Abstract
IMPORTANCE
Facial paralysis (FP) after surgery has substantial functional, emotional, and financial consequences. Most iatrogenic FP is managed by watchful waiting, with the expectation of facial function recovery. A potential treatment is physical therapy (PT).
OBJECTIVE
To investigate whether noninvasive PT compared with no PT or other intervention improves facial nerve outcomes in adults with iatrogenic FP.
LP;&-2QEVIDENCE REVIEW
Patients with noniatrogenic FP, facial reanimation surgery, and invasive adjunctive treatments (acupuncture or botulinum toxin injection) were excluded. A systematic review was conducted for records discussing iatrogenic FP and PT; a search for these records was performed using Ovid MEDLINE (1946–2019), Embase (1947–2019), Scopus (1823–2019), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, World Health Organization International Clinical Trials Registry Platform (2004–2019), and ClinicalTrials.gov (1997–2019). The references of all the included articles were also assessed for eligible studies. All human participant, English-language study designs with at least 2 cases were included. Quality assessment was performed using the Methodological Index for Non-randomized Studies (MINORS) and the revised Cochrane Risk of Bias 2 (RoB 2) tool for randomized controlled trials. All search strategies were completed on May 16, 2019, and again on October 1, 2019.
FINDINGS
Fifteen studies (7 of which were retrospective cohort studies) and 313 patients with iatrogenic FP were included in the systematic review. Most iatrogenic FP (166 patients [53%]) was associated with parotidectomy; traditional PT (ie, facial massage) was the most common intervention (196 patients [63%]). The use of various facial grading systems and inconsistent reporting of outcomes prevented direct comparison of PT types.
CONCLUSIONS AND RELEVANCE
Because of heterogeneity in reported outcomes of facial nerve recovery, definitive conclusions were unable to be made regarding the association between PT and outcomes of iatrogenic FP. Physical therapy probably has benefit and is associated with no harm in patients with iatrogenic FP.
Facial paralysis (FP) secondary to surgery has a reported incidence of 11% to 40%.1 Rates of iatrogenic FP vary by surgery type: oral-maxillofacial surgery is the most common (40%), followed by head and neck surgery (25%), otologic surgery (17%),and cosmetic surgery(11%).1For patients, FP is associated with substantial functional, emotional, and financial consequences.2–4 Given its detrimental impact, iatrogenic FP is an important complication for otolaryngologists, plastic surgeons, oral surgeons, and dermatologists to manage. Most iatrogenic FP is expected to be temporary5 and is managed by watchful waiting.
Treatment options to encourage facial function recovery are varied, including medical therapy (ie, corticosteroids) and physical therapy (PT).5 Physical therapy is advantageous because it is noninvasive and encourages patients to actively participate in their recovery.6 However, there is a paucity of literature on the effectiveness of PT. Although a 2011 Cochrane review by Teixeira et al7 found no high-quality evidence supporting the benefit or harm of PT for idiopathic FP, studies of PT for iatrogenic FP are limited to a few randomized controlled trials (RCTs)5,8 and observational studies.9 Iatrogenic FP can occur despite meticulous surgical dissection and intraoperative confirmation of facial nerve continuity likely because of local manipulation.1 Therefore, iatrogenic FP represents a pathogensis unique from idiopathic FP, which is often stated to result from inflammation after viral infection.7 The primary objective of this systematic review was to investigate whether noninvasive PT compared with no PT or other intervention improves facial nerve outcomes in adults with iatrogenic FP.
Methods
Study Design
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)10 guidelines were followed in this systematic review (Figure). A systematic review protocol was published on PROSPERO.11 Using the PICOS (population, interventions, comparators, outcomes, and study design) format, the population of interest was adults (≥18years old)with iatrogenic FP of any degree (complete or partial), the intervention was noninvasive PT of any modality compared with no PT (or other intervention), and the outcome was description of facial nerve outcomes. All human participant, English-language study designs and case series with at least 2 cases were included. The exclusion criteria were noniatrogenic FP (ie, any cause of FP not after a surgical procedure), any invasive intervention (nerve repair, facial reanimation, acupuncture, or botulinum toxin injection), pediatric patients, and intraoperative facial nerve transection because this type of injury is unlikely to resolve via nonsurgical intervention alone. Electrical stimulation was not excluded as an invasive form of PT because it is available over the counter and does not require an additional procedure by a specialist, unlike botulinum toxin injection or acupuncture. If studies did not report facial nerve transection, we assumed that the facial nerve was in continuity. Patients with noniatrogenic FP, facial reanimation surgery, and invasive adjunctive treatments (acupuncture or botulinum toxin injection) were excluded. Case reports, review articles, nonhuman studies, non–English-language text, and unavailable full text were excluded.
Figure.
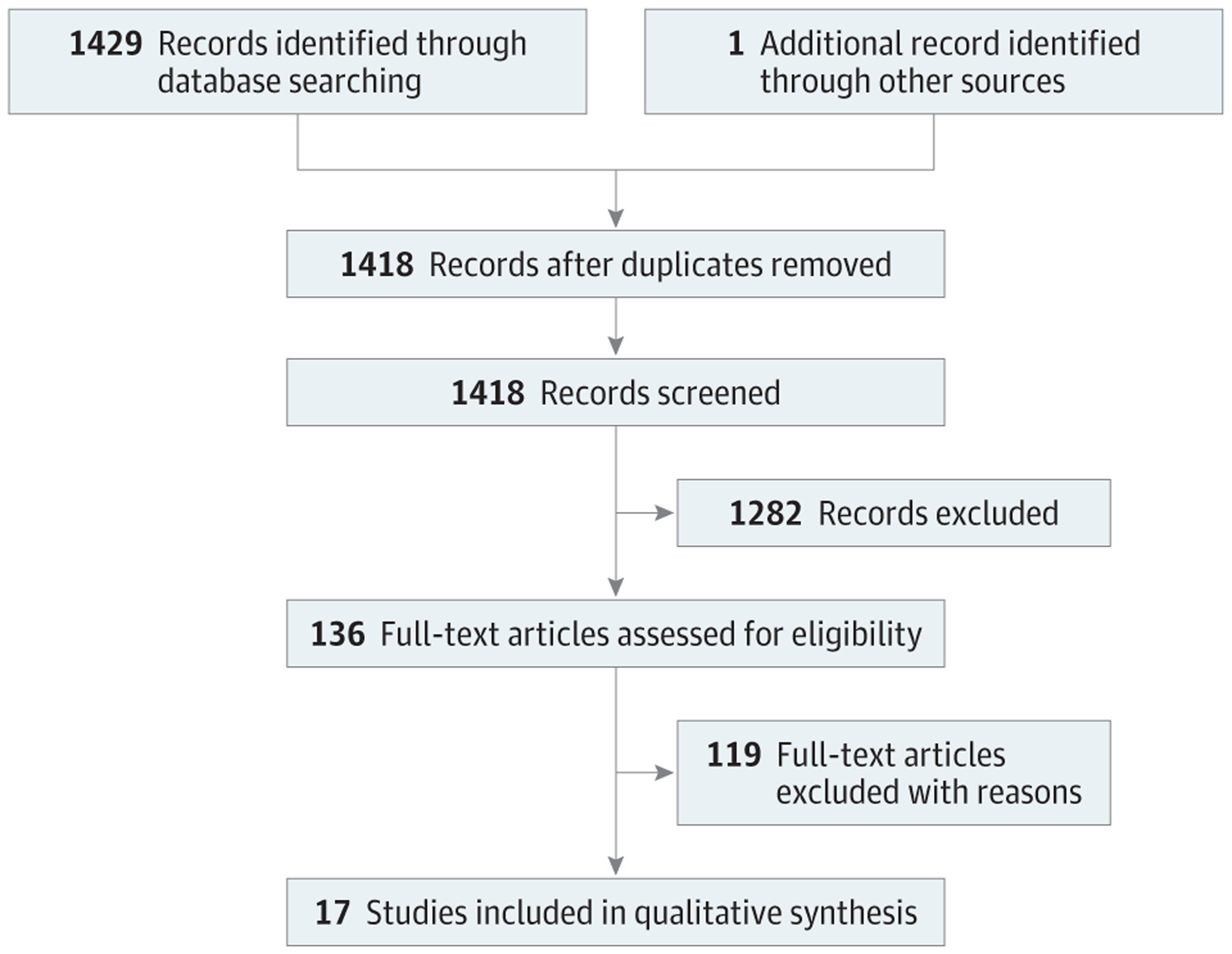
PRISMA Flow Diagram of Studies in the Systematic Review
A medical librarian (L.E.S.) created search strategies for FP and PT to identify records discussing iatrogenic FP and PT in Ovid MEDLINE (1946–2019), Embase (1947–2019), Scopus (1823–2019), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, World Health Organization International Clinical Trials Registry Platform (2004–2019), and ClinicalTrials.gov (1997–2019). Database search results were not limited to publication year or study type. All search strategies were completed on May 16, 2019, and again on October 1, 2019. A total of 1429 records were identified through database searching, with 1418 records included after removal of duplicate records.12 Fully reproducible search strategies can be found in the eAppendix in the Supplement.
Two of us (N.S.W. and J.J.C.) first independently screened titles and abstracts for inclusion. Next, 2 independent reviewers (N.S.W. and L.J.) screened the full texts. Study quality was appraised by the first author (N.S.W.) using the Methodological Index for Non-randomized Studies (MINORS) criteria13 and the revised Cochrane Risk of Bias 2 (RoB 2) tool for RCTs.14 Any discrepancies were addressed and resolved by consensus with the senior author (J.J.C.); only 2 articles15,16 required further discussion with the senior reviewer (J.J.C.).
The primary outcome was facial function after PT, which was assessed by clinician-graded instruments (House-Brackmann [HB] facial grading system),17 the Sunnybrook Facial Grading System (SFGS),18 alternative facial grading systems (Facial Paralysis Recovery Profile and Recovery Index19 and facial measurements8,20–22), or narrative description of facial function. A secondary outcome was synkinesis. If data were not available in the published article, authors were contacted via email.
Statistical Analysis
Descriptive statistics characterized the type of surgery, PT, and facial nerve outcome measures. Mean differences between pretreatment and posttreatment facial nerve outcome grades were calculated. Heterogeneity in the facial nerve outcome measures used precluded meta-analysis.
Results
Characteristics of Included Studies
Table 1 summarizes the included studies. There were 17 articles5,8,9,15,20,21,23–33 published on 15 studies; a total of 313 patients with iatrogenic FP were included in the systematic review. One study resulted in 3 articles,15,20,24 and each was assessed for unique patient data for this systematic review. One study23 was added after reviewing the references of all of the included studies. The studies originated from all over the world, published from 1991 to 2019. Four studies5,8,15,20,23,24 were RCTs, 7 studies9,25–30 were retrospective cohort studies, 3 studies31–33 were case series, and 1 was a prospective cohort study.21
Table 1.
Characteristics of Included Studies
| Source | Location | Study design (single cohort or comparative) | Type of surgery (No. of patients) | Type of intervention (No. of patients) | Primary outcome metric used |
|---|---|---|---|---|---|
| Ross et al,23 1991 | Toronto, Ontario, Canada | RCT |
|
|
HB |
| Beurskens and Heymans,20 2003; Beurskens et al,15 2006; Beurskens and Heymans,24 2006 | Nijmegen, the Netherlands | RCT |
|
|
HB, SFGS |
| Infante-Cossio et al,5 2016 | Seville, Spain | RCT | Superficial parotidectomy (n = 79) |
|
HB |
| Paolucci et al,8 2020 | Rome, Italy | RCT | Acoustic neuroma resection (n = 22) |
|
HB, SFGS |
| Coulson et al,21 2006 | Sydney, Australia | Prospective cohort (single cohort) |
|
Video self-modeling, structured implementation intentions | HB, SFGS |
| Gittins et al,25 1999 | Colchester, United Kingdom | Retrospective cohort (single cohort) | Acoustic neuroma resection (n = 8) | Transcutaneous electrical nerve stimulation of the orbicularis oculi | HB |
| Segal et al,26 1995 | Montreal, Quebec, Canada | Retrospective cohort (single cohort) |
|
EMG biofeedback therapy, conscious awareness, mirror therapy | HB |
| Brach et al,27 1997 | Pittsburgh, Pennsylvania | Retrospective cohort (single cohort) |
|
EMG biofeedback, self-directed home PT | HB |
| Barbara et al,9 2003 | Rome, Italy | Retrospective cohort (comparative) | Acoustic neuroma resection via translabyrinthine approach (n = 29) |
|
HB |
| VanSwearingen and Brach,28 2003 | Pittsburgh, Pennsylvania | Retrospective cohort (single cohort) |
|
EMG biofeedback, neuromuscular reeducation | SFGS |
| García-Purriños,29 2011 | Murcia, Spain | Retrospective cohort (single cohort) | Partial superficial parotidectomy (n = 14) | PT method unspecified | Description of recoveryb |
| Costa et al,30 2019 | Rio de Janeiro, Brazil | Retrospective cohort (single cohort) | Parotidectomy: superficial, total, with or without wide local resection (n = 73) | Facial massage, mirror exercises, stretching of contralateral muscles | SFGS |
| Hussain et al,31 1994 | England | Case series |
|
Electrical stimulation | FPRP/I |
| Choi et al,32 2010 | Taipei, Taiwan | Case series | Bilateral sagittal split osteotomy (n = 4); 5 total patients (4 treated with some form of PT plus 1 treated without any PT) | PT method unspecified, including electrical stimulation | Description of recoveryb |
| Barbara et al,33 2014 | Rome, Italy | Case series | Middle ear implant via mastoidectomy and posterior tympanotomy (n = 3) | PT method unspecified | HB |
Abbreviations: EMG, electromyography; FP, facial paralysis; FPRP/I, Facial Paralysis Recovery Profile and Recovery Index; HB, House-Brackmann Facial Grading System; PT, physical therapy; RCT, randomized controlled trial; SFGS, Sunnybrook Facial Grading System.
This study had participants with mixed etiologies of FP and did not specify into which groups individuals with iatrogenic FP were assigned. Thirty-one total patients were randomized in this study (25 patients with iatrogenic FP and 6 patients with idiopathic FP or herpes zoster virus). Twenty-four patients of the total population were randomized to receive some form of PT, and 7 patients were observed as a control group.
Studies with “description of recovery” used no facial grading system.
Quality Assessment
eTable 1 in the Supplement (listing 11 studies9,21,25–33) and eTable 2 in the Supplement (listing 6 studies5,8,15,20,23,24) summarize the study quality assessment. Eleven observational studies9,21,25–33 were assessed with the MINORS criteria. The maximum sum scores for noncomparative observational studies21,25–33 and for comparative observational studies9 are 16 and 24, respectively. In this systematic review, 4 noncomparative studies29,30,32,33 had sum scores at the lower end (range, 4–6), and 6 noncomparative studies21,25–28,31 had sum scores at the higher end (range, 9–12). The only comparative observational study9 had a low sum score of 9 (eTable 1 in the Supplement). Four RCTs5,8,15,20,23,24 assessed with the RoB 2 tool ranged in quality from low to some concern for bias (eTable 2 in the Supplement).
Type of Surgery, Intervention, and Outcome Measure
Table 2 summarizes the types of surgical procedures in the included studies and cited incidences of FP.1,8,30,32,33 The procedures were classified based on location of the surgery as extratemporal (170 patients [54%]), temporal (4 patients [1%]), and intracranial (126 patients [40%]). If the location of surgery was not specified, the data were classified as incomplete reporting (13 patients [4%]). Most iatrogenic FP (166 patients [53%]) was associated with parotidectomy.
Table 2.
Types of Surgery in the Included Studies, With Cited Incidence Rates of FP
| Surgery by location of facial nerve | Description | Patients, No. (%) (N = 313) | Reported incidence rate of FP in the literature |
|---|---|---|---|
| Extratemporal | Includes parotidectomy, face-lift, TMJ arthroplasty, styloidectomy, facial fracture repair | 170 (54) | |
| Temporal | Includes mastoidectomy, cochlear implantation, lateral temporal bone resection | 4 (1) | |
| Intracranial | Includes approaches for acoustic neuroma/vestibular schwannoma resection (translabyrinthine, retrosigmoid craniotomy, middle fossa craniotomy); middle fossa approach for temporal bone CSF leak or meningocele repair | 126 (40) | “Immediately after removal of a vestibular schwannoma or neuroma of the acoustic nerve, various degrees of facial nerve palsy arise in 20–70% of patients”8(p58) |
| Incomplete reportinga | NA | 13 (4) | NA |
Abbreviations: CSF, cerebrospinal fluid; FP, facial paralysis; NA, not applicable; TMJ, temporomandibular joint.
Incomplete reporting refers to patients for whom the type of surgery was not specifically stated.
Table 3 summarizes the type of intervention, classified as traditional PT (196 patients [63%]), electromyography (EMG) biofeedback (39 patients [12%]), electrical stimulation (10 patients [3%]), and observation (19 patients [6%]). Observation involved no PT intervention and included treatment with corticosteroids or antiviral agents alone. Studies that did not specify the type of PT were classified as incomplete reporting (49 patients [16%]). The use of various facial grading systems and inconsistent reporting of outcomes prevented direct comparison of PT types.
Table 3.
Types of Interventions in the Included Studies
| Intervention | Description | Patients, No. (%) (N = 313)a |
|---|---|---|
| Traditional PT | Includes massage therapy, mime therapy, mirror therapy, neuromuscular retraining | 196 (63) |
| EMG biofeedback | Includes conversion of EMG activity into auditory or visual feedback to guide facial movements alone or with traditional PT | 39 (12) |
| Electrical stimulation | Includes electrical stimulation with direct current administered transcutaneously or percutaneously | 10 (3) |
| Incomplete reporting | The article did not specify the type of PT received but stated only that the individual was treated with PT | 49 (16) |
| Observationb | Groups for which observation or medical therapy only was prescribed | 19 (6) |
Abbreviations: EMG, electromyography; PT, physical therapy.
Summarizes the number of patients who received a given type of intervention.
Observation includes patients who received no physical therapy, including patients who received medical therapy with corticosteroids and antiviral agents without PT.
The facial nerve outcome measures used are summarized in eTable 2 in the Supplement. Among the studies reporting use of one or more facial grading scales, the outcome measures were classified as HB Facial Grading System grade (7 studies5,9,23,25–27,33), SFGS grade (2 studies28,30), both HB Facial Grading System grade and SFGS grade (3 studies8,21,24), alternative facial grading system (8 studies8,15,20,21,25,27,31,32), and no facial grading system used (2 studies29,32). Of note, only 4 of the 17 included articles reported quality of life outcomes using a validated disease-specific scale, either the Facial Disability Index15,20,21 or the Facial Clinimetric Evaluation8; however, for the 3 studies15,20,21 using the Facial Disability Index, data were mixed among noniatrogenic and iatrogenic causes, precluding adequate comparisons.
Qualitative Synthesis
PT vs Observation
Four studies9,23,24,32 compared PT with observation. The retrospective cohort study by Barbara et al9 of 29 patients with FP after acoustic neuroma resection compared massage therapy with a historical cohort who did not receive PT. Physical therapy was initiated immediately after surgery and continued for 12 months. Both groups had an initial median HB grade of IV of VI. The study did not report final HB grades for either group, but a postintervention subgroup analysis was performed for the patients with postoperative HB IV and V. This subgroup analysis found that, after 1 year, 67% of patients in the PT group improved from HB IV to II and 67% from HB V to III. In contrast, the control group had only 10% of patients improve from HB IV to III and 70% from HB V to IV. Although postintervention data are incomplete, Barbara et al9(p935) asserted that “facial nerve function improved to a much greater extent and more quickly when early physical rehabilitation was applied.” Beurskens and Heymans24 compared mime therapy with observation in an RCT that included 48 patients with chronic FP (>9 months) of mixed origins; however, individual HB and SFGS scores for 7 patients with iatrogenic FP were not reported. The authors concluded that “facial symmetry improved in 100% of the PT group versus 38% of the observation group.”24(p182) In a case series of FP after sagittal split osteotomy, Choi et al32 described 1 patient treated with PT, 3 patients treated with PT and electrical stimulation, and 1 patient treated with no PT. No facial grading system was used. All 3 patients who were treated with PT and electrical stimulation achieved “complete recovery” by 6 months. The patient treated with PT alone had persistent weakness in “the frontal branch” of the facial nerve. The patient treated with no PT achieved “complete recovery” by 6 months. Finally, Ross et al23 performed an RCT with 31 patients, 25 of whom had iatrogenic FP for at least 9 months. There were 3 groups, including patients who received (1) EMG biofeedback with mirror therapy, (2) mirror therapy alone, and (3) observation only. Preintervention HB grades were reported for groups 1 and 2 but were unreported for group 3. The authors did not specify into which groups the patients with iatrogenic FP were randomized, nor did they report any postintervention HB grades. They concluded that there is “significant beneficial effect of feedback training with either mirror feedback therapy alone or with the addition of EMG [bio]feedback.”23(p748)
Traditional PT
Traditional PT was the most commonly used type of intervention. An RCT by Infante-Cossio et al5 compared the effectiveness of supervised PT vs self-directed home PT after superficial parotidectomy in 79 patients with FP. Outcomes were reported using a modified HB Facial Grading System scale. The authors found no difference in outcomes between the 2 groups at 12 months. Paolucci et al8 performed an RCT comparing types of traditional PT in 22 patients after acoustic neuroma resection. For 3 months, 1 group performed mirror therapy, and the comparator group performed mime therapy, self-massage, speaking exercises, and breathing and relaxation exercises. At the 5-month follow-up, the median HB grade and SFGS grade had improved for both groups. The authors concluded that both groups showed “global and progressive” improvement in facial function.8 Other studies9,24 did not report outcomes; however, their conclusions suggested improvement after PT.
Electrical Stimulation
Three studies25,31,32 reported on the use of electrical stimulation of facial muscles. The case series by Choi et al32 described 3 patients who achieved full recovery of facial function after treatment with PT and electrical stimulation. The case series by Hussain et al31 reported on the use of electrical stimulation alone in 2 patients after otologic surgery. Outcomes were described using the Facial Paralysis Recovery Profile and Recovery Index.19 Patients were treated for 60 weeks, and all demonstrated improvement. In the case series by Choi et al32 and by Hussain et al,31 the method of electrical stimulation (percutaneous or transcutaneous) and the target muscles were not reported. In a retrospective cohort study, Gittins et al25 described transcutaneous electrical stimulation of the orbicularis oculi among 8 patients with an HB grade of IV or worse after acoustic neuroma resection. Postintervention HB grades were not reported. However, patients described some subjective improvement, including decreased use of artificial tears and improved facial tone. None of these 3 studies25,31,32 describe specific symptoms of synkinesis or worsening of synkinesis as a result of electrical stimulation.
EMG Biofeedback
Four studies23,26–28investigated the use of EMG bio feedback with traditional PT. In the retrospective cohort study of 6 patients by Brach et al,27 outcomes included HB grade and facial synkinesis. The HB grades did not change; however, improvement in oral synkinesis was noted. In their retrospective cohort study of 19 patients, VanSwearingen and Brach28 described similar findings with EMG bio feedback. The retrospective cohort study of 14 patients by Segal et al26 demonstrated an improved HB grade over time; however, individual patient HB grades were not reported. As previously noted, the RCT by Ross et al23 reported improved outcomes with EMG biofeedback.
PT and Synkinesis
Synkinesis is abnormal, involuntary facial movement that occurs when trying to perform a voluntary facial movement.27 It is an unwanted sequelae of facial nerve injury resulting from abnormal facial nerve reinnervation during nerve regeneration.34,35 Four studies24,26–28 analyzed synkinesis outcomes after PT. In their retrospective cohort studies, Brach et al27 and VanSwearingen and Brach28 observed that most individuals had reduced synkinesis after PT. However, the data are incomplete because patients with mixed causes of FP were included in both studies, and it is unclear how many of the patients who experienced reduced synkinesis had iatrogenic FP. The RCT by Beurskens and Heymans24 found that patients in the mime therapy group improved in synkinesis, and patients in the observation group experienced worsening of synkinesis after 3 months. The retrospective cohort study by Segal et al26 described variable synkinesis outcomes.
Discussion
This systematic review investigated 313 patients with iatrogenic FP treated with PT. The 17 included articles5,8,9,15,20,21,23–33 ranged from low quality to high quality. The most commonly reported surgical procedure resulting in FP was parotidectomy, and the most commonly used PT was traditional PT. Overall, PT was associated with improved FP outcomes. Heterogeneity in outcome reporting precluded direct comparisons of the different types of PT interventions and meta-analysis of the FP outcomes. The inherent challenges of studying this surgical complication have contributed to a gap in the literature examining treatment options (specifically PT for iatrogenic FP).7,36,37 This gap was the basis for our systematic review.
The goals of PT for FP are to maintain muscle trophism, tone, and proprioception.5 Physical therapy aims to maximize intentional facial movements and minimize synkinesis, often by using the contralateral side of the face to facilitate motor learning.21 The successful end point of PT is the use of motor learning to control neuroplasticity such that facial movements appear more symmetric with little conscious effort.23,26
This systematic review analyzed cases of iatrogenic FP managed with traditional PT, EMG biofeedback, electrical stimulation, or observation. Traditional PT, such as mime therapy, mirror therapy, or neuromuscular training, centers on a combination of massage, relaxation, and expression of emotional and functional facial movements.5,8,9,20 Techniques are applied to the paralyzed side of the face to improve static and dynamic symmetry while simultaneously controlling synkinesis.20 In EMG biofeedback, individuals use audio or visual feedback from EMG activity to attempt deliberate, symmetric facial movements, stopping the exercise immediately if facial asymmetry or synkinesis occurs.26 Electromyography biofeedback is beneficial because immediate feedback is given about facial movement, and the individual is redirected to a more appropriate movement. Advocates for EMG biofeedback hypothesize that, over time, motor learning and neuroplasticity allow for the unconscious production of desired facial movements without the need for feedback.23,27 Electrical stimulation centers on the theory that muscle fibers are dependent on neural activity; therefore, inactivity leads to atrophy, whereas electrical stimulation of denervated muscle reverses atrophic changes, especially for small, fast-twitch muscles, such as the facial mimetic muscles. Many experiments with electrical stimulation are performed on animal models; demonstrable success in humans has not been definitively achieved.25 Given the heterogeneity in types of PT, lack of standardized reporting of outcomes, and the paucity of prospective RCTs, we cannot conclude which PT modality is the most effective with the current evidence from this systematic review.
Synkinesis is primarily treated with chemodenervation of hyperactive muscles using botulinum toxin.38 Surgical denervation of these muscles may also be considered.39 Physical therapy may be combined with botulinum toxin to improve facial symmetry.40–43 Only a few of the included studies of PT alone reported synkinesis outcomes, with variable results.24,26–28
The most appropriate timing for the initiation of PT is an important clinical consideration. Some argue that premature assessment of facial movement could draw inaccurate conclusions about a recovering facial nerve.23 However, inaction may leave patients with decreased quality of life and greater uncertainty about recovery.8 Numerous studies15,20,21,23–25,27,31 in this systematic review examined the association of PT with FP of at least 9 months’ duration and demonstrated advantageous results. Therefore, PT may be beneficial regardless of timing, although substantial uncertainty remains.
A further question is whether supervised PT or self-directed home PT is more effective. Self-directed home PT typically involves a set of facial exercises, either individualized to the patient or standardized. Patients are instructed to practice for varied periods (ranging from 30 minutes to 1 hour) and frequencies (once a day to multiple times a day).5,23,26 Electromyography biofeedback cannot be performed at home because of equipment needs, but it is possible to do electrical stimulation at home.25 There is evidence to suggest that self-directed home PT is as effective as supervised PT.5 In addition, a long-term treatment regimen that can be self-administered at home may have the advantages of health care cost savings24 and patient convenience for those who may have difficulty receiving in-person treatment because of travel limitations, medical comorbidities, or socioeconomic factors. Some argue that the heterogeneity of presentation of FP necessitates individualized PT directed by a physical therapist,43,44 but it is unknown if self-directed PT is more beneficial in cost and effectiveness than supervised PT.
The varied and nuanced types of PT, as well as the lack of uniformity in their administration, represent a major impediment to the study of the effectiveness of PT. Consistency in the outcome metric used (HB Facial Grading System, SFGS) was absent among the articles included in our systematic review. Moreover, despite the substantial burden of FP on quality of life, only 4 of the 17 included articles reported outcomes using a validated disease-specific scale (the Facial Disability Index15,20,21 or the Facial Clinimetric Evaluation Scale8), and heterogeneous reporting precluded adequate quality-of-life comparisons. A standardized PT protocol for FP would allow for improved assessment of the outcomes. A consensus among physical therapists, surgeons, and patients would be the appropriate next step to investigate the effectiveness of PT in FP. In addition, standardized reporting of outcomes for facial function with established follow-up times would allow for meta-analyses in the future to advance our understanding of this challenging clinical scenario.
Limitations
Despite a thorough systematic search and analysis, this study has a number of limitations. Heterogeneity in outcome reporting was a major limitation. Different facial grading systems were implemented in almost all included articles; most studies8,15,20,21,25,27,31,32 used an alternative grading system. For articles using the HB Facial Grading System and the SFGS, 2 studies5,30 applied a modified version of these systems that has not been validated. Inadequate reporting was the greatest challenge to this systematic review. Five observational studies21,26,27,30,33 and 1 RCT23 lacked post intervention outcome reporting. Numerous studies included participants with various causes of FP (iatrogenic, idiopathic, or infectious), and frequently the individualized outcomes before and after PT were not reported separately for the patients with iatrogenic FP. Missing data from participants, entire cohort groups, or potentially eligible but non–English-language studies possibly impacted the conclusions of our systematic review. Requests for complete information were made to all corresponding authors of the included studies via email. In all cases, the requested information was unavailable or inadequate, or there was no reply. Issues with potential bias were also present. Two RCTs5,23 had insufficient blinding of participants and therapists. None of the included studies reported adverse effects from PT, which could either indicate reporting bias or that PT is harm free. Despite the inclusion of fair-quality and poor-quality studies, the benefit of performing a systematic review for this clinical scenario is the ability to counsel patients and clinicians that the utility of PT is uncertain based on available evidence rather than anecdotal conjecture. In addition, studies did not consistently specify whether systemic corticosteroids or antiviral agents were administered; however, given the literature on their use for acute FP, including iatrogenic FP, it can be assumed that corticosteroids are widely administered to patients with FP.7,45,46 Therefore, the amount of benefit from medical therapy vs from PT could not be assessed in our systematic review. Therefore, were commend that future studies use standardized reporting measures to better stratify and compare outcomes in facial nerve care and research.47
Conclusions
Because of the incomplete and heterogeneous reporting of outcomes for facial function, definitive conclusions were unable to be made regarding the effectiveness of PT for iatrogenic FP. Physical therapy probably has benefit and is associated with no harm in patients with iatrogenic FP, but additional studies are needed. Standardized reporting of outcomes for facial function is critically important to inform clinicians, patients, and researchers on how to best manage this challenging clinical scenario.
Supplementary Material
Key Points.
Question
In adults with iatrogenic facial paralysis (FP), is noninvasive physical therapy (PT) compared with no PT or other intervention associated with improved facial nerve outcomes?
Findings
Among 15 studies and 313 patients with iatrogenic FP in this systematic review, PT overall was associated with improved FP outcomes in patients with iatrogenic FP. Heterogeneity in outcome reporting precluded direct comparisons of the different types of PT interventions.
Meaning
Physical therapy may provide improvement for iatrogenic FP rather than watchful waiting; however, standardized facial function outcome reporting is critically important to inform clinicians, patients, and researchers on how to best manage this challenging clinical scenario.
Funding/Support:
Research for this work was supported by the National Institute on Deafness and Other Communication Disorders within the National Institutes of Health and by a training grant from the Development of Clinician/Researchers in Academic ENT (award 5T32DC000022-30 to Dr Wamkpah).
Role of the Funder/Sponsor:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Ms Simon reported serving on the New England Journal of Medicine Library Advisory Board for the 1-year term beginning January 2020 (which includes a $500 stipend and annual meeting travel fees). However, because of COVID-19, the annual meeting did not occur, and travel expenses were not incurred. No other disclosures were reported.
Meeting Presentation: This article was accepted for poster presentation at the 2020 American Academy of Facial Plastic and Reconstructive Surgery Annual Meeting; September 10–12, 2020; Boston, Massachusetts.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Hohman MH, Bhama PK, Hadlock TA. Epidemiology of iatrogenic facial nerve injury: a decade of experience. Laryngoscope. 2014;124(1): 260–265. doi: 10.1002/lary.24117 [DOI] [PubMed] [Google Scholar]
- 2.Goines JB, Ishii LE, Dey JK, et al. Association of facial paralysis–related disability with patient and observer-perceived quality of life. JAMA Facial Plast Surg. 2016;18(5):363–369.doi: 10.1001/jamafacial.2016.0483 [DOI] [PubMed] [Google Scholar]
- 3.Nellis JC, Ishii M, Byrne PJ, Boahene KDO, Dey JK, Ishii LE. Association among facial paralysis, depression, and quality of life in facial plastic surgery patients. JAMA Facial Plast Surg. 2017;19(3): 190–196. doi: 10.1001/jamafacial.2016.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruins TE, van Veen MM, Mooibroek-Leeuwerke T, Werker PMN, Broekstra DC, Dijkstra PU. Association of socioeconomic, personality, and mental health factors with health-related quality of life in patients with facial palsy. JAMA Otolaryngol Head Neck Surg. 2020;146(4):331–337. doi: 10.1001/jamaoto.2019.4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Infante-Cossio P, Prats-Golczer VE, Lopez-Martos R, Montes-Latorre E, Exposito-Tirado JA, Gonzalez-Cardero E. Effectiveness of facial exercise therapy for facial nerve dysfunction after superficial parotidectomy: a randomized controlled trial. Clin Rehabil. 2016;30(11):1097–1107. doi: 10.1177/0269215515617309 [DOI] [PubMed] [Google Scholar]
- 6.Novak CB. Rehabilitation strategies for facial nerve injuries. Semin Plast Surg. 2004;18(1):47–52. doi: 10.1055/s-2004-823123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira LJ, Valbuza JS, Prado GF. Physical therapy for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2011;(12):CD006283. doi: 10.1002/14651858.CD006283.pub3 [DOI] [PubMed] [Google Scholar]
- 8.Paolucci T, Cardaroia A, Colonnelli P, et al. Give me a kiss! an integrative rehabilitative training program with motor imagery and mirror therapy for recovery of facial palsy. Eur J Phys Rehabil Med. 2020;56(1):58–67. doi: 10.23736/S1973-9087.19.05757-5 [DOI] [PubMed] [Google Scholar]
- 9.Barbara M, Monini S, Buffoni A, et al. Early rehabilitation of facial nerve deficit after acoustic neuroma surgery. Acta Otolaryngol. 2003;123(8): 932–935. doi: 10.1080/00016480310000629 [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Physical therapy for facial paralysis after iatrogenic injury. PROSPERO identifier: CRD42020154437. Updated April 28, 2020. Accessed April 2020. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020154437 [Google Scholar]
- 12.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–243. doi: 10.3163/1536-5050.104.3.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 14.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 15.Beurskens CH, Heymans PG, Oostendorp RA. Stability of benefits of mime therapy in sequelae of facial nerve paresis during a 1-year period. Otol Neurotol. 2006;27(7):1037–1042. doi: 10.1097/01.mao.0000217350.09796.07 [DOI] [PubMed] [Google Scholar]
- 16.Karp E, Waselchuk E, Landis C, Fahnhorst J, Lindgren B, Lyford-Pike S. Facial rehabilitation as noninvasive treatment for chronic facial nerve paralysis. Otol Neurotol. 2019;40(2):241–245. doi: 10.1097/MAO.0000000000002107 [DOI] [PubMed] [Google Scholar]
- 17.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2): 146–147. doi: 10.1177/019459988509300202 [DOI] [PubMed] [Google Scholar]
- 18.Ross BG, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. Otolaryngol Head Neck Surg. 1996;114(3):380–386. doi: 10.1016/S0194-5998(96)70206-1 [DOI] [PubMed] [Google Scholar]
- 19.Adour KK, Boyajian JA, Kahn ZM, Schneider GS. Surgical and nonsurgical management of facial paralysis following closed head injury. Laryngoscope. 1977;87(3):380–390. doi: 10.1288/00005537-197703000-00011 [DOI] [PubMed] [Google Scholar]
- 20.Beurskens CH, Heymans PG. Positive effects of mime therapy on sequelae of facial paralysis: stiffness, lip mobility, and social and physical aspects of facial disability. Otol Neurotol. 2003;24(4):677–681. doi: 10.1097/00129492-200307000-00024 [DOI] [PubMed] [Google Scholar]
- 21.Coulson SE, Adams RD, O’Dwyer NJ, Croxson GR. Physiotherapy rehabilitation of the smile after long-term facial nerve palsy using video self-modeling and implementation intentions. Otolaryngol Head Neck Surg. 2006;134(1):48–55. doi: 10.1016/j.otohns.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 22.Jansen C, Jennekens FG, Wokke JH, Leppink GJ, Wijnne HJ. Lip-length and snout indices: methods for quantitative assessment of peri-oral facial muscle strength. J Neurol Sci. 1990;97(2–3): 133–142. doi: 10.1016/0022-510X(90)90212-6 [DOI] [PubMed] [Google Scholar]
- 23.Ross B, Nedzelski JM, McLean JA. Efficacy of feedback training in long-standing facial nerve paresis. Laryngoscope. 1991;101(7, pt 1):744–750. doi: 10.1288/00005537-199107000-00009 [DOI] [PubMed] [Google Scholar]
- 24.Beurskens CH, Heymans PG. Mime therapy improves facial symmetry in people with long-term facial nerve paresis: a randomised controlled trial. Aust J Physiother. 2006;52(3):177–183. doi: 10.1016/S0004-9514(06)70026-5 [DOI] [PubMed] [Google Scholar]
- 25.Gittins J, Martin K, Sheldrick J, Reddy A, Thean L. Electrical stimulation as a therapeutic option to improve eyelid function in chronic facial nerve disorders. Invest Ophthalmol Vis Sci. 1999; 40(3):547–554. [PubMed] [Google Scholar]
- 26.Segal B, Zompa I, Danys I, et al. Symmetry and synkinesis during rehabilitation of unilateral facial paralysis. J Otolaryngol. 1995;24(3):143–148. [PubMed] [Google Scholar]
- 27.Brach JS, VanSwearingen JM, Lenert J, Johnson PC. Facial neuromuscular retraining for oral synkinesis. Plast Reconstr Surg. 1997;99(7):1922–1931. doi: 10.1097/00006534-199706000-00017 [DOI] [PubMed] [Google Scholar]
- 28.VanSwearingen JM, Brach JS. Changes in facial movement and synkinesis with facial neuromuscular reeducation. Plast Reconstr Surg. 2003;111(7):2370–2375. doi: 10.1097/01.PRS.0000061007.36637.88 [DOI] [PubMed] [Google Scholar]
- 29.García-Purriños FJ. Thirteen years’ experience with superficial partial parotidectomy as treatment for benign parotid tumours [in Spanish]. Acta Otorrinolaringol Esp. 2011;62(1):10–13. doi: 10.1016/S2173-5735(11)70002-3 [DOI] [PubMed] [Google Scholar]
- 30.Costa MGESTD, Maranhão-Filho PA, Santos IC, Luiz RR, Vincent MB. Parotidectomy-related facial nerve lesions: proposal for a modified Sunnybrook Facial Grading System. Arq Neuropsiquiatr. 2019;77(7):460–469. doi: 10.1590/0004-282x20190074 [DOI] [PubMed] [Google Scholar]
- 31.Hussain SS, Winterburn SJ, Grace AR. Eutrophic electrical stimulation in long-standing facial palsy. Eur Arch Otorhinolaryngol. 1994;S133–S134. doi: 10.1007/978-3-642-85090-5_40 [DOI] [PubMed] [Google Scholar]
- 32.Choi BK, Goh RC, Chen PK, Chuang DC, Lo LJ, Chen YR. Facial nerve palsy after sagittal split ramus osteotomy of the mandible: mechanism and outcomes. J Oral Maxillofac Surg. 2010;68(7):1615–1621. doi: 10.1016/j.joms.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 33.Barbara M, Volpini L, Monini S. Delayed facial nerve palsy after surgery for the Esteem fully implantable middle ear hearing device. Acta Otolaryngol. 2014;134(4):429–432. doi: 10.3109/00016489.2013.868602 [DOI] [PubMed] [Google Scholar]
- 34.Jowett N A general approach to facial palsy. Otolaryngol Clin North Am. 2018;51(6):1019–1031. doi: 10.1016/j.otc.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 35.Jowett N, Hadlock TA. An evidence-based approach to facial reanimation. Facial Plast Surg Clin North Am. 2015;23(3):313–334. doi: 10.1016/j.fsc.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 36.Cardoso JR, Teixeira EC, Moreira MD, Fávero FM, Fontes SV, Bulle de Oliveira AS. Effects of exercises on Bell’s palsy: systematic review of randomized controlled trials. Otol Neurotol. 2008; 29(4):557–560. doi: 10.1097/MAO.0b013e31816c7bf1 [DOI] [PubMed] [Google Scholar]
- 37.Ferreira M, Santos PC, Duarte J. Idiopathic facial palsy and physical therapy: an intervention proposal following a review of practice. Phys Ther Rev. 2011;16(4):237–243. doi: 10.1179/1743288X11Y.0000000024 [DOI] [Google Scholar]
- 38.Cabin JA, Massry GG, Azizzadeh B. Botulinum toxin in the management of facial paralysis. Curr Opin Otolaryngol Head Neck Surg. 2015;23(4):272–280. doi: 10.1097/MOO.0000000000000176 [DOI] [PubMed] [Google Scholar]
- 39.Azizzadeh B, Irvine LE, Diels J, et al. Modified selective neurectomy for the treatment of post–facial paralysis synkinesis. Plast Reconstr Surg. 2019;143(5):1483–1496. doi: 10.1097/PRS.0000000000005590 [DOI] [PubMed] [Google Scholar]
- 40.Mandrini S, Comelli M, Dall’angelo A, et al. Long-term facial improvement after repeated BoNT-A injections and mirror biofeedback exercises for chronic facial synkinesis: a case-series study. Eur J Phys Rehabil Med. 2016;52(6):810–818. [PubMed] [Google Scholar]
- 41.Maria CM, Kim J. Individualized management of facial synkinesis based on facial function. Acta Otolaryngol. 2017;137(9):1010–1015. doi: 10.1080/00016489.2017.1316871 [DOI] [PubMed] [Google Scholar]
- 42.Wei LA, Diels J, Lucarelli MJ. Treating buccinator with botulinum toxin in patients with facial synkinesis: a previously overlooked target. Ophthalmic Plast Reconstr Surg. 2016;32(2):138–141. doi: 10.1097/IOP.0000000000000449 [DOI] [PubMed] [Google Scholar]
- 43.Lindsay RW, Robinson M, Hadlock TA. Comprehensive facial rehabilitation improves function in people with facial paralysis: a 5-year experience at the Massachusetts Eye and Ear Infirmary. Phys Ther. 2010;90(3):391–397. doi: 10.2522/ptj.20090176 [DOI] [PubMed] [Google Scholar]
- 44.Brach JS, VanSwearingen JM. Physical therapy for facial paralysis: a tailored treatment approach. Phys Ther. 1999;79(4):397–404. doi: 10.1093/ptj/79.4.397 [DOI] [PubMed] [Google Scholar]
- 45.Nam KJ, Han MS, Jeong YJ, Rah Y, Choi J. Comparison of the efficacy of various doses of steroids for acute facial palsy. Acta Otolaryngol. 2019;139(5):451–455. doi: 10.1080/00016489.2019.1578411 [DOI] [PubMed] [Google Scholar]
- 46.Yadav S, Panda NK, Verma R, Bakshi J, Modi M. Surgery for post-traumatic facial paralysis: are we overdoing it? Eur Arch Otorhinolaryngol. 2018;275 (11):2695–2703. doi: 10.1007/s00405-018-5141-y [DOI] [PubMed] [Google Scholar]
- 47.Vila PM, Kallogjeri D, Yaeger LH, Chi JJ. Powering the gracilis for facial reanimation: a systematic review and meta-analysis of outcomes based on donor nerve. JAMA Otolaryngol Head Neck Surg. 2020;146(5):1–8. doi: 10.1001/jamaoto.2020.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


