Abstract
The obesity epidemic has increased risk for non-alcoholic fatty-liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), advanced fibrosis and cirrhosis. We hypothesized that metabolic syndrome (MetS) severity would correlate with markers of NAFLD and NASH fibrosis. We evaluated cross-sectional data from 5,463 participants of the National Health and Nutrition Examination Survey 1999–2012, age 20–64 years with and without diabetes, excluding those with heavy drinking and infectious liver serologies. We used linear and logistic regression to evaluate links between MetS-severity (using a race/ethnicity-specific MetS-severity-Z-score, MetS-Z) and apparent NALFD sequelae, using elevated ALT to determine presence of NAFLD and elevated NAFLD Fibrosis Score to identify advanced fibrosis (NASH Clinical Research Network scoring stage 3–4). The prevalences of unexplained ALT elevations and advanced fibrosis were 11.4% and 1.37%, respectively. MetS-Z-scores were higher among those with elevated ALT (0.7, 95% confidence interval [CI]: 0.6,0.8) and advanced fibrosis (1.7, CI:1.5,1.9), compared to those without liver abnormalities (0.2, CI:0.2,0.3). For every 1-standard-deviation unit increase in MetS-Z, there were higher odds of elevated ALT (OR=1.58, CI:1.44,1.72) and advanced fibrosis (OR=1.96, CI:1.77,2.18), with some attenuation after adjustment for age, sex, race/ethnicity and diabetes status. Significant differences were noted by race/ethnicity, with stronger links among whites vs. blacks. The degree of MetS-severity was associated with progressive increase in apparent NAFLD and advanced fibrosis; as MetS-severity has also been linked to future cardiovascular disease, diabetes and chronic kidney disease, this provides support for use of a MetS-severity score to screen for general health, with high levels triggering further assessment for liver abnormalities.
Keywords: metabolic syndrome, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, fibrosis, risk
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD)—comprised of non-alcoholic steatosis and non-alcoholic steatohepatitis (NASH)—is associated with risk for progressive fibrosis, cirrhosis and cardiovascular disease (CVD)[1,2,3,4]. This elevates the need to discern which adults have NAFLD—and in particular advanced fibrosis and cirrhosis (fibrosis stage 3 or 4 based on the NASH Clinical Research Network scoring system[5,6]), as these individuals are at the highest risk for mortality[2] and can then be targeted for more intensive treatment[7]. While liver biopsy is the gold standard for diagnosing NASH and advanced fibrosis, population-based studies have evaluated disease prevalence using fibrosis scoring systems such as the NAFLD Fibrosis Score (NFS) based on clinically-measured factors[4,8,9,10]. In addition, unexplained elevations of ALT in the absence of infectious hepatitis or alcoholism have been used to estimate the prevalence of NAFLD[11,12]. Studies have used these approaches in cohorts of the National Health and Nutrition Examination Survey (NHANES) from variable time frames to estimate prevalences among US adults for NAFLD of 7.3% (’99-’02)[11] and fibrosis stage 3 or 4 of 3.2% (’88-‘94)[13]. Each of these processes have been demonstrated to have variations in prevalence by sex and race/ethnicity, with higher prevalence in men and Hispanics and lower prevalence in blacks compared to whites[11,14].
An additional diagnosis associated with increased risk for NALFD is the metabolic syndrome (MetS), a constellation of cardiovascular risk factors associated with insulin resistance[15,16]. Traditional MetS criteria, such as those of the Adult-Treatment-Panel III (ATP-III) utilize cut-offs to identify abnormalities in the individual MetS components, including elevated waist circumference, high blood pressure, low HDL, high fasting triglycerides, and elevated fasting glucose—with individuals exhibiting >3 of these being classified as having MetS[17]. Using ATP -III criteria, a diagnosis of MetS is associated with an odds ratio (OR) of 7.8 for the presence of suspected NAFLD in adolescents[18] and 1.43 for NASH among adults with NAFLD[19]. Among individuals with hepatitis C, MetS is associated with an OR of 1.7 for cirrhosis[20]; however the role of MetS in the progression from NASH to advanced fibrosis is not known Use of MetS criteria is limited by their binary nature and by racial/ethnic discrepancies, in which black individuals are less likely to be classified as having MetS despite having a higher prevalence of Type 2 diabetes mellitus (T2DM) and greater CVD mortality. Because of these limitations, we previously derived a continuous MetS-severity Z-score (MetS-Z) that is sex- and race/ethnicity specific[21]. This MetS-severity score is associated with increased risk of future T2DM[22,23], CVD[23,24], and chronic kidney disease[25]—raising potential for MetS-Z to have a role in clinical care for identifying individuals at risk for multiple MetS-associated chronic diseases. The association of MetS-Z with unexplained ALT elevations (as an estimate of NAFLD in adults) and fibrosis scoring systems (as estimates of fibrosis and cirrhosis)—is not known.
Given the reciprocal associations of MetS and NAFLD and given known risks for progression from NAFLD to advanced fibrosis, we hypothesized that 1) MetS-Z would be higher among individuals with unexplained elevations in ALT and fibrosis stage 3 or 4 by elevated NFS >0.676[8], 2) MetS-Z would be correlated with fibrosis scoring systems, 3) higher MetS Z would be associated with an increased risk of unexplained ALT elevations and advanced fibrosis, even after adjustment for diabetes. Our research objective was thus to use non-invasive measures to determine the potential presence of NAFLD and advanced fibrosis in a nationally representative sample and to further assess the degree of MetS severity among these individuals in comparison to individuals without apparent liver abnormalities. Because of known differences by race/ethnicity in NAFLD and MetS, we further set out to assess whether any associations between MetS-Z and apparent liver disease varied by race/ethnicity. Finally, because of known associations among diabetes, MetS severity and age, we further assessed for odds of apparent NAFLD or advanced fibrosis using models adjusting for diabetes These data may have implications for the need for further liver assessments among individuals found to have elevations in MetS severity.
METHODS AND MATERIALS
NHANES
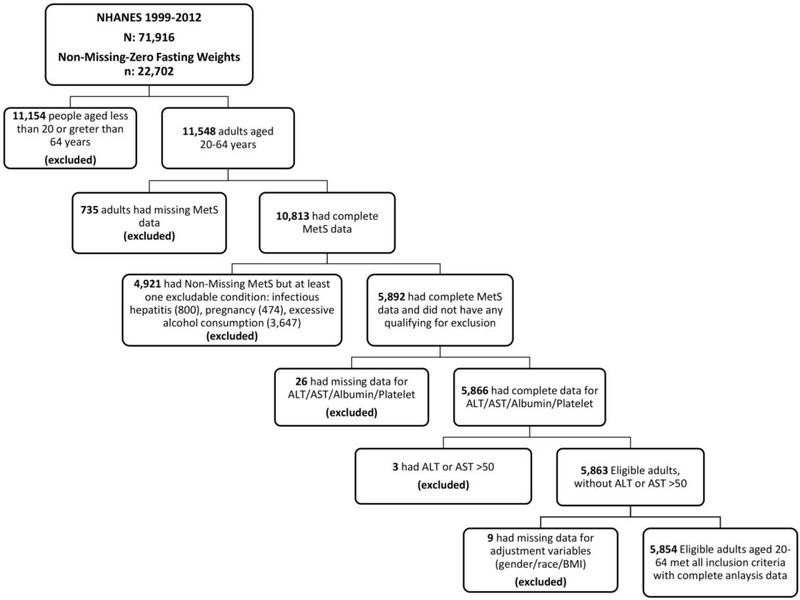
We evaluated data from the National Health and Nutrition Examination Survey (NHANES), a nationally-representative sample of the US population. This study was approved by the National Center for Health Statistics Research Ethics Review Board and all participants gave informed consent or assent; the Institutional Review Board at the University of Florida approved the analysis. Data are available at https://www.cdc.gov/nchs/nhanes/index.htm. As a retrospective analysis, this study was not registered in ClinicalTrials.gov. Laboratory assessments used in our analyses have been described previously[18]. We analyzed data between 1999 and 2012 in adults age 20–64 years who had available data for population weights (Figure 1). We excluded participants who had missing fasting data regarding metabolic syndrome factors (N=735) and participants who were pregnant (N=474) or had known causes of liver disease that may result in elevations in ALT or cirrhosis or variables required for assessment of liver disease, including infectious hepatitis (as evidenced by positive serum measures of Hepatitis B surface antigen or core antibody, Hepatitis C confirmed antibody or RNA, or Hepatitis D antibody, N=800) or excess alcohol intake (defined as consuming greater than 2 servings of alcohol daily for men or 1 serving daily for women, N=3,647). We also excluded those with missing variables for liver assessment (N=26) or extreme elevations in ALT or AST >500, as performed previously[26] (N=3) or were missing variables required for adjustment (N=9).
Figure 1:
Inclusion and exclusion flow chart.
In total, 16,868 participants were non-eligible, with many participants meeting multiple exclusion criteria (Figure 1). Our final pool of participants (n=5,854) was weighted to be nationally representative of US adults based on survey design and fasting status.
NAFLD and fibrosis disease definitions
Acknowledging that liver biopsy is the gold standard for diagnosis of NAFLD, we used unexplained elevations in ALT to identify probable NAFLD, using cut offs of >40 for males and >31 for females, as performed previously[12]. As a sensitivity analysis, we also assessed for those with ALT measures outside the 95% limits of the cohort (i.e., the 97.5 percentile or an ALT z-score of 1.96).
We defined advanced fibrosis as performed previously[8], by an NFS > 0.676, with NFS calculated as follows:
In calculating the diabetes component of this score, individuals with fasting glucose values ≥100 mg/dL or a diagnosis of diabetes were assigned a value of 1 and those with fasting glucose <100 mg/dL and no diabetes were assigned a value of 0.
We further defined liver fibrosis as individuals who had both an elevated NFS and an elevated Fib4 (value ≥ 2.67), with Fib4 calculated as follows:
Metabolic Syndrome Assessment
We used a sex- and race/ethnicity-specific MetS severity Z-score formulated previously[21]. To derive this set of scores, confirmatory factor analysis was performed using data from NHANES ’99-’10 separately for male and female non-Hispanic -whites, non-Hispanic -blacks, and Hispanic adults ages 20–64 years. This approach yielded differential loading factors for each MetS component among these 6 sex- and race/ethnicity-specific subgroups. These loading factors were then used to generate equations for each sex- and racial/ethnic sub-group with weights for the individual MetS components based on how MetS was manifest in that subgroup. These equations were used to calculate the MetS Z-scores. correlate with other MetS risk markers,[21] such as insulin[23] and adiponectin[23], and is predictive of long-term risk of diabetes[22,23] and CVD[23,24]. Importantly, the MetS-Z score also has been validated to predict future CVD among individuals with diabetes, which is important, since the current study included individuals with diabetes[22]. For individuals who were not non-Hispanic-white, non-Hispanic-black, or Hispanic, we calculated the MetS-Z score using the equation for non-Hispanic whites, as performed previously[16].
Additionally, MetS was defined using the ATP-III criteria, where individuals needed to have at least 3 of the following abnormalities: elevated WC (≥102 cm for men, ≥88 cm for women), elevated fasting triglyceride (≥150 mg/dl), reduced HDL (<40 mg/dl for men, <50 mg/dl for women), elevated BP (≥130 mmHg systolic or ≥85 mmHg diastolic or drug treatment for hypertension) and elevated fasting blood glucose (≥100 mg/dl)[17].
Impaired fasting glucose was determined by having fasting glucose 100–125.9 mg/dL.
Homeostasis model of insulin resistance (HOMA-IR) as calculated as (Fasting insulin × Fasting glucose)/405, where fasting insulin is measuring in mU/L and glucose is mg/dL[28]. Diabetes was defined by a fasting glucose value >125 mg/dL or prior physician-reported diagnosis of diabetes.
Statistical Analysis
Statistical analysis was performed using SAS (SAS 9.4, Cary, NC) survey procedures to account for the complex survey design of NHANES. We analyzed all data that fit the inclusion/exclusion criteria and thus did not perform a priori power analysis. Significance was determined at a 95% confidence level, P <0.05. Linear regression was used to create models correlating MetS-Z with markers of insulin resistance and liver health. Initial comparisons of MetS and these factors were unadjusted to generate Pearson’s r for these relationships. Subsequent logistic regression assessing odds of elevated ALT, and advanced fibrosis by MetS-Z and ATP-III MetS was performed, both unadjusted and adjusted for age, sex, and race/ethnicity.
RESULTS
Participant characteristics
We evaluated data from 5,854 individuals who met inclusion criteria. Of these, 691 (weighted percent 11.4%) had unexplained elevations in ALT[12] but not advanced fibrosis, 140 (2.2%) of whom had elevations beyond the 97.5 percentile; another 131 (1.4%) had fibrosis stage 3–4 by virtue of elevated NFS and 18 (0.14%) had both elevated NFS and Fib4 (Table 1). Compared to the normal group, those with unexplained ALT elevations[12] (but without advanced fibrosis) had a lower proportion of non-Hispanic black individuals (8.0% vs. 12.4%) and a higher proportion of Hispanic individuals (19% vs. 12%). Those in the unexplained ALT elevation group had higher mean values for BMI, WC, triglycerides, glucose and hsCRP and a higher proportion of those with diabetes and ATP-III MetS. Those in the advanced fibrosis group (compared to the normal group) exhibited similar trends in their differences as the ALT elevation group, but the only differences without overlapping CI’s were in lower HDL cholesterol and higher hsCRP. The group with both elevated NFS and Fib4 was too small for meaningful comparisons, with wide confidence intervals.
Table 1.
Participant Characteristicsa
| Overall | Normal ALT, no advanced fibrosis | Unexplained elevated ALT,b without advanced fibrosis | Elevated ALT above 97.5% without advanced fibrosis | Fibrosis stage 3 or 4c | Elevated NFS and Fib4d | |
|---|---|---|---|---|---|---|
| Total | 5,854 | 5,032 (87.26) | 691 (11.37) | 140 (2.15) | 131 (1.37) | 18 (0.14) |
| Age (years) | 43.1 [42.5, 43.6] | 43.2 [42.6, 43.8] | 42.1 [41.0, 43.1] | 38.8 [36.5, 41.1] | 56.0 [54.0, 58.0] | 58.2 [54.7, 61.7] |
| Male | 2,851 (50.6)[49.3, 51.8] | 2,403 (49.5)[48.2, 50.8] | 393 (59.2)[54.4, 64.0] | 69 (60.3)[50.5, 70.1] | 55 (48.5)[37.0, 60.0] | 11 (70.5)[45.9, 95.2] |
| Race/Ethnicity | ||||||
| NH White | 2,641 (70.0)[67.4, 72.7] | 2,314 (70.6)[67.9,73.3] | 286 (66.9)[62.6, 71.3] | 52 (65.5)[56.5, 74.4] | 41 (60.3)[48.4, 72.1] | 2 (23.2)[0, 54.9] |
| NH Black | 1,204 (11.3)[9.7, 13.0] | 1,062 (11.6)[9.9, 13.3] | 92 (7.5)[5.7, 9.4] | 14 (6.3)[2.7, 10.0] | 50 (25.0)[16.2, 33.8] | 8 (37.7)[9.6, 65.8] |
| Hispanic | 1,618 (12.6)[10.8, 14.5] | 1,311 (11.8)[10.0,13.5] | 270 (19.3)[15.6, 23.0] | 66 (22.7)[14.8, 30.6] | 37 (12.1)[6.8, 17.4] | 5 (13.4)[0, 31.0] |
| Other | 391 (6.0)[5.0, 7.0] | 345 (6.0)[5.0, 7.0] | 43 (6.2)[3.5, 8.9] | 8 (5.5)[0.9, 10.1] | 3 (2.6)[0, 6.8] | 3 (25.7)[0, 59.2] |
| Income: Poverty Ratioe | ||||||
| < 2.0 | 2,284 (30.6)[28.4, 32.8] | 1,922 (30.0)[27.8,32.2] | 300 (33.5)[29.4, 37.6] | 64 (35.8)[25.8, 45.8] | 62 (45.1)[33.8, 56.5] | 9 (40.4)[9.9, 70.9] |
| 2.0–4.0 | 1,443 (28.7)[26.8, 30.6] | 1,252 (29.1)[27.1,31.2] | 154 (24.4)[20.3, 28.4] | 27 (16.5)[7.5, 25.4] | 37 (38.3)[26.0, 50.7] | 6 (55.4)[23.8, 86.9] |
| > 4.0 | 1,692 (40.7)[38.1, 43.2] | 1,478 (40.9)[38.2,43.5] | 194 (42.1)[37.5, 46.7] | 40 (47.7)[35.4, 60.1] | 20 (16.5)[7.8, 25.3] | 1 (4.2)[0, 12.6] |
| Educationf | ||||||
| Less than HS | 855 (15.3)[13.6, 17.1] | 715 (15.1)[13.2,17.0] | 104 (15.7)[11.5, 20.0] | 24 (19.2)[10.1,28.4] | 36 (24.2)[14.8, 33.5] | 4 (11.4)[0. 24.5] |
| HS Diploma | 736 (21.0)[18.9, 23.0] | 630 (21.0)[18.9,23.0] | 87 (20.4)[15.0, 25.8] | 14 (20.3)[8.2, 32.3] | 19 (26.6)[12.9, 40.3] | 3 (31.5)[0, 68.7] |
| More than HS | 1,998 (63.7)[60.9, 66.5] | 1,733 (63.9)[61.1,66.8] | 220 (63.9)[58.1, 69.6] | 34 (60.2)[45.4, 74.9] | 45 (49.3)[36.5,62.0] | 7 (57.1)[18.4, 95.8] |
| Current Smoker | 1,020 (17.4)[16.0, 18.8] | 917 (18.3)[16.8,19.8] | 87 (11.4)[8.4, 14.5] | 16 (9.7)[4.2, 15.2] | 16 (8.9)[3.1, 14.8] | 2 (7.3)[0, 18.0] |
| Alcohol(drinks/day)g | 2.0 [1.4,2.7] | 2 [1.3, 2.8] | 1.4 [1.4, 1.5] | 1.6 [1.4, 1.7] | 1.4 [1.2, 1.6] | 1.2 [0.8, 1.5] |
| Diabetes | 695 (8.7) [7.7, 9.7] | 481 (6.9)[6.0,7.8] | 114 (14.2)[10.8, 17.5] | 29 (20.9)[12.3, 29.5] | 100 (77.7)[69.3, 86.1] | 10 (44.5)[13.5, 75.6] |
| CVD | 329 (5.0)4.1, 5.7] | 257 (4.5)[3.7,5.3] | 42 (6.1)[3.6, 8.6] | 9 (6.2) [1.2, 11.1] | 30 (20.9)[11.9, 30.0] | 4 (46.8)[14.2, 79.5] |
| MetS Metrics | ||||||
| BMI | 28.7 [28.4, 28.9] | 28.3 [28, 28.5] | 31.3 [30.7, 31.8] | 31.5 [30.2, 32.8] | 42.3 [39.7, 44.8] | 31.6 [29.8, 33.4] |
| Waist Circ. | 98.0 [96.9, 98.1] | 96.5 [96,97.1] | 104.9 [103.5, 106.4] | 106.0 [102.7, 109.3] | 127.0 [122.5, 131.5] | 108.6 [102.4, 114.7] |
| SBP | 119.0[118.5, 119.5] | 118.6 [118,119.2] | 121.0 [119.8, 122.3] | 120.4 [117.9, 122.9] | 131.8 [127.9, 135.6] | 123.1 [117.7, 128.4] |
| HDL Cholesterol | 51.6 [51.1, 52.1] | 52.3 [51.8,52.9] | 46.0 [44.8, 47.3] | 43.4 [41.0, 45.7] | 47.9 [44.8, 51.1] | 51.7 [45.9, 57.4] |
| Triglycerides | 136.1 [132.0,140.2] | 129.1 [125,133.2] | 180.9 [165.8, 196.0] | 179.5 [151.6, 207.4] | 162.9 [138.2, 187.6] | 129.7 [69.4, 189.9] |
| Glucose | 105.0 [104.1, 105.9] | 103.5 [102.6,104.4] | 111.4 [108.1, 114.8] | 114.9 [108.2, 121.5] | 142.2 [131.4, 153.0] | 123.0 [102.1, 143.9] |
| ATP-III MetS | 2,250 (36.5)[34.6, 38.2] | 1,750 (32.7)[30.9,34.6] | 391 (59.5)[55.6, 63.5] | 83 (63.5)[53.3, 73.7] | 109 (82.5)[72.1, 92.9] | 10 (56.5)[23.9, 89.1] |
| hsCRPh | 0.4 [0.4, 0.4] | 0.4 [0.36, 0.42] | 0.5 [0.4, 0.5] | 0.4 [0.4, 0.5] | 0.7 [0.5, 0.9] | 1.0 [0.1, 1.9] |
Presented: Means [95% CI] or N (%) [95% CI]
ALT >40 in males and >31 in females [12]
NASH Fibrosis Score > 0.676
NASH Fibrosis Score > 0.676 and Fib4 ≥ 2.67
n missing/not reported = 435
n missing/not reported = 2,265
n missing/not reported = 2,924
n missing/not reported = 965
Compared to excluded participants, the included individuals were older, more likely to be male, more likely to be white and had higher income and education levels, and had fewer servings of alcohol per day (Supplemental Table 1).
MetS severity and liver-related factors
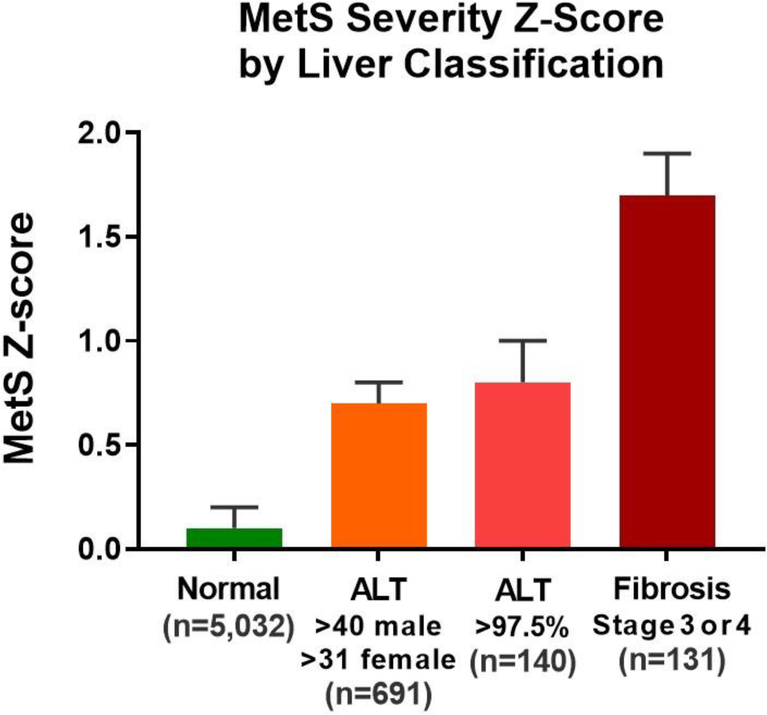
Figure 2 displays MetS-Z levels and Table 2 provides levels of variables related to MetS and liver disease, overall and by sub-group for individuals who were normal, had unexplained ALT elevations or had advanced fibrosis by NFS elevation. Compared to normal individuals, those with unexplained ALT elevations and cirrhosis had a higher degree of MetS severity, assessed both using the standard MetS-Z score and the no-glucose MetS-Z score. Expressed as a percentile of MetS-Z for the US adult population these values corresponded to: normal group, 54th percentile; unexplained ALT elevations, 76th percentile; and advanced fibrosis, 96th percentile. The ALT elevation group (compared to the normal group) also had higher insulin resistance as assessed using fasting insulin and HOMA-IR, and had higher levels of ALT, AST, GGT and APRI and a lower ALT:AST ratio. Compared to the ALT elevation groups, the advanced fibrosis group had higher values for HOMA-IR and ALT:AST ratio but lower values for ALT and AST.
Figure 2: MetS severity Z-score by liver categorization.
MetS-Z (means and 95% confidence interval) is shown for participants without liver abnormalities (normal), unexplained ALT elevations, advanced fibrosis (NAFLD Fibrosis Score >0.676).
Table 2:
Mean levels of MetS severity and liver-related factorsa
| Overall | Normal ALT, No Elevated NFS | Unexplained elevated ALT,b without advanced fibrosis | Elevated ALT above 97.5%tile without advanced fibrosis | Fibrosis stage 3 or 4c | Elevated NFS and Fib4 d | |
|---|---|---|---|---|---|---|
| MetS & insulin resistance | ||||||
| MetS-Z | 0.2 [0.2, 0.3] | 0.1 [0.1, 0.2] | 0.7 [0.6, 0.8] | 0.8 [0.7, 1.0] | 1.7 [1.5, 1.9] | 0.6 [−0.1, 0.7] |
| No-glucose MetS-Z | 0.1 [0.1, 0.1] | 0.0 [0.0, 0.04] | 0.5 [0.5, 0.6] | 0.6 [0.4, 0.8] | 0.8 [0.7, 1.0] | 0.2 [−0.4, 0.7] |
| Insulin | 9.8 [9.4, 10.1] | 8.8 [8.5, 9.1] | 15.1 [13.9, 16.3] | 14.8 [12.5, 17.0] | 25.6 [17.1, 34.2] | 24.7 [3.7, 45.8] |
| HOMA-IR | 2.7 [2.6, 2.8] | 2.3 [2.3, 2.4] | 4.5 [4.0, 4.9] | 4.4 [3.6, 5.2] | 8.8 [6.1, 11.5] | 6.5 [2.1, 10.8] |
| Liver and other factors | ||||||
| ALT | 25.4 [24.9, 25.8] | 21.5 [21.3, 21.7] | 54.9 [52.6, 57.1] | 93.0 [86.5, 99.1] | 26.7 [22.4, 30.9] | 31.8 [19.5, 44.1] |
| AST | 24.3 [24.0, 24.6] | 22.3 [22.1, 22.5] | 39.3 [37.3, 41.4] | 64.1 [56.5, 71.6] | 28.4 [23.5, 33.2] | 56.7 [26.0, 87.4] |
| ALT :AST ratio | 1.1 [1.0, 1.1] | 1.1 [1.1, 1.1] | 0.7 [0.7, 0.8] | 0.7 [0.6, 0.8] | 1.1 [1, 1.2] | 1.8 [1.2, 2.3] |
| GGT | 25.5 [24.7, 26.3] | 22.7 [22.0, 23.4] | 45.2 [41.8, 48.5] | 62.2 [50.5, 74.0] | 41.3 [32.1, 50.5] | 84.9 [27.3, 142.5] |
| hsCRPe | 0.4 [0.4, 0.4] | 0.4 [0.4, 0.4] | 0.5 [0.4, 0.5] | 0.4 [0.4, 0.5] | 0.7 [0.5, 0.9] | 1.0 [0.1, 1.9] |
| NFS | −2.4 [−2.5, −2.4] | −2.5 [−2.5, −2.4] | −2.6 [−2.7, −2.5] | −2.7 [−3.0, −2.4] | 1.3 [1.1, 1.4] | 1.8 [1.3, 2.2] |
Presented: Means [95% CI]
ALT >40 in males and >31 in females [12]
NASH Fibrosis Score > 0.676
NASH Fibrosis Score > 0.676 and Fib4 > 2.67
n missing/not reported = 777
Supplemental Table 2 displays the values by race/ethnicity and diabetes status, adjusted for sex and age; the advanced fibrosis group was not included because of the small total number. MetS-Z levels were higher among those with vs. without diabetes (e.g., among white non-Hispanic individuals with ALT elevations 0.6 vs. 1.8). However, both among those with and without diabetes, there remained higher MetS-Z scores among individuals with vs. without ALT elevations (e.g., among white non-Hispanic individuals without diabetes 0.6 vs. 0.03). Among individuals with ALT elevations but without diabetes, MetS-Z levels were higher among white individuals compared to black individuals (0.6 vs. 0.1). Values were otherwise not different between racial/ethnic groups with and without diabetes.
Correlation of MetS-Z with liver factors
We next assessed associations of MetS-Z with factors related to liver disease (Table 3). While MetS-Z was overall positively associated with ALT (r=0.25) and multiple additional factors, these associations were driven almost entirely by individuals with ALT levels in the normal range; among those with unexplained ALT elevations[12], there were weaker correlations of MetS-Z with ALT (r=0.11) and GGT (r=0.13), with associations that were similar to the overall group with respect to hsCRP and NFS, and factors related to insulin resistance. Those with advanced fibrosis only exhibited significant correlations for MetS-Z with the ALT:AST ratio (-0.30) and HOMA-IR as a marker of insulin resistance (r=0.16).
Table 3:
Correlations of MetS-Z with liver factorsa
| Overall | Normal ALT, No advanced fibrosis | Unexplained elevated ALT,b without advanced fibrosis | Elevated ALT above 97.5%tile without advanced fibrosis | Fibrosis stage 3 or 4c | Elevated NFS and Fib4 d | |
|---|---|---|---|---|---|---|
| ALT | 0.25 | 0.26 | 0.11 | 0.12 | 0.00 | 0.04 |
| AST | 0.10 | −0.02 | 0.05 | 0.05 | −0.20 | −0.17 |
| ALT:AST ratio | −0.33 | −0.33 | −0.06 | −0.00 | −0.30 | −0.03 |
| GGT | 0.25 | 0.25 | 0.13 | 0.09 | −0.04 | 0.08 |
| hsCRP | 0.21 | 0.21 | 0.22 | 0.27 | 0.13 | 0.10 |
| NFS | 0.39 | 0.37 | 0.37 | 0.28 | 0.09 | 0.01 |
| Insulin | 0.39 | 0.48 | 0.35 | 0.38 | 0.02 | −0.44 |
| HOMA-IR | 0.47 | 0.59 | 0.47 | 0.62 | 0.14 | −0.25 |
Presented: Means [95% CI]
ALT >40 in males and >31 in females [12]
NASH Fibrosis Score > 0.676
NASH Fibrosis Score > 0.676 and Fib4 ≥ 2.67
Odds of liver abnormality by MetS-Z
Finally, we assessed the odds of liver abnormalities, overall and by race/ethnicity (Table 4). Overall, each one SD increase in MetS-Z was associated with higher odds of unexplained ALT elevations (OR=1.58), and elevated NFS (OR=1.96). Following adjustment for age, gender, race/ethnicity, and diabetes status, there were slight increases in the associations between MetS-Z and elevated ALT (OR=1.64) but a partial attenuation in the association with NFS (OR=1.16).
Table 4:
Odds of elevated ALT and advanced fibrosis by MetS-Z, overall and by race/ethnicity.a
| Overall | White | Black | Hispanic | Other | Mets-Z × Race Interaction p-value | |
|---|---|---|---|---|---|---|
| Elevated ALTb | ||||||
| Unadjusted | 1.58 [1.44, 1.72] | 1.82 [1.57, 2.1] | 1.15 [1.02, 1.31] | 1.39 [1.21, 1.61] | 1.48 [1.15, 1.91] | < 0.0001 |
| Adjusted for age, sex, race/ethnicity, diabetes status | 1.64 [1.46, 1.83] | 1.87 [1.60, 2.2] | 1.20 [1.05, 1.37] | 1.46 [1.24, 1.72] | 1.56 [1.18, 2.10] | 0.0002 |
| Elevated ALT above 97.5%tile | ||||||
| Unadjusted | 1.49 [1.34, 1.65] | 1.60 [1.40, 1.84] | 1.14, [0.76, 1.70] | 1.38 [1.19, 1.60] | 1.91 [1.00, 3.69] | 0.2122 |
| Adjusted for age, sex, race/ethnicity, diabetes status | 1.48 [1.25, 1.77] | 1.62 [1.34, 1.95] | 1.14 [0.80, 1.63] | 1.38 [1.11, 1.73] | 1.93 [1.03, 3.60] | 0.0931 |
| Fibrosis stage 3 or 4c | ||||||
| Unadjusted | 1.96 [1.77, 2.18] | 2.2 [1.78, 2.72] | 1.79 [1.46, 2.18] | 1.74 [1.5, 2.02] | 0.36 [0.18, 0.74] | 0.0001 |
| Adjusted for age, sex, race/ethnicity, diabetes status | 1.16 [1.0, 1.3] | 1.24 [1.04, 1.46] | 1.17 [0.98, 1.39] | 1.06 [0.84, 1.34] | 0.12 [0.05, 0.27] | <0.0001 |
| Elevated NFS and Fib4 d | ||||||
| Unadjusted | 1.29 [0.90, 1.85] | 1.61 [1.41, 1.85] | 1.24 [1.01, 1.52] | 1.73 [1.51, 1.99] | 0.36 [0.18, 0.74] | 0.0006 |
| Adjusted for age, sex, race/ethnicity, diabetes status | 0.70 [0.3, 1.6] | 1.08 [0.62, 1.91] | 0.75 [0.35, 1.62] | 1.47 [1.03, 2.10] | 0.08 [0.02, 0.37] | 0.0048 |
Presented: Odds ratio [95% CI]
ALT >40 in males and >31 in females [12]
NASH Fibrosis Score > 0.676
NASH Fibrosis Score > 0.676 and Fib4 ≥ 2.67
There were significant interactions between MetS-Z and race in the association with elevated ALT, with stronger associations among white individuals (OR=1.87) compared to black individuals (OR=1.20), and with intermediate values among individuals in the Hispanic(1.46) and Other group (1.48). Following adjustment, associations with elevated NFS remained significant in whites (OR=1.24) but not in blacks or Hispanics and with a protective association in the Other group (OR=0.12).
When using binary ATP-III criteria, there were associations between those with MetS and elevated ALT[12] (OR=3.00, CI 2.54,3.55) and advanced fibrosis (8.44, CI 4.11,17.3)(Supplemental Table 3). Following adjustment, those with ATP-III MetS had higher odds of elevated ALT among whites compared to blacks.
DISCUSSION
In the setting of the worldwide obesity epidemic, non-invasive approaches are needed to identify individuals at risk for NAFLD and its sequelae. We evaluated associations between current non-invasive estimates of liver disease and a continuous MetS severity score that has shown potential for clinical use as a predictor of other diseases, including future diabetes[22,23], CVD[23,24], and chronic kidney disease[25]. The current study confirmed our hypothesis of heightened odds of elevated ALT and advanced fibrosis with rising MetS severity. For every 1 SD increase in the MetS Z-score there was a 58.5% increase in odds of unexplained ALT elevations and a 96% increase in fibrosis stage 3/4—providing the potential to prompt more focused liver assessment among patients with particularly high levels of MetS-Z. We assessed a cohort of individuals both with and without diabetes, which itself is known to be associated with greater metabolic disarray; however, our findings in the current study persisted in models that adjusted for diabetes status. While MetS by ATP-III criteria had been linked to increased risk of liver disease[19], the current findings are the first to provide evidence that risk continues to climb as the severity of MetS abnormalities worsens.
We noted clear linear associations between MetS-Z and levels of ALT as a marker of liver disease, both overall and among individuals whose ALT remained in the normal range; however, this linear association was weaker among those with ALT elevations and was not significantly associated among those with advanced fibrosis. These findings were not unexpected, since the degree of elevations in ALT levels themselves are not strongly associated with the degree of severity of liver disease. Indeed it should be noted that frequently there is a decrease in ALT levels in cases of cirrhosis, where diminished numbers hepatocytes can lead to lower ALT levels overall[29]. Because of this, elevations in ALT levels are not ideal screening tests for fibrosis or cirrhosis risk, further underscoring that a MetS score like this may overcome some of the limitations of ALT alone in identifying risk for sequelae of NAFLD. Another possibility for lower ALT values in the fibrosis group is because the individuals in the fibrosis group were enriched with individuals with diabetes (77% vs. 14.2% among those with unexplained ALT elevations), they could have received additional treatments that led to a reduction in ALT levels. While abnormalities noted in a non-invasive assessment like NFS could further highlight need for evaluation, hepatic scores such as this are not as familiar to primary care providers. A more general score of metabolic dysfunction such as MetS-Z—potentially calculated via the electronic medical record and with application for other chronic diseases[22,23,24,25]—may prove more useful for identifying patients in the general population who would benefit from further hepatic evaluation, including referral, imaging or ultimately biopsy.
A continuous marker of MetS severity may also reveal clues to underlying mechanisms. We noticed overall rising odds of ALT elevation and advanced fibrosis with rising metabolic dysfunction. This continues questions of cause and effect related to MetS. Prior studies have noted reciprocal relationships between MetS and NAFLD, with elevations in ALT levels preceding development of diabetes independent of weight change[30] and individuals with MetS exhibiting systemic inflammation and hepatocyte dysfunction that contribute to worsening NAFLD[31]. Because of the cross-sectional nature of the current study, we were unable to make conclusions about any mechanistic relationships behind our findings, including whether existing MetS contributed to NAFLD or advanced fibrosis or whether evolving liver disease was responsible for the higher levels of MetS severity. Nevertheless, a score such as this could be evaluated further in longitudinal studies assessing for whether worsening MetS severity precedes or follows diagnoses of NAFLD or advanced cirrhosis—with a hypothesis that some degree of both cause and effect would be noted. In addition, there remains the potential for use of a score like MetS-Z as a non-invasive means of tracking response to treatment for liver disease, with potential decreases in MetS severity with declining severity of liver disease[16]. The ability to track response over time is more limited using traditional MetS criteria, given their binary nature.
We noted discrepancies in the relationship between MetS severity and liver disease by race/ethnicity, with the association between MetS-Z and ALT elevations strongest among whites (OR=1.87) and significantly lower among blacks (OR=1.20). This is interesting, given that whites (compared to blacks) have both a higher prevalence of MetS as categorized using traditional criteria[32] and a higher prevalence of NAFLD[11,14] and measures of hepatic steatosis[14] despite similar amount of insulin resistance[32]. The continuous score that we used to assess MetS severity was formulated on a race/ethnicity-specific basis and appears to accurately assess the CVD risks of MetS among black individuals[24]. The lower association of this score with risk for liver disease thus may relate to the lower likelihood among blacks to develop NAFLD, which is thought in part to relate to variability in the presence of NAFLD-related genes including PNPLA3, a gene that confers risk for higher hepatic fat content and subsequent disease and is less common among blacks[33]. These differences in MetS by race/ethnicity may be able to assist medical providers in interpreting these results and guiding care.
We found a prevalence of unexplained ALT elevations[12] of 11.4% and advanced fibrosis of 1.37% in this cohort following exclusions related to alcohol consumption and hepatitis serologies. This compared to an estimate of ALT elevations of 7.3% from ’99-’02[11] suggesting and interval increase prevalence due to the ongoing obesity epidemic. However we found a lower proportion of advanced fibrosis compared to 3.2% from a study using a similar approach on data from NHANES III ’88-’94; this difference is of unclear etiology[13]. As expected, the proportion of those with advanced fibrosis stage 3 or 4 in our study was significantly higher (3.2%) among those excluded—who likely included individuals with alcohol and infectious causes of fibrosis.
While this study benefitted from a large, nationally representative cohort, we also recognize multiple limitations of these analyses besides their cross-sectional nature. Liver biopsy remains the gold standard for assessment of liver disease, and unexplained ALT elevations, NFS and Fib4 are merely estimates of underlying disease and lack the additional precision of imaging techniques to assess hepatic fat content and liver biopsy to assess fibrosis. We further lacked data on ultrasound, which would provide a more accurate estimate of steatosis. We excluded a large number of individuals, primarily because of lacking data from a fasting blood sample, which is needed for assessment of MetS. We included individuals with diabetes, which itself is associated with a higher severity of MetS[22], though we included diabetes in our models of associations between MetS-Z and liver disease. The equation used to calculate NFS includes the presence of diabetes as a factor, meaning that MetS-Z may have been expected to be higher in this group. Nevertheless, high MetS-Z levels could be used to raise suspicion for fibrosis, even if used among individuals whose diabetes status is not initially known. We noted a low prevalence of advanced fibrosis by NFS and a particularly low prevalence of individuals with both NFS and Fib4. This may have limited some assessment of associations of MetS severity and advanced fibrosis by sex or racial/ethnic group— analyses which may be more easily performed in case-control assessments of individuals in gastroenterology clinics.
CONCLUSION
NAFLD has been referred to as the hepatic manifestation of MetS; consistent with this concept, we found associations between a MetS severity Z-score and risk for unexplained elevations in ALT (as a surrogate for NAFLD), elevated NFS (as a surrogate for advanced fibrosis). This extends prior research demonstrating associations seen for binary MetS criteria, revealing that this risk worsens along the spectrum of increasing MetS severity. This MetS Z-score, which has been previously validated in assessment of risk for future CVD, diabetes, and chronic kidney disease, could be used to identify individuals who should be screened further for liver disease, followed over time, and targeted for treatment with intensive lifestyle modification and potentially pharmacologic treatment.
Supplementary Material
HIGHLIGHTS.
NAFLD & advanced fibrosis risk increases on the spectrum of worsening MetS severity
MetS severity is higher in those with advanced fibrosis vs. those with only NAFLD.
These risks remained higher among white vs. black individuals.
A MetS score could be used to identify individuals needing further hepatic evaluation.
ACKNOWLEDGEMENTS
The authors declare that they have no conflicts of interest. This work was supported by the National Institutes of Health [grant number 1R01HL120960 (MJG and MDD)].
Abbreviations:
- ATP-III
Adult Treatment Panel III
- BP
blood pressure
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- MetS
metabolic syndrome
- NHANES
National Health and Nutrition Examination Survey
- NAFLD
non-alcoholic fatty-liver disease
- NASH
non-alcoholic steatohepatitis
- NFS
NAFLD Fibrosis Score
- OR
odds ratio
- T2DM
Type 2 diabetes mellitus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from s[teatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–55. [DOI] [PubMed] [Google Scholar]
- [2].Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
- [3].Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138–53. [DOI] [PubMed] [Google Scholar]
- [4].Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011;43:617–49. [DOI] [PubMed] [Google Scholar]
- [5].Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis C. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- [6].Brunt EM, Kleiner DE, Wilson LA, Sanyal AJ, Neuschwander-Tetri BA. Improvements in Histologic Features and Diagnosis Associated With Improvement in Fibrosis in Nonalcoholic Steatohepatitis: Results From the Nonalcoholic Steatohepatitis Clinical Research Network Treatment Trials. Hepatology 2019;e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association (vol 107, pg 811, 2012). American Journal of Gastroenterology 2012;107:1598–98. [DOI] [PubMed] [Google Scholar]
- [8].Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. [DOI] [PubMed] [Google Scholar]
- [9].Siddiqui MS, Yamada G, Vuppalanchi R, Van Natta M, Loomba R, Guy C, Brandman D, Tonanscia J, Chalasani N, Neuschander-Tetri B, Sanyal AJ, Network NCR. Diagnostic Accuracy of Noninvasive Fibrosis Models to Detect Change in Fibrosis Stage. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed]
- [10].Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019;156:1264–81.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol 2006;101:76–82. [DOI] [PubMed] [Google Scholar]
- [12].Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003;98:960–7. [DOI] [PubMed] [Google Scholar]
- [13].Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bril F, Portillo-Sanchez P, Liu IC, Kalavalapalli S, Dayton K, Cusi K. Clinical and Histologic Characterization of Nonalcoholic Steatohepatitis in African American Patients. Diabetes Care 2018;41:187–92. [DOI] [PubMed] [Google Scholar]
- [15].Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014;2:901–10. [DOI] [PubMed] [Google Scholar]
- [16].Gurka MJ, Mack JA, Chi X, DeBoer MD. Use of metabolic syndrome severity to assess treatment with vitamin E and pioglitazone for non-alcoholic steatohepatitis. J Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome - An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- [18].Deboer MD, Wiener RC, Barnes BH, Gurka MJ. Ethnic differences in the link between insulin resistance and elevated ALT. Pediatrics 2013;132:e718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA, (CRN) NCRN. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011;53:810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut 2009;58:111–7. [DOI] [PubMed] [Google Scholar]
- [21].Gurka MJ, Lilly CL, Norman OM, DeBoer MD. An Examination of Sex and Racial/Ethnic Differences in the Metabolic Syndrome among Adults: A Confirmatory Factor Analysis and a Resulting Continuous Severity Score. Metabolism 2014;63:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gurka MJ, Golden SH, Musani SK, Sims M, Vishnu A, Guo Y, Cardel M, Pearson TA, DeBoer MD. Independent associations between a metabolic syndrome severity score and future diabetes by sex and race: the Atherosclerosis Risk In Communities Study and Jackson Heart Study. Diabetologia 2017;60:1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].DeBoer MD, Gurka MJ, Morrison JA, Woo JG. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes (Lond) 2016;40:1353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].DeBoer MD, Gurka MJ, Hill Golden S, Musani SK, Sims M, Vishnu A, Guo Y, Pearson TA. Independent Associations between Metabolic Syndrome Severity & Future Coronary Heart Disease by Sex and Race. J Am Coll Card 2017;69:1204–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].DeBoer MD, Filipp SL, Musani SK, Sims M, Okusa MD, Gurka M. Metabolic Syndrome Severity and Risk of CKD and Worsened GFR: The Jackson Heart Study. Kidney Blood Press Res 2018;43:555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, Carey W, Alkhouri N. Prevalence of Nonalcoholic Steatohepatitis-Associated Cirrhosis in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol 2017;112:581–87. [DOI] [PubMed] [Google Scholar]
- [27].Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M, Investigators AC. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- [28].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- [29].Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G, Fargion S. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 2008;48:792–8. [DOI] [PubMed] [Google Scholar]
- [30].Sattar N, McConnachie A, Ford I, Gaw A, Cleland SJ, Forouhi NG, McFarlane P, Shepherd J, Cobbe S, Packard C. Serial metabolic measurements and conversion to type 2 diabetes in the west of Scotland coronary prevention study: specific elevations in alanine aminotransferase and triglycerides suggest hepatic fat accumulation as a potential contributing factor. Diabetes 2007;56:984–91. [DOI] [PubMed] [Google Scholar]
- [31].Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis 2010;42:320–30. [DOI] [PubMed] [Google Scholar]
- [32].Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutrition Metabolism and Cardiovascular Diseases 2012;22:141–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.