Abstract
Objectives:
Nonspecific signs and symptoms combined with positive urinalysis results frequently trigger antibiotic therapy in frail older adults. However, there is limited evidence about which signs and symptoms indicate urinary tract infection (UTI) in this population. We aimed to find consensus among an international expert panel on which signs and symptoms, commonly attributed to UTI, should and should not lead to antibiotic prescribing in frail older adults, and to integrate these findings into a decision tool for the empiric treatment of suspected UTI in this population.
Design:
A Delphi consensus procedure.
Setting and Participants:
An international panel of practitioners recognized as experts in the field of UTI in frail older patients.
Measures:
In 4 questionnaire rounds, the panel (1) evaluated the likelihood that individual signs and symptoms are caused by UTI, (2) indicated whether they would prescribe antibiotics empirically for combinations of signs and symptoms, and (3) provided feedback on a draft decision tool.
Results:
Experts agreed that the majority of nonspecific signs and symptoms should be evaluated for other causes instead of being attributed to UTI and that urinalysis should not influence treatment decisions unless both nitrite and leukocyte esterase are negative. These and other findings were incorporated into a decision tool for the empiric treatment for suspected UTI in frail older adults with and without an indwelling urinary catheter.
Conclusions/Implications
A decision tool for suspected UTI in frail older adults was developed based on consensus among an international expert panel. Studies are needed to evaluate whether this decision tool is effective in reaching its aim: the improvement of diagnostic evaluation and treatment for suspected UTI in frail older adults.
Keywords: Urinary tract infection, frail older adults, antibiotic prescribing, nonspecific signs and symptoms, urinalysis
INTRODUCTION
Mrs X, a frail, 85-year-old woman, is a “little agitated” and “just not herself today.” After assessing Mrs X, her nurse decides to perform a dipstick test on a urine sample, which is found to be positive for nitrite and leukocyte esterase. Mrs X's physician is contacted and told of the positive dipstick results. The physician orders a urine culture and begins empiric antibiotic therapy.
This clinical scenario occurs routinely in older adults residing in long-term care facilities (LTCF)1, 2, 3, 4 and is similarly prevalent in noninstitutionalized frail older adults.5 Symptoms like “agitation,” “not being him/herself,” and a broad range of other nonspecific signs and symptoms (S&S) are frequently attributed to urinary tract infections (UTIs).1, 2, 3, 4, 5, 6 If a dipstick test or urinalysis is positive, this is interpreted as confirming the diagnosis, and antibiotic prescribing often follows.4, 5
There are, however, serious concerns regarding this practice. First, many conditions in the frail older patient present atypically, so a broad range of possible causes should be considered when any nonspecific S&S are present.4 Furthermore, at any time, up to 50% of urine samples from nursing home residents who are not unwell test positive for nitrite (100% of catheterized patients), and up to 90% for leukocyte esterase.1, 5, 7, 8, 9 Thus, there is a high a priori likelihood of positive results when performing urine tests (ie, dipstick, urinalysis, or urine culture) in this population. Hence, there is no gold standard to distinguish between asymptomatic bacteriuria and true UTI.8 Consequently, the practice of attributing nonspecific S&S to possible UTI and performing urine tests to confirm this diagnosis promotes inappropriate antibiotic prescribing.
Inappropriate antibiotic prescribing is undesirable both on the patient level, because of potential side effects and drug interactions, and on the societal level because of its contribution to antimicrobial resistance. In a recent guideline-based evaluation of treatment decisions for UTI in Dutch nursing home residents, 32% of antibiotic prescriptions were judged as inappropriate.10 Other reports describe even higher percentages of inappropriate antibiotic prescribing for presumed UTI, ranging from 35% to 93%.11, 12, 13, 14, 15, 16
Minimum criteria for the initiation of antibiotics for UTI in frail older adults have been previously developed.4, 17 These criteria have in common that they focus on the presence of urinary tract related S&S and do not incorporate nonspecific S&S, whereas these nonspecific S&S commonly trigger a UTI suspicion in practice, as illustrated by the above scenario.1, 2, 3, 4, 5, 6 However, the role of nonspecific S&S in the diagnosis of UTI in frail older adults remains poorly understood.4, 17, 18
This article reports results from an international Delphi process with the following aims: (1) reach expert consensus on which S&S, commonly attributed to UTI in frail older adults, should and should not result in empiric antibiotic prescribing; and (2) produce a practical decision tool for diagnostic evaluation and empiric antibiotic treatment of suspected UTI in frail older adults with and without an indwelling urinary catheter.
METHODS
Study Design
A Delphi procedure was performed to reach consensus on antibiotic prescribing for S&S that are attributed to UTI in frail older adults. In this procedure, a group facilitation technique is used to transform expert opinion into group consensus through a series of structured questionnaire rounds. Each questionnaire contains the anonymized results from the previous round(s), and participants are asked to consider these results in their replies in the subsequent questionnaire rounds.19 Consensus was defined as an agreement level of at least 75%.20
Expert Panel
The Delphi moderators (L.B., H.V., C.H.) selected international experts based on their multiple research activities and clinical expertise in UTI in frail older patients. They were invited to participate by e-mail in April 2016. They were also asked whether they had suggestions for other experts whom they believed should be part of the panel. Experts were not informed about the other persons invited for the study or about those participating until completion of the study.
Delphi Rounds
Four Delphi rounds were conducted between May 2016 and March 2017. Questionnaires were prepared by the research team (L.B., H.V., C.H.) and tested for content, clarity, and lay-out by 2 or more members of a pilot panel. This panel consisted of four elderly-care physicians (ie, a medical specialty in The Netherlands focused on care of the frail older patient).
Round 1 and 2: Individual S&S
The research team prepared a list with 38 S&S (Table 1) recognized as being attributed to UTI (defined as cystitis and pyelonephritis) in practice.4, 5, 18, 21, 22, 23, 24, 25 These S&S were presented to the expert panel in random order in the first round, and grouped into 4 categories in the second round: S&S related to the urinary tract, nonspecific S&S, S&S related to the character of urine, and systemic S&S. Experts were asked to evaluate each sign or symptom individually (ie, regardless of context and the presence of other S&S), for the likelihood that it is caused by UTI (not likely/more unlikely than likely/more likely than unlikely/likely). Specific and general comments relevant to S&S were invited, and panel members were asked if there were any other potential S&S that should also be included.
Table 1.
S&S Included in the First Round Delphi Questionnaire
|
S&S Related to the Urinary Tract • Dysuria • Frequency of urination (new/worsening) • Hematuria • Prostate pain • Urethral purulence • Urgency (new/worsening • Urinary incontinence (new/worsening) • Urinary retention S&S related to the character of urine • Cloudy urine • Urine color change • Urine odor change Systemic S&S • Delirium (clear-cut) • Fever • Hypotension • Hypothermia • Tachycardia • Rigors/shaking chills |
Nonspecific S&S • Agitation/aggression (new/worsening) • Alertness/consciousness decrease • Confusion (new/worsening) • Costovertebral angle pain/tenderness (new/worsening) • Diarrhea • Dietary intake/appetite decrease • Dizziness (new/worsening) • Fatigue (new/worsening) • Fluid intake decrease • Functional status/ADL decrease • General lack of well-being • Malaise • Mobility decrease • Nausea, with vomiting • Nausea, without vomiting • Nocturia • Scrotum pain (new) • Suprapubic pain/tenderness (new/worsening) • Syncope • Urinary output decrease • Weakness (new/worsening) |
ADL, activities of daily living
Round 3 and 4: Clinical scenarios
In the third and fourth round, combinations of S&S were presented to the panel members and they were asked whether they would prescribe antibiotics empirically for each clinical scenario (yes/no); each question separately asked about men and women. General comments and comments regarding alternative treatment policies were invited for each clinical scenario. The full questionnaire for the third round is presented in Appendix 1. In the fourth Delphi round, 2 additional questions were asked: (1) what best defines urinary tract-related S&S that are relevant in evaluating a possible UTI? (“new” or “new or significantly increased” urgency, frequency, incontinence, dysuria, (visible) urethral purulence); and (2) which option best represents the place of dipstick results in a patient presenting with urinary tract related S&S? (it should not influence the treatment decision unless both nitrite and leukocyte esterase are negative/antibiotics should only be prescribed if both nitrite and leukocyte esterase are positive/antibiotics should be prescribed if at least nitrite is positive/antibiotics should be prescribed if at least leukocyte esterase is positive/antibiotics should be prescribed if either nitrite or leukocyte esterase is positive). In addition, a preliminary decision tool for the empiric treatment of suspected UTI in frail older adults was provided. The section for the noncatheterized frail older adult was developed by the research team based on the outcomes of the first 3 Delphi rounds. The section for the frail older adult with an indwelling urinary catheter comprised a Delphi study outcome-based adaptation of previously described criteria.17 Expert panel members were asked to comment on specific elements of the decision tool and on the decision tool in general.
Development of the Final Decision Tool
After the fourth Delphi round, the decision tool for the empiric treatment of suspected UTI in frail older adults was adjusted and sent to the expert panel, along with a description of the alterations made to the draft version and the final results of the Delphi questionnaire rounds. Expert panel members were invited to provide any final comments on the adjusted version of the decision tool; agreement was assumed if no comments were received. Final comments of the expert panel members were considered by the research team. The final version of the decision tool for the empiric treatment of UTI in frail older adults with and without an indwelling urinary catheter was sent to the expert panel, together with a description of how the final comments had been addressed.
RESULTS
Of 16 invited experts, 15 were willing to participate in the study; one expert did not accept the invitation because of time constraints. One expert suggested an additional expert to invite. This expert was invited and accepted, so the number participating was 16. The mean age was 56 years (range 42–71 years), and there were 5 different nationalities represented (US - 8; The Netherlands - 3; Canada - 2; Sweden - 2; Australia - 1). Medical disciplines represented, with several experts having multiple specialties, were infectious disease specialists (10), internists (6), geriatricians (6), general practitioners (3), elderly care physicians (2), and a medical microbiologist (1). The average number of years of experience as a specialized physician was 24 (range 14–37 years). All experts were experienced in care of frail older patients. Response rates to the 4 Delphi questionnaires were 100%, 88%, 94%, and 88%, respectively.
First and Second Delphi Round: Individual S&S
Table 2 presents the combined results of the first 2 Delphi rounds: an overview of consensus on the likelihood that S&S are being caused by UTI. Systemic S&S (ie, fever, rigors/shaking chills, delirium, hypotension, hypothermia, and tachycardia) are not included in the table: experts indicated that these are possibly caused by UTI, but there may also be an alternative explanation. Whether these systemic S&S are associated with a UTI depends, therefore, on the presence of other S&S. Experts additionally commented that distinction should be made between “slight” and “moderate to severe” decrease in alertness/consciousness; “no clinical suspicion of delirium” should be added to mental status change symptoms other than delirium; “new” urgency, frequency and incontinence are more likely caused by UTI than “worsening” symptoms; urethral purulence is less likely indicative of UTI in catheterized patients; and hematuria is more likely caused by UTI if it is macroscopic and in patients who do not use oral anticoagulants.
Table 2.
Combined Results of the First and Second Delphi Round; Consensus* on the Likelihood that S&S Are Caused by a UTI
| Consensus on (More) Likely (than Unlikely) Caused by UTI |
Consensus on (More) Unlikely (than Likely) Caused by UTI |
Proportion S&S for Which Consensus was Reached |
No Consensus | |
|---|---|---|---|---|
| S&S related to urinary tract (n ¼ 8) | Dysuria (recent onset), frequency of urination (new/worsening), urgency (new/worsening), (visible) urethral purulence | Urinary retention | 5/8 (62.5%) | Hematuria (69%: not likely caused by UTI), prostate pain (54%: not likely caused by UTI), urinary incontinence (new/worsening; 62%: likely caused by UTI) |
| Nonspecific S&S (n ¼ 23) | Costovertebral angle pain/tenderness (new/worsening) | Agitation/aggression (new/worsening), diarrhea, dietary intake decrease, dizziness (new/worsening), fatigue (new/worsening), fluid intake decrease, functional status/ADL decrease, general lack of well-being, malaise, mental status change: confusion (new/worsening) without a clinical suspicion of delirium, mental status change: moderate to severe decrease in alertness/consciousness without a clinical suspicion of delirium, mental status change: slight decrease in alertness/consciousness without a clinical suspicion of delirium, mobility decrease, nausea with vomiting, nausea without vomiting, nocturia, syncope, urinary output decrease, weakness (new/worsening) | 20/22 (90.9%) | Scrotum pain (new; 69%: not likely caused by UTI), suprapubic pain/tenderness (new/worsening; 69%: not likely caused by UTI) |
| S&S related to the character of urine (n ¼ 3) | Cloudy urine, urine color change, urine odor change | 3/3 (100%) - | - |
Consensus was defined as an agreement level of _75%.20
Third and Fourth Delphi Round: Clinical Scenarios
Table 3 shows the combined results of the third and fourth Delphi round: an overview of consensus on antibiotic prescribing for clinical scenarios. Consensus was reached on not prescribing antibiotics for 8 clinical scenarios and on antibiotic prescribing for 5. The consensus level was not reached in the remaining 5 clinical scenarios, but there was a tendency toward antibiotic prescribing in 4 of these. The results are described for men and women combined, as there were no gender-based differences in consensus levels.
Table 3.
Combined Results of the Third and Fourth Delphi Round; Consensus* on Antibiotic Prescribing for Clinical Presentations of S&S in Which it was Assumed that UTI was not Ruled out by Additional Diagnostics
| Clinical Presentations with Consensus on Antibiotic Nonprescribing |
Clinical Presentations with Consensus on Antibiotic Prescribing |
Clinical Presentations without Consensus |
|---|---|---|
| One nonsevere UTI-specific S&S,† irrespective of the presence of nonspecific S&S‡ | One very bothersome UTI-specific S&S† with nonspecific S&S‡ | One very bothersome UTI-specific S&S† without any other S&S (57% would prescr be +7% if no other cause could be found) |
| Macroscopic hematuria, irrespective of the presence of nonspecific S&S‡ and whether the patients takes oral anticoagulants | At least 3 UTI-specific S&S†, irrespective of the presence of nonspecific S&S‡ | Two UTI-specific S&S†, irrespective of the presence of nonspecific S&S‡ (71% would prescribe +21% only if moderate/severe) |
| Prostate pain, irrespective of the presence of nonspecific S&S‡ | UTI-specific S&S† with new costovertebral angle pain/tenderness | New costovertebral angle pain/tenderness with systemic S&S (71% would prescribe +7% depending on the severity of the S&S) |
| New costovertebral angle pain/tenderness, irrespective of the presence of nonspecific S&S‡ | UTI-specific S&S† with new suprapubic pain | New suprapubic pain with systemic S&S (57% would not prescribe) |
| New suprapubic pain, irrespective of the presence of nonspecific S&S‡ | New costovertebral angle pain/tenderness with systemic S&S and nonspecific S&S‡ | Urinary retention with UTI-specific S&S† (69%would prescribe) |
| New scrotum pain, irrespective of the presence of nonspecific S&S‡ | ||
| Urinary retention, irrespective of the presence of nonspecific S&S‡ | ||
| One or more nonspecific S&S‡ |
Consensus was defined as an agreement level of _75%.20
UTI-specific S&S, after the first 2 Delphi rounds defined as dysuria, new urgency, new frequency, new incontinence, and (visible) urethral purulence.
Nonspecific S&S, after the first 2 Delphi rounds defined as: agitation/aggression (new or worsening), decreased dietary intake, decreased fluid intake, decreased functional status/ADL, decreased mobility, decreased urinary output, diarrhea, dizziness (new or worsening), fatigue (new or worsening), general lack of well-being, malaise, mental status change without a clinical suspicion of delirium, nausea (with or without vomiting), nocturia, syncope, and weakness (new or worsening).
Considering the definition of urinary tract related S&S relevant to the evaluation of a possible UTI, 8 out of 14 experts preferred “new” over “new or significantly increased” urgency, frequency, incontinence, dysuria, and (visible) urethral purulence. Further, 13 out of 14 the experts stated that dipstick results should not influence the treatment decision in a patient presenting with urinary tract related S&S, unless both nitrite and leukocyte esterase are negative, which rules out a UTI.
Decision Tool for the Empiric Treatment of Suspected UTI in Frail Older Adults
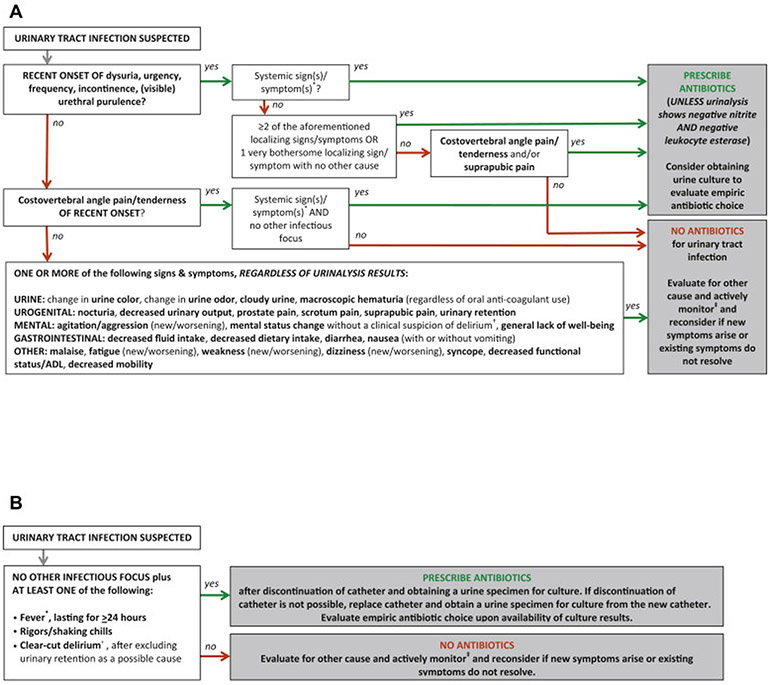
Table 4 describes how the research team dealt with nonconsensus items in the adjusted version of the decision tool. Additional alterations to the draft version based on expert comment were the inclusion of alternative treatment advice in situations where antibiotics should not be prescribed, the adjustment of the definition of systemic S&S, the adoption of the Infectious Diseases Society of America definition for fever in LTCF residents,23 the inclusion of the Diagnostic and Statistical Manual of Mental Disorders-V definition for delirium,26 the inclusion of the advice to obtain urine cultures in situations when antibiotics should be prescribed, the addition that fever should be present for at least 24 hours in frail older patients with an indwelling urinary catheter,27 the inclusion of advice regarding urinary catheter removal or replacement, and the addition that delirium in catheterized patients should only result in antibiotic prescribing if urinary retention is excluded as a possible cause. The final version of the decision tool for the empiric treatment of suspected UTI in frail older adults with and without an indwelling urinary catheter is presented in Figure 1A and B.
Table 4.
Description of How Nonconsensus* Items after the Fourth Round Delphi Questionnaire Were Processed by the Research Team in the Adjusted Version of the Decision Tool for the Empiric Treatment of UTI in Frail Older Adults
| Nonconsensus Items | Decision | Rationale |
|---|---|---|
| Should antibiotics be prescribed if there was one very bothersome of the following recent-onset of S&S: dysuria, urgency, frequency, incontinence, (visible) urethral purulence? | Antibiotics should be only prescribed if there is no other cause | The majority (57%) of panel members would prescribe antibiotics in case of one very bothersome of these S&S, 64% if also an expert is considered who would only prescribe if no other cause could be found. |
| Should there be a minimum of 2 or 3 urinary tract related S&S to proceed to antibiotic prescribing? | A minimum of 2 urinary tract related S&S justifies antibiotic prescribing | The majority (71%) of panel members would prescribe antibiotics in case of 2 urinary tract related S&S, 93% if also the experts that would conditionally prescribe are considered (the conditions being moderate or severe signs and symptoms). |
| Should costovertebral angle pain/tenderness result in antibiotic prescribing if combined with systemic S&S? | Costovertebral angle pain/tenderness combined with systemic S&S justifies antibiotic prescribing | The majority (71%) of panel members would prescribe antibiotics in case of costovertebral angle pain/tenderness combined with systemic S&S, 78% if also an expert is considered who would prescribe if the systemic S&S would include fever with hypotension and tachycardia. |
| Should suprapubic pain result in antibiotic prescribing if combined with systemic S&S? | Suprapubic pain combined with systemic S&S does not justify antibiotic prescribing | The majority (57%) of panel members would not prescribe antibiotics for possible UTI in case of suprapubic pain combined with systemic S&S. Four experts pointed at the non-specific nature of suprapubic pain and the importance of evaluating for other causes (which could be a different infectious source justifying antibiotic prescribing). |
| Should antibiotics be prescribed in case of urinary retention combined with one or more of the following recent-onset S&S: dysuria, urgency, frequency, incontinence, (visible) urethral purulence? | Antibiotics should be prescribed in case there are 2 or more localizing S&S or one very bothersome localizing S&S with no other cause | The listed urinary tract related S&S should be guiding here, not the urinary retention, so decisions on antibiotic prescribing for those S&S e resulting from the Delphi procedure – apply here. |
| Should urinary tract related S&S relevant in evaluating a possible UTI be defined as “new” or “new/significantly increased”? | Only “new” urinary tract related S&S are considered in evaluating a possible UTI, rephrased as “of recent onset” | The majority (57%) of panel members had a preference for “new” over “new/significantly increased.” In addition, 3-panel members commented that the term “significantly increased” is ambiguous (i.e., can differ between persons). |
Consensus was defined as an agreement level of _75%.20
Fig. 1.
(A) Decision tool for the empiric treatment of suspected UTI in frail older adults without an indwelling urinary catheter. (B) Decision tool for the empiric treatment of suspected UTI in frail older adults with an indwelling urinary catheter.
DISCUSSION
We describe a Delphi procedure in which 4 consecutive questionnaire rounds resulted in the development of a consensus-based decision tool for the empiric treatment of suspected UTI in frail older adults. The most notable study findings were that the vast majority of nonspecific S&S should be evaluated for other causes instead of being attributed to UTI and that urinalysis should not influence treatment decisions unless both nitrite and leukocyte esterase are negative. Implementation of the decision tool has the potential to improve diagnostic evaluation and treatment for suspected UTI in the frail older patient and may contribute to better management of nonspecific S&S and more appropriate antibiotic use in this population. This would fit well into antibiotic stewardship programs, which aim at promoting prudent antibiotic use and are increasingly being established in LTCF.28, 29 Prudent antibiotic use is beneficial for individual patients, to reduce unnecessary exposure to side effects and drug interactions, but even more importantly in the light of the worldwide emergence of antimicrobial resistance.30
Because of high prevalence of asymptomatic bacteriuria and pyuria in the frail older patient,1, 5, 7, 8, 9 the Delphi expert panel emphasized the importance of only using urinalysis to rule out UTI in the presence of S&S consistent with this infection. This is reflected in the decision tool (section for noncatheterized frail older adults), by placing urinalysis at the end of the diagnostic process in situations where antibiotic prescribing may be justified based on clinical assessment. The decision tool also indicates that it is not helpful to use a urine dipstick or urinalysis to “screen” for UTI in patients with nonspecific S&S, as these should not result in antibiotic prescribing for UTI even if the urinalysis results are abnormal.
One may argue that a scenario not covered by the decision tool is a patient with fever and no other S&S. In current practice, a dipstick test or urinalysis may be performed to identify UTI as a possible cause for the fever. Here as well, we believe that a dipstick test or urinalysis should not be used since a study found that only 1 out of 10 patients with fever and bacteriuria will have a UTI.31 Therefore, if fever persists and no infectious source can be identified based on the results of other diagnostic tests such as a chest film, antibiotics may be prescribed for “nonfocal infection,” but not for a UTI based on dipstick or urinalysis results. Hence, fever (like other systemic S&S) is not included as a single item in the decision tool, as it is considered only relevant for the evaluation of a possible UTI if combined with other localizing S&S (ie, dysuria, urgency, frequency, incontinence, urethral purulence) or costovertebral angle pain/tenderness.
Similar to dipstick tests and urinalysis, urine cultures should not be used to rule-in a UTI diagnosis, as positive urine culture results often indicate asymptomatic bacteriuria in frail older patients without localizing S&S.8, 32 Instead, the value of a urine culture is to guide the choice of antibiotic therapy in cases where treatment is indicated based on the clinical presentation. It is recommended that a urine culture be obtained before the start of antibiotic treatment, and to discontinue antibiotic treatment if the culture is subsequently negative, or switch to an appropriate drug based on antibiotic sensitivity testing (ie, the narrowest-spectrum drug for which the causative micro-organism is sensitive, while taking into account the patient's renal function, potential drug interactions, and medication allergies).
The decision tool developed in the current study is unique in its incorporation of a broad range of nonspecific S&S that are known to trigger a UTI suspicion in practice. The widely known McGeer criteria (1991), in 2012 revised by Stone et al, and Loeb criteria (2005) focus on the presence of UTI specific S&S but do not give guidance with regard to the role of nonspecific S&S.4, 17 The same is true for the criteria incorporated in the “suspected UTI SBAR” form developed by the Agency for Healthcare Research and Quality.33 A decision tool developed by Crnich and Drinka shows several similarities with the tool developed in the current study.34 For example, it provides advice regarding diagnostic evaluation in situations where nonspecific S&S trigger the UTI suspicion, and it discourages urinalysis if only nonspecific S&S are present. Differences include that the decision tool developed in the current study specifies specific and nonspecific S&S, and that urinalysis is advised at a later stage in the diagnostic process.
Strengths and Limitations
A strength of the current study is that we included a panel of experts who are leading UTI research in frail older patients, as demonstrated by publications and other research activities relevant to this topic. In addition, all panel members are physicians with clinical expertise in the diagnosis and treatment of UTI in this population. Another strength is that the response rate remained high over the 4 questionnaire rounds. Finally, the anonymous nature of the Delphi procedure facilitated equal input of all participants, which is an advantage over regular consensus procedures where dominant individuals may have more influence in the decision-making process.19 A limitation of the study is that the consensus level of >75% was not reached on antibiotic prescribing for 5 clinical scenarios, although agreement levels were close to the consensus threshold in 3 of these (ie, 69%-71%). The study moderators motivated their decision to follow majority opinion in these cases to the expert panel, and concluded acceptance based on the invited responses of panel members to these decisions. Another limitation is that we did not define “frail older adults” in the questionnaire rounds. There is discussion about the concept of frailty in the literature, with definitions varying from the one developed by Fried to the one developed by Rockwood.35 The patient population that the study moderators (L.B., H.V., C.H.) had in mind include older adults with increased vulnerability, multiple conditions simultaneously, and increased healthcare needs. There may be concerns that this differs from the population that the expert panel members had in mind when completing the questionnaires. However, on retrospective inquiry, all panel members indicated that the study moderators' description of the population corresponds with the population they had in mind when participating in the questionnaire rounds.
Implications for Research and Practice
In current practice, antibiotics are frequently prescribed for clinical scenarios that according to the decision tool developed in this study do not justify antibiotic treatment.10, 11, 12, 13, 14, 15, 16 A previous study found that the implementation of a diagnostic and treatment algorithm for UTI reduced antibiotic use in LTCF.36 In line with these findings, we hypothesize that implementation of the current decision tool will result in reduced inappropriate antibiotic use and more attention for other possible causes for nonspecific S&S in the frail older patient. Future studies are needed to test this hypothesis both in community-dwelling and institutionalized frail older adults. In addition, the applicability of the decision tool to older patients with advanced dementia or other severe cognitive impairments should be evaluated, as these patients often have problems expressing S&S.
The decision tool is intended to support clinical decision-making regarding antibiotic prescribing for suspected UTI, and should be used in addition to clinical judgment. Although the most common clinical scenarios are covered by the decision tool, physicians should be aware that a few clinical scenarios may not be covered. For example, a patient with a fever, localizing S&S, and a dipstick negative for both nitrite and leukocyte esterase may have an obstructed pyelonephritis and antimicrobial therapy pending further investigations may be appropriate.
CONCLUSIONS
A Delphi process with an international expert panel resulted in the development of a consensus-based decision tool for the empiric treatment of suspected UTI in frail older adults with and without an indwelling urinary catheter. The implementation and use of this decision tool in practice should be evaluated for different subgroups of frail older adults, including cognitively impaired individuals. Successful implementation of the decision tool has the potential to improve diagnostic evaluation and treatment of suspected UTI in the frail older patient and may promote more appropriate antibiotic use and more attention for other causes of nonspecific S&S in this population.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Herman J.M. Cools for his participation in the Delphi procedure; the other Delphi panel members accepted the invitation to contribute to this publication and are listed as authors. We also thank the pilot panel members for their review of the Delphi questionnaires: Jobje Haaijman (all rounds), Jos van Berkel (first round), Paul van Houten (first round), and Martin Smalbrugge (second, third, and fourth rounds).
Support:
This work was supported by the Dutch Ministry of Health, Welfare and Sport, by providing a grant to the Dutch Association of Elderly Care Physicians (Verenso).
Footnotes
CONFLICTS OF INTEREST:
L.B. reports personal fees from the Dutch Association of Elderly Care Physicians, during the conduct of the study; H.V. reports grants from the Dutch Ministry of Health, Welfare and Sport, during the conduct of the study; S.G.s reports personal fees from InfectoPharm, outside the submitted work; D.N. reports grants from the Agency for Healthcare Research and Quality and grants from the National Institute of Aging, during the conduct of the study; P.U. reports personal fees from the Swedish Medical Products Agency, outside the submitted work; the other authors have no competing interests to disclose.
REFERENCES
- 1.Nicolle LE, Urinary tract infection in long-term-care facility residents. Clin Infect Dis, 31 (2000), pp. 757–761 [DOI] [PubMed] [Google Scholar]
- 2.Schweizer AK, Hughes CM, Macauley DC, O'Neill C, Managing urinary tract infections in nursing homes: A qualitative assessment. Pharm World Sci, 27 (2005), pp. 159–165 [DOI] [PubMed] [Google Scholar]
- 3.D'Agata E, Loeb MB, Mitchell SL, Challenges in assessing nursing home residents with advanced dementia for suspected urinary tract infections. J Am Geriatr Soc, 61 (2013), pp. 62–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nace DA, Drinka PJ, Crnich CJ, Clinical uncertainties in the approach to long-term care residents with possible urinary tract infection. J Am Med Dir Assoc, 15 (2014), pp. 133–139 [DOI] [PubMed] [Google Scholar]
- 5.Mody L, Juthani-Mehta M, Urinary tract infections in older women: A clinical review. JAMA, 311 (2014), pp. 844–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eke-Usim AC, Rogers MA, Gibson KE, et al. , Targeted Infection Prevention Study Team. Constitutional symptoms trigger diagnostic testing before antibiotic prescribing in high-risk nursing home residents. J Am Geriatr Soc, 64 (2016), pp. 1975–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker S, McGeer A, Simor AE, et al. , Why are antibiotics prescribed for asymptomatic bacteriuria in institutionalized elderly people? A qualitative study of physicians' and nurses' perceptions. CMAJ, 163 (2000), pp. 273–277 [PMC free article] [PubMed] [Google Scholar]
- 8.Juthani-Mehta M, Datunashvili A, Tinetti M, Tests for urinary tract infection in nursing home residents. JAMA, 312 (2014), pp. 1687–1688 [DOI] [PubMed] [Google Scholar]
- 9.Detweiler K, Mayers D, Fletcher SG, Bacteruria and urinary tract infections in the elderly. Urol Clin North Am, 42 (2015), pp. 561–568 [DOI] [PubMed] [Google Scholar]
- 10.Van Buul LW, Veenhuizen RB, Achterberg WP, et al. , Antibiotic prescribing in Dutch nursing homes: How appropriate is it?. J Am Med Dir Assoc, 16 (2015), pp. 229–237 [DOI] [PubMed] [Google Scholar]
- 11.Zimmer JG, Bentley DW, Valenti WM, Watson NM, Systemic antibiotic use in nursing homes. A quality assessment. J Am Geriatr Soc, 34 (1986), pp. 703–710 [DOI] [PubMed] [Google Scholar]
- 12.Loeb M, Simor AE, Landry L, et al. , Antibiotic use in Ontario facilities that provide chronic care. J Gen Intern Med, 16 (2001), pp. 376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim CJ, McLellan SC, Cheng AC, et al. , Surveillance of infection burden in residential aged care facilities. Med J Aust, 196 (2012), pp. 327–331 [DOI] [PubMed] [Google Scholar]
- 14.Jones SR, Parker DF, Liebow ES, et al. , Appropriateness of antibiotic therapy in long-term care facilities. Am J Med, 83 (1987), pp. 499–502 [DOI] [PubMed] [Google Scholar]
- 15.Montgomery P, Semenchuk M, Nicolle LE, Antimicrobial use in nursing homes in Manitoba. J Geriatr Drug Ther, 9 (1995), pp. 55–74 [Google Scholar]
- 16.Ryan S, Gillespie E, Stuart RL, Urinary tract infection surveillance in residential aged care. Am J Infect Control, 46 (2018), pp. 67–72 [DOI] [PubMed] [Google Scholar]
- 17.Loeb M, Bentley DW, Bradley S, et al. , Development of minimum criteria for the initiation of antibiotics in residents of long-term-care facilities: Results of a consensus conference. Infect Control Hosp Epidemiol, 22 (2001), pp. 120–124 [DOI] [PubMed] [Google Scholar]
- 18.Lim CJ, Kong DC, Stuart RL, Reducing inappropriate antibiotic prescribing in the residential care setting: Current perspectives. Clin Interv Aging, 9 (2014), pp. 165–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenna HP, The Delphi technique: A worthwhile research approach for nursing?. J Nurs Adm, 19 (1994), pp. 1221–1225 [DOI] [PubMed] [Google Scholar]
- 20.Diamond IR, Grant RC, Feldman BM, et al. , Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol, 67 (2014), pp. 401–409 [DOI] [PubMed] [Google Scholar]
- 21.Juthani-Mehta M, Quagliarello V, Perrelli E, et al. , Clinical features to identify urinary tract infection in nursing home residents: A cohort study. J Am Geriatr Soc, 57 (2009), pp. 963–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavazzi G, Delerce E, Cambau E, et al. , Diagnostic criteria for urinary tract infection in hospitalized elderly patients over 75 years of age: A multicenter cross-sectional study. Med Mal Infect, 43 (2013), pp. 189–194 [DOI] [PubMed] [Google Scholar]
- 23.High KP, Bradley SF, Gravenstein S, et al. , Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. J Am Geriatr Soc, 57 (2009), pp. 375–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone ND, Ashraf MS, Calder J, et al. , Surveillance definitions of infections in long-term care facilities: Revisiting the McGeer criteria. Infect Control Hosp Epidemiol, 33 (2012), pp. 965–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juthani-Mehta M, Tinetti M, Perrelli E, et al. , Role of dipstick testing in the evaluation of urinary tract infection in nursing home residents. Infect Control Hosp Epidemiol, 28 (2007), pp. 889–891 [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders (5th ed.), American Psychiatric Publishing, Arlington, VA: (2013) [Google Scholar]
- 28.Nicolle LE, Catheter-related urinary tract infection: Practical management in the elderly. Drugs Aging, 31 (2014), pp. 1–10 [DOI] [PubMed] [Google Scholar]
- 29.Feldstein D, Sloane PD, Feltner C, Antibiotic stewardship programs in nursing homes: A systematic review. J Am Med Dir Assoc, 19 (2018), pp. 110–116 [DOI] [PubMed] [Google Scholar]
- 30.Jump RLP, Gaur S, Katz MJ, et al. , Template for an antibiotic stewardship policy for post-acute and long-term care settings. J Am Med Dir Assoc, 18 (2017), pp. 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arias CA, Murray BE, Antibiotic-resistant bugs in the 21st century—A clinical super-challenge. N Engl J Med, 360 (2009), pp. 439–443 [DOI] [PubMed] [Google Scholar]
- 32.Orr PH, Nicolle LE, Duckworth H, et al. , Febrile urinary infection in the institutionalized elderly. Am J Med, 100 (1996), pp. 71–77 [DOI] [PubMed] [Google Scholar]
- 33.Sundvall PD, Ulleryd P, Gunnarsson RK, Urine culture doubtful in determining etiology of diffuse symptoms among elderly individuals: A cross-sectional study of 32 nursing homes. BMC Fam Pract, 12 (2011), p. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The American Institutes for Research and the Texas A & M University School of Rural Public Health, TMF Health Quality Institute. Suspected UTI SBAR Toolkit, Available at: https://www.ahrq.gov/nhguide/toolkits/determine-whether-to-treat/toolkit1-suspected-uti-sbar.html, Accessed 30th Mar 2018 [Google Scholar]
- 36.Crnich CJ, Drinka P, Improving the management of urinary tract infections in nursing homes: It's time to stop the tail from wagging the dog. Ann Long-Term Care Clin Care Aging, 22 (2014), pp. 32–36 [Google Scholar]
- 37.Suskind AM, Frailty and lower urinary tract symptoms. Curr Urol Rep, 18 (2017), p. 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loeb M, Brazil K, Lohfeld L, et al. , Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: Cluster randomised controlled trial. BMJ, 331 (2005), p. 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.