Abstract
Metal ligand cooperativity is a powerful strategy in transition metal chemistry. This type of mechanism for the activation of O2 is best exemplified by heme centers in biological systems. While aerobic oxidations with Fe and Cu are well precedented, Ni-based oxidations are frequently less common due to less-accessible metal-based redox couples. Some Ni enzymes utilize special ligand environments for tuning the Ni(II)/(III) redox couple such as strongly donating thiolates in Ni superoxide dismutase. A recently characterized example of a Ni-containing protein, however, suggests an alternative strategy for mediating redox chemistry with Ni by utilizing ligand-based reducing equivalents to enable oxygen binding. While this mechanism has little synthetic precedent, we show here that Ni complexes of the redox-active ligand tBu,TolDHP (tBu,TolDHP = 2,5-bis((2-t-butylhydrazono)(p-tolyl)methyl)-pyrrole) activate O2 to generate a Ni(II) superoxo complex via ligand-based electron transfer. This superoxo complex is competent for stoichiometric oxidation chemistry with alcohols and hydrocarbons. This work demonstrates that coupling ligand-based redox chemistry with functionally redox-inactive Ni centers enables oxidative transformations more commonly mediated by metals such as Fe and Cu.
Graphical Abstract

Introduction
Ligand-based storage of electrons is an important motif in transition-metal reactivity and catalysis.1-12 An archetypal example is found in nature, where oxidation of the porphyrin ligand in heme systems enables the activation of O2 for further oxidative reactivity.13-20 Despite the utility of ligand-based redox chemistry, most aerobic oxidations in biological and synthetic systems still require metals such as Fe and Cu with accessible redox couples such as Fe(II)/(III)/(IV) and Cu(I)/(II).21-28 In contrast to these cases, accessing Ni (II/III) or (II/IV) redox couples is less facile and corresponding Ni mediated aerobic oxidations are comparatively rare.29-32 In biological systems such as Ni superoxide dismutase, the Ni(II)/(III) redox potential must be specifically tuned by the ligand environment, mainly via strongly donating thiolate ligands.33,34 Recently, however, an alternative mechanism involving an ancillary ligand to O2 electron transfer to generate a Ni(II) superoxo complex has been invoked in the Ni-containing enzyme quercetin dioxygenase.35,36 Nickel-superoxo species are generally rare even in synthetic systems, and Ni mediated oxygen activation without accessing Ni(I) or Ni(III) oxidation states has little synthetic precedent.37-46 Therefore, the proposed enzymatic mechanism for quercetin dioxygenase motivates studies to examine whether ligand cooperativity is a viable strategy for Ni systems to activate O2 and mediate oxidative transformations.
The use of ligand-based redox couples for aerobic oxidative reactivity with synthetic systems is comparatively underexplored.25,47-50 We have been interested in investigating ligands which can store both protons and electrons and have demonstrated this reactivity on dihydrazonopyrrole (DHP) scaffolds.51,52 Specifically, this functionality in Ph,TolDHP (Ph,TolDHP = 2,5-bis((2-phenylhydrazono)(p-tolyl)methyl)-pyrrole) complexes of Ni enables the homolytic cleavage of H2O and subsequent oxidative reactivity, but also results in aerobic decomposition via ligand-based C─H oxidation.53 To avoid this decomposition pathway,tBu,TolDHP (tBu,TolDHP = 2,5-bis((2-t-butylhydrazono)(p-tolyl)methyl)-pyrrole) was targeted as a variant where the less reactive C─H bonds on the tBu substituent should prevent ligand-based oxidation and subsequently facilitate oxidative reactivity. Herein we report that this ligand enables the activation of O2 to superoxide via ligand-based electron transfer, leading to a Ni(II) superoxo complex which can mediate oxidative reactivity including alcohol oxidation, O-atom transfer, and activation of benzylic C─H bonds.
Results and Discussion
Synthesis and Electronic Structure of a T-shaped Ni Complex
The dihydrazonopyrrole ligand, tBu,TolDHP, is synthesized by condensation of excess t-butyl hydrazine with the previously reported ditolylacyl pyrrole at high temperature for 5 days, after which it is isolated in 66% yield as a doubly protonated hydrochloride salt.51,52 Deprotonation with four equivalents of n-BuLi and subsequent addition to (DME)NiCl2 (DME = dimethoxyethane) yields a deep purple complex in 43% yield, assigned as [tBu,TolDHP•]Ni (1) (Scheme 1) which can be purified by passage through a silica plug. Complex 1 is paramagnetic, and Evan’s method gives a μeff of 2.02 B.M., indicating an S = ½ spin system. The X-band EPR spectrum of 1 in benzene is also consistent with this assignment, as a broad axial signal is observed with features at g = 2.24 and 2.12 (Figure S9). When the EPR spectrum was recorded in THF, a much more complicated signal arises, likely from a solvent-binding equilibrium in the fourth coordination site of the Ni center (Figure S10). Crystals of 1 were obtained to allow for more detailed structural analysis of this complex, and single crystal X-ray diffraction (SXRD) revealed a T-shaped Ni complex bound to tBu,TolDHP through the pyrrole nitrogen and both β-nitrogens of the hydrazone arms of the ligand (Figure 1). The structure is symmetric within the estimated standard deviation, with the two Ni-Nhydrazone bond lengths being 1.843(4) Å and 1.843(7) Å. The Npyrrole-Ni-Nhydrazone angles of 93.78(1)° and 93.79(1)° indicate a nearly perfect T-shaped geometry. T-shaped complexes are uncommon with Ni(II). While the DHP scaffold may have some electronic influence in enforcing a T-shaped geometry in 1, this coordination is likely facilitated by steric crowding from the tBu groups as well.54-58
Scheme 1.
Synthesis of 2 and 3 from 1 and interconversion of 2 and 3
Figure 1.
SXRD structure of 1. Ellipsoids are set to 50% probability. Hydrogen atoms have been omitted for clarity, and one p-tol ring was modeled for 3-component disorder but only one orientation is shown for clarity. Selected bond lengths and angles: Ni-N1 1.843(4) Å, Ni-N3 1.877(3) Å, Ni-N5 1.843(7) Å, N1-N2 1.302(5) Å, N5-N4 1.309(4) Å, C5-N2 1.349(6) Å, N1-Ni-N3 93.78(1)°, N5-Ni-N3 93.79(1)°.
This complex is analogous to the previously published [Ph,TolDHP•]Ni T-shaped complex which was assigned as a high spin S = 1 Ni(II) center antiferromagnetically coupled to a ligand-based radical based on structural and spectroscopic data. The bond lengths in 1 are very similar to those in [Ph,TolDHP•]Ni with both the Ni─N bond distances and the bond lengths within the ligand backbone nearly identical between the two structures. The similarity in bond lengths within the DHP scaffold and in the Ni-N distances indicates that the ligands in both complexes have a similar electronic structure. The deviation from g = 2 in the EPR spectrum of 1 is similar to that of [Ph,TolDHP•]Ni and can be explained by a significant amount of spin density localized on Ni as would be expected for a high-spin Ni(II) center.
To further investigate this proposed electronic configuration, 1 was modeled with a variety of DFT calculations including broken symmetry treatments (see SI). All calculations on 1 illustrate a similar picture which is consistent with an S = 1 Ni center antiferromagnetically coupled to a ligand radical. The Ni center has a Mulliken spin of 1.12, with a mixture of positive and negative spin densities of −0.32 and 0.22 on the ligand backbone. The negative spin density is distributed on both hydrazones and through the pyrrole carbons of the ligand while the positive spin density is largely centered on the pyrrole nitrogen and is likely due to covalency between Ni and N (Figure S42). Here too, the spin density is qualitatively identical to that observed for the Ph-substituted system (Figure S44). In sum, all of these data are consistent with the assignment of 1 as a high-spin Ni(II) center coupled antiferromagnetically to a ligand-based radical, directly analogous to the electronic structure of [Ph,TolDHP•]Ni.
Ligand-Based Redox Chemistry
With the concrete assignment of a ligand-based radical bound to Ni(II) we wanted to examine whether ligand based redox chemistry akin to what is proposed in biological systems is accessible in 1. The cyclic voltammogram (CV) of 1 shows two reversible redox events: an oxidation at −0.3 V vs. Fc/Fc+ and a reduction at −1.7 V vs Fc/Fc+ (Figure S28), which we assign as ligand-based oxidation and reduction respectively based on comparison with similar couples in the Ph substituted system. These couples are shifted approximately 300 mV negative of those in [Ph,TolDHP•]Ni, suggesting a more reducing ligand environment putatively due to the more donating tBu groups.
We then attempted to chemically access these ligand-based redox events. While we have been unable to isolate more reduced complexes, addition of one equivalent of AgOTf to 1 results in an immediate color change from purple to deep blue. The 1H NMR spectrum of this reaction product shows the disappearance of the characteristic broad peaks for 1, with new paramagnetic features growing into the spectrum, assigned as the four-coordinate triflate adduct [tBu,TolDHP]NiOTf (2, Figure S4), which was isolated in 80% yield. The 19F NMR spectrum of 2 indicates that the triflate anion remains bound to the metal center in solution, as demonstrated by the chemical shift and broadening of the triflate signal (Figure S5). Complex 2 is best assigned as a high-spin Ni center with an oxidized DHP ligand. The paramagnetism of the complex and an Evans method magnetic moment of μeff = 2.87 B.M. support the assignment of an S = 1, high-spin Ni(II) center. The solid-state structure of 2 was obtained by SXRD and confirms the connectivity of the complex, but poor diffraction precluded analysis of specific bond lengths. The structure does show a notable chain structure where each Ni center is bridged to an adjacent Ni through the triflate ligands, leading to 1-D chains in the solid state (Figure S51). This binding mode is unlikely in solution due to the complex’s solubility in benzene and other non-polar solvents. The chemical accessibility and reasonable potentials of this ligand-based redox event suggested to us that reactivity with O2 would be viable.
Formation and Characterization of a Ni Superoxide Complex
We then turned to investigate the aerobic reactivity of 1 to determine whether the tBu groups effectively stabilize this complex towards oxidative degradation. Complex 1 undergoes a distinct color change from purple to red when exposed to air or pure O2 in a benzene solution resulting in a product with no discernible 1H NMR signals. We have assigned this new species as a Ni superoxo complex [tBu,TolDHP]NiO2 (3) based on a variety of characterization methods. Tracking the conversion of 1 to 3 in benzene by UV-vis spectroscopy reveals an isosbestic transition suggesting a clean 1:1 transformation (Figure S20). Complex 3 has a solution magnetic moment of μeff = 1.65 B.M., confirming that the complex is still an S = ½ species despite the lack of 1H NMR signals. X-band EPR indicates the formation of a new species that displays a rhombic S = ½ signal with g = 2.23, 2.10, and 2.07 (Figure S11). These values are consistent with other reported Ni(II) superoxo complexes.37-49
We have extensively attempted to collect Raman data on 3 with no success. We have been, however, able to record infrared (IR) spectra of 3 as a KBr pellet and in solution with both 16O2 and 18O2. The solid state spectrum of 3 shows a isotope sensitive feature assigned as an O─O stretch which appears at 1045 cm−1 upon labeling, similar to other published vibrational data for superoxo species.37-46 The feature with natural abundance O2 is difficult to directly assign due to overlap with other features in that area, but is likely underneath a feature at ~1105 cm−1 (Figure S34) to give a shift of 60 cm−1 that is within experimental error of the predicted shift of 64 cm−1 from a simple harmonic oscillator approximation. In an effort to obtain a better subtraction dataset, the IR spectrum of 3 was also recorded as a C6H6 solution. A 16O2 ─ 18O2 subtraction spectrum shows the appearance of a feature at 1097 cm−1 and a decrease in intensity of the features at 1170 cm−1 (Figure S35). This gives a shift of 73 cm−1, which is also within error of the predicted shift of 68 cm−1. Taken together, these vibrational analyses are consistent with the assignment of a superoxo complex.
Complex 3 is extremely oily and soluble which has precluded the determination of its structure via SXRD analysis. To circumvent this issue Ni K-edge X-ray absorption spectroscopy (XAS) was performed to probe the geometry and electronic structure of Ni (Figure 2). Complexes 1 and 3 have distinct shapes in their edge regions consistent with a change in coordination number upon exposure to air (Figure 2A). The intense rising edge in the spectrum of 1 at 8338.9 eV corresponds to a 1s→4p transition due to the expected non-degenerate 4p levels in a T-shaped geometry. Similar features are observed in reported XAS spectra of trigonal planar Cu(I) complexes.59 In contrast, this feature in 3 is assigned as a shakedown transition by comparison to similar features observed in square planar 3d transition metal complexes with ligand to metal charge transfer transitions (LMCT).59 The lowered intensity of this feature is likely due to the deviation from ideal D4h symmetry as predicted in the DFT calculated structure of 3 (Figure 3).
Figure 2.
A. Ni K-edge X-ray absorption data for 1 and 3, showing the normalized energies of the XANES region. B. R-space EXAFS fitting for 3, with K-space shown in inset.
Figure 3.
Calculated structure of 3 (A) and spin density plot of 3 (B) showing the unpaired spin density in the complex. Iso values are set at 0.005.
In addition to this feature, a 1s→3d transition is observed at 8333.6 eV in both 1 and 3. The intensity of 1s→3d pre-edge features is dependent on the degree of p-d mixing. The coordination of an O atom to the T-shaped geometry of 1 will lead to a distorted square-planar geometry. This higher coordination number should increase the energy gap between the d and p levels, effectively decreasing p-d mixing and causing a drop in the intensity of this pre-edge feature. Similar drops in intensity have been observed for other series with increasing coordination numbers.60-64 DFT calculations also support this assignment, as the p-orbital contribution to the highest lying d-orbital drops from 8.8% to 1.9% in the conversion from 1 to 3 (Table S3). Taken together, these pre-edge data support the assigned structure of 3.
We have also examined the Extended Absorption Fine Structure (EXAFS) region of the XAS data for 3. (Figure 2B, Figures S40-42, Table S7). The EXAFS data was fit for the first two shells around Ni and confirms the assignment of an end-on Ni superoxo complex, showing a Ni-O bond length of 2.08(1) Å. This bond length is longer than that typically found in Ni superoxo complexes, but is similar to the Ni-O bond length of another end-on Ni superoxo complex that has been characterized by EXAFS.40,41,44 The second shell fitting also distinguished the second O of the superoxo ligand at 2.70(1) Å from the Ni, indicating there is no interaction with the metal but confirming the presence of the superoxo ligand. The second shell fit also included the nitrogen of the hydrazone and carbons of the t-butyl group to confirm that these signals are not interfering with the superoxo oxygen, and the C and N scatterers are distinct at longer distances of at least 0.17 Å further from Ni. The Ni-N bond lengths are slightly lengthened compared to 1, with a Ni-Npyrrole distance of 1.873(6) Å and Ni-Nhydrazone distances of 1.920(6) Å, suggesting ligand-based oxidation to a monoanionic ligand in 3 weakens the ligand binding to Ni compared to the dianionic ligand in 1. Attempts to fit the second shell of 3 without the second O of the superoxo ligand resulted in a significantly worse fit, indicating the second O is necessary for describing the structure (Table S9). Overall the EXAFS data and analysis verifies the structure of 3 as a Ni superoxo species.
The XAS data on 1 and 3 also provide an opportunity to probe the oxidation state of the Ni center and verify the proposed ligand centered electron transfers. There is effectively no energy shift (less than the 0.4 eV resolution) for the K-edge or pre-edge features in the generation of 3 from 1. Although the Ni K-edge does not show a large change with oxidation state, a 1.8 eV shift in the K-edge and 0.7 eV shift in the pre-edge energy has been observed between Ni(III) peroxo and Ni(II) superoxo complexes.44,65 The absence of similar shifts in the conversion from 1 to 3 indicates that no metal centered redox occurs.
Finally, we have examined the geometry and electronic structure of 3 with DFT calculations (Figure 3). The geometry of 3 in the DFT computed structure is that of an end-on superoxide ligand consistent with our assignment. Attempts to minimize an η2 structure resulted in dissociation of the second O ligand to generate an end-on structure. The bond lengths of 3 are reasonably close to the values obtained from EXAFS fitting, supporting that the end-on structure is assigned correctly (Table S8). The computed spin density in 3 is predicted to lie nearly entirely on O2 (Figure 3), consistent with our spectroscopic data. TD-DFT was also performed to compare the calculated UV-vis data with the experimentally determined data. The shape of the UV-vis spectrum of 3 is reliably reproduced, with the peaks of the calculated spectrum within a reasonable error of those in the experimentally determined spectrum (Figures S46 and S47). In sum, all of these data are consistent with the proposed structural assignment of 3 as an end-on Ni superoxo complex which is formed by ligand centered electron transfer with no formal oxidation state change on Ni.
Oxidative Reactivity
With the characterization of 3 in hand, we then examined the reactivity of this system both to confirm its composition and to probe whether 3 can mediate oxidative conversions. Stirring 2 with one equivalent of KO2 for one hour results in a color change from blue to dark red (Scheme 1). Analysis of this reaction mixture by EPR and UV-vis spectroscopy reveals signals consistent with conversion to 3 (Figures S15 and S22). These data also support the assignment of 3 as a Ni superoxo complex as using an external superoxide source produces the same product. Electrochemistry of 3 was also investigated to probe the reversibility of O2 binding and activation. CV of 3 shows an irreversible oxidation and reduction at 0.32 and −1.7 V vs. Fc/Fc+ respectively (Figure S29). Chemical oxidation was targeted with the hypothesis that oxidation of 3 could convert back to 2. Addition of one equivalent of AgOTf to a stirring THF solution of 3 indeed results in the reappearance of 2 as indicated by UV-vis spectroscopy, albeit in a slightly lower yield of ~50% (Figure S21). This is likely due to oxidation of the superoxide ligand to dioxygen, which then dissociates as the triflate binds to give 2.
We then investigated the reactivity of 3 with the H-atom donors diphenylhydrazine (DPH) and 9,10-dihydroanthracene (DHA). Complex 3 somewhat surprisingly shows little to no reactivity with DHA, at room temperature, as determined by 1H NMR and GC/MS monitoring, but does generate 0.6 eq of azobenzene within 10 minutes when reacted with DPH at room temperature under N2 (Figure S6). We rationalize that the muted reactivity with DHA implies a very weak O─H BDE for the putative Ni hydroperoxo product of C─H abstraction. Computations support this hypothesis and suggest an O─H BDE of ~63 kcal/mol (Table S5).
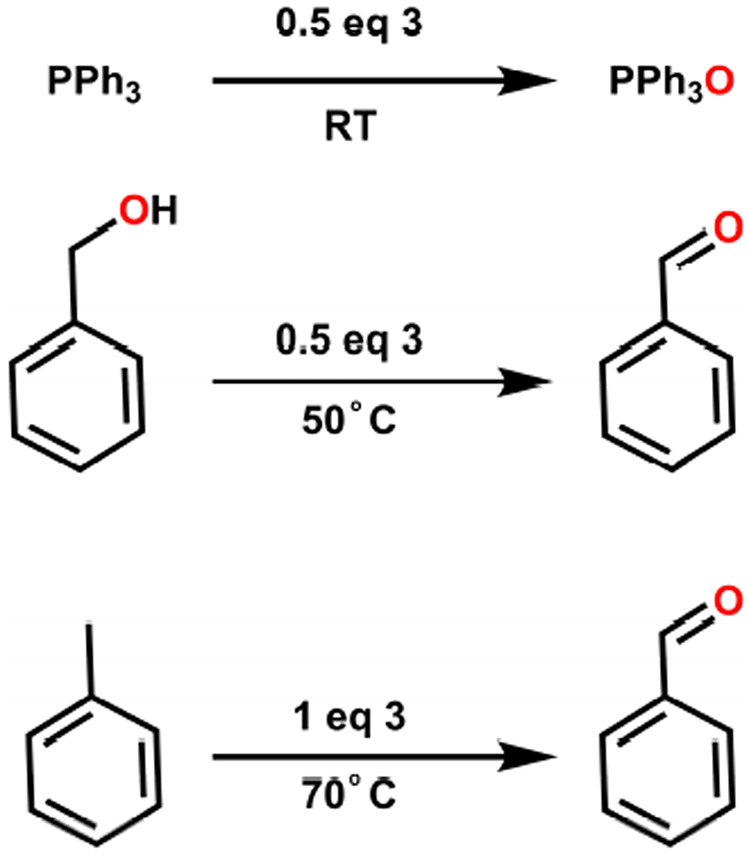
Despite this muted H-atom abstraction reactivity, complex 3 still mediates oxidative reactivity with organic substrates. Complex 3 reacts with triphenylphosphine under N2 to produce two equivalents of triphenylphosphine oxide by 31P NMR at room temperature (Scheme 2). Oxidation of alcohols to aldehydes was also investigated and NMR analysis indicates that complex 3 converts two equivalents of benzyl alcohol to benzaldehyde at 50 °C over three hours under air (Scheme 2). Finally, C─H activation was studied using toluene as a test substrate. At 70 °C for three hours under air, 3 produces about one equivalent of benzaldehyde from neat toluene, likely via C─H activation to benzyl alcohol then oxidation of the benzyl alcohol to benzaldehyde. This reaction was also performed using 3 enriched with 18O from reaction of 1 with 18O2. GC/MS analysis indicates that the benzaldehyde product is enriched with 18O2 as expected (Figure S39). There are very few examples of C─H oxidation reactivity with Ni,66-70 though superoxo species in general have been suggested as intermediates in C─H oxidations 48 The consistent appearance of two equivalents of oxidized products (or double oxidation in the case of toluene) suggests that the reaction is stoichiometric in oxygen equivalents. Furthermore, the fact that 3 generates two equivalents of triphenylphosphine oxide under N2 also implies that both of the O-equivalents from the superoxide ligand can be transferred to substrates. It is currently unclear why the complex cannot turn over to bind another equivalent of O2 and enable catalytic oxidations.
Scheme 2.
Oxidative reactivity of 3.
The muted C─H abstraction reactivity of 3 with DHA raises interesting mechanistic questions about the oxidative activity of this complex. The resulting mixtures after the oxidation reactions are complicated and further study is needed to understand potential decomposition routes. It is possible if not likely that the elevated temperatures required for this oxidative reactivity lead to the formation of another species, potentially dissociated superoxide, which is the active oxidant. Kinetic analysis of the reaction of 3 with benzyl alcohol shows a second order dependence on the concentration of 3, indicating a more complex mechanism than direct substrate oxidation by the superoxo species (Figure S24-27). The low reactivity of 3 is unusual in that many other Ni(II) superoxo species are much more unstable and reactive. Because complex 3 is formed by a comparatively mild, ligand-based redox event, the resulting superoxo is not very oxidizing. Therefore, the ligand-based nature of the electron transfer may play an important role in the isolation and study of this species, suggesting the viability of ligand-based redox events for stabilizing highly reactive compounds. While still stoichiometric, the oxidative reactivity of 3 is unusual and demonstrates that the ligand-based activation of O2 enables oxidative reactivity for Ni centers as has been proposed in quercetin dioxygenase.
Conclusion
Herein we have demonstrated ligand-based redox activation of O2 to generate an unusual Ni superoxo species. This complex can mediate oxidative reactions including stoichiometric C─H activations. This ligand-based electron transfer to O2 is related to that in biological systems proposed to utilize a similar Ni(II) superoxo intermediate. The superoxo complex 3 was characterized with EPR spectroscopy, XAS, IR spectroscopy, and computational studies to validate the proposed ligand-based reactivity pathway. This work represents an important step forward in leveraging ligand-based redox for inexpensive and benign aerobic oxidations with Ni.
Experimental
General Considerations:
All reagents were purchased from commercial suppliers and used without further purification unless otherwise specified. The t-butyl hydrazine was synthesized by deprotonating t-butyl hydrazine hydrochloride All manipulations were carried out under an atmosphere of N2 using standard Schlenk and glovebox techniques. Glassware was dried at 180 °C for a minimum of two hours and cooled under vacuum prior to use. Solvents were dried on a solvent purification system from Pure Process Technology and stored over 4 Å molecular sieves under N2. Tetrahydrofuran was stirred over NaK alloy and run through an additional activated alumina column prior to use to ensure dryness. Solvents were tested for H2O and O2 using a standard solution of sodium-benzophenone ketyl radical anion. C6D6 was dried by passage over a column of activated alumina and stored over 4 Å molecular sieves in the glovebox. 1H and 13C{1H} spectra were recorded on Bruker DRX 400 or 500 MHz spectrometers. Chemical shifts are reported in ppm units referenced to residual solvent resonances for 1H and 13C{1H} spectra, UV-vis spectra were recorded on a Thermo Evolution 300 spectrometer and addition of gases was performed by injecting via syringe into a cuvette sealed with a septum. UV-vis spectra at elevated temperature were done using a Unisoku Cryostat. IR was recorded on a Bruker Tensor II. EPR spectra were recorded on an Elexsys E500 Spectrometer with an Oxford ESR 900 X-band cryostat and a Bruker Cold-Edge Stinger and were simulated using the Easyspin suite in Matlab software.71 GC/MS was collected on an Agilent SQ GCMS with 5977A single quad MS and 7890B GC. Elemental analysis was performed by Midwest Microlabs. Electrochemical measurements were performed using a BAS Epsilon potentiostat and analyzed using BAS Epsilon software version 1.40.67NT. Cyclic voltammetry measurements were made using a glassy carbon working electrode, platinum wire counter electrode, and silver wire pseudo-reference electrode, and referenced to internal Fc/Fc+.
X-Ray Structure Determination
Crystal Structure Determination.
The diffraction data were measured at 100 K on a Bruker D8 VENTURE with PHOTON 100 CMOS detector system equipped with a Mo-target micro-focus X-ray tube (λ = 0.71073 Å). Data reduction and integration were performed with the Bruker APEX3 software package (Bruker AXS, version 2015.5-2, 2015). Data were scaled and corrected for absorption effects using the multi-scan procedure as implemented in SADABS (Bruker AXS, version 2014/5, 2015, part of Bruker APEX3 software package). The structure was solved by the dual method implemented in SHELXT and refined by a full-matrix least-squares procedure using OLEX2 software package (XL refinement program version 2014/7).72-75 Suitable crystals were mounted on a cryo-loop and transferred into the cold nitrogen stream of the Bruker D8 Venture diffractometer. Most of the hydrogen atoms were generated by geometrical considerations and constrained to idealized geometries and allowed to ride on their carrier atoms with an isotropic displacement parameter related to the equivalent displacement parameter of their carrier atoms. Compound 1 was modeled for three component disorder of one of the p-tol rings. Compound 2 was modeled for two component disorder of the bridging triflate group.
X-ray Absorption Measurements.
Powder samples were prepared by grinding finely. A Teflon washer (5.3 mm internal diameter) was sealed on one side with Kapton tape and powder was then transfer transferred to the inside of this ring before compacting with a Teflon rod and sealing the remaining face with Kapton tape. All sample preparation was performed under an inert atmosphere. X-ray absorption near-edge spectra (XANES) and Extended Absorption Fine Structure (EXAFS) were employed to probe the local environment around Ni. Data were acquired at the Advanced Photon Source at Argonne National Laboratory with a bending magnet source with ring energy at 7.00 GeV. Ni K-edge (8332.8 eV) data were acquired at the MRCAT 10-BM beam line in transmission. The incident, transmitted and reference X-ray intensities were monitored using gas ionization chambers. A metallic nickel foil standard was used as a reference for energy calibration and was measured simultaneously with experimental samples. X-ray absorption spectra were collected at room temperature.
Data collected was processed using the Demeter76-78 software suite by extracting the EXAFS oscillations χ(k) as a function of photoelectron wavenumber k. The theoretical paths were generated using FEFF6 and the models were determined using the fitting program Artemis.
Synthesis of 2,5-bis((2-t-butylhydrazono)(p-tolyl)methyl)-pyrrole) (tBu,TolDHP•2HCl).
In the glovebox, 500 mL 3-neck round bottom flask equipped with two septa and a reflux condenser was charged with 2,5-ditolylacylpyrrole51,52 (3.0 g, 9.9 mmol), t-butyl hydrazine (6.1 g, 70 mmol, 7.0 eq), toluene (250 mL), molecular sieves, catalytic 2 M HCL etherate (0.01 mL) and a stir bar. This was removed from the glovebox and refluxed on the Schlenk line for 5 days at 115 °C. The resulting yellow solution was cooled to room temperature, returned to the glovebox, and filtered through Celite to remove the molecular sieves, giving a clear yellow solution. This was evaporated to dryness to give an orange oil, which was taken up in benzene, then 2 M hydrogen chloride in diethyl ether (10 mL, 2 eq) was added, resulting in precipitation of a yellow solid. The yellow solid was collected by filtration, then the benzene filtrate was evaporated to dryness, taken up in petroleum ether (100 mL) and filtered to collect more yellow solid. The two batches of solid were combined to give tBu,TolDHP•2HCl (3.4 g, 6.6 mmol, 66%). 1H NMR (CDCl3, 500 MHz, 25° C) δ = 12.96 (s, 1H, N-H pyrrole), 11.46 (s, 4H, N-H hydrazone), 7.63 (d, 4H, J = 8 Hz, tol C-H), 7.27 (d, 4H, J = 8 Hz, tol C-H), 6.48 (d, 2H, J = 4 Hz, Pyrrole C-H), 2.46 (s, 6H, tol-Me), 1.71 (s, 18H, tbu). 13C {1H} NMR (CDCl3, 125 MHz 25 °C) δ = 164.6 (C=N), also 142.6, 133.3, 129.9, 128.8, 126.9, 119.8, 62.5, 25.3, 21.3. IR (nujol mull, cm−1): 3457 (m, N-H), 3131 (m, N-H), 3088 (m, N-H), 1598 (s, C=N). Anal calcd: C 65.1, H 7.6, N 13.5 Found: C 65.8, H 7.7, N 11.8
Synthesis of [tBu,TolDHP•]Ni (1).
To a stirring THF solution of tBu,TolDHP•2HCl (0.47 g 0.92 mmol, 40 mL) was added 2.5 M n-BuLi in hexanes (1.5 mL, 4 eq), turning from yellow to red. The red mixture was stirred for 5 minutes, then added to a stirring slurry of NiCl2DME in THF, turning deep purple. After stirring overnight for 12 hours, all volatiles were removed under vacuum, and the resulting purple residue was taken up in benzene and passed through a silica plug, then evaporated to give 1 as a purple powder. Yield: 0.20 g, 0.40 mmol, 43%. Single crystals can be obtained by crystallization from concentrated Et2O at −35° C. EPR (frozen toluene/petroleum ether, 15 K, gz, gx, gy) 2.24, 2.10, 2.07. Evans method (C6D6, 25 °C, μB) μeff = 2.02. IR (KBr pellet, cm−1) 3026 (w), 2963 (s), 2919 (s), 1905, 1797, 1521 (s, C=N), 1466, (m) 1460 (s), 1364 (s), 1011 (m), 820 (s), 719 (m) UV-vis (Benzene solution) 275 nm, 570 nm, 80 nm. Anal calcd: C 67.3; H 6.8; N 14.0. Found: C 66.5; N 7.6; H 13.7.
Synthesis of [tBu,TolDHP]NiOTf (2).
To a stirring THF solution of 1 (0.050 g, 0.1 mmol, 5 mL) was added a THF solution of AgOTf (0.026 g, 0.1 mmol, 2 mL, 1 eq) resulting in an immediate color change from dark purple to dark blue. The reaction was stirred for 30 min, then filtered and the solvent was evaporated. The resulting blue residue was washed with petroleum ether (5 mL) then taken up in benzene (10 mL), filtered, and evaporated to give 2 as a blue powder (0.052 g, 0.08 mmol, 80%). Single crystals could be obtained by layering a toluene solution of 2 with petroleum ether at −35 °C. 1H NMR (C6D6, 500 MHz, 25° C) δ = 46.06, 14.13, 12.54, 12.40, −1.36. IR (KBr Pellet, cm−1) 2973 (s), 2920 (m), 2867 (w). 1614 (m), 1508 (m), 1468 (m) 1366 (s), 1261 (vs), 1174 (s) 1100 (m), 1032 (m), 822 (w). Anal Calc’d C 53.7; H 5.2; N 10.8. Found: C 53.8; H 5.6; N 10.6
Synthesis of [tBu,TolDHP]NiO2 (3).
A solution of 1 in benzene (0.023 g, 0.046 mmol, 5 mL) was removed from the glovebox and air was bubbled through the solution for 30 seconds, resulting in a color change from purple to red. All volatiles were removed under vacuum, giving 3 as a red oil. Yield: 0.022 g, 0.041 mmol, 90%. EPR (frozen benzene, 15 K, gz, gx, gy) 2.23, 2.09, 2.00. Evans method (C6D6, 25 °C, μB) μeff = 1.65. IR (KBr Pellet, cm−1) 2965 (s), 2919 (m), 2861 (m), 1607 (m), 1512 (m), 1440 (s), 1361 (s), 1303 (m), 1266 (s), 1183 (m), 1115 (m), 1103 (m), 1014 (s), 833 (m), 805 (m). UV-vis (Benzene solution) 350 nm, 550 nm, 870 nm. ESI-MS (MeCN solution): m/z = 539.2 [tBu,TolDHP]Ni(NCMe). Due to decomposition of the material over time, satisfactory elemental analysis could not be obtained.
Synthesis of 3 by reaction of 2 with KO2.
To a stirring THF solution of 2 (0.013 g, 0.02 mmol, 3 mL) was added a THF slurry of KO2 (0.0015 g, 0.02 mmol, 1 mL, 1 eq). This was stirred for 1 hour, slowing turning from deep blue to dark red. The solution was filtered and evaporated under vacuum to give 3 as a red oil.
Oxidation of benzyl alcohol.
In a nitrogen glovebox, to a solution of 1 in C6D6 (0.005 g, 0.01 mmol, 0.5 mL) was added benzyl alcohol (0.002 mg, 0.02 mmol, 2 eq) and naphthalene (6 mg). This was added to an NMR tube and removed from the glovebox and exposed to air, turning from purple to red as 1 reacted to form 3. The tube was resealed and heated to 50 °C for 5 hours and the appearance of 1.9 equivalents of benzaldehyde was observed by integration against the internal naphthalene standard. The rate of the reaction was tracked via UV-Vis on a 1 mM solution of 3, and monitored at 550 nm. The absorbance was fit to a non-linear curve to determine the rate constant of the reaction, where the final absorbance was also fit as a variable.
Oxidation of toluene.
To 5 mL of toluene was added 1 (0.005 g, 0.01 mmol), which was then removed from the glovebox, exposed to air to convert the 1 to 3, resealed under air, and then heated to 70 °C for 3 hours, at which point it was tested by GC/MS and the resulting benzaldehyde peak was integrated versus a benzaldehyde standard curve and indicated the formation of 0.9 equivalents of benzaldehyde.
Oxidation of PPh3.
To a C6D6 solution of 1 (0.003 g, 0.006 mmol, 0.5 mL) was added PPh3 (0.030 g, 0.012 mmol, 20 eq) and removed from the glovebox, exposed to air overnight, then tested by 31P NMR. The amount of OPPh3 was quantified by integration compared to the PPh3 peak to give the percentage of the PPh3 that had converted indicating the formation of 2.0 equivalents of OPPh3. An identical experiment using 3 under N2 also converted PPh3 to OPPh3, and control experiments with PPh3 under air in the absence of 3 did not show oxidation under the same timescale.
Reaction of 3 with DPH.
To a stirring C6D6 solution of 3 (0.010 g, 0.019 mmol, 1 mL) was added freshly recrystallized DPH (0.007 g, 0.037 mmol, 1.9 eq) and stirred at room temperature in the glovebox. After 10 minutes the reaction was sampled via NMR and showed the appearance of 0.6 equiv. of azobenzene, indicating slow H-atom transfer reactivity.
Reaction of 3 with DHA.
To a stirring C6D6 solution of 3 (0.010 g, 0.019 mmol, 1 mL) was added freshly recrystallized DHA (0.004 g, 0.022 mmol, 1.1 eq) and stirred at room temperature in the glovebox overnight. Monitoring by NMR and GC/MS indicated no change to the DHA.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institute of Health (R35 GM133470). We thank the University of Chicago for funding as well as 3M corporation for a NTFA to John S. Anderson. We would like to thank Dr. Ethan Hill for assistance with EPR measurements. We also thank the Research Computing Center at the University of Chicago for providing computing resources. We thank Dr. John Katsoudas and Dr. Joshua Wright for assistance with XAS collection at beamline 10-BM. MRCAT operations are supported by the Department of Energy and the MRCAT member institutions. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Footnotes
Supporting Information.
Experimental Procedures, NMR, IR, GC/MS, UV-Vis, EPR, XAS, Electrochemistry, SXRD data and DFT. (PDF)
Crystal Data (CIF)
This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- (1).Chirik PJ Iron- and Cobalt-Catalyzed Alkene Hydrogenation: Catalysis with Both Redox-Active and Strong Field Ligands. Acc. Chem. Res 2015, 48 (6), 1687–1695. [DOI] [PubMed] [Google Scholar]
- (2).Luca OR; Crabtree RH Redox-active Ligands in Catalysis. Chem. Soc. Rev 2013, 42 (4), 1440–1459. [DOI] [PubMed] [Google Scholar]
- (3).Lyaskovskyy V; de Bruin B Redox Non-Innocent Ligands: Versatile New Tools to Control Catalytic Reactions. ACS Catal. 2012, 2 (2), 270–279. [Google Scholar]
- (4).Chirik PJ; Wieghardt K Radical Ligands Confer Nobility on Base-Metal Catalysts. Science, 2010, 327, 794–795. [DOI] [PubMed] [Google Scholar]
- (5).Bhutto SM; Holland PL Dinitrogen Activation and Functionalization Using β-Diketiminate Iron Complexes. Eur. J. Inorg. Chem 2019, 2019 (14), 1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Coughlin EJ; Qiao Y;Lapsheva E; Zeller M; Schelter EJ; Bart SC Uranyl Functionalization Mediated by Redox-Active Ligands: Generation of O-C Bonds via Acylation. J. Am. Chem. Soc 2019, 141 (2), 1016–1026. [DOI] [PubMed] [Google Scholar]
- (7).Zhanaidarova A; Ostericher AL; Miller CJ; Jones SC; Kubiak CP Selective Reduction of CO2 to CO by a Molecular Re(ethynyl-bpy)(CO)3Cl Catalyst and Attachment to Carbon Electrode Surfaces. Organometallics 2019, 38 (6), 1204–1207. [Google Scholar]
- (8).Hollas AM; Ziller JW.; Heyduk AF Three oxidation states of the bis(3,5-di-tert-butyl-2-phenolato)azanido pincer ligand on chromium(III). Polyhedron 2018, 143, 111–117. [Google Scholar]
- (9).Lindley BM; Bruch WJ; White PS; Hasanayn F; Miller AJM Ammonia Synthesis from a Pincer Ruthenium Nitride via Metal-Ligand Cooperative Proton-Coupled Electron Transfer. J. Am. Chem. Soc 2017, 139 (15), 5305–5308. [DOI] [PubMed] [Google Scholar]
- (10).Atienza CCH; Diao T; Weller KJ; Nye SA; Lewis KM; Delis JGP; Boyer JL; Roy AK; Chirik PJ Bis(imino)pyridine Cobalt-Catalyzed Dehydrogenative Silylation of Alkenes: Scope, Mechanism, and Origins of Selective Allylsilane Formation. J. Am. Chem. Soc 2014, 136 (34), 12108–18. [DOI] [PubMed] [Google Scholar]
- (11).Myers TW; Berben LA, Aluminum-ligand cooperation promotes selective dehydroegnation of formic acid to H2 and CO2. Chem. Sci 2014, 5 (7), 2771–2777. [Google Scholar]
- (12).Blackmore KJ; Lal N; Ziller JW; Heyduk AF Catalytic Reactivity of a Zirconium(IV) Redox-Active Ligand Complex with 1,2-Diphenylhydrazine. J. Am. Chem. Soc. 2008, 130 (9), 2728–2729. [DOI] [PubMed] [Google Scholar]
- (13).Liu JJ; Marinescu SC Harnessing the Oxidative Power of Monooxygenases through Electrochemistry. ACS Cent. Sci 2019, 5(4), 577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Pegis ML; Martin DJ; Wise CF; Brezny AC; Johnson S,I; Johnson LE; Kumar N Raugei S; Mayer JM Mechanism of Catalytic O2 Reduction by Iron Tetraphenylporphyrin. J. Am. Chem. Soc 2019, 141 (20), 8315–8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Bhunia S; Rana A; Roy P; Martin DJ; Pegis ML; Roy B; Dey A Rational Design of Mononuclear Iron Porphyrins for Facile and Selective 4e−/4H+ O2 Reduction: Activation of O─O Bond by 2nd Sphere Hydrogen Bonding. J. Am. Chem. Soc 2018, 140 (30), 9444–9457. [DOI] [PubMed] [Google Scholar]
- (16).Pereira MM; Dias LD; Calvete MJF Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 8 (11), 10784–10808. [Google Scholar]
- (17).Huang X; Groves JT Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev 2018, 118 (5), 2491–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Meunier B; de Visser SP; Shaik S Mechanism of Oxidation Reactions Catalyzed by Cytochrome P450 Enzymes. Chem. Rev 2004, 104 (9), 3947–3980. [DOI] [PubMed] [Google Scholar]
- (19).Sono M; Roach MP; Coulter ED; Dawson JH Heme-Containing Oxygenases. Chem. Rev 1996, 96 (7), 2841–2887. [DOI] [PubMed] [Google Scholar]
- (20).Collman JP Synthetic models for the oxygen-binding hemoproteins. Acc. Chem. Res 1977, 10 (7), 265–72. [Google Scholar]
- (21).Adam SM; Wijeratne GB; Rogler PJ; Diaz DE; Quist DA; Liu JJ; Karlin KD Synthetic Fe/Cu Complexes: Toward Understanding Heme-Copper Oxidase Structure and Function. Chem. Rev 2018, 118 (22), 10840–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Allen SE; Walvoord RR; Padilla-Salinas R; Kozlowski MC Aerobic Copper-Catalyzed Organic Reactions. Chem. Rev 2013, 113 (8), 6234–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Costas M; Mehn MP; Jensen MP; Que L Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates. Chem. Rev 2004, 104 (2), 939–986. [DOI] [PubMed] [Google Scholar]
- (24).Costas M; Chen K; Que L Biomimetic nonheme iron catalysts for alkane hydroxylation. Coord. Chem. Rev 2000, 200-202, 517–544. [Google Scholar]
- (25).Rajabimoghadam K; Darwish Y; Bashir U; Pitman D; Eichelberger S; Siegler MA; Swart M; Garcia-Bosch I Catalytic Aerobic Oxidation of Alcohols by Copper Complexes Bearing Redox-Active Ligands with Tunable H-Bonding Groups. J. Am. Chem. Soc 2018, 140 (48), 16625–16634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hoover JM; Ryland BL; Stahl SS Mechanism of Copper(I)/TEMPO-Catalyzed Aerobic Alcohol Oxidation. J. Am. Chem. Soc 2013, 135 (6), 2357–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hoover JM; Stahl SS Highly Practical Copper(I)/TEMPO Catalyst System for Chemoselective Aerobic Oxidation of Primary Alcohols. J. Am. Chem. Soc 2011, 133 (42), 16901–16910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Groves JT High-valent Iron in Chemical and Biological Oxidations. Inorg. Biochem 2006, 100, 434–447 [DOI] [PubMed] [Google Scholar]
- (29).Marko IE; Gautier A; Dumeunier R; Doda K; Philippart F; Brown SM; Urch CJ Efficient, Copper-Catalyzed, Aerobic Oxidation of Primary Alcohols. Angew. Chem., Int. Ed 2004, 43 (12), 1588–1591. [DOI] [PubMed] [Google Scholar]
- (30).Weiss CJ; Wiedner ES; Roberts JAS; Appel AM Chem. Commun 2015, 51 (28), 6172–6174. [DOI] [PubMed] [Google Scholar]
- (31).Weiss CJ; Das P; Miller DL; Helm ML; Appel AM Nickel phosphine catalysts with pendant amines for electrocatalytic oxidation of alcohols. ACS Catal. 2014, 4 (9), 2951–2958. [DOI] [PubMed] [Google Scholar]
- (32).Sikari R; Sinha S; Jash U; Das S; Brandao P; de Bruin B; Paul ND Deprotonation Induced Ligand Oxidation in a Ni(II) Complex of a Redox Noninnocent N1-(2-Aminophenyl)benzene-1,2-diamine and Its Use in Catalytic Alcohol Oxidation. Inorg. Chem 2016, 55(12) 6114–6123 [DOI] [PubMed] [Google Scholar]
- (33).Broering EP; Truong PT; Gale EM; Harrop TC Synthetic Analogues of Nickel Superoxide Dismutase: A New Role for Nickel in Biology. Biochemistry 2013, 52 (1), 4–18. [DOI] [PubMed] [Google Scholar]
- (34).Barondeau DP; Kassmann CJ; Bruns CK; Tainer JA; Getzoff ED Nickel Superoxide Dismutase Structrue and Mechanism. Biochemistry 2004, 43 (25), 8038–8047. [DOI] [PubMed] [Google Scholar]
- (35).Li H; Wang X; Tian G; Liu Y Insights into the dioxygen activation and catalytic mechanism of the nickel-containing quercetinase. Catal. Sci. Technol 2018, 8 (9), 2340–2351. [Google Scholar]
- (36).Jeoung J-H; Nianios D; Fetzner S; Dobbek H Quercetin 2,4-Dioxygenase Activates Dioxygen in a Side-On O2─Ni Complex. Angew. Chem., Int. Ed 2016, 55 (10), 3281–3284. [DOI] [PubMed] [Google Scholar]
- (37).Noh H; Cho J Synthesis, characterization and reactivity of non-heme 1st row transition metal-superoxo intermediates. Coord. Chem. Rev 2019, 382, 126–144. [Google Scholar]
- (38).Duan P-C; Manz D-H; Dechert S; Demeshko S; Meyer F Reductive O2 Binding at a Dihydride Complex Leading to Redox Interconvertible μ-1,2-Peroxo and μ-1,2-Superoxo Dinickel(II) Intermediate. J. Am. Chem. Soc 2018, 140 (14), 4929–4939. [DOI] [PubMed] [Google Scholar]
- (39).Panda C; Chandra A; Corona T; Andris E; Pandey B; Garai S; Lindenmaier N; Kuenstner S; Farquhar ER; Roithova J; Rajaraman G; Driess M; Ray K Nucleophilic versus Electrophilic Reactivity of Bioinspired Superoxido Nickel(II) Complexes. Angew. Chem., Int. Ed 2018, 57 (45), 14883–14887. [DOI] [PubMed] [Google Scholar]
- (40).Corona T; Company A Spectroscopically Characterized Synthetic Mononuclear Nickel–Oxygen Species. Chem. - Eur. J 2016, 22 (38), 13422–13429. [DOI] [PubMed] [Google Scholar]
- (41).Cho J; Kang HY; Liu LV; Sarangi R; Solomon EI; Nam W Mononuclear nickel(II)-superoxo and nickel(III)-peroxo complexes bearing a common macrocyclic TMC ligand, Chem. Sci 2013, 4 (4), 1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Company A; Yao S; Ray K; Driess M Dioxygenase-Like Reactivity of an Isolable Superoxo–Nickel(II) Complex. Chem. - Eur. J 2010, 16 (31), 9669–9675. [DOI] [PubMed] [Google Scholar]
- (43).Yao S; Bill E; Milsmann C; Wieghardt K; Driess MA “Side-on” Superoxonickel Complex [LNi(O2)] with a Square-Planar Tetracoordinate Nickel(II) Center and Its Conversion into [LNi(μ-OH)2NiL]. Angew. Chem., Int. Ed 2008, 47 (37), 7110–7113. [DOI] [PubMed] [Google Scholar]
- (44).Kieber-Emmons MT; Annaraj J; Seo MS; Van Heuvelen KM; Tosha T; Kitagawa T; Brunold TC; Nam W; Riordan CG Identification of an “End-on” Nickel–Superoxo Adduct, [Ni(tmc)(O2)]. J. Am. Chem. Soc 2006, 128 (44), 14230–14231. [DOI] [PubMed] [Google Scholar]
- (45).Fujita K; Schenker R; Gu W; Brunold TC; Cramer SP; Riordan CG A Monomeric Nickel–Dioxygen Adduct Derived from a Nickel(I) Complex and O2. Inorg. Chem 2004, 43 (11), 3324–3326. [DOI] [PubMed] [Google Scholar]
- (46).Kimura E; Sakonaka A; Machida R Novel Nickel (II) Complexes with Doubly Deprotonated Dioxopentaamine Macrocycli Ligands for Uptake and Activation of Molecular Oxygen. J. Am. Chem. Soc, 1982, 104, 4255–4257 [Google Scholar]
- (47).Wang D; Weinstein AB; White P-B; Stahl SS Ligand-Promoted Palladium-Catalyzed Aerobic Oxidation Reactions. Chem. Rev 2018, 118 (5), 2636–2679. [DOI] [PubMed] [Google Scholar]
- (48).Corcos AR; Villanueva O; Walroth RC; Sharma SK; Bacsa J; Lancaster KM; MacBeth CE; Berry JF Oxygen Activation by Co(II) and a Redox Non-Innocent Ligand: Spectroscopic Characterization of a Radical–Co(II)–Superoxide Complex with Divergent Catalytic Reactivity. J. Am. Chem. Soc 2016, 138 (6), 1796–1799. [DOI] [PubMed] [Google Scholar]
- (49).Garrido-Barros P; Funes-Ardoiz I; Drouet S; Benet-Buchholz J; Maseras F; Llobet A Redox Non-innocent Ligand Controls Water Oxidation Overpotential in a New Family of Mononuclear Cu-Based Efficient Catalysts. J. Am. Chem. Soc 2015, 137 (21), 6758–6761. [DOI] [PubMed] [Google Scholar]
- (50).Henthorn JT; Lin S Agapie T Combination of Redox-Active Ligand and Lewis Acid for Dioxygen Reduction with π-Bound Molybdenum–Quinonoid Complexes. J. Am. Chem. Soc. 2015, 137 (4), 1458–1464. [DOI] [PubMed] [Google Scholar]
- (51).Chang M-C; McNeece AJ; Hill EA; Filatov AS; Anderson JS Ligand-Based Storage of Protons and Electrons in Dihydrazonopyrrole Complexes of Nickel. Chem. - Eur. J 2018, 24 (31), 8001–8008. [DOI] [PubMed] [Google Scholar]
- (52).McNeece AJ; Chang M-C; Filatov AS; Anderson JS Redox Activity, Ligand Protonation, and Variable Coordination Modes of Diimino-Pyrrole Complexes of Palladium. Inorg. Chem, 2018, 57, 7044–7050. [DOI] [PubMed] [Google Scholar]
- (53).Chang M-C; Jesse KA; Filatov AS; Anderson JS Reversible homolytic activation of water via metal–ligand cooperativity in a T-shaped Ni(ii) complex Chem. Sci 2019, 10 (5), 1360–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Yoo C; Lee A T-Shaped Nickel(I) Metalloradical Species. Y. Angew. Chem., Int. Ed., 2017, 56, 9502–9506. [DOI] [PubMed] [Google Scholar]
- (55).Wenz J; Kochan A; Wadepohland H; Gade LH A Readily Accessible Chiral NNN Pincer Ligand with a Pyrrole Backbone and Its Ni(II) Chemistry: Syntheses, Structural Chemistry, and Bond Activations. Inorg. Chem, 2017, 56 , 3631–3643. [DOI] [PubMed] [Google Scholar]
- (56).Rettenmeier CA; Wenz J; Wadepohl H; Gade LH Activation of Aryl Halides by Nickel(I) Pincer Complexes: Reaction Pathways of Stoichiometric and Catalytic Dehalogenations. Inorg. Chem 2016, 55 , 8214–8224 [DOI] [PubMed] [Google Scholar]
- (57).Andrella NO; Sicard AJ; Gorelsky SI; Korobkov I; Baker RT A T-shaped Ni[κ2-(CF2)4−] NHC complex: unusual Csp3─F and M─CF bond functionalization reactions. Chem. Sci 2015, 6, 6392–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Eckert NA; Dinescu A; Cundari TR; Holland PL A T-Shaped Three-Coordinate Nickel(I) Carbonyl Complex and the Geometric Preferences of Three-Coordinate d9 Complexes. Inorg. Chem 2005, 44, 7702–7704. [DOI] [PubMed] [Google Scholar]
- (59).Sarangi R X-ray absorption near-edge spectroscopy in bioinorganic chemistry: Application to M─O2 systems. Coord Chem. Rev 2013, 257, 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Mitsuru S; Komorita S; Yamatera H XANES spectra of copper(II) complexes: correlation of the intensity of the 1s .fwdarw. 3d transition and the shape of the complex. Inorg. Chem 1992, 31, 459. [Google Scholar]
- (61).Wirt MD; Sagi I; Chen E; Frisbie SM; Roxane L; Chance MR J. Am. Chem. Soc 1991, 113, 5299. [Google Scholar]
- (62).Colpas GJ; Maroney MJ; Bagyinka C; Kumar M; Willis WS; Suib SL; Baidya N; Mascharak PK Geometric conformations of intermediates of B12 catalysis by x-ray edge spectroscopy: cobalt(I) B12, cobalt(II) B12, and base-off adenosylcobalamin. Inorg. Chem 1991, 30, 920. [Google Scholar]
- (63).Penner-Hahn JE; Fronko RM; Pecraro VL; Yocum CF; Betts SD; Bowlby NR Structural characterization of the manganese sites in the photosynthetic oxygen-evolving complex using x-ray absorption spectroscopy. J. Am. Chem. Soc 1990, 112, 2549. [Google Scholar]
- (64).Roe AL Schneder DJ; Mayer RJ; Pyrz JW; Widom J; Que L X-ray absorption spectroscopy of iron-tyrosinate proteins. J. Am. Chem. Soc 1984, 106, 1676. [Google Scholar]
- (65).Cho J; Sarangi R; Annaraj J; Kim SY; Kubo M; Ogura R; Solomon EI; Nam W Geometric and electronic structure and reactivity of a mononuclear ‘side-on’ nickel(III)–peroxo complex. Nat. Chem 2009, 1, 568–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Rettenmeier CA; Wadepohl H; Gade LH Electronic structure and reactivity of nickel(i) pincer complexes: their aerobic transformation to peroxo species and site selective C─H oxygenation. Chem. Sci 2016, 7 (6), 3533–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Ansari A; Jayapal P; Rajaraman G C─H Bond Activation by Metal–Superoxo Species: What Drives High Reactivity? Angew. Chem., Int. Ed 2015, 54 (2), 564–568. [DOI] [PubMed] [Google Scholar]
- (68).Fabry DC; Rueping M Merging Visible Light Photoredox Catalysis with Metal Catalyzed C─H Activations: On the Role of Oxygen and Superoxide Ions as Oxidants. Acc. Chem. Res 2016, 49 (9) , 1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Latifi R; Tahsini L; Kumar D; Sastry GN; Nam W; De Visser SP Oxidative properties of a nonheme Ni(II)(O2) compelx: Reactivity patterns for C-H Activation, aromatic hydroxylation, and heteroatom oxidation. Chem. Comm, 2011, 47, 10674–10676. [DOI] [PubMed] [Google Scholar]
- (70).Chen D; Martell AE Oxygen Insertion in the Ni(II) Complexes of Dioxopentaza Macrocyclic Ligands. J. Am. Chem. Soc 1990, 112 (25), 9411–9412. [Google Scholar]
- (71).Stoll S; Schweiger A EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson 2006, 178(1), 42–55 [DOI] [PubMed] [Google Scholar]
- (72).Sheldrick GM SHELXT - Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Dolomanov OV; Bourhis LJ; Gildea RJ; Howard AK; Puschmann H OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst 2009, 42, 339. [Google Scholar]
- (74).Sheldrick GM Acta Cryst. A short history of SHELX. 2008, A64, 112–122. [DOI] [PubMed] [Google Scholar]
- (75).Sheldrick GM Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Ravel B; Newville M ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchr. Radn, 2005, 12, 537–541. [DOI] [PubMed] [Google Scholar]
- (77).Newville M IFEFFIT: interactive XAFS analysis and FEFF fitting J. Synchr. Radn 2001, 8, 322–324. [DOI] [PubMed] [Google Scholar]
- (78).Rehr JJ; Albers RC Theoretical approaches to x-ray absorption fine structure. Rev. Mod. Phys 2000, 72, 621–654. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.