Supplemental Digital Content is available in the text
Keywords: angiotensin-converting enzyme, atopy, gastrointestinal, inflammation, severe acute respiratory syndrome coronavirus-2, eosinophilic esophagitis, pediatric
ABSTRACT
Infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) can lead to coronavirus-induced disease 2019 (COVID-19). The gastrointestinal (GI) tract is now an appreciated portal of infection. SARS-CoV-2 enters host cells via angiotensin-converting enzyme-2 (ACE2) and the serine protease TMPRSS2. Eosinophilic gastrointestinal disorders (EGIDs) are inflammatory conditions caused by chronic type 2 (T2) inflammation. the effects of the T2 atopic inflammatory milieu on SARS-COV-2 viral entry gene expression in the GI tract is poorly understood. We analyzed tissue ACE2 and TMPRSS2 gene expression in pediatric eosinophilic esophagitis (EoE), eosinophilic gastritis (EG), and in normal adult esophagi using publicly available RNA-sequencing datasets. Similar to findings evaluating the airway, there was no difference in tissue ACE2/TMPRSS2 expression in EoE or EG when compared with control non-EoE/EG esophagus/stomach. ACE2 gene expression was significantly lower in esophagi from children with or without EoE and from adults with EoE as compared with normal adult esophagi. Type 2 immunity and pediatric age could be protective for infection by SARS-CoV-2 in the gastrointestinal tract because of decreased expression of ACE2.
What Is Known/What Is New
What Is Known
Coronavirus-induced disease 2019 has rapidly spread around the world, infecting millions of people and causing both respiratory and gastrointestinal symptoms.
Angiotensin-converting enzyme 2 and TMPRSS2 are the major portals of entry that allow severe acute respiratory syndrome coronavirus-2 entry at epithelial barriers.
The gastrointestinal tract is a potential nidus of severe acute respiratory syndrome coronavirus-2 infection with high levels of angiotensin-converting enzyme-2 expression in the esophagus reported.
What Is New
Normal adult esophagi have higher expression of angiotensin-converting enzyme-2 compared with adults with eosinophilic esophagitis.
Children with and without eosinophilic esophagitis have lower levels of esopahgeal angiotensin-converting enzyme-2 gene expression compared with normal adult esophagi.
Adults and children with eosinophilic esophagitis express less TMPRSS2 compared with noneosinophilic esophagitis adults.
Since December 2019, a fast-moving global pandemic, coronavirus-induced disease 2019 (COVID-19), has ensued with over 1 million deaths worldwide (1). Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) enters host cells via angiotensin-converting enzyme-2 (ACE2), a member of the renin-angiotensin system (R-A system), and the serine protease, TMPRSS2 (2). New evidence demonstrates that SARS-CoV-2 can infect and be harbored in the gastrointestinal (GI) tract (3).
Although T2 inflammatory atopic diseases are common, they do not necessarily associate with worsening COVID-19 risk (4,5). Jackson et al demonstrated that ACE2 expression in nasal airway epithelium was significantly decreased in atopic asthmatic patients compared with nonatopic asthmatic patients. Children also are relatively protected, with substantially lower morbidity and mortality rates from COVID-19 (6,7). Eosinophilic gastrointestinal disorders, especially EoE and EG, are increasing in prevalence and caused by T2 bowel inflammation. The current clinical data demonstrate that patients with eosinophilic gastrointestinal disorders (EGIDs) do not have worse COVID-19 and, despite the ability of SARSCoV-2 to infect the esophagus (3), there are no reports of COVID-19 infection in EoE. It is not currently clear if T2 inflammation associated with EGIDs could decrease the ability of SARSCoV-2 to infect the esophagus, stomach or bowel. Given the increasing prevalence of EGIDs, this is a salient question (8), however.
While ACE2 classically works in the renin-angiotensin (R-A) system to regulate blood pressure, it is also an important mediator of inflammation (Fig. 1A) (9). The differential expression of ACE2 and the R-A system by age, organ system, and in the presence of inflammation has become of heightened interest due to the COVID-19 pandemic. ACE1 conversion of angiotensinogen to angiotensin 2 and its subsequent binding to angiotensin receptors-1 and -2 (AGTR1, 2) leads to inflammation. ACE2 mediated conversion of angiotensin 2 to angiotensin 1–7 results in anti-inflammatory effects. For this reason, we aimed to understand the expression of not only ACE2 but also R-A system genes in the tissue of EoE and EG patients as compared to non-inflamed control subjects. We further evaluated TMPRSS2 expression in these patient populations since spike protein cleavage by TMPRSS2 is required for ACE2 mediated cell entry of SARSCoV-2 (2).
FIGURE 1.
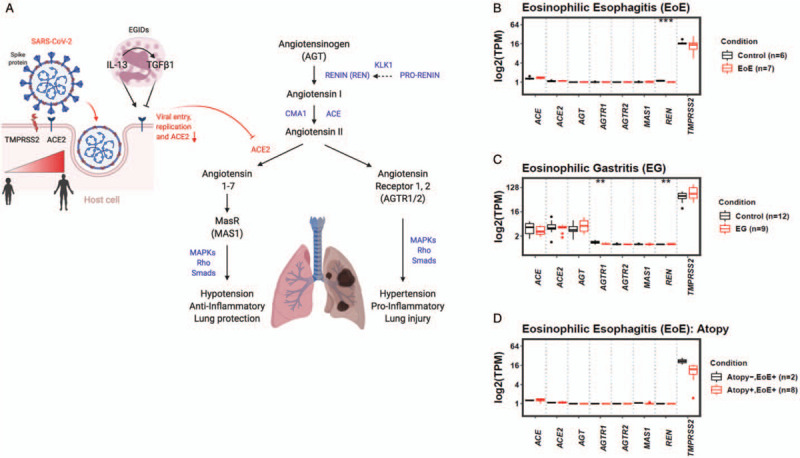
Expression of renin-angiotensin system genes and in eosinophilic gastrointestinal disorders. (A) Eosinophilic gastrointestinal disorders (EGIDs) and associated Th2 inflammation as well as age effect on TMPRSS2/ACE2 expression. ACE1 (ACE) conversion of angiotensin I to angiotensin II causes inflammation and hypertension. ACE2 cleavage of angiotensin II to angiotensin 1–7 and binding to Mas receptor (MAS1) decreases blood pressure lowering and inflammation. Angiotensinogen (AGT) is cleaved by renin (REN) and nonrenin EoE-associated proteases, chymase (CMA1) and kallikrein 1 (KLK1) (dashed line). (B--D) renin-angiotensin (R-A) system and TMPRSS2 transcriptomes in eosinophilic esophagitis (EoE) (B) and EG (C) biopsies. TPM = transcripts per million.
Herein, we study the influence of age and EoE or eosinophilic gastritis (EG) disease state on ACE-2, TMPRSS-2, and R-A system gene expression using RNA sequencing data from patients who were, in part, from the multicenter Consortium for Eosinophilic Gastrointestinal Research (CEGIR). We hypothesize that the pediatric esophagus may be less vulnerable than the adult esophagus to infection by SARSCoV-2 and that T2 inflammation associated with EGIDs can influence esophageal ACE-2 and TMPRSS2 expression.
METHODS
Datasets
Publicly available RNA-Seq data were used for esophageal biopsy specimens of children with EoE (n = 7) or control subjects (n = 6) (GSE58640), gastric biopsy specimens from patients with EG (n = 9) or control (n = 12) subjects (EGID express: https://egidexpress.research.cchmc.org/data/). Patients who were designated as controls had no pathologic findings on their endoscopies. The available RNA-Seq data were stored in different formats, such as read count, transcripts per million (TPM), and fragments per kilobase of transcript per million (FPKM). This hinders the integrative analysis of the RNA-Seq data. To ensure fair comparisons across different datasets, we converted RNA-Seq count data (GSE58640) to TPM. Eight total genes were analyzed in the RNAseq dataset. The esophagus of normal adults transcriptomic data were downloaded from GTEx Portal (https://gtexportal.org/home/datasets). Specifically, the gene read counts of the RNA-Seq GTEx version 8 data set (GTEx_Analysis_2017-06-05_v8_RNASeQCv1.1.9_gene_tpm.gct.gz) were downloaded. The sample annotations were also downloaded from the GTEx Portal (https://gtexportal.org/home/histologyPage) by selecting the Tissue = “Esophagus – Mucosa.” Data was further selected to include normal esophageal mucosa with no histologic abnormalities from people ages 20 to 59 (n = 184).
Atopy was defined as in the original publications (10,11). In the EG and EoE cohorts, atopy was defined as the presence of asthma, allergic rhinitis, atopic dermatitis, and/or food allergy.
Quantification
For the raw data of esophageal biopsy specimens of patients with EoE or control subjects (GSE58640), RNA-Seq quality was assessed using FastQC. Adapter sequences and low-quality bases were trimmed using Trimmomatic. Sequence alignment was performed using STAR against the Human genome (GRCH.38; GCF_000001405.38) with the default parameters. The expression of each gene was quantified using HTSeq and converted the count data to TPM for further analysis.
Statistics
Differential gene expression was performed between different pairwise disease states comparisons for the 8 genes (ACE, ACE2, AGT, AGTR1, AGTR2, MAS1, REN, and TMPRSS2) of the R-A pathway using the limma package in R (12). in which the P values were derived for all genes. The P values were further adjusted to multiple testing for each gene using false discovery rate (FDR) method by Benjamini-Hochberg (13). Genes with an FDR-adjusted P value less than 0.05 were defined as differentially expressed genes. For the comparisons of ACE2 and TMPRSS2 genes across different conditions, we applied the Wilcoxon rank-sum test on their gene expressions (TPM) in the studied conditions. Genes were considered as statistically significant differential expression at P < 0.05.
RESULTS
RNA-sequencing data from children with EoE (mean age = 6 years, range 3–10 years) or noninflamed control esophagus (mean age = 13 years, range 2–17 years) and EG patients (mean age = 16 years, range 7–31 years) or noninflamed control stomach (mean age = 15 years, range 8–28 years) was compared for R-A system and TMPRSS2 gene expression. Comparative transcriptomics of active EoE and EG tissue demonstrated that EoE and EG patients had similar ACE2 and TMPRSS2 expressions when compared with noninflamed esophagi or stomach from control subjects (Fig. 1B and C). Of the R-A system genes, REN expression was significantly lower in patients with active EoE (Fig. 1B). Within the R-A system, AGTR1 and REN were differentially expressed and downregulated in active EG as compared with noninflamed stomach from control subjects (Fig. 1C). On single gene pairwise comparison, EoE patients with concurrent atopy (n = 8) had significantly lower esophageal expression of TMPRSS2 as compared with nonatopic EoE patients (n = 2) (P < 0.05); however, this was not significant using multiple gene comparisons, likely because of the small sample size (Fig. 1D).
COVID-19 is a milder disease in children, perhaps because of differences in the expression level of SARS-CoV-2 entry proteins (7). Therefore, we evaluated expression levels of viral receptors in the context of age. As control patients with noninflamed esophagi have symptoms that warrant endoscopy and as our dataset contained only pediatric control subjects, we utilized RNA sequence data from normal adult esophagi. Normal pediatric samples are not available in this database. We compared the esophageal expression of ACE2 and TMPRSS2 in normal adults as compared with non-EoE children. ACE2 was expressed at significantly higher levels in the adult normal, as compared with the pediatric control, esophagus (Fig. 2A).
FIGURE 2.
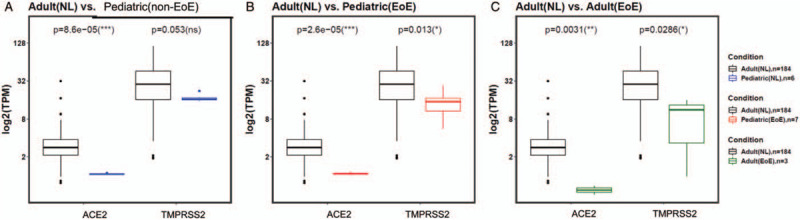
Age and eosinophilic esophagitis status effect ACE2/TMPRSS2 gene expression. Expression of severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) receptors in (A) normal adult versus pediatric noninflamed controls, (B) normal adult versus pediatric eosinophilic esophagitis (EoE), and (C) normal adult versus adult EoE.
To assess the expression of ACE2 and TMPRSS2 in the context T2 eosinophilic inflammation as compared with a histologically normal esophagus, we compared ACE2 and TMPRSS2 gene expressions in the esophagus of normal and EoE adults. We also compared the expression level of ACE2 and TMPRSS2 in children with EoE to that in the normal adult esophagus. ACE2 was expressed at significantly higher levels in the esophagus of normal adults as compared with adult or pediatric EoE patients (Fig. 2B and C). Interestingly, esophageal specimens from both adult and pediatric EoE subjects also expressed significantly less TMPRSS2 as compared with normal adults (Fig. 2B and C). Together, these data support the hypothesis that the esophagus from children may be relatively protected from SARS-CoV-2 GI infectivity because of lower ACE2 expression and that esophageal Th2 type inflammation in children or adults may be particularly protective for infection.
DISCUSSION
Although the respiratory tract has been considered the main nidus for viral entry, the GI tract may also be a primary entry portal for COVID-19 (14). Both the ileum and esophagus are considered “high risk” for SARS-CoV-2 infection based on the level of ACE2 transcript (15,16). Herein, we provide the first analysis of the expression of ACE2, R-A system, and TMRPSS2 genes in EoE and EG versus control esophagi and stomach, using RNAseq data. We have found that age and eosinophilic T2 inflammation influence the expression of the SARS-COV-2 viral genes required for viral entry.
These results should be evaluated in organ-specific and atopic-specific contexts. Although Radzikowksa et al found no difference in ACE2 expression between bronchial biopsies of normal and asthmatic patients, when looked at in the context of the atopic state, Jackson et al found lower levels of expression of ACE2 in allergic asthmatic adults and the negative correlation of ACE2 expression with IL-13 and IgE levels (4,17). Similarly, we show here that pediatric EoE subjects had no overall differences in ACE2/TMPRSS2 expression compared with noninflamed control esophagi but that adult and pediatric EoE patients had significantly lower ACE2 and TMPRSS2 expression compared with normal tissue. It is possible that the distinct phenotypes of EoE (18) and asthma may also lead to differential risk of infection.
The most serious consequence of COVID-19 is severe life-threatening pulmonary edema and acute respiratory distress syndrome (ARDS). During ARDS type lung injury, the R-A system acts to increase capillary permeability contributing to pulmonary edema. ACE2, on the other hand, acts to negatively regulate the effects of the classical R-A system and induces an anti-inflammatory effects (19). Although ACE2 is seemingly protective against severe ARDS in the setting of lung injury, it is also the gateway by which SARS-CoV-2 enters the mucosal surface.
We acknowledge that a limitation of this work is that it evaluates only RNA expression. Future work should evaluate ACE2/TMPRSS2 protein-level expression in these populations as well as the severity and acquisition rate of SARS-CoV2 in EGID patients across ages. Although we have tried to control for differing methodologies across studies, a direct comparison of pediatric and adult gastrointestinal tissue would provide superior results. In addition, our EGID sample sizes were small and normal tissue RNA sequence data was available only in adults.
CONCLUSIONS
We have demonstrated the ACE2/TMPRSS2 has limited gene expression in the GI tract and provided potential mechanisms whereby young age and EoE-associated Th2 inflammation could confer advantages for lower infection rates from COVID-19 by decreasing the expression of its receptor and protease. These insights may be of utility when designing novel SARS-CoV-2 therapies and understanding the clinical course and risks of COVID-19.
Acknowledgments
We thank CEGIR investigators, clinical coordinators, support staff, and patients for their support. We thank Sandeep Gupta, MD and Amanda Rudman-Spergel, MD for critical reading of the manuscript and Stephanie Dong, BS for her assistance.
Footnotes
Amanda B. Muir and Seema S. Aceves are co-senior authors.
Austin W.T. Chiang and Loan D. Duong are co-first authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
The names of the members of the CEGIR Investigator Group/CEGIR participants are mentioned in Appendix 1 (Supplemental Digital Content, http://links.lww.com/MPG/C219).
This work was supported by R01AI092135 (S.S.A.), K24AI135034 (S.S.A.), U54AI117804 (M.E.R.). R01DK124266 (A.B.M.), R03DK118310 (A.B.M.), CHOP Gastrointestinal Epithelial Modeling (GEM) Program, CHOP GI Epithelial Biology Center (A.B.M.), R35 GM119850 (N.E.L.).
CEGIR (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including American Partnership for Eosinophilic Disorders (APFED), Campaign Urging Research for Eosinophilic Diseases (CURED), and Eosinophilic Family Coalition (EFC). As a member of the RDCRN, CEGIR is also supported by its Data Management and Coordinating Center (DMCC) (U2CTR002818).
T.S. has received research support from JSPS Overseas Research Fellowships and is a co-inventor of patents owned by Cincinnati Children's Hospital Medical Center. M.E.R. is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Celgene, Astra Zeneca, Allakos, Arena Pharmaceuticals, GlaxoSmith Kline, Guidepoint and Suvretta Capital Management, and has an equity interest in the first 4 listed, and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust) and UpToDate. M.E.R. is an inventor of patents owned by Cincinnati Children's Hospital. S.S.A. is a co-inventor of oral viscous budesonide patented by UCSD and licensed by Takeda, has royalties from UpToDate and is a consultant for Aimmune, Astra Zeneca, Astellas, and Gossamer. The other authors claim no conflict of interest.
REFERENCES
- 1.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271.e8–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng SC, Tilg H. COVID-19 and the gastrointestinal tract: more than meets the eye. Gut 2020; 69:973–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol 2020; 146:203.e3–206.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savarino E, Lorenzon G, Ghisa M, et al. Lack of complications in patients with eosinophilic gastrointestinal diseases during SARS-CoV-2 outbreak. J Allergy Clin Immunol Pract 2020; 8:2790.e1–2792.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz AT, Zeichner SL. COVID-19 in children: initial characterization of the pediatric disease. Pediatrics 2020; 145:e20200834. [DOI] [PubMed] [Google Scholar]
- 7.Lingappan K, Karmouty-Quintana H, Davies J, et al. Understanding the age divide in COVID-19: why are children overwhelmingly spared? Am J Physiol Lung Cell Mol Physiol 2020; 319:L39–L44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonsalves N. Eosinophilic gastrointestinal disorders. Clin Rev Allergy Immunol 2019; 57:272–285. [DOI] [PubMed] [Google Scholar]
- 9.Gaddam RR, Chambers S, Bhatia M. ACE and ACE2 in inflammation: a tale of two enzymes. Inflamm Allergy Drug Targets 2014; 13:224–234. [DOI] [PubMed] [Google Scholar]
- 10.Sherrill JD, Kiran KC, Blanchard C, et al. Analysis and expansion of the eosinophilic esophagitis transcriptome by RNA sequencing. Genes Immun 2014; 15:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoda T, Wen T, Caldwell JM, et al. Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). Molecular, endoscopic, histologic, and circulating biomarker-based diagnosis of eosinophilic gastritis: Multi-site study. J Allergy Clin Immunol 2020; 145:255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser 1995; 57:289–300. [Google Scholar]
- 14.Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020; 69:997–1001. [DOI] [PubMed] [Google Scholar]
- 15.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 2020; 14:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deinhardt-Emmer S, Wittschieber D, Sanft J, et al. Early postmortem mapping of SARS-CoV-2 RNA in patients with COVID-19 and correlation to tissue damage. bioRxiv 2020; 395: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 2020; 75:2829–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkins D, Furuta GT, Liacouras CA, et al. Eosinophilic esophagitis phenotypes: ready for prime time? Pediatr Allergy Immunol 2017; 28:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia HP, Look DC, Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 2005; 79:14614–14621. [DOI] [PMC free article] [PubMed] [Google Scholar]


