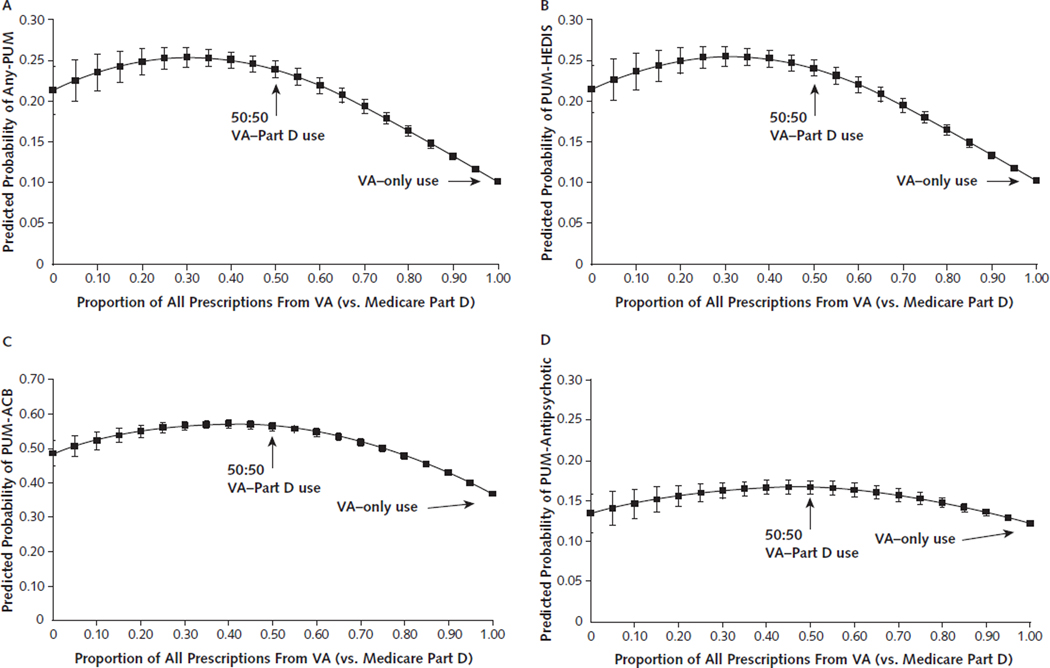
Sensitivity Analysis Results: Alternative Definition of Dual Use.
Analyses of dual VA/Part D use measured as the proportion of prescriptions received from the VA revealed the expected curvilinear relationship. The risk of PUM exposure was highest when seeking prescriptions in near-equal proportions from VA and Part D (Appendix Figures 2A-2D).
Appendix Figure 2.
Predicted probability of PUM exposure, by the proportion of all VA prescriptions.
Error bars represent 95% CIs. ACB = Anticholinergic Cognitive Burden; Any-PUM = includes exposure to PUM-HEDIS, PUM-ACB, and PUM-antipsychotic; HEDIS = Healthcare Effectiveness Data and Information Set; PUM = potentially unsafe medication; PUM-ACB = daily exposure to drugs that have a cumulative ACB scale score of ≥3; PUM-antipsychotic = any prescription for antipsychotic medication; PUM-HEDIS = exposure to any high-risk drug based on the 2010 HEDIS list of potentially harmful drugs in older adults; VA = U.S. Department of Veterans Affairs; A. Any-PUM exposure. B. PUM-HEDIS exposure. C. PUM-ACB exposure. D. PUM-antipsychotic exposure.
Sensitivity Analysis Results: Potential Effect Modification by VA Service Network.
While minor variation was observed across VISNs (Appendix Tables 3A-3D), stratified-pooled results did not differ substantively from non-stratified results.
DISCUSSION
Our analysis of a national cohort of over 75,000 veterans with dementia revealed higher rates of potentially unsafe prescribing for veterans receiving prescriptions from both VA and Medicare providers (dual users). The overall prevalence of exposure to potentially unsafe medications was high overall (44%), but was particularly high in dual users (59%) compared to VA-only users (39%). Adjusted results revealed similar results. Compared to VA-only users, dual VA/Medicare Part D use more than doubled the odds of exposure any potentially unsafe medication and exposure to HEDIS “medications to avoid in older adults”. Dual VA/Part D use also doubled the odds of exposure to medications with high anticholinergic cognitive burden. Similarly, dual-using dementia patients were at greater risk of receiving prescriptions for antipsychotic medications. Compared to VA-only users, dual users had an average of one additional week of exposure to antipsychotics and HEDIS medications, and an additional month of high anticholinergic exposure.
While this study is the first to our knowledge to examine the impact of dual-system use on prescribing safety among veterans, our results are consistent with previous research on dual use of other health services, which found poorer quality of care and health outcomes among veteran dual users (9–11, 30–34). Although future research should investigate the mechanisms by which dual VA/non-VA drug benefit use increases the risk of unsafe prescribing, there are several possible explanations for our findings. First, dual use of VA and non-VA systems may disrupt key aspects of care continuity necessary for safe prescribing to dementia patients (35). Research by Byrne et al (36), for example, suggests that VA and private systems are not sufficiently integrated to facilitate exchange of crucial patient diagnostic, laboratory, and prescribing information (12). The absence of electronic information exchanges places much of the burden on the cognitively-impaired veteran and/or their family caregiver(s) to communicate complex information across systems where veterans are presumably seeing different prescribers. Second, VA-only users may have been protected by the evidence-based, national VA formulary and other medication restrictions VA has in place (e.g., criteria for use, prior authorization). Unlike Part D which places few restrictions on drugs, the VA uses an integrated, nationalized formulary that includes ongoing review of medication safety and effectiveness and administrative infrastructure capable of restricting potentially harmful medications when necessary (37). Finally, while PUM exposure was more common among dual users (59%), the high rate of exposure in the VA-only group (39%) is consistent with prior research (38). The persistent challenge of potential unsafe prescribing in older adults, even within an integrated healthcare system, further underscores the need for increased screening and interventions to reduce PUM.
The finding that dual VA/Part D users were exposed to an additional month of high anticholinergic burden, and an additional week of antipsychotics, is concerning. The FDA acknowledged the elevated mortality risk associated with antipsychotic prescribing to dementia patients through “black box” label warnings as early as 2005. Exposure to drugs with strong anticholinergic properties increases cognitive impairment (39), risk of falls (40), and all-cause mortality (39). Anticholinergics also may work antagonistically to dementia therapies designed to boost acetylcholine (41); increasing the risk of an anticholinergic – acetylcholinesterase inhibitor prescribing cascade (42).
Taken together, these findings have important policy and clinical implications for dementia patients. First, for the benefits of dual use (increased access) to outweigh the risks, careful co-management of care between VA and non-VA providers is critical. Successful co-management requires that health information exchange between systems – currently the responsibility of veterans and caregivers – needs to improve. Pilot programs such as VA’s Virtual Lifetime Electronic Record (VLER) have shown great potential for facilitating sharing of health information between VA and non-VA providers (43). Efforts to rapidly implement such programs, particularly to vulnerable veterans, are urgently needed to keep pace with recent policies designed to expand access to non-VA providers.
Second, pharmacist-led medication therapy management (MTM) is known to decrease use of unsafe medications (44). VA pharmacists could serve as “medication coordination managers” for high-risk, dual-using veterans. In addition to improving safety, pharmacists could help reduce patient, caregiver, and physician burden by assisting with communication of medication information across systems.
There are several limitations to our analysis. First, our sample is representative of veterans with dementia, enrolled in Medicare fee-for-service, who use VA outpatient care. While our sample represents one of the country’s largest cohorts of persons with dementia, it may not be representative of all Medicare-eligible veterans with dementia. Whether dual VA/non-VA medication use presents risks for all older veterans including those without dementia who have access to Medicare drug benefits, as well as veterans enrolled in Medicare managed care or other non-VA drug benefits, are important directions for future research. Second, while our propensity score approach addresses potential confounding from observed variables, unobserved factors may bias results. Rule-out sensitivity analyses, however, suggest any unobserved confounder(s) would need to be extremely strong to negate observed results. Third, given the lack of information on characteristics of VA and Medicare prescribers in our prescription data, we are unable to determine the role of prescriber characteristics in influencing the results. Finally, our measures do not capture the full range of potentially unsafe prescribing scenarios, nor is every instance of exposure to our chosen measures necessarily unsafe. We did not assess duplication of therapies, drug-drug interactions, drug-disease interactions, or unsafe over-the-counter medications (6), which are important areas for future investigation.
CONCLUSIONS
We report evidence that prescribing safety may be inadvertently compromised when national policies expand patient access to multiple, poorly coordinated health care systems. Policymakers should consider implementing electronic health information exchanges and additional medication therapy management services across systems to keep pace with recent policies designed to expand veterans’ access to non-VA providers and protect vulnerable patients from risks associated with dual system use.
Supplementary Material
Table 2:
Unadjusted Use of Potentially Unsafe Medication Among Older Veterans with Dementia Using VA Outpatient Services in 2010 by Source of Prescription Medications (N = 75,829)
| Medication Safety Measure | Dual VA/Part D use (n=14,941) | VA-only use (n=60,888) | Difference (95% CI)a |
|---|---|---|---|
|
Potentially Unsafe Medication
Exposure (PUM-HEDIS, PUM-ACB, or PUM-Antipsychotic) |
|||
| Any PUM exposure, % | 59.0% | 39.1% | +19.8 (19.0 – 20.7) |
| Number of PUM exposure-days in 2010,b mean (SD; Range) | 159.0 (216; 0–1095) | 114.3 (198; 0–1095) | +44.8 (40.2 – 48.4) |
| HEDIS Potentially Harmful Drugs in the Elderly | |||
| Any HEDIS drug exposure, % | 20.5% | 9.8% | +10.7 (10.0 – 11.4) |
| Number of days in 2010 with HEDIS exposure, mean (SD; Range) | 20.5 (66; 0–365) | 14.1 (58; 0–365) | +6.4 (5.2 – 7.6) |
| Anticholinergic Cognitive Burden | |||
| Any day in 2010 with Anticholinergic Cognitive Burden score ≥ 3, % | 53.8% | 35.4% | +18.4 (17.5 – 19.3) |
| Number of days in 2010 with
anticholinergic cognitive burden score ≥ 3, mean (SD; Range) |
104.7 (137; 0–365) | 74.6 (126; 0–365) | +30.1 (27.5 – 32.6) |
| Antipsychotic medications | |||
| Any antipsychotic prescription in 2010, % | 16.7% | 11.4% | +5.2 (4.6 – 5.8) |
| Number of days of antipsychotic exposure, mean (SD; Range) | 33.8 (93; 0–365) | 25.6 (83; 0–365) | 8.2 (6.3 – 9.8) |
All comparisons across Medication User Groups were statistically significant at the p<0.001 level. Pearson chi-square was used for binary outcomes. Bias-corrected bootstrapping was used for assessing number of days.
PUM exposure days is the sum of PUM-HEDIS days, PUM-ACB days, and PUM-Antipsychotic days in 2010. A day where a patient was exposed to all 3 PUMs would count as 3 exposure-days.
Abbreviations: VA, Veterans Affairs; PUM, Potentially Unsafe Medications; HEDIS, HEDIS potentially harmful medications in the elderly; ACB, Anticholinergic Cognitive Burden.
Acknowledgments
Grant Support: All authors supported by the U.S. Department of Veterans Affairs, Health Services Research & Development Merit Award (IIR 12-379). The investigators retained full independence in the conduct of this research.
Footnotes
Reproducible Research Statement:
Protocol: not available
Statistical Code: Statistical analysis code is available to interested readers by contacting Dr. Joshua Thorpe at Joshua.Thorpe@va.gov. The provision of code for variable creation will be considered on a case-by-case basis. Requests should be directed to Dr. Joshua Thorpe at Joshua.Thorpe@va.gov
Data: not available
REFERENCES
- 1.World Health Organization. Dementia: A Public Health Priority. Geneva, Switzerland. [Google Scholar]
- 2.Cooley SG, Asthana S. Dementia care for veterans: Enhancing comprehensive, coordinated services. Generations. 2011;34(42):57–63. [Google Scholar]
- 3.U.S. Department of Health and Human Services. Healthy People 2020. Washington, DC: Office of Disease Prevention and Health Promotion; 2012. [Google Scholar]
- 4.Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148–57. [DOI] [PubMed] [Google Scholar]
- 5.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187–94. [DOI] [PubMed] [Google Scholar]
- 6.Thorpe JM, Thorpe CT, Kennelty KA, Gellad WF, Schulz R. The impact of family caregivers on potentially inappropriate medication use in noninstitutionalized older adults with dementia. Am J Geriatr Pharmacother. 2012;10(4):230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blalock SJ, Byrd JE, Hansen RA, Yamanis TJ, McMullin K, DeVellis BM, et al. Factors associated with potentially inappropriate drug utilization in a sample of rural community-dwelling older adults. Am J Geriatr Pharmacother. 2005;3(3):168–79. [DOI] [PubMed] [Google Scholar]
- 8.Holmes HM, Luo R, Kuo YF, Baillargeon J, Goodwin JS. Association of potentially inappropriate medication use with patient and prescriber characteristics in Medicare Part D. Pharmacoepidemiol Drug Saf. 2013;22(7):728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gellad WF, Zhao X, Thorpe CT, Mor MK, Good CB, Fine MJ. Dual use of Department of Veterans Affairs and medicare benefits and use of test strips in veterans with type 2 diabetes mellitus. JAMA Intern Med. 2015;175(1):26–34. [DOI] [PubMed] [Google Scholar]
- 10.Wolinsky FD, An H, Liu L, Miller TR, Rosenthal GE. Exploring the association of dual use of the VHA and Medicare with mortality: separating the contributions of inpatient and outpatient services. BMC Health Serv Res. 2007;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolinsky FD, Miller TR, An H, Brezinski PR, Vaughn TE, Rosenthal GE. Dual use of Medicare and the Veterans Health Administration: are there adverse health outcomes? BMC Health Serv Res. 2006;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gellad WF. The Veterans Choice Act and Dual Health System Use. J Gen Intern Med. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute of Medicine. Priority Areas for National Action: Transforming Health Care Quality. Summary of Institute of Medicine report. . Rockville: Agency for Healthcare Research and Quality; 2003. [Google Scholar]
- 14.Atkins DC. What we need to know about dual use HSR&D FORUM: Translating Research into Quality Health Care for Veterans 2013. [Google Scholar]
- 15.Centers for Medicare and Medicaid Services. Chronic Condition Data Warehouse. 2013. [Google Scholar]
- 16.Taylor DH, Fillenbaum GG, Ezell ME. The accuracy of Medicare claims data in identifying Alzheimer’s disease. Journal of Clinical Epidemiology. 2002;55(9):929–37. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Quality Assurance. The Healthcare Effectiveness Data and Information Set (HEDIS). 2010. [Google Scholar]
- 18.Boustani MA, Campbell NL, Munger S, Maidment I, Fox GC. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–20. [Google Scholar]
- 19.West T, Pruchnicki MC, Porter K, Emptage R. Evaluation of anticholinergic burden of medications in older adults. J Am Pharm Assoc (2003). 2013;53(5):496–504. [DOI] [PubMed] [Google Scholar]
- 20.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–43. [DOI] [PubMed] [Google Scholar]
- 21.Tan L, Wang HF, Wang J, Tan CC, Tan MS, Meng XF, et al. Efficacy and safety of atypical antipsychotic drug treatment for dementia: a systematic review and meta-analysis. Alzheimers Res Ther. 2015;7(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Gellad WF, Aspinall SL, Handler SM, Stone RA, Castle N, Semla TP, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50(11):954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Department of Agriculture Economic Research Service. Measuring Rurality: Urban Influence Codes. U.S. Department of Agriculture Economic Research Service; 2003. [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 25.Bang H, Robins JM. Doubly robust estimation in missing data and causal inference models. Biometrics. 2005;61(4):962–73. [DOI] [PubMed] [Google Scholar]
- 26.Efron B. Better bootstrap confidence intervals. Journal of the American Statistical Association. 1987;82(397):171–85. [Google Scholar]
- 27.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 28.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291–303. [DOI] [PubMed] [Google Scholar]
- 29.Sturmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution—a simulation study. Am J Epidemiol. 2010;172(7):843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carey K, Montez-Rath ME, Rosen AK, Christiansen CL, Loveland S, Ettner SL. Use of VA and Medicare services by dually eligible veterans with psychiatric problems. Health Serv Res. 2008;43(4):1164–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hynes DM, Koelling K, Stroupe K, Arnold N, Mallin K, Sohn MW, et al. Veterans’ access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45(3):214–23. [DOI] [PubMed] [Google Scholar]
- 32.Tarlov E, Lee TA, Weichle TW, Durazo-Arvizu R, Zhang Q, Perrin R, et al. Reduced overall and event-free survival among colon cancer patients using dual system care. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2231–41. [DOI] [PubMed] [Google Scholar]
- 33.Weeks WB, Bott DM, Lamkin RP, Wright SM. Veterans Health Administration and Medicare outpatient health care utilization by older rural and urban New England veterans. J Rural Health. 2005;21(2):167–71. [DOI] [PubMed] [Google Scholar]
- 34.Ajmera M, Wilkins TL, Sambamoorthi U. Dual Medicare and Veteran Health Administration use and ambulatory care sensitive hospitalizations. Journal of General Internal Medicine. 2011;26 Suppl 2(0):669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. BMJ. 2003;327(7425):1219–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrne MM, Kuebeler M, Pietz K, Petersen LA. Effect of using information from only one system for dually eligible health care users. Med Care. 2006;44(8):768–73. [DOI] [PubMed] [Google Scholar]
- 37.Good CB, Valentino M. Access to affordable medications: the Department of Veterans Affairs pharmacy plan as a national model. Am J Public Health. 2007;97(12):2129–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naples JG, Marcum ZA, Perera S, Gray SL, Newman AB, Simonsick EM, et al. Concordance Between Anticholinergic Burden Scales. J Am Geriatr Soc. 2015;63(10):2120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox C, Richardson K, Maidment ID, Savva GM, Matthews FE, Smithard D, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–83. [DOI] [PubMed] [Google Scholar]
- 40.Sarter M, Albin RL, Kucinski A, Lustig C. Where attention falls: Increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol. 2014;257:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu CJ, Tune LE. Chronic exposure to anticholinergic medications adversely affects the course of Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(4):458–61. [PubMed] [Google Scholar]
- 42.Gill SS, Mamdani M, Naglie G, Streiner DL, Bronskill SE, Kopp A, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165(7):808–13. [DOI] [PubMed] [Google Scholar]
- 43.Byrne CM, Mercincavage LM, Bouhaddou O, Bennett JR, Pan EC, Botts NE, et al. The Department of Veterans Affairs’ (VA) implementation of the Virtual Lifetime Electronic Record (VLER): findings and lessons learned from Health Information Exchange at 12 sites. Int J Med Inform. 2014;83(8):537–47. [DOI] [PubMed] [Google Scholar]
- 44.Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.