Abstract
Background
Carnitine palmitoyl transferase 1A (CPT1A), the key regulator of fatty acid oxidation, contributes to tumor metastasis and therapeutic resistance. We aimed to identify its clinical significance as a biomarker for the diagnosis and prediction of breast cancer.
Methods
Western blot, ELISA and in silico analysis were used to confirm CPT1A levels in breast cancer cell lines, cell culture medium and breast cancer tissues. Four hundred thirty breast cancer patients, 200 patients with benign breast disease, and 400 healthy controls were enrolled and randomly divided into a training set and a test set with a 7:3 ratio. Training set was used to build diagnostic models and 10-fold cross validation was used to demonstrate the performance of the models. Then test set was aimed to validate the effectiveness of the diagnostic models. ELISA was conducted to detect individual serum CPT1A levels. Receiver operating characteristic (ROC) curves were generated, and binary logistic regression analyses were performed to evaluate the effectiveness of CPT1A as a biomarker in breast cancer diagnosis. CPT1A levels between post-operative and pre-operative samples were also compared.
Results
CPT1A was overexpressed in breast cancer tissues, cell lines and cell culture medium. Serum CPT1A levels were higher in breast cancer patients than in controls and were significantly associated with metastasis, TNM stage, histological grading and molecular subtype. CPT1A levels were decreased in post-operative samples compared with paired pre-operative samples. Moreover, CPT1A exhibited a higher efficacy in differentiating breast cancer patients from healthy controls (training set: area under the curve, AUC, 0.892, 95% CI, 0.872–0.920; test set, AUC, 0.904, 95% CI, 0.869–0.939) than did CA15–3, CEA, or CA125.
Conclusion
CPT1A is overexpressed in breast cancer and can be secreted out of breast cancer cell. Serum CPT1A is positively associated with breast cancer progression and could serve as an indicator for disease monitoring. Serum CPT1A displayed a remarkably high diagnostic efficiency for breast cancer and could be a novel biomarker for the diagnosis of breast cancer.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-08134-7.
Keywords: Carnitine palmitoyl transferase 1A, Breast cancer, Biomarker, Diagnosis
Background
Breast cancer is the leading cause of cancer-related death among women. In 2018, 2.1 million new breast cancer cases and 0.6 million cancer-related deaths were estimated worldwide, accounting for 24.4% of total new cancer cases and 15% of total cancer deaths in women [1]. In China, there were an estimated 268,600 new female breast cancer cases and 69,500 deaths in 2015 [2]. In the last two decades, the incidence of breast cancer in China has increased twice as fast as the global rate, particularly in urban areas, because of the prevalence of obesity, physical inactivity, and changes in reproductive pattern [3]. Although early-stage breast cancer is associated with a favorable prognosis, patients diagnosed at an advanced stage suffer from metastasis, therapeutic relapse and poor outcomes [4–6]. In view of the huge burden of breast cancer, there is an increasing demand for improved screening, diagnosis, and management of this disease.
The most widely used method for breast cancer screening is mammography [7, 8]. Although this method was reported to reduce cancer-specific mortality, it has the potential harm of overdiagnosis, which can lead to unnecessary treatment and pain [9, 10]. Further diagnosis of primary breast cancer is based on histopathological examination of tumor tissue [11, 12]. However, these invasive methods are painful, time-consuming, and sometimes tumoral heterogeneity leads to inaccurate diagnoses. Thus, it is important to develop novel, non-invasive and rapid diagnostic methods for breast cancer.
Tumor-specific proteins produced by cancer cells can be identified in the blood of cancer patients and used for cancer diagnosis [13]. At present, carcinoembryonic antigen (CEA) and cancer antigen 15–3 (CA15–3) are the most widely used serum tumor markers for the detection and assessment of treatment responses in breast cancer [14]. Cancer antigen 125 (CA125), which is primarily used for detection of ovarian cancer, has also been suggested as a tumor marker for breast cancer [15]. However, the sensitivity of these biomarkers is limited in the early stages of breast cancer [16].
Carnitine palmitoyl transferase 1A (CPT1A) controls the rate-limiting step of fatty acid oxidation (FAO) which is increasing recognized as crucial metabolic signature of cancer [17–23]. Recently CPT1A is emerged as a crucial regulator for breast cancer [24–29]. CPT1A is upregulated in MYC-overexpressing triple-negative breast cancer (TNBC), radiation-resistant breast cancer cells and radiation-derived breast cancer stem cells [26, 27]. Inhibition of CPT1A activates cell apoptosis and suppresses cell invasion in breast cancer [26, 27, 29]. Overall, these studies indicated that CPT1A plays an important role in breast cancer progression and could be a promising target in breast cancer.
Our previous study found that CPT1A promotes radiation-resistance in nasopharyngeal carcinoma, and gene ontology enrichment analysis showed that exocytosis is predicted as the most associated biological process for CPT1A-binding proteins [22]. Moreover, CPT1A was identified in extracellular vesicles (EVs) derived from breast, ovary, kidney cancer and leukemia cell lines by using proteomic profiling in two studies [30, 31]. These studies indicated that, although CPT1A is known to locate on mitochondrial outer membranes [32], it might also exist in EVs and play a role in EVs-mediated biological activities in cancer.
In this study, we aimed to identify the clinical significance of CPT1A as a biomarker for the diagnosis and prediction of breast cancer. We confirmed the expression level of CPT1A in breast cancer tissues, cell lines and culture medium. Then, we measured the serum levels of CPT1A in a large-scale study and evaluate its clinical significance and diagnostic efficiency for breast cancer.
Methods
Patients
In this large-scale study, a total of 560 breast cancer patients, 280 patients with benign breast disease and 600 healthy women were included from the Central Hospital of Wuhan between March 2017 and January 2019. Blood samples of patients were collected 1 day before surgery or breast puncture biopsy. The diagnosis of breast cancer and benign breast disease was confirmed by histopathology according to the guidelines of the National Comprehensive Cancer Network and the guidelines of the Chinese Society of Clinical Oncology.
Breast cancer patients were enrolled according to the following criteria: female patients were newly diagnosed with breast cancer and were not subjected to any other malignant disease, severe injury, or anti-neoplastic therapy before the diagnosis. We excluded 130 breast cancer patients from the study: 39 patients did not have certain histopathological diagnosis, 25 patients had breast tumor metastasized from other organs, 35 patients had received chemotherapy, 11 patients were suffered from breast cancer recurrence, and 20 patients were lost to follow-up. Finally, 430 patients newly diagnosed with breast cancer were enrolled.
Controls were cancer-free subjects, consisted of healthy controls and individuals with benign breast disease. Healthy individuals without exposure to potentially harmful chemicals and malignant disease were verified to be healthy after routine diagnostic tests, including chest X-rays, liver and kidney function tests, viral index analysis and tumor marker analysis, at the time of blood collection. Eighty-five individuals were excluded for dyslipidemia, 87 individuals were excluded for liver dysfunction and 28 individuals were excluded for coagulation disorders. Thus, 400 healthy individuals were enrolled as healthy control. Patients with benign breast disease who were not subjected to malignant disease, severe injury and acute inflammation were enrolled. Twenty-four patients were excluded because of a history of cancer, 18 patients were excluded for lactational mastitis, 22 patients were excluded for breast prosthesis-caused inflammation and 16 patients were excluded for loss to follow-up. As a result, 200 patients with benign breast disease were included.
The finally included participants were randomly divided into a training and test sets with a 7:3 ratio. The training set was used to develop the diagnostic models and the test set was aimed to validate the effectiveness of the diagnostic models. In training set, 126 paired breast cancer serum samples were collected before surgery and 1 week after surgery, to assess tumor-monitoring value. In test set, 44 paired pre-and post-treatment breast cancer serum samples were collected. Clinicopathologic information about patients, including age, pathology, tumor node metastasis (TNM) stage, and molecular subtype, was obtained from hospital pathologic records (Table 1).
Table 1.
The characteristics of the patient population
| Clinical parameters | Training set | Test set | ||||
|---|---|---|---|---|---|---|
| CPT1A level | CPT1A level | |||||
| High | Low | p Value | High | Low | p Value | |
| Age | ||||||
| ≤ 60 | 85 (28.2) | 95 (31.6) | 0.269 | 43 (33.3) | 48 (37.2) | 0.407 |
| > 60 | 65 (21.6) | 56 (18.6) | 21 (16.3) | 17 (13.2) | ||
| Menopausal status, n (%) | ||||||
| Premenopausal | 76 (25.2) | 85 (28.2) | 0.328 | 35 (27.1) | 40 (31.0) | 0.430 |
| postmenopausal | 74 (24.6) | 66 (21.9) | 29 (22.5) | 25 (19.4) | ||
| Tumor size, n (%) | ||||||
| Tis | 5 (3.9) | 20 (15.5) | 0.000 | 4 (3.1) | 8 (7.0) | 0.033 |
| T1 | 44 (34.1) | 52 (40.3) | 16 (12.4) | 30 (23.3) | ||
| T2 | 84 (65.1) | 76 (58.9) | 35 (27.1) | 24 (18.6) | ||
| T3 | 13 (10.1) | 3 (2.3) | 7 (5.4) | 3 (2.3) | ||
| T4 | 4 (3.1) | 0 (0.0) | 2 (1.6) | 0 (0.0) | ||
| Lymph node status, n (%) | ||||||
| N0 | 75 (24.9) | 108 (35.9) | 0.001 | 33 (25.6) | 49 (38.0) | 0.004 |
| N1 | 39 (13.0) | 24 (8.0) | 12 (9.3) | 11 (8.5) | ||
| N2 | 17 (5.6) | 13 (4.3) | 7 (5.4) | 4 (3.1) | ||
| N3 | 19 (6.3) | 6 (2.0) | 12 (9.3) | 1 (0.8) | ||
| TNM stage, n (%) | ||||||
| 0 | 5 (1.7) | 20 (6.6) | 0.000 | 4 (3.1) | 8 (6.2) | 0.000 |
| I | 27 (9.0) | 41 (13.6) | 6 (4.7) | 25 (19.4) | ||
| IIA | 48 (15.9) | 53 (17.6) | 24 (18.6) | 15 (11.6) | ||
| IIB | 25 (8.3) | 19 (6.3) | 7 (5.4) | 10 (7.8) | ||
| III | 39 (13.0) | 15 (5.0) | 19 (14.7) | 6 (4.7) | ||
| IV | 6 (2.0) | 3 (1.0) | 4 (3.1) | 1 (0.8) | ||
| Histological grading, n (%) | ||||||
| G1 | 9 (3.0) | 22 (7.3) | 0.045 | 2 (1.6) | 15 (11.6) | 0.000 |
| G2 | 82 (27.2) | 81 (26.9) | 25 (19.4) | 33 (25.6) | ||
| G3 | 56 (18.6) | 47 (15.6) | 35 (27.1) | 16 (12.4) | ||
| Unkown | 3 (1.0) | 1 (0.3) | 2 (1.6) | 1 (0.8) | ||
| ER, n (%) | ||||||
| Negative | 64 (21.3) | 61 (20.3) | 0.690 | 13 (10.1) | 22 (17.1) | 0.063 |
| Positive | 86 (28.6) | 90 (29.9) | 51 (39.5) | 42 (33.3) | ||
| PR, n (%) | ||||||
| ≤ 20 | 71 (23.6) | 79 (26.2) | 0.387 | 28 (21.7) | 21 (16.3) | 0.181 |
| > 20 | 79 (26.2) | 72 (23.9) | 36 (27.9) | 44 (34.1) | ||
| HER2, n (%) | ||||||
| Negative | 108 (35.9) | 85 (28.2) | 0.004 | 47 (36.4) | 36 (27.9) | 0.032 |
| Positive | 42 (14.0) | 66 (21.9) | 17 (13.2) | 29 (22.5) | ||
| Ki67, n (%) | ||||||
| < 15% | 20 (6.6) | 33 (11.0) | 0.052 | 14 (10.9) | 20 (15.5) | 0.252 |
| ≥ 15% | 130 (43.2) | 118 (39.2) | 50 (38.8) | 45 (34.9) | ||
| Molecular subtype, n (%) | ||||||
| Luminal A | 11 (3.7) | 18 (6.0) | 0.000 | 7 (5.4) | 15 (11.6) | 0.033 |
| Luminal B/HER2- | 59 (19.6) | 52 (17.3) | 23 (17.8) | 33 (25.6) | ||
| Luminal B/HER2+ | 16 (5.3) | 21 (7.0) | 12 (9.3) | 9 (7.0) | ||
| HER2+ | 23 (7.6) | 44 (14.6) | 9 (7.0) | 4 (3.1) | ||
| TNBC | 41 (13.6) | 16 (5.3) | 13 (10.1) | 4 (3.1) | ||
Pearson’s chi-square test
Sample collection
Blood samples were collected in the morning before breakfast with informed consent from patients. Four millilitre peripheral blood samples were collected in separation gel/coagulation-promoting vacuum tubes. The samples were centrifuged at 2, 000 x g for 15 min at 4 °C within 1 h after collection. The supernatants (sera) were then transferred into new tubes and stored at − 80 °C until testing.
Cell lines
The human immortalized mammary epithelial cell line HBL-100 (Cat. # GNHu10), human breast cancer cell lines SK-BR-3 (Cat. # TCHu225), T-47D (Cat. # TCHu87), MCF7 (Cat. # TCHu74), MDA-MB-453 (Cat. # SCSP-5044), MDA-MB-468 (Cat. # TCHu136), MDA-MB-231 (Cat. # TCHu227), Hs578T (Cat. # TCHu127) and BT549 (Cat. # TCHu93) were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China) in 2016. Cells were incubated in a humidified incubator with 5% CO2 at 37 °C. HBL-100, SK-BR-3 and T-47D cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM, Hyclone, USA) containing 10% fetal bovine serum (FBS, Gibco, USA). MCF7 cells were cultured in Eagle’s minimum essential medium (EMEM, Hyclone, USA) containing 10% FBS and 0.01 mg/mL bovine insulin. MDA-MB-453, MDA-MB-468 and MDA-MB-231 cells were cultured in Leibovitz’s L-15 medium containing 10% FBS. HCC1806 cells were cultured in PRMI-1640 medium containing 10% FBS. Hs578T cells were cultured in DMEM medium with 0.01 mg/ml insulin and 10% FBS. BT549 cells were cultured in PRMI-1640 medium with 0.0231 units/mL insulin and 10%FBS. Cells were routinely authenticated every year by using the following methods: cellular DNA was purified with QIAamp DNA mini kit (QIAGEN, Cat. # G51306) and used for PCR amplification with STR Multi-amplification kit (Goldeneye DNA ID System 20A, Peoplespot), then the PCR products were assayed with 3100xI DNA Analyzer (Applied Biosystems). Cells were routinely tested for mycoplasma contamination using the PCR Mycoplasma Detection Kit (ABM, Cat. # G238). Cells were grown in T25 culture flasks and passaged using 0.25% Trypsin/EDTA. Protein and cell culture medium were collected within 3 passages in 2 weeks.
Western blot analysis
Cell pellets were harvested and disrupted in IP lysis buffer (Thermo Scientific, MA, USA). Protein concentrations were measured using a BCA assay kit (Thermo Scientific, MA, USA). Then proteins were separated by SDS-PAGE and transferred onto a 0.45-μm PVDF membrane at 4 °C for 1 h (Millipore, USA). Then 5% fatty acids-free milk was used to incubate with PVDF membranes at room temperature for 2 h. After that, CPT1A primary antibody (ab102679, Abcam, MA, USA) was used as 1:1000 and β-Actin primary antibody (A2066, Sigma-Aldrich, Darmstadt, Germany) was used as 1:3000 to incubate with PVDF membranes at 4 °C overnight. Wash the membranes with PBST buffer for 3 times and 10 min for each time. Then peroxidase-conjugated secondary antibody (7076, Cell Signaling Technology, MA, USA) were used as 1:1000 to incubate with the membranes at room temperature for 1 h. Wash the membranes with PBST buffer for 3 times and 10 min for each time. Visualization and grayscale analysis were performed by using the ChemiDoc XRS system and Image Lab software (Bio-Rad, CA, USA).
Elisa
CPT1A levels of serum and cell culture medium were detected by using a commercially available ELISA kit (SEH368Hu96 Test, Cloud-Clone Corp, China) according to the manufacturer’s protocol. This kit is a sandwich enzyme immunoassay for quantitative measurement of CPT1A. serum samples were diluted in Standard Diluent as 1:5, 1:10, and 1:20 in a preliminary experiment. Then we chose the optimal sample dilution as 1:10, to guarantee the values were within the range of the standard curve. Standards, diluted serum samples and cell culture medium were run in triplicate. Blank, diluted standard series and samples in a 100 μL total volume were added to the wells of an ELISA analysis plate pre-coated with an antibody specific to CPT1A. The plate was covered with a sealer and incubated for 1 h at 37 °C. Then the liquid was removed, and 100 μL of Reagent A, containing a biotin-conjugated antibody specific to CPT1A, was added to each well. The plate was incubated for another 1 h at 37 °C and washed with Wash Buffer three times. Then 100 μL of Reagent B, containing avidin-conjugated horseradish peroxidase (HRP) was added, and the plate was incubated for 30 min at 37 °C. After five washes with Wash Buffer, a 3, 3, 5, 5-tetramethylbenzidine (TMB) substrate which reacted with the HRP enzyme was added to the plates resulting in color development. Then the plate was incubated for 15 min at 37 °C, and the reaction was terminated by addition of a sulfuric acid solution. The optical density (OD) was immediately measured at a wavelength of 450 nm in a plate reader (EnSpire 2300, PerkinElmer, USA). The concentration of CPT1A was determined by curve-fitting to an OD standard curve.
Logistic regression models
The standard logistic regression formula is:
“p” is the estimated probability of breast cancer patients, “β0” is a constant, “β” is the influence coefficient, “n” is the number of influence factors [33, 34].
Formula for predicting breast cancer was developed based on the data of breast cancer patients and controls in the training set. β0 and βn were obtained by binary logistic regression. The estimated probability of breast cancer patients and controls in the test set were calculated using the formula. Then ROC curve analysis and binary logistic regression analysis were conducted based on the estimated probability in the test set to evaluate the effectiveness of the models.
Analysis of tumor markers and lipids
Serum CA15–3, CEA, and CA125 levels were detected by using an automatic electrochemistry luminescence immunoassay system (Abbott, I2000–2, USA). Serum lipids, including triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), and non-esterified fatty acids (NEFA), were tested using an automatic biochemical analysis system (Olympus, AU5421, Japan). The reference changes of each tumor biomarker and lipid mentioned above are listed in Table S3.
Survival curve analysis
The Kaplan–Meier method was used to estimate overall survival by log-rank test according to data from the Cancer Genome Atlas (TCGA). The Kaplan–Meier curves were drawn by using the GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA).
Ten-fold cross validation
To avoid over-fitting, a 10-fold cross validation was performed. For 10-fold cross validation, the samples were randomly divided into two parts: one for training and anther for testing. This process was repeated for 10 times. The program was run by using Pathon 3.8. The code is provided on the public code-sharing website scikit-learn (https://scikit-learn.org/stable/index.html). The cross-validated ROC curves were indicated in Fig. S5.
Statistical analysis
The Mann–Whitney U test was performed to compare the differences between groups using continuous-variable and nonparametric analyses in GraphPad Prism Windows (version 5). Association between CPT1A levels and clinicopathological characteristics were estimated by a Chi-square test. The differences of CPT1A level between pre-surgery and post-surgery serum samples were analyzed by Mann–Whitney U test in GraphPad Prism 5 software. ROC curves were generated to access the sensitivity, specificity and AUCs with a 95% CI of CPT1A or tumor markers in distinguishing breast cancer patients from controls. To evaluate the diagnostic efficiency of CPT1A alone or CPT1A in combination with CA15–3, CEA, and CA125, formulas were obtained in training set by binary logistic regression analysis. The regression formulas are provided in the Table S2. Then ROC curve analysis and binary logistic regression analysis were conducted in the test set by using the formulas to validate the effectiveness of the models. ROC curve analysis and logistic regression analysis were performed using SPSS windows (version 19.0). All p-values are two-sided, and p < 0.05 is considered to be statistically significant.
Results
CPT1A levels in breast cancer tissues, cell lines and culture medium
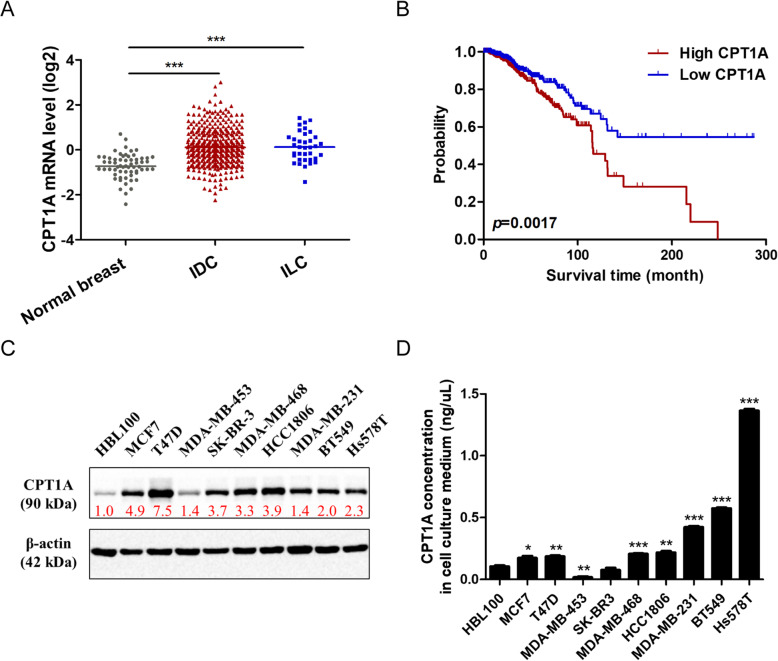
Our previous study found that CPT1A binds to several vesicular trafficking proteins, and CPT1A was indeed identified in exosomes of breast cancer cell line in another study [22, 30]. Then we moved to investigate CPT1A levels in breast cancer tissues, cell lines and cell culture medium. We found that CPT1A is overexpressed in breast cancer tissues compared with normal breast tissues. High level of CPT1A leads to a poor outcome of breast cancer patients according to the TCGA database (Fig. 1a, b). CPT1A expression levels are also elevated in breast cancer cell lines compared with immortalized mammary epithelial cells (Fig. 1c, Fig. S1). Then we analyzed the protein level of CPT1A in cell culture medium of a panel of breast cancer cell lines. We found that CPT1A levels in cell culture medium are increased in most breast cancer cell lines, especially in TNBC cell lines, compared with immortalized mammary epithelial cells (Fig. 1d). These results show that CPT1A is overexpressed in breast cancer and could be secreted extracellularly by breast cancer cells, which might be possible to act as a biomarker for breast cancer.
Fig. 1.
CPT1A levels in breast cancer tissues, cell lines and culture medium. a CPT1A mRNA levels in normal breast tissues (n = 61), invasive ductal breast cancer (IDC) tissues (n = 389) and invasive lobular breast cancer (ILC) tissues (n = 36) according to data from the Cancer Genome Atlas (TCGA) database. b Overall survival rates of breast cancer patients with low (n = 481) or high (n = 481) expression levels of CPT1A were estimated with the Kaplan–Meier method by log-rank test according to data from TCGA database. The Kaplan–Meier curves were drawn by using the GraphPad Prism 5 software. c CPT1A protein levels in immortalized breast epithelial cell line and breast cancer cell lines. β-Actin was used as a control to confirm equal loading of protein. Greyscale values of each protein bands were calculated. The relative fold change of CPT1A band relative to β-Actin band were indicated below CPT1A bands. Corresponding uncropped full-length blots are presented in Supplementary Fig. 1. The full-length blots were cropped between 55 KDa and 72 KDa to separate CPT1A and β-actin protein. d CPT1A protein levels in cell culture medium of indicated cell lines above were detected by an ELISA assay after 72 h incubation. *p < 0.05, **p < 0.001, ***p < 0.0001
CPT1A serum levels in patients with breast cancer, benign breast disease and healthy controls
The enrollment and inclusion of participants is shown in Methods and Fig. S2. All the participants were randomly divided into a training set and a test set with a 7:3 ratio. The training set includes 301 breast cancer patients, 280 healthy controls and 140 patients with benign breast disease. The test set includes 129 breast cancer patients, 120 healthy controls and 60 patients with benign breast disease. The characteristics of breast cancer patients are shown in Table 1. The training and test set populations were comparable with respect to most of the clinical parameters.
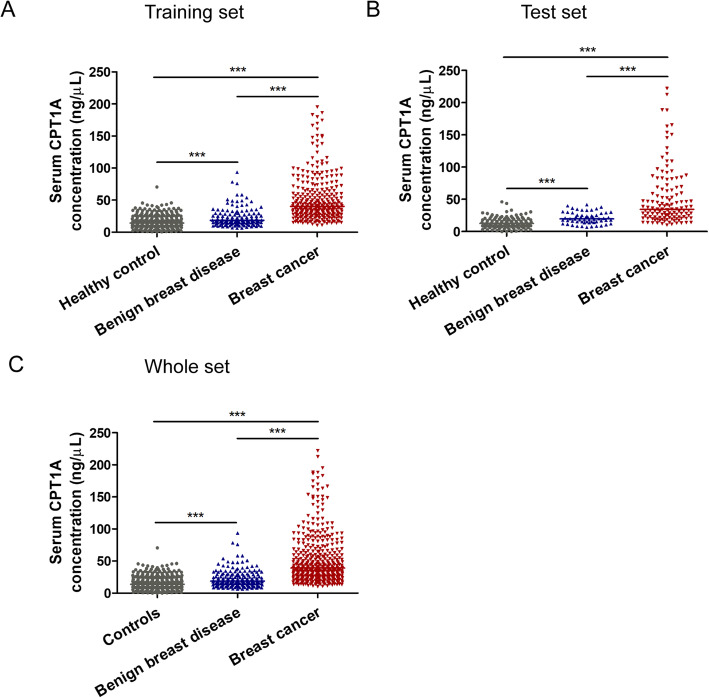
We analyzed the serum CPT1A levels (median ± IQR; IQR, interquartile range) in each participant using an ELISA assay. In the training set, the median serum CPT1A level in breast cancer patients was 40.22 ± 35.12 ng/mL, which was significantly higher than that of patients with benign breast disease (18.44 ± 14.61 ng/mL; p < 0.0001) or healthy controls (14.37 ± 14.56 ng/mL; Fig. 2a, p < 0.0001). In the test set, the median CPT1A level in breast cancer patients was 34.56 ± 42.31 ng/mL, which was notably higher than that of patients with benign breast disease (19.64 ± 12.33 ng/mL; p < 0.0001), or healthy controls (12.53 ± 11.43 ng/mL; Fig. 2b, p < 0.0001). In the whole set, the median CPT1A levels in serum from breast cancer patients was 38.99 ± 36.63 ng/mL, which was significantly higher than the levels observed in patients with benign breast disease (18.63 ± 13.91 ng/mL; p < 0.0001) or healthy controls (13.79 ± 11.97 ng/mL; Fig. 2c, p < 0.0001).
Fig. 2.
The serum CPT1A levels from healthy controls, patients with benign breast diseases and breast cancer. a Serum CPT1A levels in the training set. b Serum CPT1A levels in the test set. c Serum CPT1A levels in the whole set. The horizontal lines indicated median values. Statistical significance was determined by the Mann–Whitney U test. **p < 0.001, ***p < 0.0001
The relationship between serum CPT1A level and the clinicopathological characteristics of breast cancer patients
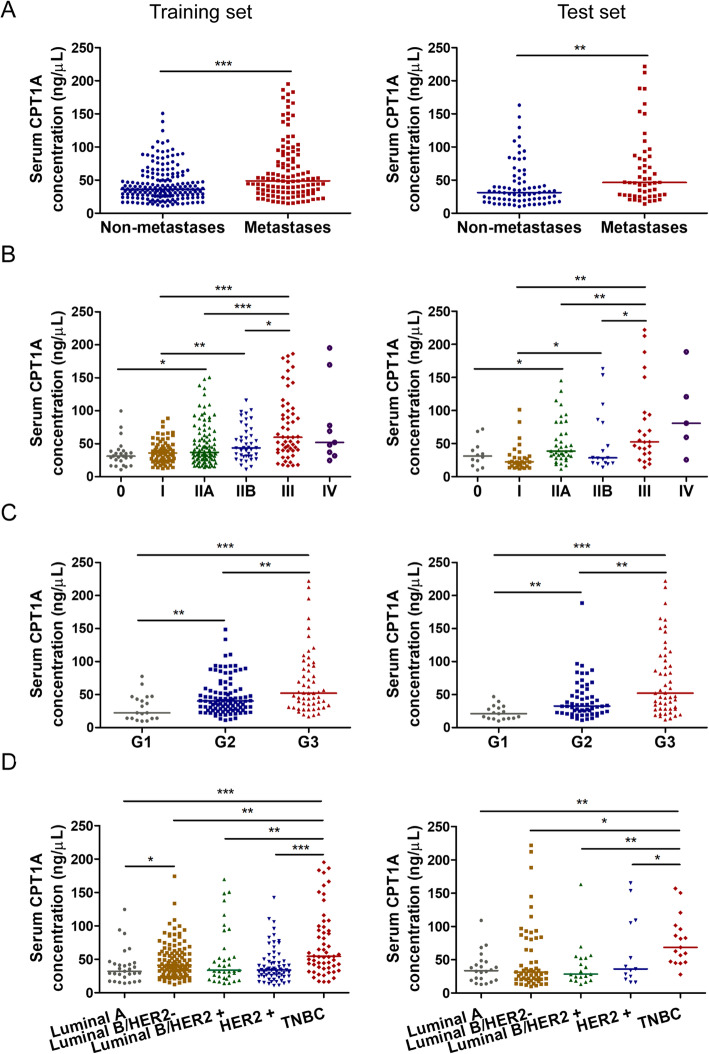
To evaluate the clinical significance of serum CPT1A, correlation between serum CPT1A level and clinical parameters 0f breast cancer patients were estimated by a Pearson’s Chi-square test (Table 1). The threshold value used to classify breast cancer patients into the high and low groups is the median value of CPT1A level in each set. We found that CPT1A level is significantly associated with lymph node status, tumor size, TNM stage, histological grading, human epidermal growth factor receptor 2 (HER2) status and molecular subtype in both the training and test set (Table 1).
The results also showed that the median value of CPT1A level in patients with lymph node or distant metastasis (training set: 48.77 ± 53.41 ng/mL; test set: 46.26 ± 57.74 ng/mL) was significantly higher than in patients without metastasis (training set: 36.23 ± 28.87 ng/mL, p < 0.0001; test set, 31.15 ± 27.80 ng/mL, p < 0.001; Fig. 3a). Relative to in situ cancer cases (training set: 31.17 ± 18.35 ng/mL; test set: 31.20 ± 25.37 ng/mL), invasive ductal carcinoma cases exhibited higher CPT1A levels (training set: 41.21 ± 36.66 ng/mL, p < 0.001; test set: 37.90 ± 42.91 ng/mL, p < 0.001; Fig. S3). Patients with advanced TNM stage III exhibited higher CPT1A serum levels (training set: 59.89 ± 63.82 ng/mL; test set: 52.68 ± 59.77 ng/mL), compared with patients with stage I (training set: 36.12 ± 24.34 ng/mL, p < 0.0001; test set: 22.46 ± 15.23 ng/mL, p < 0.001), II A (training set: 36.98 ± 35.70 ng/mL, p < 0.0001; test set: 38.61 ± 34.50 ng/mL, p < 0.001), or II B (training set: 43.73 ± 38.10 ng/mL, p < 0.05; test set: 28.41 ± 63.19 ng/mL, p < 0.05; Fig. 3b). We also found that serum CPT1A levels were remarkably higher in histological grade 3 (G3) cases (training set: 45.86 ± 51.96 ng/mL; test set: 52.16 ± 80.69 ng/mL) than in grade 1 (G1) cases (training set: 32.12 ± 29.60 ng/mL, p < 0.0001; test set: 21.17 ± 17.09 ng/mL, p < 0.0001) and grade 2 (G2) cases (training set: 40.22 ± 28.55 ng/mL, p < 0.001; test set: 32.69 ± 30.70 ng/mL, p < 0.001; Fig. 3c). Moreover, significant elevation of CPT1A levels was observed in triple-negative breast cancer patients (training set: 54.55 ± 58.06 ng/mL; test set: 59.56 ± 48.23 ng/mL) relative to luminal A (training set: 32.12 ± 23.64 ng/mL, p < 0.0001; test set: 33.66 ± 26.12 ng/mL, p < 0.001), luminal B/HER2− (training set: 41.09 ± 31.59 ng/mL, p < 0.001; test set: 31.34 ± 61.74 ng/mL, p < 0.05), luminal B/HER2+ (training set: 33.73 ± 36.63 ng/mL, p < 0.001; test set: 28.43 ± 33.27 ng/mL, p < 0.001), and HER2+ patients (training set: 34.15 ± 22.81 ng/mL, p < 0.0001; test set: 39.94 ± 100.39 ng/mL, p < 0.05; Fig. 3d).
Fig. 3.
Serum CPT1A level in different clinicopathological classifications of breast cancer. a Serum CPT1A levels from non-metastatic and metastatic breast cancer patients. b Serum CPT1A levels from breast cancer patients with different TNM stage. c Serum CPT1A levels from breast cancer patients with different histological grade. d Serum CPT1A levels from breast cancer patients of different molecular subtype. Statistical significance was were determined by the Mann–Whitney U test. *p < 0.05, **p < 0.001, ***p < 0.0001
In addition, we did not find any correlation between serum CPT1A level and age, menopausal status, or ki-67 level in either training or test sets (Table 1). As CPT1A is a key regulator in lipid metabolism, we analyzed the correlation between serum levels of CPT1A and lipids (TG, TC, HDL-C, LDL-C, and NEFA) in the diagnosis of breast cancer. Spearman correlation analysis indicated no correlation between CPT1A and TG, TC, HDL-C, LDL-C, or NEFA level respectively (Fig. S4). Moreover, univariate logistic regression analysis also revealed that CPT1A was an effective diagnostic factor independently of the lipids in differentiating breast cancer patients from healthy controls (Table S1).
Construction and validation of diagnostic models for breast cancer
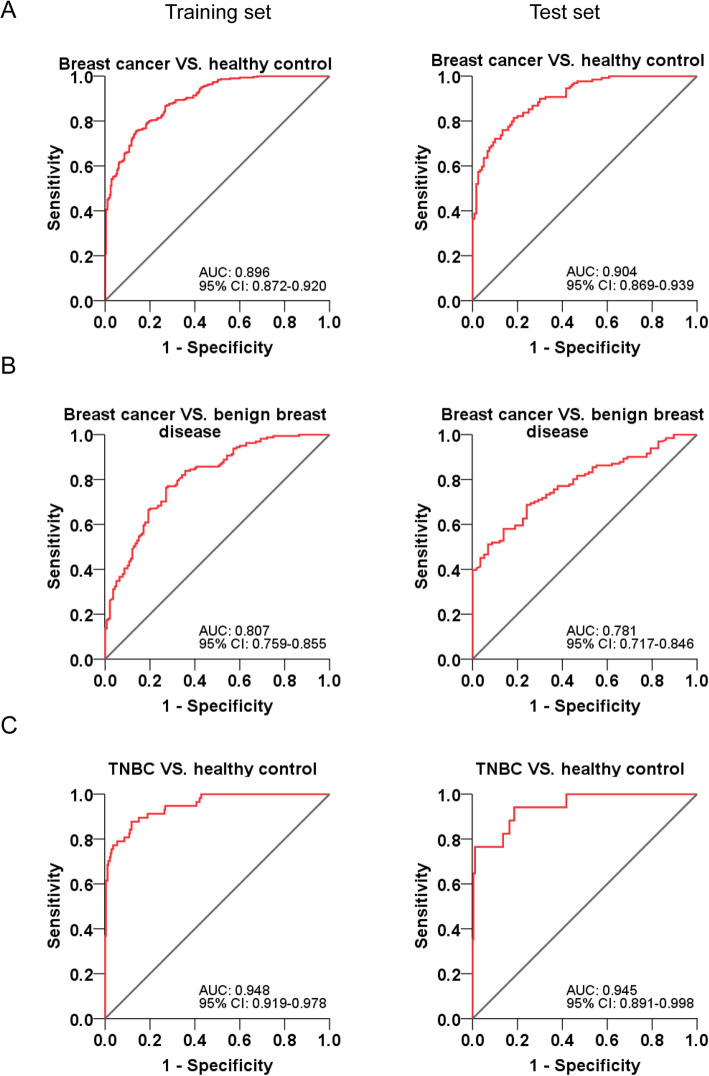
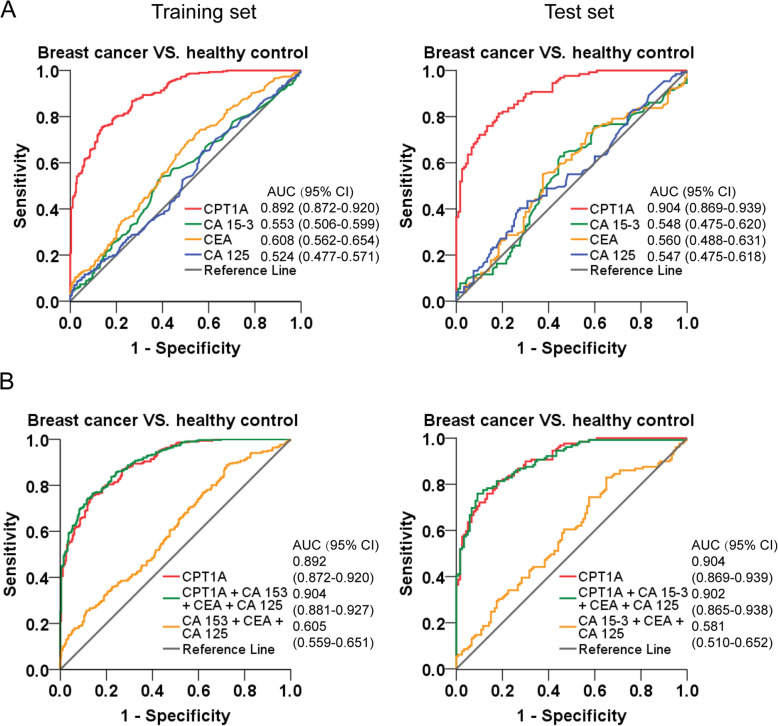
ROC curves based on the ELISA results in the training set and test set were plotted to determine the diagnostic efficiency of serum CPT1A in breast cancer (Fig. 4, Table 2). In the training set, the AUC of CPT1A in differentiating breast cancer patients from healthy controls was 0.892 (Fig. 4a, left; 95% CI, 0.872–0.920), and the optimum CPT1A cutoff value was 27.57 ng/mL for breast cancer diagnosis (training set: sensitivity, 75.4%; specificity, 86.1%). We also found that CPT1A exhibited good efficacy in differentiating patients with breast cancer from patients with benign breast disease (Fig. 4b, left; AUC, 0.807; 95% CI, 0.759–0.855). Moreover, CPT1A displayed an extremely high discriminatory capacity for differentiating TNBC patients from healthy controls (Fig. 4c, left; AUC, 0.948; 95% CI, 0.919–0.978).
Fig. 4.
ROC curve analyses using CPT1A to differentiate breast cancer from benign breast disease or healthy controls. a ROC curves of CPT1A for breast cancer patients versus healthy controls in the training set (left) and test set (right), respectively. b ROC curves of CPT1A for breast cancer patients versus benign breast cancer patients in the training set (left) and test set (right), respectively. c ROC curves of CPT1A for TNBC patients versus healthy controls in the training set (left) and test set (right), respectively
Table 2.
The diagnostic efficiency of models in differentiating breast cancer and controls
| Training set | Test set | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) |
SN (%) | SP (%) | PPV (%) | NPV (%) | +LR | -LR | AUC (95% CI) |
SN (%) | SP (%) | PPV (%) | NPV (%) | +LR | -LR | |
| Breast cancer VS. Controls | ||||||||||||||
| CPT1Aa | 0.892 (0.872–0.920) | 75.4 | 86.1 | 81.8 | 77.1 | 5.42 | 0.29 | 0.904 (0.869–0.939) | 81.4 | 81.7 | 84.2 | 76.0 | 4.45 | 0.23 |
| CA153 | 0.553 (0.506–0.599) | 52.8 | 61.4 | 56.1 | 56.8 | 1.37 | 0.77 | 0.548 (0.475–0.620) | 62.8 | 55.0 | 41.7 | 68.2 | 1.40 | 0.68 |
| CEA | 0.608 (0.562–0.654) | 69.1 | 49.3 | 62.5 | 51.2 | 1.36 | 0.63 | 0.560 (0.488–0.631) | 55.0 | 62.5 | 50.0 | 63.6 | 1.47 | 0.72 |
| CA125 | 0.524 (0.477–0.571) | 65.1 | 42.5 | 32.1 | 73.4 | 1.13 | 0.82 | 0.547 (0.475–0.618) | 39.5 | 73.3 | 45.0 | 56.6 | 1.48 | 0.83 |
| CA153 + CEA + CA125 | 0.605 (0.559–0.651) | 88.7 | 27.9 | 60.4 | 48.2 | 1.23 | 0.41 | 0.581 (0.510–0.652) | 82.9 | 35.0 | 54.2 | 58.9 | 1.28 | 0.49 |
| CPT1A + CA153 + CEA + CA125 | 0.904 (0.881–0.927) | 76.4 | 86.1 | 83.9 | 77.1 | 5.50 | 0.27 | 0.902 (0.865–0.938) | 76.0 | 90.8 | 88.3 | 77.5 | 8.26 | 0.26 |
| Breast cancer VS. Benign breast disease | ||||||||||||||
| CPT1A | 0.807 (0.759–0.855) | 74.6 | 72.9 | 75.7 | 68.3 | 2.75 | 0.35 | 0.781 (0.717–0.846) | 68.7 | 75.9 | 44.8 | 85.5 | 2.85 | 0.41 |
| TNBC VS. Controls | ||||||||||||||
| CPT1A | 0.948 (0.919–0.978) | 87.7 | 88.2 | 98.2 | 70.2 | 7.43 | 0.14 | 0.945 (0.891–0.998) | 94.1 | 81.4 | 99.6 | 52.9 | 5.06 | 0.07 |
Abbreviations: +LR positive likelihood ratio, −LR negative likelihood ratio, PPV positive predictive value, NPV negative predictive value
aThe diagnostic cut-off value was 26.08 ng/mL
According to the binary logistic regression analysis in the training set, diagnostic models of CPT1A for breast cancer diagnosis were constructed. The formulas were list in Table S2. Then the validation of the logistic regression models was assessed in the test set. As a result, in the test set, the AUC of CPT1A in differentiating breast cancer patients from healthy controls was 0.904 (Fig. 4a, right; 95% CI, 0.869–0.939). Additionally, CPT1A was also effective in differentiating patients with breast cancer from patients with benign breast disease (Fig. 4b, right; AUC, 0.781; 95% CI, 0.717–0.846), and in differentiating TNBC patients from healthy controls (Fig. 4c, right; AUC, 0.945; 95% CI, 0.891–0.998), in the test set. These results show that CPT1A satisfactorily discriminates breast cancer patients from healthy controls or patients with benign breast disease. Moreover, it is exhibited a high discriminatory capacity for differentiating TNBC patients from healthy controls.
To better understand the potential use of serum CPT1A as a clinical biomarker, we further compared the predictive value and likelihood ratios of CPT1A with three conventional tumor markers, CA15–3, CEA, and CA125, in discrimination of breast cancer patients from healthy controls. Diagnostic models of each marker and combining panels were constructed by binary logistic regression analysis based on the data in the training set. To evaluate the prediction performance of the models, 10-fold cross validation was performed. Among the four biomarkers, CPT1A (training set: AUC, 0.892; 95% CI, 0.872–0.920, Fig. 5a, left; cross-validated AUC, 0.909; 95% CI, 0.874–0.945, Fig. S5A) displayed a significantly higher AUC in differentiating breast cancer patients from healthy controls than did CA15–3 (training set: AUC, 0.553; 95% CI, 0.506–0.599, Fig. 5a, left; cross-validated AUC, 0.557; 95% CI, 0.503–0.611, Fig. S5B), CEA (training set: AUC, 0.608; 95% CI, 0.562–0.654, Fig. 5a, left; cross-validated AUC, 0.605; 95% CI, 0.529–0.680, Fig. S5C), CA 125 (training set: AUC, 0.524; 95% CI, 0.447–0.571, Fig. 5a, left; cross-validated AUC, 0.525; 95% CI, 0.469–0.582, Fig. S5D), and the combination of CA 15–3, CEA and CA 125 (training set: AUC, 0.605; 95% CI, 0.559–0.651, Fig. 5b, left; cross-validated AUC, 0.563; 95% CI, 0.511–0.617, Fig. S5E). However, the combination of CPT1A, CA15–3, CEA, and CA125 (training set: AUC, 0.904; 95% CI, 0.881–0.927, Fig. 5b, left; cross-validated AUC, 0.914; 95% CI, 0.880–0.948; Fig. S5F) did not significantly improve the classification capacity and diagnostic efficacy of breast cancer patients than CPT1A alone.
Fig. 5.
The ROC curve analyses using CPT1A, CA15–3, CEA and CA125 for the differentiation of breast cancer cases and healthy controls. a ROC curves of CPT1A, CA15–3, CEA and CA125 for breast cancer cases versus healthy controls in the training set (left) and test set (right), respectively. b ROC curves of CPT1A and a model which combined the analysis of CPT1A, CA15–3, CEA and CA125 for breast cancer cases versus healthy controls in the training set (left) and test set (right), respectively
In addition, the 10-fold cross-validated AUC values showed relatively modest difference from the AUC values we obtained from the established models based on the training set, which confirms that there is no over fitting and the models are reliable. Moreover, the models showed consistent results between training and test set. CPT1A (test set: AUC, 0.904; 95% CI, 0.869–0.939, Fig. 5a, right), and the combination of CPT1A, CA 153, CEA and CA 125 (test set: AUC, 0.902; 95% CI, 0.865–0.938, Fig. 5b, right) displayed a significantly higher AUC in differentiating breast cancer patients from healthy controls than did CA15–3 (test set: AUC, 0.548; 95% CI, 0.475–0.620, Fig. 5a, right), CEA (test set: AUC, 0.560; 95% CI, 0.488–0.618, Fig. 5a, right), CA125 (test set: AUC, 0.547; 95% CI, 0.475–0.618, Fig. 5a, right), and the combination of CPT1A, CA15–3, CEA, and CA125 (test set: AUC, 0.581; 95% CI, 0.510–0.652, Fig. 5b, right).
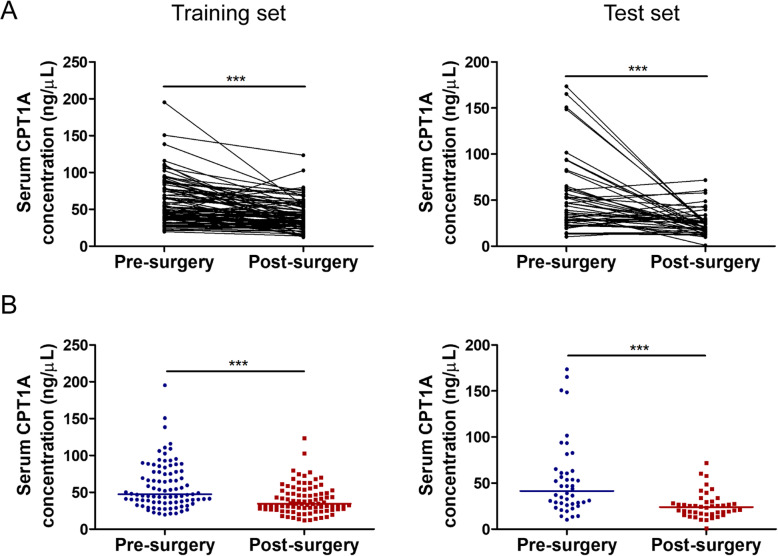
Comparison of CPT1A levels in paired pre-and post-operative breast cancer serum samples
Surgery is the primary treatment for breast cancer patients. Thus, we also collected post-surgery breast cancer serum samples. In the training set, there are 126 paired pre-and post-surgery breast cancer serum samples. The median level of CPT1A in serum from breast cancer patients after surgery was 32.16 ± 22.54 ng/mL, which was significantly lower than its level before surgery (Fig. 6a, b, left; 45.61 ± 45.89 ng/mL, p < 0.0001). In the test set, there are 44 paired pre-and post-surgery breast cancer serum samples. CPT1A levels (23.95 ± 12.70 ng/mL) consistently decreased in post-surgery serum samples of breast cancer patients compared with paired pre-surgery samples (Fig. 6a, b, right; 41.40 ± 35.02 ng/mL, p < 0.0001). These results indicated that CPT1A levels are positively associated with tumor burden and could serve as an indicator for disease monitoring after tumor resection.
Fig. 6.
Serum CPT1A levels of pre-surgery and post-surgery breast cancer patients. a CPT1A levels in serum from the same breast cancer patient before and after surgery, in the training set (left, n = 126) and test set (right, n = 44), respectively. b Serum CPT1A levels from breast cancer patient before and after surgery, in the training set (left, n = 126) and test set (right, n = 44), respectively. Statistical significance was determined by the Mann–Whitney U test. ***p < 0.0001
Discussion
Early diagnosis has significant implications for the treatment of breast cancer patients and is associated with favorable prognosis. Although mammography is the most commonly used screening method for breast cancer, it has limitations, including false-positive results and overdiagnosis. Currently, serum tumor markers such as CA15–3, CEA, and CA125 have been developed as non-invasive tools for the detection and prediction of outcomes in breast cancer [16, 35, 36]. However, less than 50% of breast cancer patients are detected with the elevation of serum CA15–3 and CEA levels, and CA125 is used mainly for detection of ovarian cancer, which is less specific in other cancer types [37, 38]. An ideal serologic marker for breast cancer is expected to serve as a diagnostic and prognostic tool with high sensitivity and specificity.
In the present study, we investigated the potential of CPT1A to serve as a diagnostic biomarker for breast cancer. Recently CPT1A has been considered as a key regulator of cancer metabolism reprogramming [39]. It was reported to be upregulated and associated with poor prognosis in several cancers, including breast cancer [20, 25, 39, 40]. According to previous studies, CPT1A amplification was found in 20% of ER-positive breast cancer cases, and the CPT1A protein level is elevated in most breast cancer cell lines [41]. CPT1A is regulated by c-MYC or AMPK in breast cancer, and promotes breast cancer metastasis or therapeutic resistance through several oncogenic signaling pathways, such as VEGF, ERK and Src pathways [25–27, 29, 42].
Although CPT1A is a mitochondrial protein, it was identified in exosomes of breast cancer cell lines in a study exploring the proteomic profile of extracellular vesicles derived from 60 cell lines of the National Cancer Institute (NCI-60) [30]. In our study, we found that CPT1A is overexpressed in breast cancer and can be detected in the cell culture medium. However, the expression patterns of intracellular and secreted CPT1A are not consistent among a panel of breast cancer cell lines. The TNBC cell lines have the highest CPT1A levels in cell culture medium, but not in cytoplasm. As we known, the generation and transport of extracellular vesicles are complex processes, which need the collaboration of sorting machineries, membrane-trafficking processes and vesicle-associated proteins [43]. Our previous study showed that CPT1A binds to several vesicular trafficking proteins in therapeutic resistant cancer cell lines, but not in therapeutic responsive cancer cell lines [22], which indicated that the vesicle-associated proteins and their interaction with CPT1A could also contribute to the extracellular level of CPT1A. Thus, the vesicular trafficking proteins in TNBC cells may promote the secretion of CPT1A. Above all, these different lines of evidence led to our investigation of CPT1A as a possible serum biomarker for breast cancer.
In this study, we found that the serum CPT1A level was significantly elevated in 430 breast cancer patients compared with either 200 patients with benign breast disease or 400 healthy controls. Several benign breast diseases, such as atypical hyperplasia, are regarded as premalignant lesions [44]. Compared with healthy controls, serum CPT1A level was elevated in patients with benign breast diseases, which indicated that CPT1A might play a significant role in the tumorigenesis and early development of breast cancer. These results further suggest that serum CPT1A could be used as a biomarker in the screening and early detection of breast cancer.
In addition, we found that serum CPT1A levels were positively correlated with metastasis, advanced TNM stage and histological grade, and may enable discrimination between different breast cancer molecular subtypes. Tumor heterogeneity has led to breast cancer classification into four intrinsic subtypes, luminal A, luminal B, HER2-enriched, and basal-like breast cancer, and these are closely related to the development and prognosis of breast cancer [45–48]. The basal-like subtype, also known as TNBC, is characterized by a lack of estrogen receptor, progesterone receptor, and HER2 expression. It comprises 10–15% of all breast cancers and leads to worse clinical outcomes than other subtypes [49–51]. According to our study, patients with TNBC exhibited significantly higher serum CPT1A levels than those with other subtypes, and CPT1A exhibited significantly high efficacy in differentiating TNBC patients from healthy controls. These results indicate that CPT1A might be a useful diagnostic and serum biomarker for breast cancer, especially for TNBC cases.
To determine the diagnostic efficacy of CPT1A, we performed ROC curve analysis and compared the AUC, sensitivity, and specificity of CPT1A and three conventional clinically used biomarkers, CA15–3, CEA, and CA125, in breast cancer samples and healthy subjects. Analysis showed that serum CPT1A displayed a higher AUC in differentiating breast cancer patients from healthy controls, with a higher and more balanced sensitivity and specificity than the other three tumor markers. Our study has identified a novel biomarker that provides a remarkable improvement in distinguishing breast cancer patients from healthy controls.
By performing a comparison of CPT1A levels in 170 paired serum samples obtained before and after surgery, we found that serum CPT1A levels decreased in breast cancer patients after surgical removal of the tumor. Thus, the serum CPT1A level was found to be an effective indicator for evaluating the surgical outcome or tumor recurrence of breast cancer patients.
Although abnormal lipid turnover has been accepted as a vital mechanism in breast cancer progress, studies investigating lipids, including TG, TC, and HDL-C, in relation to breast cancer risk have shown conflicting results [52, 53]. In our study, univariate logistic regression analysis showed that none of the lipids TG, TC, HDL-C, LDL-C, or NEFA was an effective diagnostic indicator in breast cancer patients overall, and serum CPT1A showed significant effectiveness in breast cancer diagnosis independently of these lipids.
In our study, all the participants originated from China. Although breast tumors in Asian and Caucasian women were reported to share similar molecular and genetic characteristics [54], further studies in different populations are needed. Although the CPT1A levels in breast cancer patients and healthy controls were significantly different in our study, some overlap was still observed in patients and controls (Fig. 2). Nevertheless, CPT1A could be used as an adjunct biomarker for breast cancer diagnosis. It is also necessary to determine whether serum CPT1A is a specific biomarker of breast cancer only or is also an accurate biomarker for other cancer types.
Conclusions
Our large-scale study provided initial data about the clinically diagnostic relevance of CPT1A as a serum marker for breast cancer in both a training set and a test set. Our study showed that CPT1A could be released into cell culture medium by breast cancer cells, and serum CPT1A could be employed to distinguish breast cancer patients from healthy controls and assist in the diagnosis of breast cancer. It is positively associated with tumor burden and could serve as an indicator for disease monitoring after tumor resection. Serum CPT1A displayed a remarkably high diagnostic efficiency for breast cancer and could be a novel biomarker for the diagnosis of breast cancer.
Supplementary Information
Additional file 1: Table S1. Univariate logistic regression analysis of serum CPT1A and lipids in differentiating breast cancer and controls. Table S2. Formulas of logistic regression models built based on the data in the training set. Table S3. The reference ranges of serum tumor markers and lipids. Figure S1. Merged original blot showing CPT1A and β-Actin bands with protein markers in a panel of non-malignant cell lines and breast cancer cell lines. β-Actin was used as a control to confirm equal loading of protein. Figure S2. Subject inclusion and study profile. Figure S3. Serum CPT1A levels in different pathologic types of breast cancer. Serum CPT1A levels of breast cancer patients with cancer in situ, invasive ductal carcinoma, invasive lobular carcinoma, invasive papillary carcinoma, mucinous carcinoma and other types, in the training set (left) and test set (right), respectively. Statistical significance was determined by the Mann–Whitney U test. **p < 0.001. Figure S4. The correlation of CPT1A levels and lipids concentrations in serum of breast cancer patients. Co-expression analysis of CPT1A versus TG (A), TC (B), HDL-C (C), LDL-C (D) and NEFA (E) levels in serum of breast cancer patients in the training set (left) and test set (right), respectively. The Spearman’s correlation coefficient was calculated using the GraphPad software program. Figure S5. The ROC curve analyses of CPT1A, CA15–3, CEA and CA125 in the differentiation of breast cancer cases from healthy controls by using 10-fold cross validation in training set. 10-fold cross validated ROC curves of CPT1A (A), CA15–3 (B), CEA (C), CA125 (D), the combination of CA 15–3, CEA and CA 125 (E), and the combination of the four markers (F).
Acknowledgments
The authors thank Dr. Zhixiong Xia for his support in pathology.
Abbreviations
- AMPK
Adenosine 5′-monophosphate-activated protein kinase
- CA15–3
Cancer antigen 15–3
- CEA
Carcinoembryonic antigen
- CPT1A
Carnitine palmitoyl transferase 1A
- AUC
Area under the curve
- ERK
Extracellular regulated protein kinase
- EVs
Extracellular vesicles
- FAO
Fatty acid oxidation
- HER2
Human epidermal growth factor receptor 2
- HDL-C
High-density lipoprotein-cholesterol
- IQR
Interquartile range
- LDL-C
Low-density lipoprotein-cholesterol
- NEFA
Non-esterified fatty acids
- NPV
Negative predictive value
- PPV
Interquartile range
- ROC
Receiver operating characteristic
- TC
Total cholesterol
- TNBC
Triple-negative breast cancer
- TNM
Tumor node metastasis
- TG
Triglyceride
- -LR
Negative likelihood ratio
- +LR
Positive likelihood ratio
Authors’ contributions
ZT and YZ designed the project, interpreted data and wrote the manuscript. ZT, YZ, CZ and AX collected samples. ZT, MZ, ZL and TW performed the statistical analyses. HW, SF and SL contributed to helpful discussion. YL and ZL revised the manuscript. All authors reviewed and approved the manuscript.
Funding
This research was funded by National Science Foundation of China (81802653), Research Fund of Hubei Province Health Bureau (WJ2015MB144) and Research Fund of Wuhan Public Health Bureau (WX15A12 and WX18Y11). The funders had no role in the design of the study, data collection, analysis, interpretation of data, or in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki of 1975, revised in 2013. The protocol was approved by the ethics committee of the Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology (2017IECS062). All individual participants in the study provided written informed consent. No cell lines required ethics approval for their use in the study.
Consent for publication
No applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zheqiong Tan and Yaru Zou contributed equally to this work.
Contributor Information
Zheqiong Tan, Email: ztan9@jhmi.edu.
Zhongxin Lu, Email: lzx71@yahoo.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. 10.3322/caac.21338. [DOI] [PubMed]
- 3.Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–89. 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 5.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310(4):389–97. 10.1001/jama.2013.8272. [DOI] [PubMed]
- 6.Waks AG, Winer EP. Breast Cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 7.Peairs KS, Choi Y, Stewart RW, Sateia HF. Screening for breast cancer. Semin Oncol. 2017;44(1):60–72. doi: 10.1053/j.seminoncol.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Gourd E. Mammography deficiencies: the result of poor positioning. Lancet Oncol. 2018;19(8):e385. doi: 10.1016/S1470-2045(18)30489-3. [DOI] [PubMed] [Google Scholar]
- 9.Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-Cancer tumor size, Overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438–1447. doi: 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
- 10.Loberg M, Lousdal ML, Bretthauer M, Kalager M. Benefits and harms of mammography screening. Breast Cancer Res. 2015;17(1):63. doi: 10.1186/s13058-015-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakha EA, Green AR. Molecular classification of breast cancer: what the pathologist needs to know. Pathology. 2017;49(2):111–119. doi: 10.1016/j.pathol.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Rakha EA, Pareja FG. New Advances in Molecular Breast Cancer Pathology. Semin Cancer Biol. 2020;S1044-579X(20):30080–8. 10.1016/j.semcancer.2020.03.014. [DOI] [PubMed]
- 13.Hanash SM, Baik CS, Kallioniemi O. Emerging molecular biomarkers--blood-based strategies to detect and monitor cancer. Nat Rev Clin Oncol. 2011;8(3):142–150. doi: 10.1038/nrclinonc.2010.220. [DOI] [PubMed] [Google Scholar]
- 14.Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54(12):e11–79. 10.1373/clinchem.2008.105601. [DOI] [PubMed]
- 15.Norum LF, Erikstein B, Nustad K. Elevated CA125 in breast cancer--a sign of advanced disease. Tumour Biol. 2001;22(4):223–228. doi: 10.1159/000050620. [DOI] [PubMed] [Google Scholar]
- 16.Fang C, Cao Y, Liu X, Zeng XT, Li Y. Serum CA125 is a predictive marker for breast cancer outcomes and correlates with molecular subtypes. Oncotarget. 2017;8(38):63963–63970. doi: 10.18632/oncotarget.19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16(1):76. 10.1186/s12943-017-0646-3. [DOI] [PMC free article] [PubMed]
- 18.Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M, et al. Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell Stem Cell. 2016;19(1):23–37. 10.1016/j.stem.2016.06.001. [DOI] [PMC free article] [PubMed]
- 19.Zaugg K, Yao Y, Reilly PT, Kannan K, Kiarash R, Mason J, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25(10):1041–51. 10.1101/gad.1987211. [DOI] [PMC free article] [PubMed]
- 20.Carracedo A, Weiss D, Leliaert AK, Bhasin M, de Boer VC, Laurent G, et al. A metabolic prosurvival role for PML in breast cancer. J Clin Invest. 2012;122(9):3088–3100. doi: 10.1172/JCI62129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlaepfer IR, Joshi M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology. 2020;161(2). 10.1210/endocr/bqz046. [DOI] [PubMed]
- 22.Tan Z, Xiao L, Tang M, Bai F, Li J, Li L, et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics. 2018;8(9):2329–47. 10.7150/thno.21451. [DOI] [PMC free article] [PubMed]
- 23.Du Q, Tan Z, Shi F, Tang M, Xie L, Zhao L, et al. PGC1alpha/CEBPB/CPT1A axis promotes radiation resistance of nasopharyngeal carcinoma through activating fatty acid oxidation. Cancer Sci. 2019;110(6):2050–2062. doi: 10.1111/cas.14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pucci S, Zonetti MJ, Fisco T, Polidoro C, Bocchinfuso G, Palleschi A, et al. Carnitine palmitoyl transferase-1A (CPT1A): a new tumor specific target in human breast cancer. Oncotarget. 2016;7(15):19982–96. 10.18632/oncotarget.6964. [DOI] [PMC free article] [PubMed]
- 25.Park JH, Vithayathil S, Kumar S, Sung PL, Dobrolecki LE, Putluri V, et al. Fatty acid oxidation-driven Src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast Cancer. Cell Rep. 2016;14(9):2154–65. 10.1016/j.celrep.2016.02.004. [DOI] [PMC free article] [PubMed]
- 26.Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. 2016;22(4):427–32. 10.1038/nm.4055. [DOI] [PMC free article] [PubMed]
- 27.Han S, Wei R, Zhang X, Jiang N, Fan M, Huang JH, et al. CPT1A/2-mediated FAO enhancement-a metabolic target in Radioresistant breast Cancer. Front Oncol. 2019;9:1201. 10.3389/fonc.2019.01201. [DOI] [PMC free article] [PubMed]
- 28.Aiderus A, Black MA, Dunbier AK. Fatty acid oxidation is associated with proliferation and prognosis in breast and other cancers. BMC Cancer. 2018;18(1):805. doi: 10.1186/s12885-018-4626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong Y, Liu Z, Zhao X, Ruan S, Zhang X, Wang S, et al. CPT1A regulates breast cancer-associated lymphangiogenesis via VEGF signaling. Biomed Pharmacother. 2018;106:1–7. 10.1016/j.biopha.2018.05.112. [DOI] [PubMed]
- 30.Hurwitz SN, Rider MA, Bundy JL, Liu X, Singh RK, Meckes DG Jr. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget. 2016;7(52):86999–7015. 10.18632/oncotarget.13569. [DOI] [PMC free article] [PubMed]
- 31.Clark DJ, Fondrie WE, Liao Z, Hanson PI, Fulton A, Mao L, et al. Redefining the breast Cancer exosome proteome by tandem mass tag quantitative proteomics and multivariate cluster analysis. Anal Chem. 2015;87(20):10462–9. 10.1021/acs.analchem.5b02586. [DOI] [PMC free article] [PubMed]
- 32.Lee K, Kerner J, Hoppel CL. Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J Biol Chem. 2011;286(29):25655–25662. doi: 10.1074/jbc.M111.228692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li T, Li H, Wang A, Su X, Zhao J, Cui Y, et al. Development and validation of a simple model for detection of early hepatocellular carcinoma in a liver cirrhosis cohort. Cancer Manag Res. 2019;11:9379–86. 10.2147/CMAR.S221050. [DOI] [PMC free article] [PubMed]
- 34.Xu L, Huo X, Liu Y, Zhang Y, Qin Q, Xu X. Hearing loss risk and DNA methylation signatures in preschool children following lead and cadmium exposure from an electronic waste recycling area. Chemosphere. 2020;246:125829. doi: 10.1016/j.chemosphere.2020.125829. [DOI] [PubMed] [Google Scholar]
- 35.Shao Y, Sun X, He Y, Liu C, Liu H. Elevated levels of serum tumor markers CEA and CA15-3 are prognostic parameters for different molecular subtypes of breast Cancer. PLoS One. 2015;10(7):e0133830. doi: 10.1371/journal.pone.0133830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Liu L, Feng Z, Wang X, Huang Y, Dai H, et al. Tumor markers CA15-3, CA125, CEA and breast cancer survival by molecular subtype: a cohort study. Breast Cancer. 2020;27(4):621–30. 10.1007/s12282-020-01058-3. [DOI] [PubMed]
- 37.Lauro S, Trasatti L, Bordin F, Lanzetta G, Bria E, Gelibter A, et al. Comparison of CEA, MCA, CA 15-3 and CA 27-29 in follow-up and monitoring therapeutic response in breast cancer patients. Anticancer Res. 1999;19(4C):3511–5. [PubMed]
- 38.Einhorn N, Sjovall K, Knapp RC, Hall P, Scully RE, Bast RC Jr, et al. Prospective evaluation of serum CA 125 levels for early detection of ovarian cancer. Obstet Gynecol. 1992;80(1):14–8. [PubMed]
- 39.Qu Q, Zeng F, Liu X, Wang QJ, Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 2016;7(5):e2226. doi: 10.1038/cddis.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corbet C, Pinto A, Martherus R, Santiago de Jesus JP, Polet F, Feron O. Acidosis drives the reprogramming of fatty acid metabolism in Cancer cells through changes in mitochondrial and histone acetylation. Cell Metab. 2016;24(2):311–323. doi: 10.1016/j.cmet.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Balaban S, Shearer RF, Lee LS, van Geldermalsen M, Schreuder M, Shtein HC, et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017;5(1):1. 10.1186/s40170-016-0163-7. [DOI] [PMC free article] [PubMed]
- 42.Hampsch RA, Wells JD, Traphagen NA, McCleery CF, Fields JL, Shee K, et al. AMPK activation by metformin promotes survival of dormant ER(+) breast Cancer cells. Clin Cancer Res. 2020;26(14):3707–3719. doi: 10.1158/1078-0432.CCR-20-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 44.Allred DC, Mohsin SK, Fuqua SA. Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer. 2001;8(1):47–61. doi: 10.1677/erc.0.0080047. [DOI] [PubMed] [Google Scholar]
- 45.Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12(7):381–394. doi: 10.1038/nrclinonc.2015.73. [DOI] [PubMed] [Google Scholar]
- 46.Januskeviciene I, Petrikaite V. Heterogeneity of breast cancer: the importance of interaction between different tumor cell populations. Life Sci. 2019;239:117009. doi: 10.1016/j.lfs.2019.117009. [DOI] [PubMed] [Google Scholar]
- 47.Kalinowski L, Saunus JM, McCart Reed AE, Lakhani SR. Breast Cancer heterogeneity in primary and metastatic disease. Adv Exp Med Biol. 2019;1152:75–104. doi: 10.1007/978-3-030-20301-6_6. [DOI] [PubMed] [Google Scholar]
- 48.Odle TG. Precision medicine in breast Cancer. Radiol Technol. 2017;88(4):401M–421M. [PubMed] [Google Scholar]
- 49.Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121(1):8–16. doi: 10.1002/cncr.28914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vagia E, Mahalingam D, Cristofanilli M. The Landscape of Targeted Therapies in TNBC. Cancers (Basel). 2020;12:916. [DOI] [PMC free article] [PubMed]
- 51.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lofterod T, Mortensen ES, Nalwoga H, Wilsgaard T, Frydenberg H, Risberg T, et al. Impact of pre-diagnostic triglycerides and HDL-cholesterol on breast cancer recurrence and survival by breast cancer subtypes. BMC Cancer. 2018;18(1):654. doi: 10.1186/s12885-018-4568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin LJ, Melnichouk O, Huszti E, Connelly PW, Greenberg CV, Minkin S, et al. Serum lipids, lipoproteins, and risk of breast cancer: a nested case-control study using multiple time points. J Natl Cancer Inst. 2015;107(5):djv032. 10.1093/jnci/djv032. [DOI] [PMC free article] [PubMed]
- 54.Yu K, Lee CH, Tan PH, Tan P. Conservation of breast cancer molecular subtypes and transcriptional patterns of tumor progression across distinct ethnic populations. Clin Cancer Res. 2004;10(16):5508–5517. doi: 10.1158/1078-0432.CCR-04-0085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Univariate logistic regression analysis of serum CPT1A and lipids in differentiating breast cancer and controls. Table S2. Formulas of logistic regression models built based on the data in the training set. Table S3. The reference ranges of serum tumor markers and lipids. Figure S1. Merged original blot showing CPT1A and β-Actin bands with protein markers in a panel of non-malignant cell lines and breast cancer cell lines. β-Actin was used as a control to confirm equal loading of protein. Figure S2. Subject inclusion and study profile. Figure S3. Serum CPT1A levels in different pathologic types of breast cancer. Serum CPT1A levels of breast cancer patients with cancer in situ, invasive ductal carcinoma, invasive lobular carcinoma, invasive papillary carcinoma, mucinous carcinoma and other types, in the training set (left) and test set (right), respectively. Statistical significance was determined by the Mann–Whitney U test. **p < 0.001. Figure S4. The correlation of CPT1A levels and lipids concentrations in serum of breast cancer patients. Co-expression analysis of CPT1A versus TG (A), TC (B), HDL-C (C), LDL-C (D) and NEFA (E) levels in serum of breast cancer patients in the training set (left) and test set (right), respectively. The Spearman’s correlation coefficient was calculated using the GraphPad software program. Figure S5. The ROC curve analyses of CPT1A, CA15–3, CEA and CA125 in the differentiation of breast cancer cases from healthy controls by using 10-fold cross validation in training set. 10-fold cross validated ROC curves of CPT1A (A), CA15–3 (B), CEA (C), CA125 (D), the combination of CA 15–3, CEA and CA 125 (E), and the combination of the four markers (F).
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.