Abstract
Background
Diagnostic biomarkers for detecting chronic obstructive pulmonary disease (COPD) in acute coronary syndrome (ACS) patients are not available. SERPINA1, coding for the most potent circulating anti-inflammatory protein in the lung, has been found to be differentially methylated in blood cells from COPD patients. This study aimed to investigate the methylation profile of SERPINA1 in blood cells from ACS patients, with (COPD+) or without COPD (COPD−).
Methods
Blood samples were from 115 ACS patients, including 30 COPD+ and 85 COPD− according to lung function phenotype, obtained with spirometry. DNA treated with sodium bisulfite was PCR-amplified at SERPINA1 promoter region. Methylation analysis was carried out by sequencing the PCR products. Lymphocytes count in ACS patients was recorded at hospital admission and discharge.
Results
SERPINA1 was hypermethylated in 24/30 (80%) COPD+ and 48/85 (56.5%) COPD− (p < 0.05). Interestingly, at hospital discharge, lymphocytes count was higher in COPD− patients carrying SERPINA1 hypermethylated (1.98 × 103 ± 0.6 cell/µl) than in COPD− carrying SERPINA1 hypomethylated (1.7 × 103 ± 0.48 cell/µl) (p < 0.05).
Conclusions
SERPINA1 is hypermethylated in blood cells from COPD+ patients. COPD− carrying SERPINA1 hypermethylated and high lymphocytes count may be at risk of COPD development. Therefore, SERPINA1 hypermethylation may represent a potential biomarker for predicting COPD development in ACS patients.
Keywords: Chronic obstructive pulmonary disease, Acute coronary syndrome, COPD, ACS, SERPINA1, Alpha 1-antitrypsin
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung disease that causes obstructed airflow from the lungs [1]. It is caused by long-term exposure to particulate matter, most often from cigarette smoke [1]. Acute coronary syndrome (ACS) results from acute obstruction of a coronary artery [2]. Several studies suggest that ACS patients with concomitant COPD have a poor prognosis [3–5]. However, COPD is frequently undiagnosed in ACS patients, with a significant delay in the treatment and a negative impact on short- and long-term prognosis [5]. The gold standard for COPD diagnosis is the spirometry.
SERPINA1 gene encodes for Alpha-1 antitrypsin (AAT), a blood protease of 52 kDa constitutively released from the hepatocytes [6]. AAT is involved in inflammatory processes [7, 8]. Indeed, this protein plays a protective role on the healthy cells adjacent to the inflamed tissue where it inhibits different proteases, including the elastase produced by neutrophils [7]. The activity of AAT is very high in the lower respiratory tract where it provides more than 90% of the defenses against the elastolytic load of neutrophils [9, 10]. The relevance of AAT in COPD is evident in individuals carrying mutations in SERPINA1 gene where absence or alteration of the protein in association with cigarette consumption predisposes to the risk of developing COPD [11].
SERPINA1 gene is 12.2 kb and maps on chromosome 14q32.1 [12]. It consists of 7 exons called IA, IB, IC and II–III–IV–V, and six introns [13]. Transcriptional regulation occurs in exons IA, IB and IC, in a tissue-specific manner [13]. IA and IB regulate transcription in the monocytes and macrophages and IC in hepatocytes [14, 15]. SERPINA1 is also an inducible gene upon activating its inflammation-responsive promoter in hepatocytes, monocytes and macrophages [7, 16, 17]. High levels of circulating AAT, fourfold to sixfold higher than baseline levels, are present during the course of inflammation, infections and late pregnancy [18–21].
In a recent study we have performed a comprehensive assessment of SERPINA1 gene promoter methylation profile in peripheral blood mononuclear cells (PBMCs) from healthy subjects [21]. We showed that SERPINA1 gene promoter is hypermethylated in healthy individuals, such as blood donors, but hypomethylated in pregnant women at the third trimester of pregnancy. These findings suggest that SERPINA1 gene is normally silenced in blood cells of healthy individuals, but it is induced under emergency conditions, such as late pregnancy, when women are highly exposed to the risk of inflammations and infections [21].
Based on our recent findings, in this study we aimed to verify whether methylation of SERPINA1 gene promoter may differ between ACS patients with COPD (COPD+) and without COPD (COPD−). To this aim, the methylation profile of SERPINA1 gene promoter was investigated in blood cells from COPD+ and COPD− patients.
Methods
Sample population
Blood samples were collected from 115 ACS patients (mean age ± standard deviation [SD], 65 ± 9 yrs) at the University Hospital of Ferrara, Ferrara, Italy. The inclusion criteria comprised smokers or former smokers (≥ 10 pack/years) patients hospitalized with a clinical diagnosis of ACS, as defined by current European guidelines [22]. Exclusion criteria included previous diagnosis of COPD and/or asthma, known pulmonary diseases other than COPD, ongoing pneumonia, ongoing heart failure, documented or suspicion of malignant disease, life expectancy < 1 year, recent thoracic trauma.
Definition of undiagnosed COPD
Clinical and laboratory data as well as blood samples were collected for all patients during hospitalization. Two months after discharge, all patients underwent spirometry to verify the presence of COPD. A spirometry was performed by two expert pulmonologists and revised by an independent reviewer blinded to patients’ clinical conditions and outcomes. Spirometry was performed according to standardized procedures [23]. Briefly, COPD was diagnosed in the presence of: (1) exposure to risk factors for the disease (all patients enrolled in the study were current or former smokers); (2) presence of chronic respiratory symptoms (mainly shortness of breath, cough and sputum) and (3) post-bronchodilator fixed ratio forced expiratory volume at first second (FEV1)/forced vital capacity (FVC) < 0.7.
Blood collection and leukocyte count
A venous blood sample was collected from all ACS patients at the time of both admission and hospital discharge. Leukocyte count was performed by flow cytometry with the automated cell counter Sysmex XN. When needed, a blood smear was prepared for microscopic evaluation and accurate quantification of total lymphocytes count.
DNA extraction and DNA PCR suitability
DNA was isolated from total blood using QIAamp DNA Blood Mini Kit (Qiagen, Milan, Italy), as described [24]. After purification, DNA was quantified by spectrophotometric reading (NanoDrop 2000, Thermo Scientific) [21] and then evaluated for its PCR suitability by amplification for β-globin gene sequence [25].
Treatment of DNAs with sodium bisulfite and SERPINA1 PCR
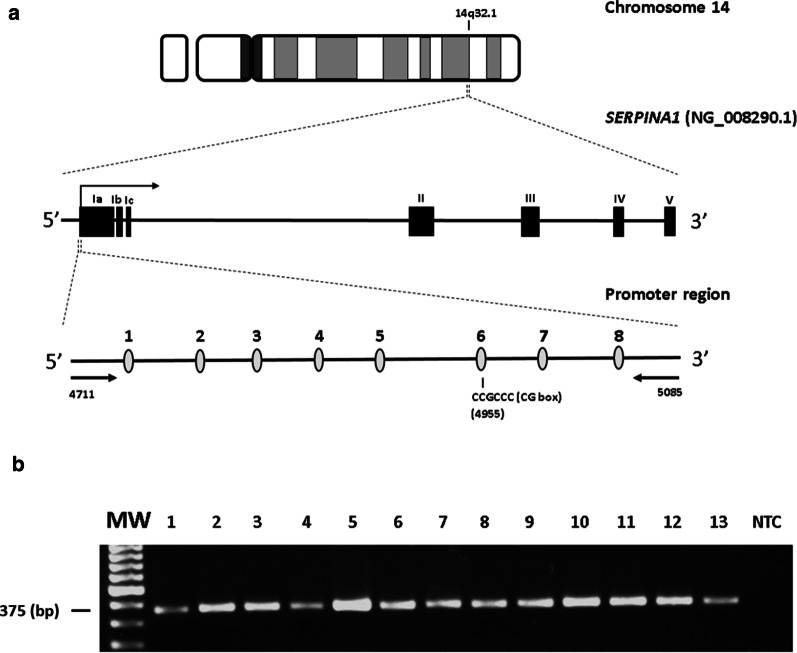
Methylation analysis was carried out by DNA treatment with sodium bisulfite, using the Epitect Bisulfite kit (Qiagen, Milan, Italy), as reported [21]. Treated DNA was purified with DNA purification columns (Epitect Bisulfite kit, Qiagen, Milan, Italy) and then subjected to PCR amplification for SERPINA1 gene promoter (GenBank accession number: NG_008290.1). Briefly, 150 ng of DNA was amplified using the primers forward 5′-TTTTGGTTTAGTTTAGGATTTTGAGG-3′ and reverse 5′-ACCTACCAATTATTAATACCAAATCTATAC-3′ [21, 26]. These primers amplify a promoter region of 375 bp (GenBank accession number: NG_008290.1, position 4711–5085), which contains 8 CpG dinucleotides. Among these CpGs, CpG number 1 (CpG-1) and CpG-8 have been previously found hypomethylated in association with lower average lung function phenotypes and COPD [26], while CpG-6 belongs to a CCGCCC-box regulatory consensus sequence of the promoter region (Fig. 1a) [21]. PCR program was: 10 min of denaturation at 95 °C followed by 40 cycles of 1 min at 95 °C, 1 min at 65 °C and 2 min at 72 °C and a final extension for 5 min at 72 °C [21]. A PCR-negative control containing distilled water without DNA, was included per reaction. PCR products were run onto 2% agarose gel electrophoresis and stained with ethidium bromide.
Fig. 1.
a Genomic structure of chromosome 14, SERPINA1 gene and SERPINA1 promoter region. Upper line: graphical representation of chromosome 14. SERPINA1 gene is located on the long arm of the chromosome at 14q32.1. Middle line: graphical representation of SERPINA1 gene (GenBank accession number NG_008290.1). The gene includes seven exons (Ia-Ic, II, III, IV and V) and six introns. Filled-in boxes and horizontal arrow indicate gene exons and orientation, respectively. Bottom line: analyzed SERPINA1 promoter sequence with 8 CpGs including the 6th CpG of the amplified region located at the CG-box consensus sequence. The horizontal arrows represent the primers. b Bisulfite PCR of SERPINA1 promoter region. MW, molecular weight; NTC, no template control. Columns 1–13, representative bisulfite-treated DNAs from acute coronary syndrome (ACS) patients with chronic obstructive pulmonary disease (COPD+, columns 1–6) and without COPD (COPD−, columns 7–13)
DNA sequencing of SERPINA1 PCR products
To evaluate the methylation levels of SERPINA1 promoter, PCR products were sequenced by direct sequencing [27]. Briefly, PCR products were purified using the QIAquick PCR Purification Kit (Qiagen, Milan, Italy) and then subjected to direct sequencing using the automated ABI-Prism-3130X DNA sequencer (Applied Biosystems, Monza, Italy) [25], as performed before [28]. SERPINA1 sequences showing more than or equal to 5 out of 8 methylated CpGs (≥ 50%) were considered hypermethylated [27, 29].
Statistical analysis
Continuous data were tested for normal distribution with the Kolmogorov–Smirnov test. Normally distributed values were presented as mean ± SD and compared by t test [30]. Otherwise, median [interquartile range] and Mann–Whitney U were used. The Chi-square trend test with Yate’s correction [31] was used to compare the observed SERPINA1 epigenotypes, i.e., DNA hypermethylation and hypomethylation, among COPD+ and COPD− groups. Lymphocyte values were analyzed with D'Agostino-Pearson test for normality, and groups were compared employing the nonparametric Mann–Whitney U test [32, 33]. Spearman correlation analysis was applied to evaluate age-related variations in SERPINA1 promoter methylation by matching the level of methylation to the age of each individual [30]. Multiple linear regression model has been used to estimate the associations between SERPINA1 epigenotypes and lymphocytes count. Statistical analyses were carried out employing the R software package (version 3.5.0) and using the GraphPad Prism for Windows (version 5.0, GraphPad) [34]. p values less than 0.05 (p < 0.05) were considered statistically significant [35].
Results
ACS patients
Population characteristic of ACS patients stratified according to the presence of COPD are reported in Table 1. Overall, 115 patients were included in the analysis. Of those, after spirometry, 30 (26%) were confirmed with COPD. Of note, only age, respiratory health screening questionnaire (RSHQ), FEV1 and pack-year resulted to be significantly different between COPD− (n = 85) and COPD+ (n = 30): age 63 ± 9 versus 70 ± 8 years, p = 0.001; RSHQ > 19 29% versus 57%, p = 0.008; FEV1 (mean L ± SD) 2.9 ± 0.7 versus 2.0 ± 0.5, p < 0.001; pack-year, mean ± SD 34 ± 23 vs 45 ± 38, p < 0.05 in COPD− vs. COPD+, respectively.
Table 1.
Population characteristics according to the presence of undiagnosed chronic obstructive pulmonary disease (COPD)
| General characteristics, mean ± SD | COPD− (n = 85) |
COPD+ (n = 30) |
p value |
|---|---|---|---|
| Age, years | 63 ± 9 | 70 ± 8 | 0.001 |
| BMI, Kg/m2 | 27.7 ± 4.2 | 27 ± 3.6 | 0.420 |
| Male, n° (%) | 73 (86) | 23 (77) | 0.243 |
| CV risk factors and comorbidities, n° (%) | |||
| Diabetes | 19 (22) | 9 (30) | 0.401 |
| Hypertension | 58 (68) | 18 (60) | 0.413 |
| Family history of CAD | 23 (27) | 8 (27) | 0.967 |
| Dyslipidemia | 47 (55) | 13 (43) | 0.260 |
| Pre-MI | 10 (12) | 8 (27) | 0.053 |
| Pre-PCI | 17 (20) | 3 (10) | 0.214 |
| Pre-CABG | 3 (4) | 2 (7) | 0.469 |
| AF | 8 (9) | 3 (10) | 0.925 |
| PAD | 4 (5) | 2 (7) | 0.678 |
| COPD parameters | |||
| RHSQ > 19, n° (%) | 25 (29) | 17 (57) | 0.008 |
| FEV1, L, mean ± SD | 2.9 ± 0.7 | 2.0 ± 0.5 | < 0.001 |
| Smoker, n° (%) | 39 (46) | 15 (50) | 0.698 |
| Former smokers, n° (%) | 46 (54) | 15 (50) | 0.698 |
| Pack years, mean ± SD | 34 ± 23 | 45 ± 38 | 0.049 |
| Clinical presentation and coronary vessel disease, n° (%) | |||
| STEMI | 38 (45) | 15 (50) | 0.617 |
| NSTEMI | 27 (32) | 10 (33) | 0.874 |
| Unstable angina | 20 (24) | 5 (17) | 0.433 |
| Descending artery | 67 (79) | 18 (60) | 0.051 |
| Circumflex artery | 44 (52) | 12 (40) | 0.268 |
| Right coronary artery | 61 (72) | 18 (60) | 0.232 |
| Left main | 12 (14) | 5 (17) | 0.551 |
| Therapy, n° (%) | |||
| Aspirin | 84 (99) | 30(100) | 0.553 |
| P2Y12 inhibitor | 85 (100) | 30(100) | 0.999 |
| Beta-blockers | 72 (85) | 28(93) | 0.228 |
| ACE inhibitors | 77 (91) | 28(93) | 0.646 |
| Statins | 83 (98) | 28(93) | 0.268 |
| Laboratory data, mean ± SD | |||
| Troponin T, ng/dl | 1.99 ± 2.7 | 2.3 ± 4.3 | 0.643 |
| CK-MB peak | 78 ± 119 | 90 ± 141 | 0.676 |
| WBC admission, u/μl | 9.4 ± 3.05 | 9.5 ± 2.94 | 0.998 |
| WBC discharge, u/μl | 8.48 ± 2.21 | 7.91 ± 1.88 | 0.209 |
| Lymphocytes, u/μl, admission | 2.14 ± 0.99 | 1.95 ± 0.96 | 0.365 |
| Lymphocytes, u/μl, discharge | 1.85 ± 0.57 | 1.74 ± 0.73 | 0.352 |
| Neutrophils, u/μl, admission | 6.11 ± 2.66 | 5.95 ± 2.06 | 0.763 |
| Neutrophils, u/μl, discharge | 5.56 ± 1.84 | 4.91 ± 1.36 | 0.081 |
| NLR, admission | 3.47 ± 2.17 | 3.78 ± 2.51 | 0.516 |
| NLR, discharge | 3.24 ± 1.44 | 3.39 ± 1.88 | 0.639 |
COPD+: ACS patients, with chronic obstructive pulmonary disease (COPD). COPD−: ACS patients without COPD. WBC: white blood cell, SD: standard deviation, ACE: angiotensin-converting enzyme, STEMI: ST-elevation myocardial infarction, NSTEMI: non-ST-elevation myocardial infarction, MI: myocardial infarction, PCI: percutaneous coronary intervention, CABG: coronary artery bypass graft, AF: atrial fibrillation, PAD: peripheral artery disease, COPD: chronic obstructive pulmonary disease, FEV1: forced expiratory volume at first second, FVC: forced vital capacity, RHSQ: respiratory health screening questionnaire, HDL: high-density lipoprotein, CAD: coronary artery disease, CV: cardiovascular, BMI: body mass index, BSA: body surface area. u/μl: cell/μl, NLR: neutrophil/lymphocyte ratio
SERPINA1 gene promoter methylation analysis
In order to profile the methylation status, SERPINA1 was PCR amplified in a promoter region containing 8 CpG dinucleotides (Fig. 1a). PCR amplifications were efficiently obtained in all blood samples from all patients (Fig. 1b).
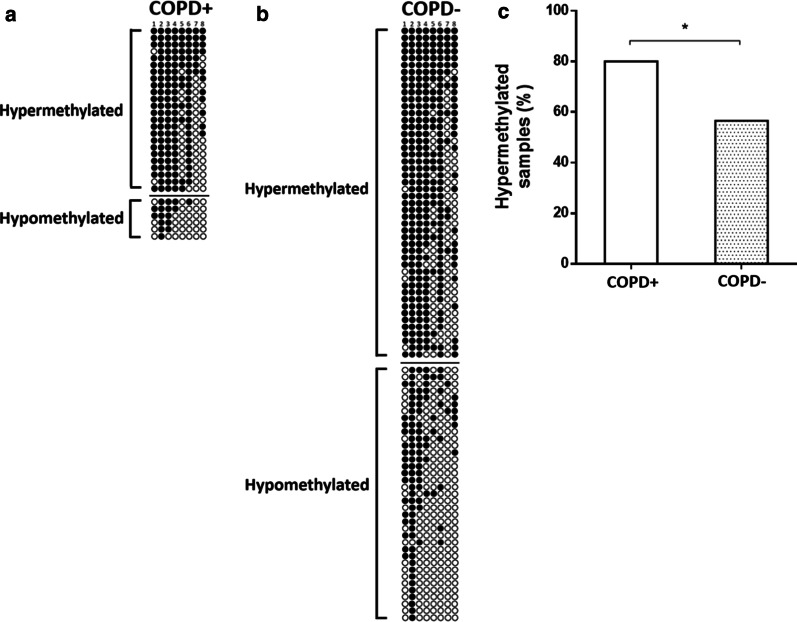
The prevalence of methylation in SERPINA1 gene promoter was evaluated by sequencing analysis of PCR products. SERPINA1 gene promoter was found to be hypermethylated in 72/115 (62.6%) of ACS patients. SERPINA1 was found to be hypermethylated in 24/30 (80%) COPD+ and in 48/85 (56.5%) COPD− (Fig. 2a, b). The difference in SERPINA1 hypermethylation between COPD+ and COPD− ACS patients resulted statistically significant (p < 0.05; Fig. 2c).
Fig. 2.
Bisulfite–polymerase chain reaction (PCR) sequencing of the SERPINA1 promoter of DNAs from blood samples belonging to acute coronary syndrome (ACS) patients a with chronic obstructive pulmonary disease (COPD+) and b without COPD (COPD−). In both panels: stratification of bisulfite–PCR sequences according to ACS subgroup and SERPINA1 hyper- and hypomethylation status. Filled-in and clear circles represent methylated and unmethylated CpGs, respectively. The CpGs within the SERPINA1 promoter are numbered across the top of the grid. Each row represents one PCR product/analyzed sequence. Samples showing more than or equal to 5 out of 8 (≥ 50%) methylated CpGs were considered hypermethylated; c frequencies of SERPINA1 promoter hypermethylation in COPD+ and COPD− samples. *p < 0.05
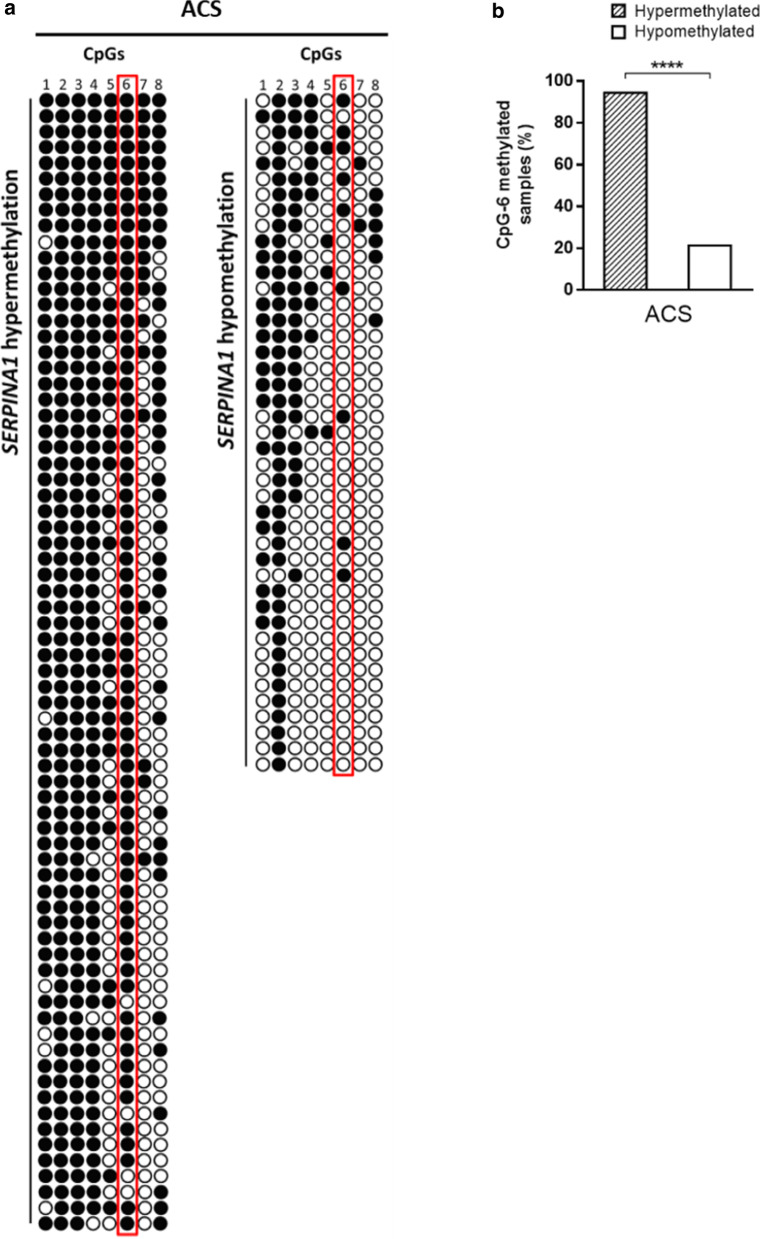
As CpG-6 dinucleotide has been recently associated with the epigenetic regulation of SERPINA1 gene by promoter methylation in blood cells from healthy individuals [21], we specifically investigated the methylation status of CpG-6 in SERPINA1 hypermethylated and SERPINA1 hypomethylated samples. To this aim, ACS patients were first stratified on the basis of the degree of methylation of SERPINA1: 5 to 8 (hypermethylated) and 1 to 4 (hypomethylated). Then, the number of CpG-6 methylated and CpG-6 un-methylated was counted in the two groups (Fig. 3a). CpG-6 was found to be methylated in 68/72 (94.4%) hypermethylated ACS samples and in 9/43 (20.9%) hypomethylated ACS samples (p < 0.0001; Fig. 3b).
Fig. 3.
a Acute coronary syndrome (ACS) patient’s stratification according to the number of CpGs methylated in SERPINA1, i.e., hypermethylation and hypomethylation. Methylated and unmethylated CpG-6 in SERPINA1 hypermethylated and hypomethylated samples, respectively, are reported. Samples showing more than or equal to 5 out of 8 (≥ 50%) methylated CpG dinucleotides were considered hypermethylated. Filled-in and clear circles represent methylated and unmethylated CpGs, respectively. The CpGs within the SERPINA1 promoter are numbered across the top of the grid. Each row represents one PCR product/analyzed sequence. Vertical red box highlights CpG-6 dinucleotides across hypermethylated and hypomethylated samples. b Frequencies of methylated CpG-6 in SERPINA1 promoter hypermethylated and hypomethylated ACS samples. ****p < 0.0001
Since hypomethylated CpG-1 and CpG-8 sites have been associated with lower average lung function phenotypes and COPD [26], we investigated the methylation status of these CpG sites in association with COPD. CpG-1 was found methylated in 24/30 (80%) and 60/85 (70.6%) of COPD+ and COPD−, respectively (p > 0.05). Furthermore, CpG-8 resulted methylated in 11/30 (36%) of COPD+ and in 34/85 (40%) of COPD− (p > 0.05). These results indicated a lack of association between CpG-1 and/or CpG-8 methylation and COPD.
Moreover, as the mean age was significantly higher in COPD+ (n = 30, mean age ± SD: 70 ± 7 years old) than in COPD− (n = 85, mean age ± SD: 63 ± 9 years old) (p < 0.001), age-related changes in SERPINA1 promoter methylation of ACS patients were assessed. Results indicate a lack of correlation between age and SERPINA1 promoter methylation in ACS patients (r = − 0.071 and p > 0.05) as well as between age and SERPINA1 methylation in both COPD+ (r = − 0.073, p > 0.05) and COPD− groups (r = − 0.166, p > 0.05). These results indicate that SERPINA1 promoter methylation is age independent in ACS patients with and without COPD. Thus, age did not represent a possible cofounder factor for SERPINA1 methylation analysis, as reported in healthy subjects [21].
In addition, in order to exclude diabetes as a confounding factor, a correlation between diabetes and SERPINA1 promoter methylation was assessed. The whole pool of ACS patients (n = 115) was stratified in patients with (n = 28) and without (n = 87) diabetes, and SERPINA1 hypermethylation rates were compared. A total of 67.9% (19/28) and 60.9% (53/87) of ACS patients with and without diabetes, respectively, presented SERPINA1 hypermethylation (p > 0.05). ACS patients were afterward stratified according to diabetes status in COPD+ with (n = 9) and without diabetes (n = 21) as well as in COPD− with (n = 19) and without diabetes (n = 66), and SERPINA1 hypermethylation rates were compared. SERPINA1 was hypermethylated in 100% (9/9) of COPD+ patients with diabetes and in 71.4% (15/21) of COPD+ patients without diabetes (p > 0.05). Moreover, 52.6% (10/19) and 57.6% (38/66) of COPD− patients with and without diabetes, respectively, presented SERPINA1 hypermethylation (p > 0.05). Thus, no differences in SERPINA1 methylation status were observed when analyzing groups according to diabetes. These results indicate that the presence of diabetes did not suppose an excluding factor in our study.
Association of lymphocytes count and SERPINA1 hypermethylation in COPD−
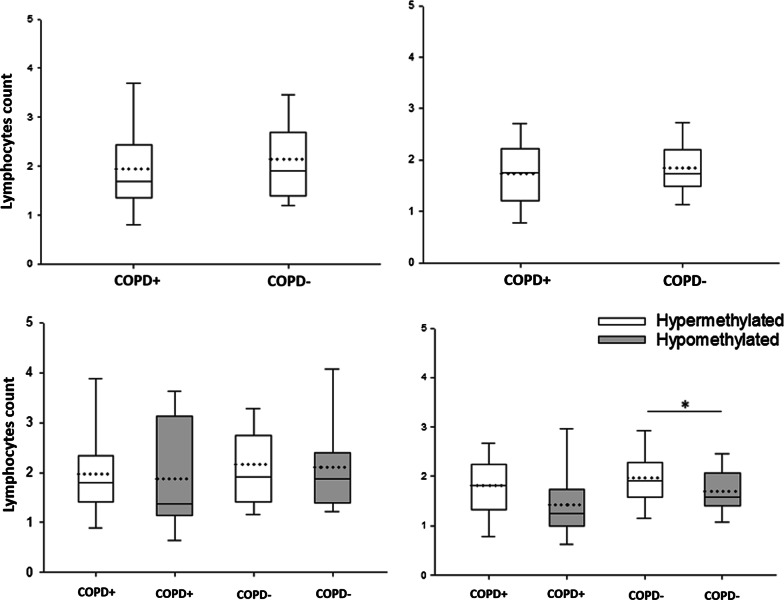
High levels of circulating lymphocytes are involved in COPD pathogenesis [36–38]. Therefore, lymphocytes count, available from the hospital database, was analyzed in COPD+ (n = 30) and COPD− (n = 85) patients at hospital admission and discharge, by multiple linear regression model. Lymphocytes count was 1.955 × 103 ± 0.960 cell/µl in COPD+ and 2.13 × 103 ± 0.98 cell/µl in COPD− at hospital admission (p > 0.05; Fig. 4a). At hospital discharge, lymphocytes count was 1.73 × 103 ± 0.73 cell/µl in COPD+ and 1.86 × 103 ± 0.57 cell/µl in COPD− (p > 0.05) (Fig. 4b).
Fig. 4.
Boxplot graph resuming the lymphocyte count in acute coronary syndrome (ACS) patients with chronic obstructive pulmonary disease (COPD+) and without COPD (COPD−) during patient a admission and b discharge. Lymphocyte count in COPD+ and COPD− patients in c admission and d discharge according to SERPINA1 promoter hypermethylated (white) and hypomethylated (grey) statuses. All panels: the lower (Q1) and upper (Q3) quartile, represent observations outside the 5–95 percentile range. The graph also shows the median (continuous middle line) and the mean (dashed line) of lymphocytes (× 103 cell/µl) per study group. *p < 0.05
Additionally, the relationship between SERPINA1 promoter methylation and lymphocytes count was investigated in admitted/discharged COPD+ and COPD− patients. At admission, lymphocytes count was similar in COPD+ with SERPINA1 hypermethylation (1.97 × 103 ± 0.92 cell/µl) and COPD+ with SERPINA1 hypomethylation (1.88 × 103 ± 1.05 cell/µl) (p > 0.05; Fig. 4c), as well as in COPD− with SERPINA1 hypermethylation (2.16 × 103 ± 0.95 cell/µl) and COPD− with hypomethylation (2.11 × 103 ± 1.01 cell/µl) (p > 0.05; Fig. 4c). At discharge, lymphocytes count was similar in COPD+ with SERPINA1 hypermethylation (1.81 × 103 ± 0.69 cell/µl) and COPD+ with hypomethylation (1.42 × 103 ± 0.73) (p > 0.05; Fig. 4d), whereas it was found to be higher in COPD− with SERPINA1 hypermethylation (1.98 × 103 ± 0.6 cell/µl) than in COPD− with SERPINA1 hypomethylation (1.7 × 103 ± 0.48 cell/µl) (p < 0.05, Fig. 4d).
Considering that the level of neutrophil count and neutrophil/lymphocyte ratio (NLR) is important prognostic predictors to assess the degree of inflammation [39, 40], these parameters were evaluated in ACS patients with and without COPD at both hospital admission and discharge. Neutrophil count was 5.95 × 103 ± 2.06 cell/μl in COPD+ and 6.11 × 103 ± 2.66 cell/μl in COPD− at hospital admission (p > 0.05). At hospital discharge, neutrophil count was 4.91 × 103 ± 1.36 cell/μl in COPD+ and 5.56 × 103 ± 1.84 cell/μl in COPD− (p > 0.05). NLR resulted to be 3.78 × 103 ± 2.51 cell/ μl in COPD+ and 3.47 × 103 ± 2.17 cell/μl in COPD− at hospital admission (p > 0.05), and 3.39 × 103 ± 1.88 cell/ μl in COPD+ and 3.24 × 103 ± 1.44 cell/μl in COPD− at hospital discharge (p > 0.05). Furthermore, no differences in both neutrophil count and NLR were found when comparing hospital admission and discharge values, for both COPD+ and COPD− groups (p > 0.05). These results indicate that measuring for neutrophil count and/or NLR in ACS patients does not enable to differentiate between COPD+ and COPD− ACS patients and cannot help in predicting which ACS patients are at risk of developing COPD.
Discussion
In the present study, we investigated the methylation status of SERPINA1 gene promoter in blood samples from ACS patients with (COPD+) and without (COPD−) COPD. Our results show that SERPINA1 promoter methylation is higher in COPD+ patients compared to COPD−. Therefore, SERPINA1 gene promoter hypermethylation may be a potential biomarker for detecting COPD in ACS patients.
SERPINA1 is constitutively expressed in hepatic cells [6]. SERPINA1 is also expressed in inducible manner in hepatocytes and blood cells during the course of the inflammation, infections and late pregnancy [16, 17, 19–21]. In PBMCs of healthy individuals the SERPINA1 promoter is hypermethylated except in women in advanced stage of pregnancy, suggesting the gene silencing under normal physiological conditions and the gene expression induction when circulating AAT is needed to be increased [19–21]. In this study, SERPINA1 gene promoter was found to be hypermethylated in 62.6% of ACS patients. The prevalence of SERPINA1 hypermethylation was higher in COPD+ (80%) than in COPD− (56.5%) patients (p < 0.05). This result suggests an association between SERPINA1 hypermethylation and COPD+ leading to the hypothesis that lack of AAT expression in blood cells may play a role in the development of COPD in ACS patients. The relevance of AAT in COPD is well known in smokers carrying mutations in SERPINA1 gene where protein deficiency synergizes with smoking in exerting strong adverse effects on lung function, causing COPD [11]. As ACS patients enrolled in this study are all smokers, the adverse effects on lung functions may be mediated by altering promoter methylation in SERPINA1.
In addition, we specifically analyzed methylation status at CpG-6 site. The dinucleotide CpG-6 falls within a CCGCCC box regulatory consensus region of SERPINA1 promoter. The CCGCCC box is located 46 nucleotides upstream of the transcription start site in SERPINA1 promoter, and it is recognized by transcription factors (TFs) to recruit RNA polymerase, thereby inducing transcription [41]. Methylation at CpG-6 could prevent CG-box-TFs interaction, thereby inducing gene transcriptional repression [42]. In our previous study, we have found that methylation status of CpG-6 reflects the methylation status of SERPINA1, being methylated in SERPINA1 hypermethylated samples and unmethylated in SERPINA1 hypomethylated samples [21]. In this study, nearly 95% of SERPINA1 hypermethylated samples had CpG-6 methylated, and 79% SERPINA1 hypomethylated samples had CpG-6 unmethylated. These results extend our previous observations and confirm that CpG-6 may play a pivotal role in epigenetic regulation of SERPINA1 in blood cells [21]. Therefore, the methylation status of CpG-6 may represent a useful marker of expression of SERPINA1 in blood cells.
As high levels of circulating lymphocytes play a role in COPD pathogenesis [36–38], lymphocytes count was analyzed in COPD+ and COPD− patients at hospital admission and discharge. Lymphocytes levels were similar in COPD+ and COPD− patients, both at hospital admission and discharge. Nevertheless, COPD− patients carrying SERPINA1 hypermethylated had circulating lymphocytes higher than COPD− patients carrying SERPINA1 hypomethylated, at hospital discharge (p < 0.05). This result suggests that COPD− patients carrying SERPINA1 hypermethylated may counteract inflammation less efficiently than COPD− patients carrying SERPINA1 hypomethylated. Therefore, we hypothesize that SERPINA1 hypermethylation may represent a risk factor for developing COPD in ACS patients at risk (smokers) with high levels of circulating lymphocytes in the presence of pharmacological treatments following percutaneous coronary intervention. In this view, ACS patients at risk of developing COPD might be identified by combining epigenetic and hematologic parameters. Follow-up studies should be carried out to verify whether hypermethylated SERPINA1 and lymphocyte count may be useful markers to predict the development of COPD in COPD− patients.
Few studies have explored SERPINA1 methylation in COPD pathogenesis. Our findings are partially in line with a recent meta-analysis study of tobacco-smoke exposed children and adult smokers, which reported association between methylated CpGs located about 32 kb downstream of SERPINA1 gene and decline in lung functions [26, 43]. Our data are discordant compared to a previous family-based study of smoking subjects with and without a history of COPD, which found two hypomethylated CpG sites, the CpG-1 and CpG-8 of this study, associated with lower average lung function phenotypes and COPD [26]. It should be pointed out that comparisons between our data and those reported above are difficult to be made, as both patient enrolment criteria and methodological approaches used herein are different. Indeed, we enrolled ACS patients, who were stratified according to the presence/absence of COPD, while previous data were obtained from predominantly smoking adults with/without COPD [26]. About methods, unlike previous investigations that studied epigenome-wide associations, our study was specifically designed to assess the methylation profile of SERPINA1 gene promoter in blood cells from ACS patients.
Our study presents some limitations. First, we studied the epigenetic regulation of SERPINA1 through promoter methylation without validation analyses, such as AAT mRNA and protein expression. However, in our previous study conducted on healthy subjects we determined an association between variations in SERPINA1 promoter methylation in PBMCs and changes in AAT circulating levels [21], while promoter methylation of SERPINA1 in association with gene expression inhibition has been demonstrated in animal models [44–46]. Second, we did not analyze mutations in SERPINA1 gene. Since about 1% of individuals carry mutations in SERPINA1, it is possible that some COPD+ may be associated with mutations in this gene. Considering that most of the gene mutations occur in the coding region [47], we may infer that SERPINA1 gene regulation by methylation in blood cells is not affected.
In conclusion, the present study shows, for the first time, that SERPINA1 gene promoter is hypermethylated in blood cells from COPD+ patients, compared to COPD−. Further, we show that CpG-6 methylation status reflects the methylation status of SERPINA1 promoter. We also found that COPD− patients with SERPINA1 hypermethylated present higher lymphocytes levels, in the presence of pharmacological treatments following percutaneous coronary intervention, than COPD− patients with SERPINA1 hypomethylated. Collectively, our data indicate that SERPINA1 hypermethylation may play a role in the development of COPD in ACS patients. Therefore, SERPINA1 promoter methylation may be a potential biomarker for detecting/predicting COPD in ACS patients.
Acknowledgements
We thank Professor Georgia Emma Gili for revising the English text of the manuscript.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- ACS
Acute coronary syndrome
- AAT
For alpha1-antitrypsin
- CpG-6
CpG number 6
- TFs
Transcription factors
Authors' contributions
FM helped in conceptualization; JCR contributed to methodology, visualization and validation; LOG and JCR helped in software and investigation; JCR, RS and GA formally analyzed; RP and GCC helped in resources; JCR, LOG and RP curated the data and statistically analyzed; FM and JCR wrote the original draft; FM, PR and MDM wrote the review and edited; FM and MT supervised the study and acquired the funding; FM and GGC administrated the project. All authors have read and agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by University of Ferrara, FAR (M.T. and F.M.) and FIR (F.M.) grants; MIUR PRIN 2017 grant (F.M.).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the County Ethical Committee (Authorization number 131295, March 21, 2014).
Consent for publication
Written informed consent was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gianluca Calogero Campo and Fernanda Martini contributed equally
Contributor Information
Gianluca Calogero Campo, Email: cmpglc@unife.it.
Fernanda Martini, Email: mrf@unife.it.
References
- 1.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet Lond Engl. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4:256. doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campo G, Pavasini R, Barbetta C, Maietti E, Mascetti S, Biscaglia S, et al. Predischarge screening for chronic obstructive pulmonary disease in patients with acute coronary syndrome and smoking history. Int J Cardiol. 2016;222:806–812. doi: 10.1016/j.ijcard.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Rothnie KJ, Smeeth L, Pearce N, Herrett E, Timmis A, Hemingway H, et al. Predicting mortality after acute coronary syndromes in people with chronic obstructive pulmonary disease. Heart Br Card Soc. 2016;102:1442–1448. doi: 10.1136/heartjnl-2016-309359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mooe T, Stenfors N. The Prevalence of COPD in individuals with acute coronary syndrome: a Spirometry-Based Screening Study. COPD. 2015;12:453–461. doi: 10.3109/15412555.2014.974742. [DOI] [PubMed] [Google Scholar]
- 6.Torres-Durán M, Lopez-Campos JL, Barrecheguren M, Miravitlles M, Martinez-Delgado B, Castillo S, et al. Alpha-1 antitrypsin deficiency: outstanding questions and future directions. Orphanet J Rare Dis. 2018;13:114. doi: 10.1186/s13023-018-0856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Y, DiCiaccio B, Li Y, Elshikha AS, Titov D, Brenner B, et al. Anti-inflammaging effects of human alpha-1 antitrypsin. Aging Cell. 2018;17:e12694. doi: 10.1111/acel.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martini F, De Mattei M, Contini C, Tognon MG. Potential use of alpha-1 anti-trypsin in the Covid-19 treatment. Front Cell Dev Biol [Internet]. 2020 [cited 2020 Dec 24];8. Available from: 10.3389/fcell.2020.577528/full [DOI] [PMC free article] [PubMed]
- 9.Dunlea DM, Fee LT, McEnery T, McElvaney NG, Reeves EP. The impact of alpha-1 antitrypsin augmentation therapy on neutrophil-driven respiratory disease in deficient individuals. J Inflamm Res. 2018;11:123–134. doi: 10.2147/JIR.S156405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouhani F, Paone G, Smith NK, Krein P, Barnes P, Brantly ML. Lung neutrophil burden correlates with increased pro-inflammatory cytokines and decreased lung function in individuals with alpha(1)-antitrypsin deficiency. Chest. 2000;117:250S–S251. doi: 10.1378/chest.117.5_suppl_1.250S. [DOI] [PubMed] [Google Scholar]
- 11.Kalsheker NA. Molecular pathology of alpha 1-antitrypsin deficiency and its significance to clinical medicine. QJM Mon J Assoc Physicians. 1994;87:653–658. [PubMed] [Google Scholar]
- 12.Billingsley GD, Walter MA, Hammond GL, Cox DW. Physical mapping of four serpin genes: alpha 1-antitrypsin, alpha 1-antichymotrypsin, corticosteroid-binding globulin, and protein C inhibitor, within a 280-kb region on chromosome I4q321. Am J Hum Genet. 1993;52:343–353. [PMC free article] [PubMed] [Google Scholar]
- 13.Matamala N, Martínez MT, Lara B, Pérez L, Vázquez I, Jimenez A, et al. Alternative transcripts of the SERPINA1 gene in alpha-1 antitrypsin deficiency. J Transl Med. 2015;13:211. doi: 10.1186/s12967-015-0585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalsheker N, Morley S, Morgan K. Gene regulation of the serine proteinase inhibitors alpha1-antitrypsin and alpha1-antichymotrypsin. Biochem Soc Trans. 2002;30:93–98. doi: 10.1042/bst0300093. [DOI] [PubMed] [Google Scholar]
- 15.Perlino E, Cortese R, Ciliberto G. The human alpha 1-antitrypsin gene is transcribed from two different promoters in macrophages and hepatocytes. EMBO J. 1987;6:2767–2771. doi: 10.1002/j.1460-2075.1987.tb02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoell DL, Ralston DR, Coulter KR, Wewers MD. Alpha 1-antitrypsin and protease complexation is induced by lipopolysaccharide, interleukin-1 β , and tumor necrosis factor- α in monocytes. Am J Respir Crit Care Med [Internet]. 1998 [cited 2020 Sep 3];157:246–55. Available from: 10.1164/ajrccm.157.1.9702033 [DOI] [PubMed]
- 17.Boutten A, Venembre P, Seta N, Hamelin J, Aubier M, Durand G, et al. Oncostatin M Is a potent stimulator of α 1 -antitrypsin secretion in lung epithelial cells: modulation by transforming growth factor- β and interferon- γ. Am J Respir Cell Mol Biol [Internet]. 1998 [cited 2020 Sep 3];18:511–20. Available from: 10.1165/ajrcmb.18.4.2772 [DOI] [PubMed]
- 18.Paczek L, Michalska W, Bartlomiejczyk I. Trypsin, elastase, plasmin and MMP-9 activity in the serum during the human ageing process. Age Ageing. 2008;37:318–323. doi: 10.1093/ageing/afn039. [DOI] [PubMed] [Google Scholar]
- 19.Twina G, Sheiner E, Shahaf G, Yaniv Salem S, Madar T, Baron J, et al. Lower circulation levels and activity of α-1 antitrypsin in pregnant women with severe preeclampsia. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2012;25:2667–2670. doi: 10.3109/14767058.2012.705397. [DOI] [PubMed] [Google Scholar]
- 20.Baron J, Sheiner E, Abecassis A, Ashkenazi E, Shahaf G, Salem SY, et al. α1-antitrypsin insufficiency is a possible contributor to preterm premature rupture of membranes. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2012;25:934–937. doi: 10.3109/14767058.2011.600369. [DOI] [PubMed] [Google Scholar]
- 21.Rotondo JC, Oton-Gonzalez L, Selvatici R, Rizzo P, Pavasini R, Campo GC, et al. SERPINA1 gene promoter is differentially methylated in peripheral blood mononuclear cells of pregnant women. Front Cell Dev Biol. 2020;8:550543. doi: 10.3389/fcell.2020.550543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 24.Contini C, Rotondo JC, Magagnoli F, Maritati M, Seraceni S, Graziano A, et al. Investigation on silent bacterial infections in specimens from pregnant women affected by spontaneous miscarriage. J Cell Physiol. 2018;234:100–107. doi: 10.1002/jcp.26952. [DOI] [PubMed] [Google Scholar]
- 25.Malagutti N, Rotondo JC, Cerritelli L, Melchiorri C, De Mattei M, Selvatici R, et al. High human papillomavirus DNA loads in inflammatory middle ear diseases. Pathogens. 2020;9(3):224. doi: 10.3390/pathogens9030224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu W, Baccarelli A, Carey VJ, Boutaoui N, Bacherman H, Klanderman B, et al. Variable DNA methylation is associated with chronic obstructive pulmonary disease and lung function. Am J Respir Crit Care Med. 2012;185:373–381. doi: 10.1164/rccm.201108-1382OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotondo JC, Borghi A, Selvatici R, Magri E, Bianchini E, Montinari E, et al. Hypermethylation-induced inactivation of the IRF6 gene as a possible early event in progression of vulvar squamous cell carcinoma associated with Lichen Sclerosus. JAMA Dermatol. 2016;152:928. doi: 10.1001/jamadermatol.2016.1336. [DOI] [PubMed] [Google Scholar]
- 28.Rotondo JC, Candian T, Selvatici R, Mazzoni E, Bonaccorsi G, Greco P, et al. Tracing males from different continents by genotyping JC polyomavirus in DNA from semen samples. J Cell Physiol. 2017;232:982–985. doi: 10.1002/jcp.25686. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell SM, Ross JP, Drew HR, Ho T, Brown GS, Saunders NFW, et al. A panel of genes methylated with high frequency in colorectal cancer. BMC Cancer. 2014;14:54. doi: 10.1186/1471-2407-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotondo JC, Oton-Gonzalez L, Mazziotta C, Lanzillotti C, Iaquinta MR, Tognon M, et al. Simultaneous detection and viral DNA load quantification of different Human Papillomavirus types in clinical specimens by the high analytical droplet digital PCR method. Front Microbiol. 2020;19(11):591452. doi: 10.3389/fmicb.2020.591452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzoni E, Di Stefano M, Fiore JR, Destro F, Manfrini M, Rotondo JC, et al. Serum IgG antibodies from pregnant women reacting to Mimotopes of Simian virus 40 large T antigen, the viral oncoprotein. Front Immunol. 2017;8:411. doi: 10.3389/fimmu.2017.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tognon M, Tagliapietra A, Magagnoli F, Mazziotta C, Oton-Gonzalez L, Lanzillotti C, et al. Investigation on spontaneous abortion and human papillomavirus infection. Vaccines. 2020;8:473. doi: 10.3390/vaccines8030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tognon M, Luppi M, Corallini A, Taronna A, Barozzi P, Rotondo JC, et al. Immunologic evidence of a strong association between non-Hodgkin lymphoma and simian virus 40. Cancer. 2015;121:2618–2626. doi: 10.1002/cncr.29404. [DOI] [PubMed] [Google Scholar]
- 34.Corazza M, Oton-Gonzalez L, Scuderi V, Rotondo JC, Lanzillotti C, Di Mauro G, et al. Tissue cytokine/chemokine profile in vulvar lichen sclerosus: An observational study on keratinocyte and fibroblast cultures. J Dermatol Sci. 2020;100(3):223–226. doi: 10.1016/j.jdermsci.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Preti M, Rotondo JC, Holzinger D, Micheletti L, Gallio N, McKay-Chopin S, et al. Role of human papillomavirus infection in the etiology of vulvar cancer in Italian women. Infect Agent Cancer. 2020;15:20. doi: 10.1186/s13027-020-00286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langer D, Ciavaglia CE, Neder JA, Webb KA, O’Donnell DE. Lung hyperinflation in chronic obstructive pulmonary disease: mechanisms, clinical implications and treatment. Expert Rev Respir Med. 2014;8:731–749. doi: 10.1586/17476348.2014.949676. [DOI] [PubMed] [Google Scholar]
- 37.Gadgil A, Duncan SR. Role of T-lymphocytes and pro-inflammatory mediators in the pathogenesis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:531–541. doi: 10.2147/COPD.S1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodge SJ, Hodge GL, Reynolds PN, Scicchitano R, Holmes M. Increased production of TGF-beta and apoptosis of T lymphocytes isolated from peripheral blood in COPD. Am J Physiol Lung Cell Mol Physiol. 2003;285:L492–499. doi: 10.1152/ajplung.00428.2002. [DOI] [PubMed] [Google Scholar]
- 39.Tahto E, Jadric R, Pojskic L, Kicic E. Neutrophil-to-lymphocyte Ratio and Its Relation with Markers of Inflammation and Myocardial Necrosis in Patients with Acute Coronary Syndrome. Med Arch Sarajevo Bosnia Herzeg. 2017;71:312–315. doi: 10.5455/medarh.2017.71.312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jasper AE, McIver WJ, Sapey E, Walton GM. Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Research. 2019;8. [DOI] [PMC free article] [PubMed]
- 41.Zarain-Herzberg A, Rupp H. Transcriptional modulators targeted at fuel metabolism of hypertrophied heart. Am J Cardiol. 1999;83:31H–37H. doi: 10.1016/S0002-9149(99)00254-4. [DOI] [PubMed] [Google Scholar]
- 42.Ahn JK, Pitluk ZW, Ward DC. The GC box and TATA transcription control elements in the P38 promoter of the minute virus of mice are necessary and sufficient for transactivation by the nonstructural protein NS1. J Virol. 1992;66:3776–3783. doi: 10.1128/JVI.66.6.3776-3783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beckmeyer-Borowko A, Imboden M, Rezwan FI, Wielscher M, Amaral AFS, Jeong A, et al. SERPINA1 methylation and lung function in tobacco-smoke exposed European children and adults: a meta-analysis of ALEC population-based cohorts. Respir Res. 2018;19:156. doi: 10.1186/s12931-018-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zglejc-Waszak K, Waszkiewicz EM, Franczak A. Periconceptional undernutrition affects the levels of DNA methylation in the peri-implantation pig endometrium and in embryos. Theriogenology. 2018;123:185–193. doi: 10.1016/j.theriogenology.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Franczak A, Zglejc-Waszak K, Martyniak M, Waszkiewicz EM, Kotwica G. Peri-conceptional nutritional restriction alters transcriptomic profile in the peri-implantation pig embryos. Anim Reprod Sci. 2018;197:305–316. doi: 10.1016/j.anireprosci.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 46.Barton DE, Francke U. Activation of human alpha 1-antitrypsin genes in rat hepatoma x human fibroblast hybrid cell lines is correlated with demethylation. Somat Cell Mol Genet. 1987;13:635–644. doi: 10.1007/BF01534484. [DOI] [PubMed] [Google Scholar]
- 47.Milger K, Holdt LM, Teupser D, Huber RM, Behr J, Kneidinger N. Identification of a novel SERPINA-1 mutation causing alpha-1 antitrypsin deficiency in a patient with severe bronchiectasis and pulmonary embolism. Int J Chron Obstruct Pulmon Dis. 2015;10:891–897. doi: 10.2147/COPD.S80173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.