Abstract
The goal of this study was to establish a short list of zoonotic pathogens involving the domestic dog that can be prioritized for a companion animal surveillance program specific to the Prairie Provinces of Canada. A list of pathogens documented in dogs was created through a comprehensive review of infectious disease textbooks for the following taxonomical categories: bacteria, ectoparasites, fungi, helminths, protozoa, rickettsia, and viruses. This created an initial list of 594 pathogens that was then pared down through an extensive review of the literature using the following criteria: i) the pathogen is zoonotic/sapronotic/anthroponotic; ii) the dog is involved in transmission to humans, maintenance, or detection of the pathogen; and iii) there is a level of risk for occurrence of the pathogen in Canada. This process yielded a final list of 84 pathogens and 3 supplementary lists of canine zoonotic/sapronotic/anthroponotic pathogens that may become relevant to future surveillance programs.
Résumé
Définition des agents pathogènes zoonotiques canins importants dans les provinces des Prairies du Canada. Le but de cette étude était d’établir une courte liste d’agents pathogènes zoonotiques impliquant le chien domestique qui peuvent être priorisés pour un programme de surveillance des animaux de compagnie propre aux provinces des Prairies du Canada. Une liste d’agents pathogènes documentés chez les chiens a été créée grâce à un examen complet des manuels sur les maladies infectieuses pour les catégories taxonomiques suivantes : bactéries, ectoparasites, champignons, helminthes, protozoaires, rickettsies et virus. Cela a créé une liste initiale de 594 agents pathogènes qui a ensuite été réduite grâce à un examen approfondi de la littérature en utilisant les critères suivants : i) l’agent pathogène est zoonotique/sapronotique/anthroponotique; ii) le chien est impliqué dans la transmission à l’homme, le maintien ou la détection de l’agent pathogène; et iii) il existe un niveau de risque d’apparition de l’agent pathogène au Canada. Ce processus a donné une liste finale de 84 agents pathogènes et trois listes supplémentaires d’agents pathogènes zoonotiques/sapronotiques/anthroponotiques canins qui pourraient devenir pertinents pour les futurs programmes de surveillance.
(Traduit par Dr Serge Messier)
Introduction
The relationship between humans and companion animals has become increasingly intimate over time (1). According to the Canadian Animal Health Institute, there were an estimated 8.2 million dogs and 8.3 million cats residing in Canada in 2018. It is reported that 41% of Canadian households own at least 1 dog and 38% own at least 1 cat (2). Unfortunately, there is limited information on zoonotic disease prevalence in the animals with which people share the most time and closest contact (1). Thus, there is a growing need for information on companion animal diseases that can also affect humans. Of the approximately 1500 infectious organisms known to cause human disease, 60% are zoonotic and originate from animal sources (3). Exploring companion animal zoonotic pathogens from a One Health perspective becomes especially important for the most vulnerable populations. This includes those who are immunocompromised (4), and remote communities with limited access to both medical and veterinary resources (5).
There are currently little to no data on the prevalence of canine zoonotic pathogens within the Prairie Provinces of Canada. Although several studies exist in the Prairie Provinces for individual canine pathogens (5–7), those most significant from a public health standpoint, as well as prioritization of these pathogens to guide public/animal health policy, have yet to be explored. Determining pathogens of significance is a foundational step in developing a surveillance program.
Surveillance systems are already well-established in human medicine (1,3). Several examples also exist in the Canadian livestock industry from both a veterinary and public health perspective (8,9). In addition, wildlife surveillance occurs in Canada at both the provincial and federal levels (10,11). Currently, rabies is one of the only companion animal zoonotic pathogens that is routinely monitored through federal and provincial surveillance programs (12,13). The Ontario Animal Health Network is the first provincial initiative to collectively monitor companion animal disease trends in Canada (14). Although companion animal surveillance programs are well-established in other parts of the world (15), there is a clear gap that needs to be filled for companion animal health data in the Prairie Provinces. A companion animal surveillance program could provide both animal health data (demographic and disease data) and relevant public health data.
The first step to establishing a companion animal surveillance program in the location of interest was to determine which pathogens are significant, focusing on the domestic dog. The primary objective of this study was to establish a short list of pathogens seen in the domestic canine population that have public health implications within the Prairie Provinces. A secondary objective was to formulate additional lists of those pathogens that may be important to future surveillance initiatives.
Materials and methods
Initial list and stepwise approach
A list of pathogens in the taxonomical categories bacteria, ectoparasites, fungi, helminths, protozoa, rickettsia, and viruses documented in dogs was created by reviewing the most up-to-date, authoritative veterinary infectious disease textbooks (4,16–18). If a pathogen was listed as having been identified in dogs in any of these textbooks, it was included in this initial list. The list was supplemented with the addition of pathogen Sars-CoV-2 (COVID-19) (19). Several ectoparasites were also included but were limited to mites and fleas. Pathogens were classified to species level whenever possible. This was often dependent on how taxonomic ranking was documented in the literature. When several species were involved (e.g., with dog bite and enteric pathogens), or when the species level was not reported, pathogens were characterized no further than the genus level.
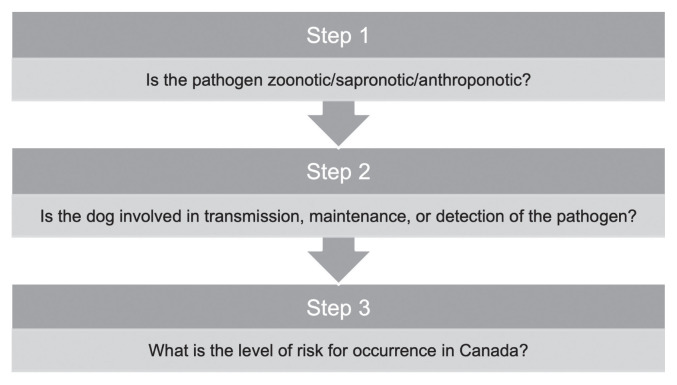
A subsequent extensive and structured literature search followed a stepwise approach (Figure 1) to narrow down this initial pathogen list. The first step was to assess any evidence that the pathogen was zoonotic, sapronotic, or anthroponotic. The second step was to assess any evidence that dogs had a role in how humans acquire the pathogen. Roles included the direct transmission of the pathogen from dogs to humans, dogs maintaining the pathogen in the environment as a definitive or reservoir host, and finally, that dogs can be used to detect the pathogen in the environment as a sentinel for human exposure. The third step was to assess any evidence for each pathogen’s level of risk for occurrence in Canada, since few publications were known to exist specifically for the Prairie Provinces. These steps were developed through in-depth discussions by the authors.
Figure 1.
Stepwise approach used to pare down the initial pathogen list to identify important canine pathogens from a public health perspective in the Prairie Provinces.
Defining zoonotic/sapronotic/anthroponotic (Step 1)
For the purposes of this study, pathogens advanced from Step 1 if they were zoonotic, sapronotic, or anthroponotic. Zoonotic pathogens are those that are transmitted from animals (or animal tissue) to humans and results in human illness (20). Transmission can include direct contact (by skin, inhalation, or ingestion), indirect contact through fomites or a contaminated environment, and vector transmission (4,17,20). Sapronotic pathogens are those that infect both animals and humans from a shared environment without transmission between hosts (4,20). Finally, anthroponotic pathogens are those that are transmitted from humans to animals (4).
An extensive search of the literature and infectious disease textbooks (4,16–18) was done to determine if a pathogen was zoonotic, sapronotic, or anthroponotic. This included searches using the pathogen name, followed by “human illness,” “in humans,” or “human disease” in research databases Google Scholar and PubMed. There were no limitations placed on publication year during searches. If at least 1 source identified the pathogen as zoonotic/sapronotic/anthroponotic, or if the pathogen was reported to cause clinical illness in humans, then the pathogen was not discarded (this included opportunistic pathogens). Pathogens were assessed in Step 1 regardless of whether or not the dog was involved in transmission of the pathogen to humans. If a pathogen did not meet the definition of zoonotic/sapronotic/anthroponotic, or if there was no evidence in the literature to support infection in humans, it was removed from the list.
Role of the dog (Step 2)
An extensive review of the literature and infectious disease textbooks (4,16–18,22) was used to evaluate the role of the dog for all of the pathogens that advanced from Step 1. This included searches using the pathogen name, followed by “dog to human,” “dog transmission,” and “dog sentinel” in research databases Google Scholar and PubMed. Pathogens advanced if the dog was involved in direct transmission of the pathogen, acted as a reservoir host, helped to maintain the pathogen in the environment (e.g., acted as a definitive host in a parasitic life cycle), or acted as a sentinel for human infection and detection of the pathogen. If the dog was historically involved in transmission to humans, maintenance or detection of the pathogen, regardless of how common the pathogen is, it advanced to Step 3. If there was not enough evidence in the texts or literature, such that the role of the dog was still largely unknown, it was discarded from the list. For several pathogens, there was not enough evidence in the literature to prove or disprove the role of the dog in transmission to humans, maintenance, or detection of the pathogen. Therefore, a supplementary list denoted Grey-Zone Pathogens was created to highlight these particular pathogens for their possible public health significance in the domestic canine population.
Presence in Canada (Step 3)
Once it was established that the dog was involved in transmission to humans, maintenance, or detection of the pathogen, Step 3 was to determine the level of risk for occurrence in Canada of each remaining pathogen using a 4-tiered approach (Figure 2). These tiers included Tier 1: the pathogen has been reported in dogs in Canada historically at least once; Tier 2: the pathogen has been reported in Canada historically at least once, but canine-specific reports are lacking; Tier 3: the pathogen has not historically been reported in Canada, or its distribution is unknown, but there is a level of risk for occurrence of the pathogen in Canada due to appropriate climate, vectors, reservoir hosts, and lifestyle; and Tier 4: the pathogen is unlikely to occur in Canada because the main reservoir host is missing, the current climate in Canada would not support survival of the pathogen or vector, or the lifestyle does not fit with contraction of the pathogen.
Figure 2.
Four-tiered approach to categorize a pathogen’s risk level of occurrence within Canada.
Using a 4-tiered approach allowed for the recognition of those pathogens that may not be significant to the Canadian canine and human population at this time, but could become relevant in the future. Information about each pathogen’s presence or capacity for occurrence in Canada was obtained through an extensive search of the literature using the pathogen name followed by “in Canada” and “in Canada in dogs” in research databases Google Scholar and PubMed. This step included reports of pathogens in all of Canada because of the limited research available for the Prairie Provinces exclusively. If a Canadian report specifically involved the dog, the pathogen was placed in Tier 1. If canine-specific reports were not identified but the pathogen has been documented in Canada (in human studies or other species, as well as environmental examples) these pathogens were placed in Tier 2. If the pathogen was not documented in Canada at the time of this study, additional searches were performed to identify whether the pathogen had the potential to occur in Canada. For example, searches were completed to determine the geographical distribution of vectors, or first and second intermediate hosts depending on the pathogen. If this information supported possible survival of the pathogen in Canada (i.e., the vector or intermediate hosts have been reported in Canada), then these pathogens were placed in Tier 3. It was assumed that bacterial pathogens not currently reported in Canada (such as several dog bite pathogens) have the capacity to occur in Canada since they are not reliant on vectors or intermediate hosts. For this reason, additional searches were not required for bacterial pathogens that did not qualify for Tier 1 or Tier 2, and these bacteria were automatically placed in Tier 3. If there was no evidence at the time of this study to support survival of the pathogen in Canada, or the distribution of vectors and intermediate hosts did not include Canada, these pathogens were placed in Tier 4. Pathogens grouped in Tier 1 represented the final shortlist. Tier 2 and Tier 3 pathogens represented supplemental shortlists to highlight pathogens that may become significant to canine surveillance initiatives in the future. Grey-Zone Pathogens identified in Step 2 were also further categorized as being present in Canada or having the potential to occur in Canada using the same search strategy.
Results
A total of 594 infectious pathogens were identified in canines (Appendix A — available from the authors upon request; Figure 3). Of these, 235 were bacteria (40%), 14 were ectoparasites (2%), 79 were fungi (13%), 109 were helminths (18%), 62 were protozoa (10%), 19 were rickettsia (3%), and 76 were viruses (13%). From this initial list, a total of 486 pathogens (82%) were then identified as zoonotic/sapronotic/anthroponotic. Of these 486 pathogens, 71 were specifically classified as sapronoses (15%).
Figure 3.
Resulting number of pathogens that fulfilled the criteria for each step. Tier 1 = final shortlist. “Grey-Zone,” Tier 2, Tier 3 = supplementary lists.
From the previous 486 pathogens, a total of 241 pathogens (50%) were further identified as involving the dog in human infection through either direct transmission, maintenance of the pathogen in the environment, or as sentinels for human exposure (Appendix A — https://wcvm.usask.ca/documents/college/appendix-a-final-.pdf). An additional 29 pathogens were classified as Grey-Zone Pathogens. This represented pathogens for which there was evidence to suggest the dog’s role in transmission to humans, maintenance, or detection of the pathogen but there was not enough evidence to prove or disprove the role of the dog at this time. Of these 29 pathogens, 19 were present in Canada and 7 had the potential to occur in Canada (Appendix B — https://wcvm.usask.ca/documents/college/appendix-b-d-final.pdf).
Of the previous 241 pathogens, 84 pathogens were identified in dogs in Canada (Tier 1; Table 1), 74 were reported in Canada but canine-specific reports were lacking (Tier 2; Appendix C — https://wcvm.usask.ca/documents/college/appendix-b-d-final.pdf), and 31 pathogens were classified as having the potential to occur in Canada (Tier 3; Appendix D — https://wcvm.usask.ca/documents/college/appendix-b-d-final.pdf). A total of 52 pathogens were identified as unlikely to occur in Canada (Tier 4).
Table 1.
Shortlist of 84 canine pathogens (Tier 1) identified as having public health implications in the Prairie Provinces of Canada.
| Taxa | Pathogen | ||
|---|---|---|---|
| Bacteria | Actinomyces viscosusc | Enterococcus faecium | Pasteurella canisc |
| Bartonella henselae | Enterococcus spp. | Pasteurella multocidac | |
| Bartonella vinsonii subsp. berkhoffii | Escherichia colia | Pasteurella spp.c | |
| Bordetella bronchiseptica | Francisella tularensis | Pseudomonas aeruginosa | |
| Borrelia burgdorferi senso stricto | Fusobacterium spp.c | Salmonella enterica | |
| Brucella canis | Helicobacter heilmannii | (enteritidis, typhimurium) | |
| Campylobacter coli | Klebsiella spp. | Staphylococcus aureusc | |
| Campylobacter jejuni | Leishmania infantum | Staphylococcus pseudintermediusc | |
| Campylobacter upsaliensis | Leptospira interrogansb | Streptococcus canis | |
| Capnocytophaga canimorsusc | MRSA | Streptococcus spp.c | |
| Clostridium difficile | MRSP | Yersinia enterocolitica | |
| Clostridium perfringens | Moraxella spp.c | Yersinia pestis | |
| Coxiella burnetii | Neisseria weaverc | ||
| Ectoparasites | Cheyletiella yasguri | Pulex irritans | |
| Ctenocephalides canis | Sarcoptes scabiei var canis | ||
| Ctenocephalides felis | |||
| Fungi | Blastomyces dermatitidis | Microsporum canis | |
| Cryptococcus gattii | Sporothrix schenckii | ||
| Histoplasma capsulatum | Trichophyton spp. | ||
| Malassezia pachydermatis | |||
| Helminths | Acanthocheilonema reconditum | Cryptocotyle lingua | Metorchis conjunctus |
| Alaria alata | Diphyllobothrium spp. | Nanophyetus salmincola | |
| Alaria americana | Dipylidium caninum | Paragonimus kellicotti | |
| Alaria canis | Dirofilaria immitis | Taenia serialis | |
| Alaria marcianae | Echinococcus granulosus | Taenia spp. | |
| Apophallus donicus | Echinococcus multilocularis | Toxocara canis | |
| Baylisascaris procyonis | Mesocestoides spp. | Uncinaria stenocephala | |
| Protozoa | Cryptosporidium canis | ||
| Giardia duodenalis assemblage A1 | |||
| Giardia duodenalis assemblage B | |||
| Trypanosoma cruzi | |||
| Rickettsia | Anaplasma phagocytophilum | ||
| Ehrlichia canis | |||
| Rickettsia rickettsia | |||
| Viruses | Rabies | ||
Escherichia coli pathovars: enterohemorrhagic E. coli (EHEC), enteropathogenic E. coli (EPEC), adherent invasive E. coli (AIEC), uropathogenic E. coli (UPEC), necrotoxigenic E. coli (NTEC), enterotoxigenic E. coli (ETEC).
Leptospira interrogans serovars autumnalis, bratislava, canicola, grippotyphosa, hardjo, icterohaemorrhagiae, pomona.
Dog bite specific pathogen.
Discussion
Using a stepwise approach, the authors were able to create an initial list of 594 pathogens identified in dogs and reduce this list to 84 pathogens (Tier 1) relevant to the Prairie Provinces from a public health perspective. In addition, 3 supplemental lists (Tier 2, Tier 3, and Grey-Zone Pathogens) were created to highlight several other groups of pathogens that may become relevant to future surveillance initiatives. To the best of the authors’ knowledge this is the first study in Canada to summarize and list important canine zoonotic/sapronotic/anthroponotic pathogens with the intent to prioritize these pathogens to help establish a companion animal surveillance program. A follow-up study will rank the pathogens on the final list using combined expert opinion to advise public/animal health policy on which pathogens should be prioritized for a companion animal surveillance program in the Prairie Provinces of Canada.
The veterinary infectious disease textbooks used to create the initial pathogen list are comprehensive and well-founded, and are likely to capture the majority of the pathogens identified in dogs. However, the initial list may not be exhaustive and there is an opportunity for rare pathogens to be missed. It is also possible that emerging pathogens will arise during the course of this research. Depending on how one chooses to define zoonotic, classification of a pathogen may be subjectively based on who is creating the list and their personal objectives. Therefore, the final short list is not rigid but is not likely to change substantially. The authors did not employ double-blind methods for paring down the list and doing so may have altered the final shortlist. Follow-up ranking exercises will provide the opportunity to revise pathogens on the list.
A significant portion of this review became an exercise on what constitutes a zoonotic pathogen. There were several instances of contradicting views in the literature on whether or not a pathogen was categorized as zoonotic (4,17,18,21). This was the reason that a clear definition of zoonotic/sapronotic/anthroponotic was determined prior to condensing the list. A priority for the authors was to ensure that if a pathogen detected in dogs impacts human health in any way that it was not overlooked if it was not zoonotic in the traditional sense (direct animal to human transmission). Several definitions were used to explore how a pathogen related to human disease. For example, sapronoses were assessed in Step 1 because although they are not directly zoonotic, dogs can serve as sentinels for sapronotic pathogens and reveal a risk for human exposure from a shared environment (4). Anthroponoses were also assessed in Step 1 to explore the idea that if a pathogen can be transmitted from human to dog, there is also a concern for transmission to occur in the other direction. Unfortunately, it is often challenging to verify zoonotic transfer versus shared environmental exposure (22), which is why it is also important to consider dogs as sentinels for many of these pathogens.
The authors chose to include several ectoparasites in the initial list of pathogens. Many ectoparasites (including fleas, ticks, and sandflies) are important from a public health perspective because they can act as vectors for zoonotic disease transmission (23–25). This exercise explored whether the ectoparasite itself should be classified as zoonotic. This became particularly relevant for the flea. Fleas are known to transmit several very serious pathogens to humans (25) but they can also directly cause clinical signs in humans, including erythema, pruritis, and dermatitis (18). Consequently, the flea was also classified as zoonotic in addition to the many pathogens this vector can harbor and transmit to humans. Although the clinical signs caused by the flea are far less severe than those caused by many of the pathogens they harbor, based on the extensive definitions employed in this study, the flea was included in the zoonotic category.
In exploring how the dog plays a role in transmission to humans, maintenance and detection of these zoonotic/sapronotic/anthroponotic pathogens, a subcategory denoted Grey-Zone Pathogens was also created. This represented pathogens for which there was some evidence in the literature that dogs are likely to contribute to human infection, either directly or as sentinels; however, nothing has been definitively proven at this time. This is either because there has not been enough research done on the pathogen, or it could not be determined whether transmission or shared environmental exposure occurred if infection in the dog was not explicitly identified (22). These pathogens may have a role in canine zoonoses in the future and should not be overlooked. Step 2 in particular exposed an overall lack of research in canine transmission of zoonotic pathogens. Several pathogens were excluded from Step 2 because there is simply not enough evidence at this time for the dog’s role in transmission, maintenance, or detection of the pathogen. These Grey-Zone Pathogens were further assessed for presence in Canada, and the potential to occur in Canada as a way to help highlight those pathogens that may become most important to the Prairie Provinces.
There was limited research available specific to the Prairie Provinces; therefore, literature encompassing all of Canada was included in Step 3 to evaluate occurrence of a pathogen in Canada. This lack of research emphasizes the need to identify pathogens relevant for surveillance programs because companion animal zoonotic disease prevalence is so limited in local regions. It is important to note that inclusion of a pathogen in Step 3 was based on what is currently available in the literature. In an evolving world, through globalization and the impacts of climate change, the authors recognize that some of the pathogens listed in the 3rd and 4th tiers may become more relevant to Canada in the future and should not be completely discounted for prospective surveillance studies. For example, non-native species of snails that act as first intermediate hosts in several parasitic lifecycles have invaded regions of the world where they were not previously detected (26). In particular, climate change also impacts the distribution of many important vectors (27). The authors chose to also highlight the pathogens in Tier 2 and Tier 3 as a reminder that just because a pathogen hasn’t been recorded in Canada, or identified specifically in the dog in Canada, doesn’t mean the pathogen isn’t relevant for canine surveillance. It could simply mean these canine pathogens haven’t yet been identified in this region because researchers haven’t been looking for them. It is important to also acknowledge the effect of canine zoonotic pathogens on rural populations within the Prairie Provinces and the challenges these areas face with limited access to both medical and veterinary services (5).
Several other interesting findings emerged during the course of this study, including dog-bite relevance in Canada. During the review process it became apparent that there was little information on dog bite pathogens and reports in Canada. Although it is suspected that several dog bite pathogens are present in Canada, for many of these pathogens there were no reports to confirm this. This is likely related to the challenges associated with bacterial isolation from contaminated bite wounds. Often initial cultures are not representative of true infection. The recommended treatment for dog bite wounds is wound management alone and cultures are only performed in persistent infections (28). In cases in which the wound persists and cultures are collected, a surveillance program to monitor pathogens isolated in unresolving dog bite wounds may be worth establishing. This is of particular importance for dog bite pathogens such as Capnocytophaga canimorsus that can be fatal (29). One particular Canadian study explored the overall occurrence of dog bites and degree of injury in children; however, the pathogens involved in these wounds were not the focus of the study (28).
Antimicrobial resistant pathogens in canines are significant in this study as this continues to be a growing area of concern and research. Canine bacterial isolates of interest on the final shortlist included methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus pseudintermedius (MRSP), vancomycin-resistant Enterococcus spp., Escherichia coli, Salmonella spp., and urinary isolates of Klebsiella spp., and Pseudomonas aeruginosa. Pathogens that are resistant to antimicrobials, particularly multi-drug resistant (MDR) pathogens, are a major public health concern. Dogs living in close contact with their owners may shed these pathogens in the environment and act as a source for human infection (30,31). For a large number of pathogens identified in this study, including antimicrobial resistant and MDR pathogens, emphasis should be placed on the consequences of these infections in immunocompromised individuals (4,22). Surveillance of antimicrobial resistance in dogs and other companion animals is an area in which additional research should be considered.
The current study focused on the domestic dog only. Other companion animals such as cats, exotic pets, small mammals, and birds were excluded. These species should be considered for future research. Because the primary goal of this research is to advise on a companion animal surveillance program, the current study focused on evidence in the literature based specifically on the domestic dog. The authors recognize that several of the zoonotic pathogens identified here are also relevant to wild canids within Canada (32,33). This is an additional area to be explored for provincial surveillance programs. The methods applied in this study can be used for any of the above-mentioned species.
Challenges in this study included reclassification of pathogen names or several names applying to the same pathogen, in particular with bacterial and parasitic species. This often made it challenging to find accurate information in the literature and many pathogens have been potentially misclassified in older studies. For example, with over 2500 serovars for Salmonella spp. and variations among authors on naming, it was difficult to find consistent reports on which Salmonella species were actually isolated in each particular case (34). For simplicity, Salmonella enterica was grouped together and emphasis was placed only on serovars enteritidis and typhimurium where distinct reports related to the dog were found (35).
In conclusion, the authors identified 84 pathogens present in dogs that are of possible public health importance within the Prairie Provinces of Canada. In addition, several other groups of pathogens were highlighted that may become important in the Prairie Provinces for future surveillance research. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This study was funded by the Public Health Agency of Canada’s Infectious Disease and Climate Change Program.
References
- 1.Day MJ, Breitschwerdt E, Cleaveland S, et al. Surveillance of zoonotic infectious disease transmitted by small companion animals. Emerg Infect Dis. 2012;18:e1. [Google Scholar]
- 2. [Last accessed March 18, 2021];Latest Canadian Pet Population Figures Released: Press Releases. Latest Canadian Pet Population Figures Released | Press Releases | Canadian Animal Health Institute (CAHI). [Internet] 2019 [cited 2020 Apr 16]. Available from: https://www.cahi-icsa.ca/press-releases/latest-canadian-pet-population-figures-released.
- 3.Moore GE, Lund E. Disease reporting and surveillance: Where do companion animal diseases fit in? Vet Clin North Am Small Anim Pract. 2009;39:225–240. doi: 10.1016/j.cvsm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Sykes J, Greene C. Infectious Diseases of the Dog and Cat. 4th ed. Philadelphia, Pennsylvania: Saunders; 2011. [Google Scholar]
- 5.Schurer JM, Ndao M, Skinner S, et al. Parasitic zoonoses: One Health surveillance in northern Saskatchewan. PLoS Negl Trop Dis. 2013;7:e2141. doi: 10.1371/journal.pntd.0002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrin BH, Peregrine AS, Goring J, Beall MJ, Little SE. Canine infection with Borrelia burgdorferi, Dirofilaria immitis, Anaplasma spp. and Ehrlichia spp. in Canada, 2013–2014. Parasit Vectors. 2017;10:1–9. doi: 10.1186/s13071-017-2184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan SJ, Ngeleka M, Philibert HM, Forbes LB, Allen AL. Canine brucellosis in a Saskatchewan kennel. Can Vet J. 2008;49:703–708. [PMC free article] [PubMed] [Google Scholar]
- 8.C3SN | Canadian Cow-Calf Surveillance Network [Internet] University of Saskatchewan; [Last accessed March 18, 2021]. [cited 2020 Apr 16]. Available from: https://research-groups.usask.ca/c3sn/index.php. [Google Scholar]
- 9. [Last accessed March 18, 2021];Canada and Alberta BSE Surveillance Program. [Internet] [cited 2020 Apr 16]. Available from: https://www.alberta.ca/canada-and-alberta-bse-surveillance-program.aspx.
- 10.Disease Surveillance. Canadian Wildlife Health Cooperative; [Last accessed March 18, 2021]. [Internet]. [cited 2020 Apr 23]. Available from: http://www.cwhc-rcsf.ca/disease_surveillance.php. [Google Scholar]
- 11.Chronic Wasting Disease. Governement of Saskatchewan; [Last accessed March 18, 2021]. [Internet]. [cited 2020 Apr 23]. Available from: https://www.saskatchewan.ca/residents/environment-public-health-and-safety/wildlife-issues/fish-and-wildlife-diseases/chronic-wasting-disease. [Google Scholar]
- 12.Wilkins W. [Last accessed March 18, 2021];Sasktachewan [Internet] [cited 2020 Apr 23]. Available from: https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/livestock/animal-health-and-welfare/rabies.
- 13.Government of Canada. Public Health Agency of Canada. [Last accessed March 18, 2021]; [Internet]. [cited 2020 Apr 23]. Available from: https://www.canada.ca/en/public-health/services/diseases/rabies/surveillance.html.
- 14.Ontario Animal Health Network [Internet] [Last accessed March 18, 2021];2018 [cited 2020 Apr 16]. Available from: https://oahn.ca/
- 15.Radford A, Tierney A, Coyne KP, et al. Developing a network for small animal disease surveillance. Vet Rec. 2010;167:472–474. doi: 10.1136/vr.c5180. [DOI] [PubMed] [Google Scholar]
- 16.Sykes JE. Canine and Feline Infectious Diseases. St. Louis, Missouri: Elsevier Saunders; 2013. [Google Scholar]
- 17.Kahn C, Line S. The Merk Veterinary Manual. 10th ed. Whitehouse Station, New Jersey: Merk & Co; 2010. [Google Scholar]
- 18.Macpherson CNL, Francois-Xavier M, Wandeler A. Dogs, Zoonoses, and Public Health. 2nd ed. Wallingford, UK: CABI; 2013. [Google Scholar]
- 19.Goumenou M, Spandidos D, Tsatsakis A. Possibility of transmission through dogs being a contributing factor to the extreme Covid-19 outbreak in North Italy. Mol Med Rep. 2020;21:2293–2295. doi: 10.3892/mmr.2020.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubalek Z, Rudolf I. Microbial Zoonoses and Sapronoses. Dordrecht, The Netherlands: Springer; 2011. [Google Scholar]
- 21.Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philos Trans R Soc B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weese J, Fulford M. Companion Animal Zoonoses Ames, Iowa: Wiley- Blackwell; 2011. [Google Scholar]
- 23.Sonenshine DE. Range expansion of tick disease vectors in north america: Implications for spread of tick-borne disease. Int J Environ Res Public Health. 2018;15:1–10. doi: 10.3390/ijerph15030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toepp AJ, Schaut RG, Scott BD, Mathur D, Berens AJ, Petersen CA. Leishmania incidence and prevalence in US hunting hounds maintained via vertical transmission. Vet Parasitol Reg Stud Reports. 2017;10:75–81. doi: 10.1016/j.vprsr.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Whitfield Y, Smith A. Household pets and zoonoses. Environ Health Rev. 2014;57:41–49. [Google Scholar]
- 26.Lounnas M, Correa AC, Vázquez AA, et al. Self-fertilization, long-distance flash invasion and biogeography shape the population structure of Pseudosuccinea columella at the worldwide scale. Mol Ecol. 2017;26:887–903. doi: 10.1111/mec.13984. [DOI] [PubMed] [Google Scholar]
- 27.Greer A, Ng V, Fisman D. Climate change and infectious diseases in North America: The road ahead. CMAJ. 2008;178:715–722. doi: 10.1503/cmaj.081325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang ME, Klassen T. Dog bites in Canadian children: A five-year review of severity and emergency department management. Can J Emerg Med. 2005;7:309–314. doi: 10.1017/s1481803500014494. [DOI] [PubMed] [Google Scholar]
- 29.Mader N, Lugrs F, Herget-Rosenthal S, Langenbeck M. Being licked by a dog can be fatal: Capnocytophaga canimorsus sepsis with purpura fulminans in an immunocompetent man. Eur J Case Reports Intern Med. 2019;6:001268. doi: 10.12890/2019_001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prescott JF, Hanna B, Reid-Smith R, Drost K. Antimicrobial drug use and resistance in dogs. Can Vet J. 2002;43:107–116. [PMC free article] [PubMed] [Google Scholar]
- 31.Guardabassi L, Schwarz S, Lloyd DH. Pet animals as reservoirs of antimicrobial-resistant bacteria. J Antimicrob Chemother. 2004;54:321–332. doi: 10.1093/jac/dkh332. [DOI] [PubMed] [Google Scholar]
- 32.Liccioli S, Catalano S, Kutz SJ, et al. Gastrointestinal parasites of coyotes (Canis latrans) in the metropolitan area of Calgary, Alberta, Canada. Can J Zool. 2012;90:1023–1030. [Google Scholar]
- 33.Schurer JM, Gesy KM, Elkin BT, Jenkins EJ. Echinococcus multilocularis and Echinococcus canadensis in wolves from western Canada. Parasitology. 2014;141:159–163. doi: 10.1017/S0031182013001716. [DOI] [PubMed] [Google Scholar]
- 34.Chlebicz A, Śliżewska K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: A review. Int J Environ Res Public Health. 2018;15:1–29. doi: 10.3390/ijerph15050863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonard EK. What could your dog be carrying? Zoonotic enteric bacteria in pet dogs in Ontario: Prevalance, risk ractors, and antimicrobial resistance. [PhD dissertation] Guelph, Ontario: University of Guelph; 2014. [Google Scholar]