Abstract
Objective:
Peripheral artery disease (PAD) is an atherothrombotic disease of the lower limbs with substantial morbidity and mortality. We used next-generation sequencing to identify genome-wide expression signatures associated with prevalent PAD and its outcomes.
Approach and Results:
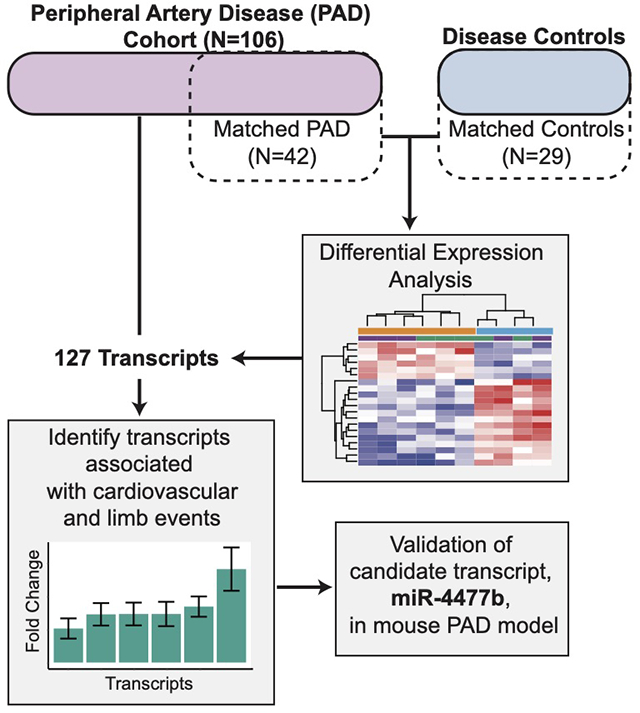
We performed whole blood RNA sequencing among severe symptomatic PAD patients undergoing lower extremity revascularization and controls. Dysregulated pathways and blood transcriptional modules were identified by comparing PAD patients (n=42) to age- and sex-matched controls (N=29). The identified signature was compared in PAD patients prior to LER with or without incident major adverse cardiac or limb events (MACLE). A novel microRNA (miRNA) associated with prevalent PAD and incident MACLE was then evaluated in a mouse hindlimb ischemia model.
127 transcripts were differentially expressed (77 upregulated and 50 downregulated; adjusted p < 0.05, |log2foldchange| > 0.5) and analyzed using Weighted Gene Co-expression Network Analysis (WGCNA). WGCNA revealed blood modules enriched for immune activation, secretory granules, and coagulation in patients with PAD. Of these 127 differentially expressed transcripts, 40 were significantly associated with MACLE (log-rank FDR < 0.1). MicroRNA (miR) miR-4477b was significantly increased in PAD patients with subsequent MACLE and in a mouse hindlimb ischemia model.
Conclusions:
A whole-blood transcript signature identified patients with symptomatic PAD and PAD patients at increased risk of MACLE. A previously uncharacterized transcript miRNA (miR-4477b) was overexpressed in prevalent PAD, incident MACLE, and in a mouse hindlimb ischemia model. Our novel transcriptomic signature provides insight into potential mechanisms of patients with severe symptomatic PAD.
Keywords: Peripheral artery disease, cardiovascular complications, gene expression/regulation, RNA transcription, micro RNA, mouse models
Subject terms: Clinical studies, computational biology, translational studies, animal models of human disease, vascular biology
Graphical Abstract
Introduction
Peripheral artery disease (PAD), a systemic atherothrombotic disease of the lower extremities, is common and associated with substantial cardiovascular (CV) morbidity and mortality. PAD is highly prevalent, affecting more than 200 million people worldwide,1 including up to 10 million persons in the United States alone.2,3 Despite recent advances in medical and device therapy, PAD is associated with impaired quality of life and significant cardiac and limb morbidity and all-cause mortality.4,5 With increasing prevalence and morbidity, lower extremity revascularization (LER) is frequently performed to improve morbidity and quality of life for patients with PAD;6 however, adverse cardiovascular events in both the short- and long-term are major concerns.7,8 It is of significant importance to prospectively identify individuals at increased risk of prevalent PAD and incident events following LER for symptomatic PAD.9,10
In contrast to coronary heart disease, relatively few gene expression changes or gene variants have been shown to influence susceptibility to PAD.11,12 A recent genome wide association study identified 19 PAD loci, 18 of which have not been previously reported and 8 of which had not yet been associated with vascular disease.13 Additionally, advances in gene expression analysis have described gene expression signatures from peripheral blood associated with risk of adverse events for patients with coronary artery disease (CAD)14 and venous thromboembolism.15 However, there have been no prior studies to prospectively identify gene expression changes associated with both prevalence and prognosis for high-risk patients with severe, symptomatic PAD. Herein we performed whole blood RNA sequencing to quantify the relative abundance of transcripts associated with prevalence of PAD and major adverse cardiovascular and limb events for PAD patients undergoing LER in the Platelet Activity and Cardiovascular Events (PACE) study.
Materials and Methods
All supporting data are available within the article and its online-only Data Supplement
Study Population and outcomes
The studies were conducted in accordance with policies of the New York University Langone Medical Center Institutional Review Board. Informed consent was obtained from each subject. Patients with PAD were recruited into the Platelet Activity and Cardiovascular Events (PACE) in peripheral artery disease study (NCT02106429). For this cohort, participants >21 years old with symptomatic PAD scheduled for non-emergent LER were recruited from New York University Langone Medical Center, Bellevue Hospital, or the Veterans Affairs NY Harbor Healthcare System. Patients with symptomatic PAD scheduled for LER were enrolled with critical limb ischemia (CLI; ulceration, gangrene or rest pain) or an ankle brachial index (ABI) < 0.6. Participants were excluded for use of non-steroidal anti-inflammatory drugs (other than aspirin) within 72 hours, platelet count <100 × 109/L or >500 × 109/L, anemia (hemoglobin <8 mg/dl) or any known hemorrhagic diathesis. All PAD patients had a blood collection immediately prior to lower extremity revascularization.
Clinical follow-up occurred at 30-days, 6 months and every 6 months thereafter. Three blinded reviewers (2 cardiologists and 1 vascular surgeon) adjudicated study events and assigned events to composite outcomes. A major adverse cardiovascular event (MACE) was defined as death, myocardial infarction (MI) or stroke. Major adverse limb event (MALE) was a composite of major unplanned amputation (any above-ankle amputation (index or contralateral limb)) or major reintervention (new bypass graft, thrombectomy/thrombolysis, or jump/interposition graft of the index limb only).16 Major adverse cardiac or limb event (MACLE) was defined as the composite of either MACE or MALE.
Age- and sex-matched controls were recruited from the New York University Langone Center for the Prevention of Cardiovascular disease registry. This ongoing registry is a repository of patients with different phenotypes of CVD (e.g. coronary artery disease, carotid artery disease) and multiple cardiovascular risk factors. Participants were excluded for use of non-steroidal anti-inflammatory drugs (other than aspirin) within 72 hours, platelet count <100 × 109/L or >500 × 109/L, anemia (hemoglobin <8 mg/dl) or any known hemorrhagic diathesis.
Sample Collection
Peripheral whole-blood RNA was collected into PAXgene™ Blood RNA tubes (Becton Dickinson and Company, Franklin Lakes, New Jersey) and stored at −80°C until microarray profiling.
RNA Sequencing and Data Generation
Automated RNA extraction was performed using the QIAsymphony PAXgene Blood RNA Kit (PreAnalytiX, Qiagen/BD). Before RNA sequencing, yield, quantity, and quality of the RNA were assessed using an Illumina HiSeq 4000 v4 chemistry single read (Illumina, Inc., San Diego, CA). RNA sequencing libraries were generated with the Illumina TruSeq (San Diego, CA) and 200 ng total RNA used as starting input per sample. Samples underwent 12 cycles of amplification. Completed libraries were quantitated, normalized, and pooled.
Differential Expression Analysis
An sex, age and race-matched cohort of PAD (N=42) and disease controls (N=29) was used (Supplemental Figure I) to complete differential expression analysis of RNA-Seq data using the Bioconductor DESeq2 R package.17 Adjusted P-values using the Benjamini-Hochberg method18 and log2 fold change (FC) with unshrunken maximum likelihood estimate were used for all downstream analysis. Gene transcripts were then filtered for expression below a background level to identify sufficiently expressed probes that would be included in downstream analysis.19 The distribution of values for each probe across participants was plotted to determine a cutoff able to differentiate expressed transcripts from those below a background intensity. The resulting distribution was bimodal, and we determined that a cutoff of 4 log2 normalized expression differentiated between the two populations (Supplemental Figure II). This resulted in the filtering out of nearly 80% of transcripts, leaving 13,219 transcripts with sufficient expression for all downstream analysis.
WGCNA Analysis
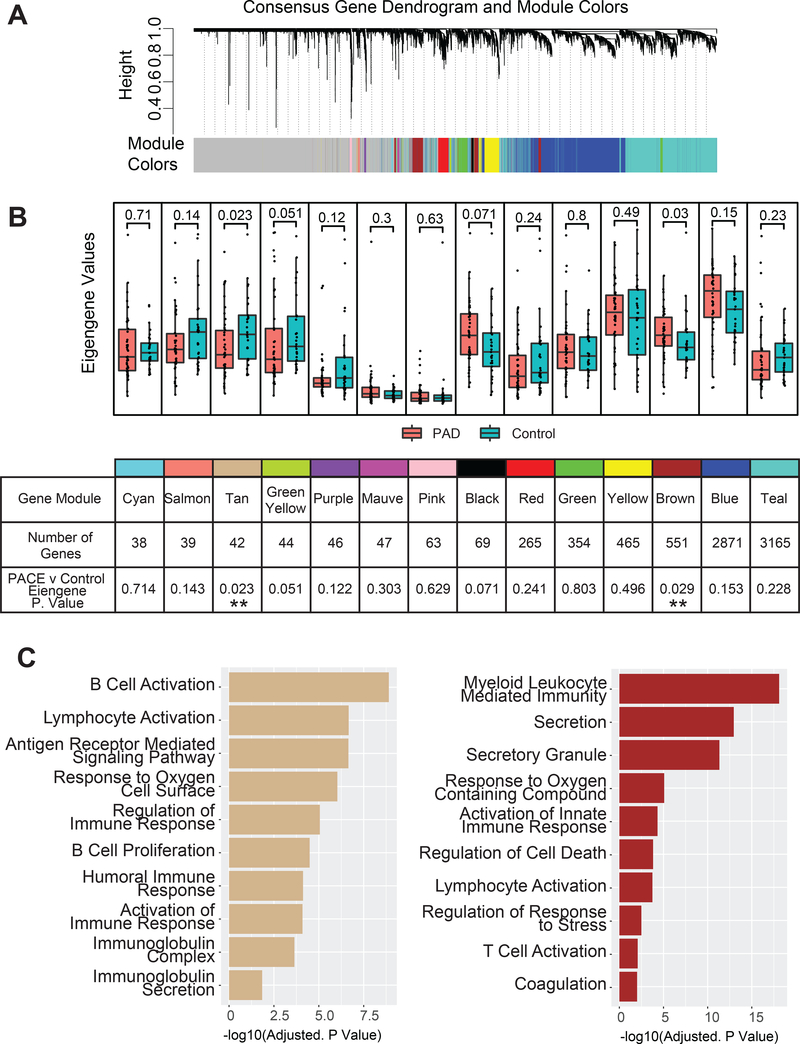
We used WGCNA (Weighted Gene Co-expression Network Analysis) to analyze transcriptional changes between our cohorts of patients with symptomatic PAD and matched controls. WGCNA analyzes population-wide gene expression patterns to identify groups of co-regulated genes. Co-regulated groups of genes were reduced to a single eigengene, represented by a color-coded module indicative of the relative expression activity for that group of genes. We then performed Wilcoxon tests to identify differentially expressed modules between patients with symptomatic PAD and matched control (Figure 2B). Last, we used a standard hypergeometric gene set enrichment test 20 to explore established gene sets within color modules that were overexpressed between patients with symptomatic PAD and matched controls.
Figure 2. WGCNA for PAD patients vs matched control.
(A) WGCNA Clustering of genes into Color Modules. (B) Boxplots for eigengene values for each color module between patients with PAD and matched controls. The table summarizes each module with the name/color, the number of genes within the module, and Wilcoxon p-value for the difference of eigengene values between cohorts. (C) Selected hypergeometric tests results for the Tan and Brown modules, x-axis is -log(adjusted p value) of the hypergeometric test.
Event outcome analysis
We assessed the prognostic relevance of the transcripts differentially expressed in 106 patients with PAD measured prior to revascularization and then followed longitudinally for a median of 26.5 months (interquartile range [IQR] 16–35) months (Supplemental Figure I). Dysregulated transcripts with a |log2FC| >0.5 and an adjusted p-value < 0.05 were included in downstream analysis. All 127 transcripts were analyzed using Kaplan-Meier analysis via the survminer package 21 in R to characterize the relationship between event-free survival and tiered expression of the differentially expressed transcripts. Each upregulated transcript was analyzed by comparing the upper quartile (Q4) of gene expression to quartiles 1–3 (Q1-Q3) of differentially expressed transcripts among all patients with PAD. For genes downregulated in PAD vs Control, the same analysis was performed but using samples with the lowest quartile of expression vs. the rest of the population. A secondary analysis examined the identified differentially expressed transcripts among only the 64 PAD patients not used in the age, sex and race-matched comparison with diseased controls.
Quality control and miRNA targeting
Quality control was conducted by using miRDB and Targetscan to determine miRNA targets.22,23 Per recommended miRDB guidelines, a target prediction score >80 was used to determine significant miRNA-mRNA associations.22 Scatterplots were generated comparing expression of miRNA vs its reported target, trendlines were plotted using simple linear regression with a 95% confidence interval. We further filtered the list to define genes that were negatively correlated with miR-4477b expression (p < 0.05) and were down regulated in patients with PAD versus matched controls. We then cross-referenced our list of candidate genes with an additional miRNA resource TargetScan23 to select for genes that fell into the top 20% of candidates predicted by TargetScan (Cumulative weighted context++ score < −0.17).
We selected miR-4477b for testing in a mouse model because it was highly dysregulated between patients with PAD and matched controls and was also significantly associated with multiple cardiovascular outcomes. Testing of miR-4477b was performed using an established mouse model of acute and sub-acute hindlimb ischemia to further evaluate differentially expressed transcripts associated with PAD and cardiovascular outcomes in our cohort.24,25 Briefly, acute ischemic injury was produced by unilateral femoral artery ligation in 10 week-old, male C57BL/6J mice as previously described.24 Gradual femoral arterial occlusion (sub-acute injury) was produced by application of two ameroid constrictors (0.25 mm × 1.00 mm; Research Instruments SW, Inc) to the proximal and distal segments of the femoral artery in male mice. We focused on male mice as sample sizes and kinetics of blood flow recovery were estimated based on results of prior studies.24,25 Mice were imaged following surgery with a 785-nm near-infrared Laser Doppler Imager-2 (Moor Instruments Inc.) to assess blood flow. Mean blood flow percentage was determined by comparing the injured to uninjured leg (Moor Instruments LDI V5.3). Skeletal muscles were harvested for RNA and expression analyses in the ischemic and non-ischemic gastrocnemius skeletal muscles after three days of acute or sub-acute femoral artery ligation. Plasma was isolated from whole blood at 1500g for 15 minutes and extracellular RNA was captured using the total RNA purification kit from Norgen Biotek Corporation. Reverse transcriptions were performed by using miScript Reverse Transcription Kit from Qiagen (218061). For miRNA expression, samples were normalized to endogenous 5S RNA. For mRNA expression, samples were normalized to endogenous mouse GAPDH. Fold changes were calculated by ΔΔCt method. Significance was determined by student’s two-tailed t-test, p<0.05. Either QuantiTect SYBR Green RT-PCR Kit (204243) or miScript SYBR Green PCR Kit (218073) from Qiagen was used for quantitative real-time qPCR analysis with the AriaMx Real-Time PCR System (Agilent Technologies) following the manufacturer’s instructions. All animal studies were approved by the Institutional Animal Care and Use Committees at the Brigham and Women’s Hospital.
Statistical analysis
Statistical testing for differential gene expression was completed using DESeq2, which uses the Wald test for estimated standard error of a log2 fold change.17 All other two group comparisons were tested using the nonparametric Mann-Whitney test. All correlations were completed using a Spearman correlation. Survival analysis was done using a Kaplan-Meier estimator with an associated log-rank test to compare between survival cohorts. The Benjamini-Hochberg method was used to generate a false discovery rate (FDR) corrected for multiple comparisons and for p-value adjustment.18 Effect size estimates and 95% Confidence Intervals (C.I.) were generated using a Cox Proportional-Hazard Model. Statistical cut-offs are noted throughout. If not stated otherwise a p-value of 0.05 was used for statistical significance.
Results
Baseline characteristics
A total of 106 PAD patients with clinical outcome data and 29 diseased controls were transcriptionally sequenced. Overall, 81 (76%) of PAD patients had critical limb ischemia. The mean ABI in the PAD cohort was 0.64 (SD, 0.43). For differential expression and predictive analysis to determine PAD-specific changes we used an age- and sex-matched cohort consisting of 42 PAD and 29 control samples (Table 1). This revealed no significant difference between matched controls and PAD patients for age, sex, race, body mass index, current or former smoking, hypertension or hyperlipidemia. Diabetes was more common among PAD patients compared to matched controls (Table 1). For downstream analysis investigating differences in outcomes within PAD we used the comprehensive PAD cohort (N=106) (Supplemental Figure I).
Table 1.
Demographics for Matched and Unmatched Cohorts.
| All PAD (N = 106) | PAD Matched (n = 42) | Control Matched (n = 29) | P Value between Matched Cohorts | |
|---|---|---|---|---|
| Age, years (±SD) | 67.48 (9.37) | 59.6 (6.42) | 57.3 (6.37) | 0.14 |
| Female, n (%) | 28 (26.4) | 11 (26.2) | 8 (27.6) | 0.9 |
| Race | ||||
| Asian, n (%) | 3 (2.8) | 1 (2.4) | 1 (3.4) | 0.8 |
| Black, n (%) | 20 (18.87) | 6 (14.3) | 3 (10.3) | 0.62 |
| White, n (%) | 68 (64.15) | 27 (64.3) | 18 (62.1) | 0.85 |
| Other, n (%) | 15(14.15) | 8 (19.0) | 7 (24.1) | 0.62 |
| Hispanic Ethnicity, n (%) | 21 (19.81) | 11 (26.2) | 10 (34.48) | 0.47 |
| Body mass index, kg/m2(±SD) | 27.38 (5.84) | 28.9 (6.8) | 30.5 (6.5) | 0.33 |
| Current cigarette smoker, n (%) | 22 (20.75) | 8 (19.0) | 4 (13.8) | 0.56 |
| Former cigarette smoker, n (%) | 61 (57.54) | 27 (64.3) | 14 (48.3) | 0.19 |
| Diabetes, n (%) | 60 (56.6) | 22 (52.4) | 8 (27.6) | 0.03 |
| Hypertension, n (%) | 94 (88.68) | 36 (85.7) | 24 (82.8) | 0.74 |
| Hyperlipidemia, n (%) | 79 (74.52) | 32 (76.2) | 21 (72.4) | 0.73 |
| Coronary artery disease, n (%) | 60 (56.6) | 20 (47.61) | 15 (51.72) | 0.74 |
| Prior stroke, n (%) | 15 (14.15) | 6 (14.3) | 2 (6.90) | 0.31 |
| Ankle brachial index (±SD) | 0.64 (0.43) | 0.61 (0.34) | NA | NA |
| Critical limb ischemia, n (%) | 81 (76.41) | 30 (71.4) | NA | NA |
| Rest pain, n(%) | 45 (42.45) | 18 (42.86) | ||
| Gangrene, n(%) | 26 (24.53) | 5 (11.90) | ||
| Ulceration, n(%) | 45 (42.45) | 19 (45.24) | ||
| Prior amputation, n (%) | 11 (10.4) | 2 (4.8) | NA | NA |
Differentially expressed genes in PAD patients
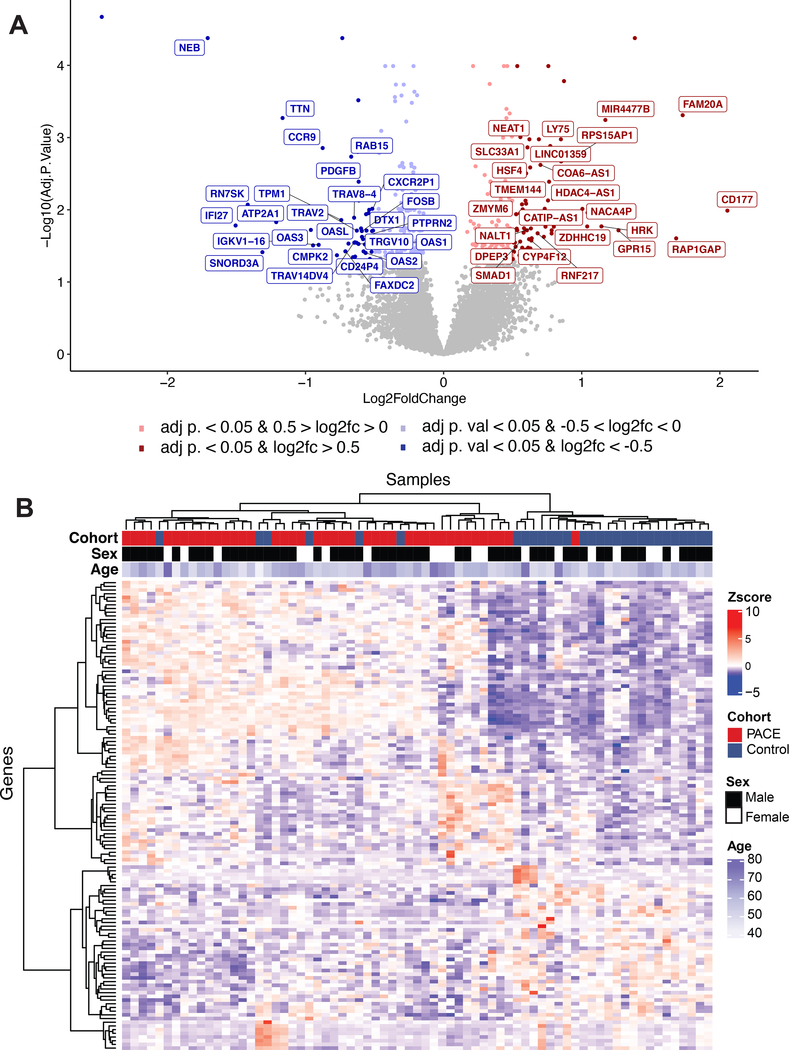
RNA-Seq data characterized 57,688 transcripts across age and sex matched PAD (N=42) and disease control (N=29) samples (Supplemental Figure I). Based on a probability density plot, transcripts below a determined expression threshold of 16 were filtered out for subsequent analysis (Supplemental Figure II). Figure 1A presents a volcano plot of the remaining 13,219 transcripts, highlighting significant (adjusted p<0.05) and/or dysregulated (|log 2 FC| >0.5) transcripts based on differential expression analysis in PAD versus controls. The detailed fold change for 127 transcripts with an adjusted p<0.05 and |log 2 FC| >0.5 is shown in Supplemental Figure IIIA, identifying upregulated a GPI-linked glycoprotein CD177, a secreted hematopoietic protein FAM20A, and a microRNA (miR-4477b). Significantly downregulated genes include the ribosomal pseudogene AC010970.1 and the sarcomeric protein nebulin, NEB. The 127 differentially expressed transcripts with an adjusted p< 0.05 and a log 2 FC |>0.5| (Supplemental Table I) were used in unsupervised hierarchical clustering to separate samples by PAD status (Figure 1B). While diabetes was more common among PAD patients than disease controls, the difference in diabetes did not influence the differential expression signature for PAD patients vs. matched disease controls (correlation coefficient for PAD with DM vs. controls compared to PAD without diabetes vs. controls was 0.68 [P<0.001], Supplemental Figure IVA).
Figure 1. Differential gene expression in matched PAD versus control.
(A) Volcano plot displaying the -log10 of the adjusted p-values vs. the log2 fold change of PAD compared to control transcript expression. Red marker indicates adjusted p < 0.05 log 2 FC > 0.5 and blue marker indicates adjusted p < 0.05 log fold change < −0.5. (B) Heatmap of 127 differentially expressed transcripts with an adjusted p-value < 0.05 and |log2FC > 0.5| identified by DESeq2 for 42PAD and 29 control samples with unsupervised clustering. Of these 127, 77 are upregulated and 50 are downregulated.
While our data identified a transcript signature associated with PAD, coronary artery disease (CAD) is frequently observed in patients with PAD and may limit specificity of the signature for PAD. However, clinical CAD did not appear to influence the PAD transcriptome signature. Patients with PAD and CAD vs controls compared to PAD without CAD vs. controls had very similar differential expression signature (r=0.76, P<0.001; Supplemental Figure IVB). To further test the specificity of our identified transcript signature for PAD, we evaluated the differential gene expression from a validated age- and sex-specific whole-blood gene expression score (ASGES) for CAD.26–28 Of the 23 genes that comprise the ASGES, 18 genes were measurably expressed in our cohort. Of these, only 2 genes were differentially expressed in PAD vs. controls (Supplemental Figure IVC). None of the differentially expressed genes in ASGES were identified as part of the 127 gene expression signature (Supplemental Table I).
WGCNA Analysis
WGCNA analysis was used to analyze transcriptional changes between patients with PAD compared to matched disease controls. Using the WGCNA algorithm, co-regulated modules of genes were assigned a color label (Figure 2A). Using these determined gene modules, we then explored the relative expression value of the entire color module in each sample by giving each sample its respective eigengene value, a relative measure of the expression of that color modules’ activity. Wilcoxon tests for each module were then performed between patients with PAD and controls to identify differentially expressed modules. Two modules differed significantly at a p value < 0.05 among patients with PAD compared to matched control: The Tan module of 42 genes was decreased (p < 0.023), whereas the Brown module composed of 551 genes was increased (p < 0.029) (Figure 2B). Hypergeometric gene set enrichment identified downregulation of genes in the Tan color block (humoral and B Cell responses) and upregulation of genes in the Brown color module (immune, secretory stress response, and coagulation pathways) among patients with PAD compared to matched control (Figure 2C).
PAD outcomes and miRNA expression
Among 106 patients with symptomatic PAD undergoing LER, 58 (55%) patients experienced a MACLE (29 (27%) MACE and 40 (38%) MALE events) at a median of 26.5 months (IQR: 16–35). Of the 127 transcripts differentially expressed between PAD patients and matched controls (adjusted p < 0.05, log 2 FC >0.5; Figure 1A), 47 transcripts had a log-rank p value < 0.05 for one of the endpoints (MACLE, MACE, MALE, Death, Death/MI, Amputation). After correction for multiple comparisons, 40 transcripts had an FDR value of < 0.1 for one or more of the endpoints. These genes, their Benjamini-Hochberg corrected false discovery rate (FDR), adjusted p-value and log2FC for patients with PAD compared to matched controls are shown in Figure 3A. The respective Hazard Ratio (HR) and 95% confidence intervals (C.I.s) by Cox Proportional-Hazards models are shown in Supplemental Figure V. PAD patients with miR-4477b expression in the top quartile were more likely to experience MACLE, MACE, Death, Death or MI, or major Amputation, FDR < 0.1 for each comparison) than PAD participants in the lower three quartiles of miR-4477b expression (Figure 3B–E). miR-4477b remained associated with MALE and MACLE in sensitivity analyses limited to events occurring within 1 year of LER (Supplemental Figure IIIB–E). Findings were similar for analyses restricted to the 64 PAD patients not included in the original test group (Supplemental Figure VI).
Figure 3. Expression differences based on incident events.
127 genes differentially expressed between patients with PAD and matched controls were analyzed to identify transcriptional changes associated with event-free survival. (A) Summary of 47 (of 127) differentially expressed genes associated with event-free survival comparing the top quartile of gene expression to the lower 3 quartiles. Side annotation indicates the log2FC and the adjusted p value for the differential expression results between patients with PAD and matched controls. (B-E) Kaplan-Meier curve and log-rank p value for miR-4477b and the outcomes of Death, MACE, Amputation, and MACLE respectively.
Abbreviations:
MACE (Major Adverse Cardiac Events): Death, myocardial infarction (MI), Stroke
MALE (Major Adverse Limb Events): Major amputation (above-ankle amputation (index or contralateral limb)) or major reoperation (new bypass graft, thrombectomy/thrombolysis, or jump/interposition graft revision of index limb only)
Major Adverse Cardiac and Limb Events (MACLE): MACE + MALE
MicroRNA Analysis
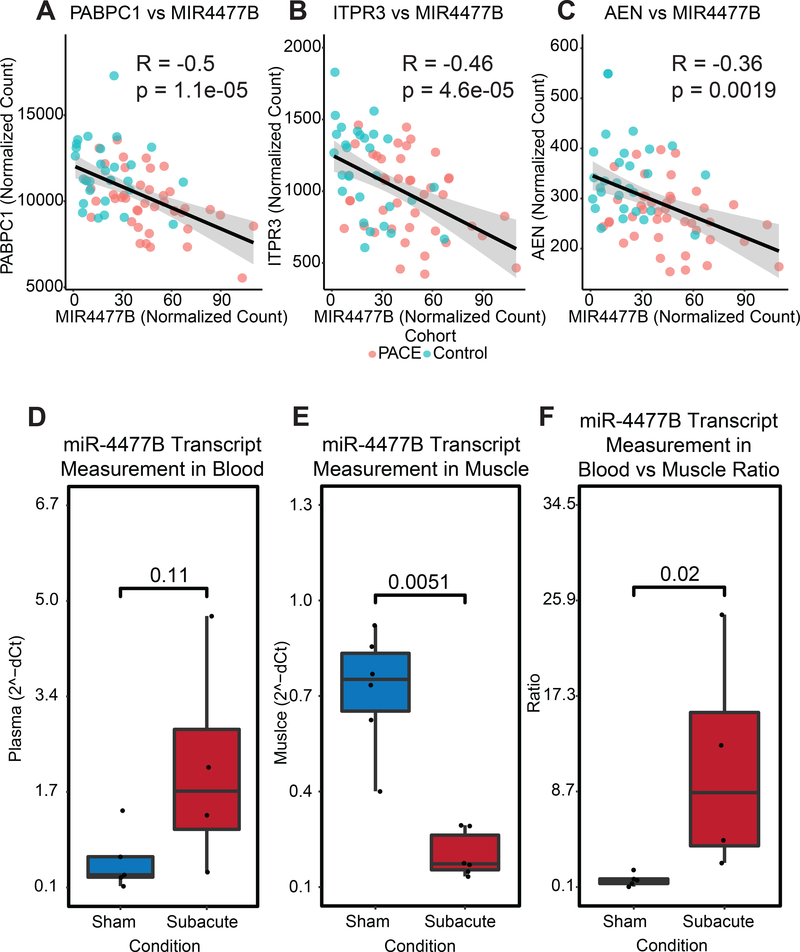
miRDB22 and Targetscan23 identified three potential messenger RNA (mRNA) targets that were significantly associated with miR-4477b and differed significantly between patients with PAD and matched controls: AEN, PABPC1, and ITPR3 (Supplemental Table II). AEN, PABPC1 and ITPR3 were significantly and negatively correlated with miR-4477b expression levels between PAD patients and diseased controls (Figure 4 A–C). Cell apoptosis and stress response has been associated with AEN;29 vascular development, protein synthesis and myocardial hypertrophy have been linked to PABPC1;30 and the role of ITPR3 in endothelial nitric oxide synthase and endothelial cell homeostasis has recently been described.31
Figure 4. miR-4477b association with possible target genes in the mouse subacute hindlimb ischemia model.
Correlation analysis of miR-4477b expression with three gene targets (A) PABPC1, (B) ITPR3, and (C) AEN. (D) Whole blood qPCR and (E) muscle expression qPCR in mouse ischemia model in the subacute setting. (F) The ratio of measurement metrics for whole blood qPCR and muscle qPCR.
miR-4477b was selected for additional testing in a mouse model because it was dysregulated between patients with PAD and matched disease controls (Figure 1) and was also significantly associated with major adverse cardiovascular events (Figure 3). To further examine the functional significance of miR-4477b, we examined its expression in skeletal muscle and plasma in a mouse hindlimb ischemia model.24,25 Compared to a sham control, skeletal muscle miR-4477b decreased significantly in subacute hindlimb ischemia (FC: 0.22, p = 0.0051) (Figure 4D). Circulating plasma levels of miR-4477b trended higher following subacute hindlimb ischemia (FC: 4.03, p = 0.11), although this increase did not reach statistical significance (Figure 4E). Prior studies have shown that skeletal muscle miRNA decreases in response to ischemia or tissue injury with accompanying increases in circulating miRNA.32–34 Therefore we investigated the ratio of circulating plasma to skeletal muscle miR-4477b in our hindlimb ischemia models, demonstrating that the ratio of blood to skeletal muscle miR-4477b expression increased with sub-acute hindlimb ischemia (p=1.5e-06, Figure 4F). Similar patterns were observed using a model of acute hindlimb ischemia (Supplemental Figure VII).
Discussion
The pathophysiological basis underlying severe PAD is not well understood and identifying which patients with phenotypically severe PAD will experience limb morbidity or mortality remains uncertain. Understanding the genetics and pathways of patients with severe PAD at risk of future events and mortality would support new therapeutic approaches and risk stratification. In this study, we performed RNA-Seq transcriptome profiling in peripheral whole blood samples for patients with severe PAD compared with age- and sex-matched controls and identified a novel microRNA (miR-4477b) that identified patients with symptomatic PAD compared to age and sex-matched patients without PAD, but with similar burdens of cardiovascular risk factors. miR-4477b also identified patients with symptomatic PAD at risk of MACLE following revascularization.
We identified 127 differentially expressed genes and used hierarchical clustering methods to separate these samples by presence or absence of PAD. Nineteen loci for PAD were recently identified in the Million Veteran Program genome wide association study of PAD.13 Ten of these genes were quantifiable in our data set, among which 3 (CELSR2, SORT1, CDKN2B-AS1) differed significantly (nominal p<0.05) between PAD patients and matched controls (Supplemental Figure VIII). CELSR2 and SORT1 are part of the sortilin complex with multiple cardiometabolic and cardiovascular effects,35 and CDKN2B-AS1 is a 9p21 chromosome variant strongly associated with PAD by ankle-brachial index.36
We used WGCNA to define co-regulated modules of genes specific to whole blood transcriptional patterns. Reduction of these gene modules to single eigengene values allowed us to assign individual relative expression values to entire modules of functionally aligned genes. The Tan and Brown modules differed significantly between patients with PAD and matched controls. The Tan module was downregulated among patients with PAD compared to matched controls and was composed of 42 genes enriched for humoral immune response pathways (adjusted p value < 7.58e-05) and B cell activation (adjusted p value <1.26e-09). In contrast, the Brown module was upregulated among patients with PAD and was composed of 551 genes enriched for myeloid-mediated immunity (adjusted p value <7.9e-19), secretory granules (adjusted p value = 4.6e-12), T cell activation (adjusted p value < 0.008) and coagulation (adjusted p value < 0.009). Altogether, our findings provide further evidence that inflammation, inflammatory cell activation and coagulation may play a role in the pathogenesis and limb outcomes for patients with symptomatic PAD.37,38 Along with inflammation and immune response, we observed a putative role for pathways of vesicle-mediated transport and secretory vesicles in the pathophysiology of PAD.39 Our network analysis highlighted a number of functional modules including B cell and lymphocyte activation, along with leukocyte mediated immunity that may play a role in understanding the complex pathobiology in PAD (Figure 2B). The pathway analysis overall suggests a decrease in regulation and moderation of an immune response, leading to a chronic inflammatory response characterized by B cell and lymphocyte activation and prolonged inflammation which could contribute to the phenotypical response seen in the PAD patients.
miR-4477b was identified as being highly significant in our differential expression analysis, highlighting the importance of this miRNA and its targets for PAD pathogenesis. miR-4477b is a novel miRNA that has not been previously associated with the pathogenesis of PAD or cardiovascular disease. Other highly significant (adjusted P < 0.01) genes with the highest log2-fold change (FC) included CD177, RAP1GAP and FAM20A. CD177 is important in formation of fibrin clot formation and platelet-neutrophil interactions at the vascular endothelium.40 RAP1GAP is a family of GTPase-activating proteins (GAP) that down-regulate the activity of the ras-related Rap1 protein. Platelets contain high levels of Rap1, a potent regulator of integrin function and platelet aggregation.41,42 The FAM20A locus encodes a secretory hematopoietic protein, which may phosphorylate proprotein convertase subtilisin-kexin 9 (PCSK9) and modulate LDL receptor activity.43
In addition to identifying gene-level differences between patients with symptomatic PAD and healthy controls, we also examined the relationships between transcript expression and cardiovascular events including death and amputation among PAD patients undergoing revascularization. Of the 127 genes differentially expressed between patients with PAD and matched controls, 40 were significantly associated with one or more major cardiovascular or limb-related endpoints. PAD patients with miR-4477b expression levels in the highest quartile were significantly more likely to experience MACE, Death, Death/MI, Amputation, and MACLE compared to PAD patients in the lower 3 quartiles of miR-4477b expression. We then used a functional microRNA database to query against targets for miR-4477b. Three targets – AEN, PABPC1, ITPR3 – were identified as being significantly negatively correlated with miR-4477b expression levels (Figure 4A–C). These transcripts have been previously implicated in pathways including cell apoptosis and stress response (AEN),29 vascular development, protein synthesis and myocardial hypertrophy (PABPC1),30 and endothelial nitric oxide synthase and endothelial cell homeostasis (ITPR3),31 providing evidence of functional significance of alterations in expression of miR-4477b.
Finally, we used a mouse hindlimb ischemia model to examine the expression of miR-4477b in tissue ischemia/hypoxia. The subacute mouse hindlimb ischemia model (Fig. 4D–F), has previously been shown to closely recapitulate the progressive vascular process of PAD in humans.44,45 The gradual occlusion of the femoral artery is induced through the application of ameroid constrictors and the mechanisms regulating blood flow recovery, gene expression, skeletal muscle necrosis, and leukocyte accumulation are distinct compared to acute arterial occlusion using ligation techniques. Histological examination of skeletal muscles in the subacute model previously demonstrated findings overlapping with human PAD including the absence of skeletal muscle necrosis and incomplete blood flow recovery with lower shear stress responsive, hypoxia, and inflammatory genes in regions of collateral arteries. In response to subacute hindlimb ischemia, skeletal muscle miR-4477b was significantly decreased, while circulating miR-4477b trended higher, leading to a significantly increased ratio of blood to skeletal muscle miR-4477b expression in our mouse model. These findings are consistent with other reports demonstrating inverse correlations of some miRNAs that are depleted in muscles and released into the circulation.46
This cross-species ischemia model suggests that increased human whole blood miR-4477b may 1) identify PAD patients compared to matched controls, and 2) indicate tissue ischemia predictive of future amputation in patients with severe PAD undergoing revascularization. Although previous studies have shown that expression of several miRNAs are significantly different between patients with stable PAD compared to age and sex matched controls,11,47 to our knowledge this is the first study to report on a specific whole blood RNA transcript (miRNA4477b) associated with amputation and a whole blood RNA signature predictive of death or amputation for patients with severe symptomatic PAD undergoing lower extremity revascularization.
There are some notable limitations to the current analysis. First, while PAD patients and disease controls were well matched, diabetes was more common among patients with PAD than matched controls (Table 1). However, the difference in diabetes did not influence the differential expression signature for PAD patients vs. matched controls (Supplemental Figure IVA). Moreover, miR-4477b remained significantly upregulated among patients with PAD and associated with MACE and MACLE in sensitivity analyses excluding patients and matched controls with DM. Together these data suggest the difference in the proportion of PAD patients and matched controls with diabetes is unlikely to explain our findings. As we recently showed, diabetes is present in ≈50% of patients undergoing revascularization for PAD,48 indicating the burden of DM among PAD patients in our study reflects the distribution of diabetes in the global population of patients with severe PAD.
While our data identified a transcript signature associated with PAD, coronary artery disease is frequently comorbid among patients with PAD and may limit specificity of the signature for PAD. However, comorbid CAD did not influence the differential expression signature for PAD patients vs. matched disease controls (correlation coefficient between patients with vs. without CAD was 0.76 (P<0.001) (Supplemental Figure IVB). To further test the specificity of the identified transcript signature for PAD, we compared differential gene expression from PAD patients in our study against a validated age- and sex-specific whole-blood gene expression score (ASGES) for CAD.26–28 We observed minimal overlap between the ASGES genes and the differential transcript signature for PAD, indicating potential specificity of our differential gene expression signature for PAD. (Supplemental Figure IVC)
Other limitations include the lack of advanced lower extremity imaging to characterize PAD severity in greater detail, which may have introduced phenotypic heterogeneity in our definition of patients with symptomatic PAD for patients undergoing LER in the current study
In conclusion, we identified a blood transcriptome signature that can discriminate between patients with and without severe, symptomatic PAD, and identify PAD patients at risk for death and amputation following lower extremity revascularization. Our novel transcriptomic signature provides insight into the mechanisms and prognosis for patients with PAD. A previously uncharacterized transcript, miR-4477b, was identified as significantly increased in patients with symptomatic PAD compared with matched controls, and among PAD patients with MACLE following lower extremity revascularization. miR-4477b was also tested in a cross-species model of hindlimb ischemia, further highlighting the potential functional significance for this transcript. Our findings may have implications for the identification and management of patients with severe PAD.
Supplementary Material
Highlights:
In a cohort with symptomatic PAD undergoing lower extremity revascularization we identified a whole blood transcript signature of 127 differentially expressed genes compared to matched diseased controls.
Co-expressed gene modules enriched for Myeloid mediated Immunity, T cell activation, and Coagulation were increased in patients with PAD, whereas gene modules decreased in PAD were enriched for B cell activation and Humoral response.
Survival analysis using the 127 differentially expressed genes between PAD patients and matched diseased controls identified 47 genes prospectively associated with major adverse cardiovascular and limb events.
miR-4477b was a top differentially expressed transcript between PAD patients and matched controls and PAD patients with miR-4477b expression in the upper quartile was significantly associated with major adverse cardiovascular and limb events.
In a subacute hindlimb ischemia in a mouse model of PAD, skeletal muscle miR-4477b decreased while whole blood miR-4477b increased, indicating that tissue ischemia and circulatory release of miR-4477b is associated with prevalent and incident PAD.
Acknowledgements:
All authors have participated in preparation, analysis and drafting of the manuscript and vouch for the content presented.
Sources of funding: NIH and the American Heart Association (AHA) Brigham and Women’s Hospital: NIH (HL115141, HL134849; and HL148207 to MWF) and AHA (18SFRN33900144; and 18SFRN33900144 to MWF) NYU: NIH (HL125991 to JDN; HL114978, HL114978 and HL144993 to JSB) and AHA 20SFRN35210252 and 20SFRN35210609 to JDN and JSB, T32GM136573 to MGC
Abbreviations:
- LER
lower extremity revascularization
- PACE
Platelet Activity and Cardiovascular Events
- WGCNA
Weighted Gene Co-expression Network Analysis
- miR
microRNA
- ASGES
age- and sex-specific whole-blood gene expression score
Footnotes
Disclosures: None
References:
- 1.Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. The Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 2.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-Specific Prevalence of Peripheral Arterial Disease in the United States. American Journal of Preventive Medicine. 2007;32:328–333. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. [DOI] [PubMed] [Google Scholar]
- 4.Emdin CA, Anderson SG, Callender T, Conrad N, Salimi-Khorshidi G, Mohseni H, Woodward M, Rahimi K. Usual blood pressure, peripheral arterial disease, and vascular risk: cohort study of 4.2 million adults. BMJ [Internet]. 2015. [cited 2020 Apr 30];351. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4586462/ [DOI] [PMC free article] [PubMed]
- 5.Criqui Michael H, Victor Aboyans. Epidemiology of Peripheral Artery Disease. Circulation Research. 2015;116:1509–1526. [DOI] [PubMed] [Google Scholar]
- 6.Nowygrod R, Egorova N, Greco G, Anderson P, Gelijns A, Moskowitz A, McKinsey J, Morrissey N, Kent KC. Trends, complications, and mortality in peripheral vascular surgery. Journal of vascular surgery. 2006;43:205–216. [DOI] [PubMed] [Google Scholar]
- 7.Hess CN, Rogers RK, Wang TY, Fu R, Gundrum J, LaPointe NMA, Hiatt WR. Major Adverse Limb Events and 1-Year Outcomes After Peripheral Artery Revascularization. Journal of the American College of Cardiology. 2018;72:999–1011. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner I, Norgren L, Fowkes FGR, Mulder H, Patel MR, Berger JS, Jones WS, Rockhold FW, Katona BG, Mahaffey K, Hiatt WR. Cardiovascular Outcomes After Lower Extremity Endovascular or Surgical Revascularization: The EUCLID Trial. Journal of the American College of Cardiology. 2018;72:1563–1572. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2017;69:e71–e126. [DOI] [PubMed] [Google Scholar]
- 10.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;64:e77–e137. [DOI] [PubMed] [Google Scholar]
- 11.Stather PW, Sylvius N, Sidloff DA, Dattani N, Verissimo A, Wild JB, Butt HZ, Choke E, Sayers RD, Bown MJ. Identification of microRNAs associated with abdominal aortic aneurysms and peripheral arterial disease. British Journal of Surgery. 2015;102:755–766. [DOI] [PubMed] [Google Scholar]
- 12.Stather PW, Sylvius N, Wild JB, Choke E, Sayers RD, Bown MJ. Differential MicroRNA Expression Profiles in Peripheral Arterial Disease. Circulation: Cardiovascular Genetics. 2018;6:490–497. [DOI] [PubMed] [Google Scholar]
- 13.Klarin D, Lynch J, Aragam K, et al. Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nature Medicine. 2019;25:1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Ghasemzadeh N, Eapen DJ, Chung NC, Storey JD, Quyyumi AA, Gibson G. Gene expression profiles associated with acute myocardial infarction and risk of cardiovascular death. Genome medicine. 2014;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis DA, Suchindran S, Beckman MG, Hooper WC, Grant AM, Heit JA, Manco-Johnson M, Moll S, Philipp CS, Kenney K, Staercke CD, Pyle ME, Chi J-T, Ortel TL. Whole blood gene expression profiles distinguish clinical phenotypes of venous thromboembolism. Thrombosis Research. 2015;135:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menard MT, Farber A, Assmann SF, Choudhry NK, Conte MS, Creager MA, Dake MD, Jaff MR, Kaufman JA, Powell RJ, Reid DM, Siami FS, Sopko G, White CJ, Rosenfield K. Design and Rationale of the Best Endovascular Versus Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST-CLI) Trial. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57:289–300. [Google Scholar]
- 19.Edelstein LC, Simon LM, Montoya RT, Holinstat M, Chen ES, Bergeron A, Kong X, Nagalla S, Mohandas N, Cohen DE, Dong J, Shaw C, Bray PF. Racial Difference in Human Platelet PAR4 Reactivity Reflects Expression of PCTP and miR-376c. Nat Med. 2013;19:1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools - PubMed [Internet]. [cited 2020 Dec 8];Available from: https://pubmed.ncbi.nlm.nih.gov/30407594/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassambara A, Kosinski M, Biecek P, Fabian S. survminer: Drawing Survival Curves using “ggplot2” [Internet]. 2020. [cited 2020 Dec 8]. Available from: https://CRAN.R-project.org/package=survminer [Google Scholar]
- 22.Wang X miRDB: A microRNA target prediction and functional annotation database with a wiki interface. RNA. 2008;14:1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimson A, Farh KK-H, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wara AK, Foo S, Croce K, Sun X, Icli B, Tesmenitsky Y, Esen F, Lee J-S, Subramaniam M, Spelsberg TC, Lev EI, Leshem-Lev D, Pande RL, Creager MA, Rosenzweig A, Feinberg MW. TGF-β1 signaling and Krüppel-like factor 10 regulate bone marrow–derived proangiogenic cell differentiation, function, and neovascularization. Blood. 2011;118:6450–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Icli B, Wu W, Ozdemir D, et al. MicroRNA-615–5p Regulates Angiogenesis and Tissue Repair by Targeting AKT/eNOS (Protein Kinase B/Endothelial Nitric Oxide Synthase) Signaling in Endothelial Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39:1458–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg S, Elashoff MR, Beineke P, et al. Investigators, PREDICT (Personalized Risk Evaluation and Diagnosis in the Coronary Tree). Multicenter Validation of the Diagnostic Accuracy of a Blood-Based Gene Expression Test for Assessing Obstructive Coronary Artery Disease in Nondiabetic Patients. Annals of Internal Medicine. 2010;153:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wingrove JA, Daniels SE, Sehnert AJ, Tingley W, Elashoff MR, Rosenberg S, Buellesfeld L, Grube E, Newby LK, Ginsburg GS, Kraus WE. Correlation of Peripheral-Blood Gene Expression With the Extent of Coronary Artery Stenosis. Circulation: Cardiovascular Genetics. 2008;1:31–38. [DOI] [PubMed] [Google Scholar]
- 28.Musunuru K, Ingelsson E, Fornage M, Liu P, Murphy AM, Newby LK, Newton-Cheh C, Perez MV, Voora D, Woo D. The Expressed Genome in Cardiovascular Diseases and Stroke: Refinement, Diagnosis, and Prediction: A Scientific Statement From the American Heart Association. Circulation: Cardiovascular Genetics. 2018;10:e000037. [DOI] [PubMed] [Google Scholar]
- 29.Kawase T, Ichikawa H, Ohta T, Nozaki N, Tashiro F, Ohki R, Taya Y. p53 target gene AEN is a nuclear exonuclease required for p53-dependent apoptosis. Oncogene. 2008;27:3797–3810. [DOI] [PubMed] [Google Scholar]
- 30.Chorghade S, Seimetz J, Emmons R, Yang J, Bresson SM, Lisio MD, Parise G, Conrad NK, Kalsotra A. Poly(A) tail length regulates PABPC1 expression to tune translation in the heart. eLife [Internet]. [cited 2020 Dec 7];6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5487213/ [DOI] [PMC free article] [PubMed]
- 31.Ming He, Huang Tse-Shun Li Shuai, et al. Atheroprotective Flow Upregulates ITPR3 (Inositol 1,4,5-Trisphosphate Receptor 3) in Vascular Endothelium via KLF4 (Krüppel-Like Factor 4)-Mediated Histone Modifications. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39:902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. [DOI] [PubMed] [Google Scholar]
- 33.Baggish AL, Park J, Min P-K, Isaacs S, Parker BA, Thompson PD, Troyanos C, D’Hemecourt P, Dyer S, Thiel M, Hale A, Chan SY. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J Appl Physiol. 2014;116:522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siracusa J, Koulmann N, Banzet S. Circulating myomiRs: a new class of biomarkers to monitor skeletal muscle in physiology and medicine. Journal of Cachexia, Sarcopenia and Muscle. 2018;9:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goettsch C, Kjolby M, Aikawa E. Sortilin and Its Multiple Roles in Cardiovascular and Metabolic Diseases. Arterioscler Thromb Vasc Biol. 2018;38:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murabito JM, White CC, Kavousi M, et al. Association Between Chromosome 9p21 Variants and the Ankle-Brachial Index Identified by a Meta-Analysis of 21 Genome-Wide Association Studies. Circ Cardiovasc Genet. 2012;5:100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. [DOI] [PubMed] [Google Scholar]
- 38.Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. The Lancet. 2018;391:219–229. [DOI] [PubMed] [Google Scholar]
- 39.Masud R, Shameer K, Dhar A, Ding K, Kullo IJ. Gene expression profiling of peripheral blood mononuclear cells in the setting of peripheral arterial disease. Journal of Clinical Bioinformatics. 2012;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai M, Grieshaber-Bouyer R, Wang J, Schmider AB, Wilson ZS, Zeng L, Halyabar O, Godin MD, Nguyen HN, Levescot A, Cunin P, Lefort CT, Soberman RJ, Nigrovic PA. CD177 modulates human neutrophil migration through activation-mediated integrin and chemoreceptor regulation. Blood. 2017;130:2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruyn K, Zwartkruis F, Rooij J, Akkerman J-W, Bos J. The Small GTPase Rap1 Is Activated by Turbulence and Is Involved in Integrin IIb 3-mediated Cell Adhesion in Human Megakaryocytes. The Journal of biological chemistry. 2003;278:22412–7. [DOI] [PubMed] [Google Scholar]
- 42.Schultess J, Danielewski O, Smolenski AP. Rap1GAP2 is a new GTPase-activating protein of Rap1 expressed in human platelets. Blood. 2005;105:3185–3192. [DOI] [PubMed] [Google Scholar]
- 43.Ali Ben Djoudi Ouadda, Marie-Soleil Gauthier, Delia Susan-Resiga, et al. Ser-Phosphorylation of PCSK9 (Proprotein Convertase Subtilisin-Kexin 9) by Fam20C (Family With Sequence Similarity 20, Member C) Kinase Enhances Its Ability to Degrade the LDLR (Low-Density Lipoprotein Receptor). Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39:1996–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClung JM, McCord TJ, Southerland K, Schmidt CA, Padgett ME, Ryan TE, Kontos CD. Subacute limb ischemia induces skeletal muscle injury in genetically susceptible mice independent of vascular density. J Vasc Surg. 2016;64:1101–1111.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y, Tang G, Yan J, Park B, Hoffman A, Tie G, Wang R, Messina LM. Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse. J Vasc Surg. 2008;48:1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Icli B, Wara AKM, Moslehi J, et al. MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling. Circ Res. 2013;113:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Signorelli SS, Volsi GL, Pitruzzella A, Fiore V, Mangiafico M, Vanella L, Parenti R, Rizzo M, Volti GL. Circulating miR-130a, miR-27b, and miR-210 in Patients With Peripheral Artery Disease and Their Potential Relationship With Oxidative Stress. Angiology. 2016;67:945–950. [DOI] [PubMed] [Google Scholar]
- 48.Bhandari N, Newman JD, Berger JS, Smilowitz NR. Diabetes Mellitus And Outcomes Of Lower Extremity Revascularization For Peripheral Artery Disease. Eur Heart J Qual Care Clin Outcomes. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.