Abstract
Purpose of review
To provide an update on the consequences of severe acute respiratory syndrome (SARS)-CoV-2 infection on the health and perinatal outcomes of pregnant women and their infants.
Recent findings
The severity of SARS-CoV-2 infection is greater in pregnant compared to nonpregnant women as measured by rates of admission to intensive care units, mechanical ventilation, mortality, and morbidities including myocardial infarction, venous thromboembolic and other thrombotic events, preeclampsia, preterm labor, and preterm birth. The risk of transmission from mother-to-infant is relatively low (1.5–5%) as quantitated by neonatal SARS-CoV-2 testing. Infants appear to be at higher risk of testing positive for SARS-CoV-2 if the mother has tested positive within 1 week of delivery or is herself symptomatic at the time of maternity admission. The rate of positivity is not higher in infants who room in with the mother compared to infants who are initially separated and cared for in a SARS-CoV-2-free environment. Infants who test positive in the hospital have no or mild signs of disease, most of which may be attributable to prematurity, and rarely require readmission for clinical signs consistent with COVID-19.
Summary
Pregnant women should take precautions to avoid infection with SARS-CoV-2. Infants born to mothers who test positive for SARS-CoV-2 can receive normal neonatal care in-hospital with their mothers if mother and staff adhere to recommended infection control practices.
Keywords: COVID-19, perinatal transmission, SARS-CoV-2
INTRODUCTION
From its onset, the severe acute respiratory syndrome (SARS)-CoV-2 pandemic strikingly affected the delivery of perinatal care in the United States due to its inordinately high transmissibility and associated morbidity and mortality. Guidelines from professional organizations on the management of mothers and infants initially differed substantially due to a lack of relevant population-based data on maternal and infant outcomes. Approximately one year after the initial outbreak in Wuhan, China some clarity has developed about the effects of this virus on pregnant women, the likelihood of perinatal transmission, and the immediate clinical outcomes of infants born to mothers who test positive for SARS-CoV-2.
Box 1.

no caption available
Severe acute respiratory syndrome-CoV-2
SARS-CoV-2 is a member of the coronaviridiae family of positive-sense single-stranded RNA viruses that were first identified in the 1960s as causative agents of upper respiratory tract infections (the common cold). Two prior coronaviruses, SARS-CoV (China; 2003) and MERS (Middle East respiratory syndrome)-CoV (Saudi Arabia; 2012), were the first coronaviruses identified that caused severe respiratory disease and high case mortality rates (approximately 9% and 30%, respectively). A variety of animal species (including bats and camels) are reservoirs for hundreds of coronaviruses. For each of the three coronaviruses associated with significant human disease, genetic mutation that enabled the virus to bind to receptors on human epithelial cells was necessary to allow zoonotic transmission.
Human infection is initiated by the binding of the ‘spike’ glycoprotein of SARS-CoV-2 to the angiotensin-converting enzyme 2 (ACE-2) embedded in the cell membranes of respiratory epithelial cells. Cleavage of the spike proteins by a transmembrane protease leads to intracellular entry of the virus by fusion or endocytosis. The host cell translates the viral mRNA to a large polyprotein that is cleaved by a viral protease into essential viral proteins whereas viral RNA-dependent RNA polymerase synthesizes viral RNA intermediaries. These viral proteins and RNA are assembled into a nucleocapsid, which upon release from the host cell can infect adjacent cells or be spread hematogenously to infect other organs (including placenta) whose cells contain ACE-2 within their membranes [1▪].
Severe acute respiratory syndrome-CoV-2 and pregnant women
At the start of the pandemic, it was unknown whether pregnant women had a greater risk of acquiring SARS-CoV-2 infection or were more likely to develop severe disease compared to nonpregnant women with similar demographics. Concern among obstetric teams about SARS-CoV-2 transmissibility was heightened because it was thought that pregnant women with active SARS-CoV-2 infection would aerosolize greater numbers of virus due to changes in breathing and vocalization during labor or due to aerosol generation if delivery under general anesthesia necessitated mechanical ventilation. In addition, the risk of infection due to exposure to amniotic fluid and maternal vaginal secretions was unknown. Hence, many hospitals opted to attempt to perform universal SARS-CoV-2 screening on every mother admitted in anticipation of delivery in order to conserve limited supplies of personal protective equipment and provide appropriate perinatal care to maternal-infant dyads where the mother tested positive.
Case series in the US early in the pandemic reported variable rates of positive maternal SARS-CoV-2 polymerase chain reaction (PCR) testing that reflected contemporaneous rates in the community. For example, among 215 women who presented for delivery at two New York City hospitals between March 22 and April 4, Sutton et al. reported that 15.4% of women had positive SARS-CoV-2 PCR testing, but importantly 88% of these positive mothers reported no symptoms of infection at the time of testing [2▪]. At Northwestern University in Chicago, 23 of 635 (3.6%) women admitted to labor and delivery between April 8 and 27 tested positive and of these 44% were asymptomatic [3]. At the same time, none of 80 asymptomatic women screened at a Los Angeles hospital during a single week in April tested positive [4]. In one study from New York City, pregnant women who lived in neighborhoods with high household membership, low assessed property values, low median incomes, and high employment rates were more likely to have tested positive for SARS-CoV-2 on admission to labor and delivery [5▪]. These findings are consistent with the observation that SARS-CoV-2 has disproportionately affected pregnant women from disadvantaged minority populations.
Large population-based studies have conclusively demonstrated that pregnant women with a history of SARS-CoV-2 infection develop more severe disease than nonpregnant women and experience worse perinatal outcomes. In a November 2020 Morbidity and Mortality Weekly Report, among 1.3 million US women of reproductive age (aged 15–44 years) who tested positive for SARS-CoV-2 between January 22 and October 3, pregnancy status was known for 409,462 symptomatic women among which 23,434 were pregnant (5.7%) [6▪▪]. After adjustments for underlying medical conditions, age, and race/ethnicity, pregnant women compared to nonpregnant women had significantly higher adjusted risk ratios (aRR) of ICU admission (aRR 3.0, 95% confidence interval (CI) 2.6–3.4), invasive ventilation (aRR 2.9, 95% CI 2.2–3.8), utilization of extracorporeal membrane oxygenation (aRR 2.5, 95% CI 1.5–4.0), and death (aRR 1.7, 95% CI 1.2–2.4). Although black women comprised 14% of the total cohort, they experienced 37% of all deaths and 27% of deaths in pregnant women. The aRRs for death and ICU admission were highest in Hispanic or Latina women and in non-Hispanic Asian and Native Hawaiian/Pacific Islander women, respectively. COVID-19 has amplified health inequities in minority populations.
More recently, investigators used data over an 8 months period from the Premier Healthcare Database (encompassing all-payer information for 20% of US hospitalizations) to construct a propensity model that compared the outcomes of pregnant women with and without COVID-19 infection [7▪]. Among 406,446 women who gave birth, 6,380 (1.6%) had a diagnosis of COVID-19. The adjusted odds ratios (aOR) for death, ICU admission, and use of mechanical ventilation for pregnant women with COVID-19 infection were 26.1, 6.5, and 23.7, respectively. Underlining findings in the general population, COVID-19 in pregnant women increased the odds of serious extrapulmonary complications including myocardial infarction (aOR 30.9), venous thromboembolism (aOR 3.4), and any thrombotic event (aOR 4.5). A number of adverse pregnancy outcomes were also significantly more common in pregnant women with COVID-19, including preeclampsia (aOR 1.21), HELLP (aOR 1.96), preterm labor (aOR 1.19), and preterm birth (aOR 1.17).
A parallel study from the Centers for Disease Control and Prevention reported on the perinatal outcomes of 4,442 women with SARS-CoV-2 with known pregnancy outcomes [8▪▪]. The rate of pregnancy loss was 0.3% before 20 weeks and 0.4% at greater than or equal to 20 weeks gestation. Of 3,912 infants with known gestational age, 12.9% were born before 37 weeks gestation (compared to a national rate of 10.2%). Among live-born infants, death before discharge occurred in 0.2%. There was no evidence of fetal growth abnormalities or a higher rate of congenital anomalies.
Whether COVID-19 directly increases the rate of stillbirth remains unclear. Although the Jering study did not find a significant increased risk of stillbirth in COVID-19 infected mothers, other studies have reported an increased rate of stillbirths during the pandemic. Although none of the stillbirths in a single-center study in the United Kingdom occurred in women known to have SARS-CoV-2 infection [9], universal screening has demonstrated that a large percentage of pregnant women with SARS-CoV-2 infection are asymptomatic. Alternatively, the increased numbers of stillbirths observed in some areas during the pandemic could be due to indirect effects leading to voluntarily reduced access to the healthcare system or decreases in the availability of routine obstetric services.
After delivery, women with COVID experience persistent symptoms including cough, myalgia, and fatigue for weeks. The UCSF Priority Registry reported a median time of symptom resolution of 37 days and 25% of women still had symptoms 8 weeks after delivery [10]. It is not known whether postpartum women have recoveries that are more extended than a comparable group of nonpregnant COVID-19 infected women. Possible long-term sequelae of COVID-19 such as accelerated cognitive decline and chronic respiratory and cardiac dysfunction are not yet defined.
Refinements of the evidence-based treatment of severe COVID-19 infection have decreased mortality. Systemic steroid therapy is now a mainstay of treatment but there are nuances when used in pregnant women. Both dexamethasone and betamethasone cross the placenta and have potent effects on fetal maturation. Extended therapy of pregnant women with these drugs beyond the traditional 2 days course used in anticipation of preterm birth may have adverse fetal and neonatal effects. For this reason, it has been recommended to transition after 48 h to a bioequivalent dose of methylprednisolone [11]. Methylprednisolone has a positive therapeutic effect when used in the treatment of adult respiratory distress, but unlike dexamethasone or betamethasone does not cross the placenta.
Transmission of severe acute respiratory syndrome-CoV-2 from mother to infant
The subject of maternal-to-infant transmission of SARS-CoV-2 has deservedly received much attention in the literature. Although rigorous case definitions of transplacental, perinatal, and postnatal transmission have been proposed [12▪], official case definitions remain under development by the World Health Organization (personal communication, David Kimberlin). The lack of a consensus framework has impeded investigation.
Early reports of potential transplacental transmission based on positive PCR testing in neonates before 12 h of age, the finding of SARS-CoV-2 IgM antibody in neonatal blood [13▪,14▪], or the detection of SARS-CoV-2 mRNA in cord blood are suspect because of potential postnatal acquisition, the unreliability of early SARS-CoV-2 IgM assays [15▪], and the lack of identification of replicable virus, respectively. All 7 infants reported by Dong and Zeng to have had SARS-CoV-2 IgM in neonatal blood had negative PCR testing and demonstrated no clinical signs of infection. Similarly, detection of SARS-CoV-2 mRNA in the placenta or observation of placental histopathology does not necessarily imply fetal infection [16].
A few reports present more persuasive evidence in support of in utero transmission [17▪–19▪]. A recent single-center study assayed SARS-CoV-2 IgG and IgM antibodies in maternal and cord blood sera in 1471 maternal/newborn dyads. Although IgG and/or IgM antibodies were detected in 83 women and IgG antibodies were found in 72 of the 83 associated cord blood specimens, IgM was not detected above a threshold concentration in any cord blood specimen [20▪▪]. This report closely paralleled the findings of Edlow et al. who reported that only 1 of 77 cord blood specimens of infants born to mothers with SARS-CoV-2 infection tested positive for detectable IgM antibody [21]. At this point, evidence warrants a limited conclusion that in utero transmission may occur but if so at a very low rate.
Although a large number of case reports and small case series and cohort studies have chronicled the results of PCR testing (primarily using nasopharyngeal specimens obtained by swab) in neonates born to mothers with positive SARS-CoV-2 tests, relatively few large population-based studies have assessed neonatal positivity in a comprehensive way. An early maternal study that accessed data in the UK Obstetric Surveillance System from 194 hospitals reported that 12 of 265 (5%) live-born infants born to mothers with confirmed SARS-CoV-2 tested positive, 6 within the first 12 h of age [22▪]. Another UK prospective population-based cohort study by Gale et al. collected data through the British Paediatric Surveillance unit on all infants admitted to a hospital by 28 days of age with a positive test for SARS-CoV-2 from March 1 to April 30, 2020 [23]. This active surveillance identified 66 infants with positive tests (an incidence of 5.6 per 100,000 live births) of which 17 (26%) were born to mothers who had tested positive for SARS-CoV-2. Only 1 of the 66 infants died but the cause of death was unrelated to SARS-CoV-2. Sanchez-Luna and colleagues in Spain have reported on the outcomes of 503 infants born between March 8 and May 26 2020 to 497 mothers who tested positive for SARS-CoV-2 [24]. Data were prospectively collected from 79 hospitals through the nationwide registry of the Spanish Society of Neonatology. These infants had a high rate of prematurity (15.7%) compared to the national rate of 6.5%. About 20% of infants had mild symptoms that investigators attributed mainly to prematurity. Only 14 of 469 (3.0%) of infants tested had a positive PCR result on the first test. A second test was performed in 157 infants, including all 14 who initially tested positive. Thirteen of these 14 infants had a negative repeat test, and 4 of 144 infants who had initially tested negative had a subsequent positive test result.
The American Academy of Pediatrics National Perinatal COVID-19 Registry is the largest prospective registry that seeks to compile demographics and outcomes of mother-infant dyads for which the mother has tested positive for SARS-CoV-2 between 14 days before through 3 days after delivery [25▪]. Preliminary findings from 4,285 mother-infant dyads entered from US hospitals in 45 states and the District of Columbia through November 8 were presented at the 2020 Hot Topics in Neonatology Conference.
Among live-born infants, 3,686 had at least one PCR test performed in hospital and of these, 57 (1.5%) had one or more positive PCR tests obtained in the hospital. Similar to findings reported by Sanchez-Luna, eight infants who tested positive initially had subsequent negative tests. For these infants, this may reflect transient colonization or perhaps false positive tests. Seventeen infants had a negative initial test and subsequently tested positive for SARS-CoV-2. The remaining 32 infants either only had a single test performed or tested positive on more than one specimen. Six infants had a first positive test on a specimen obtained after hospital discharge. False-negative tests would reduce and more consistent adherence to repeat testing would increase the infection estimate of 1.5%. These data suggest that if an infant can only be tested once during hospitalization, that test should be performed as close to 48–72 h of age as possible. As with other registries and published studies, these results cannot distinguish infants who may have acquired infection transplacentally from infants who acquired infection perinatally (that is, during labor and delivery) from infants who may have acquired infection via early horizontal transmission from mother, other family members, or caregivers.
Data in the registry also allowed examination of whether the timing of the maternal positive test in relation to delivery or the presence of clinical symptoms consistent with COVID-19 influenced the likelihood of an infant testing positive. Table 1 demonstrates that the lowest rate of positive neonatal tests occurred in mothers who had a positive test 8 or more days before delivery most of whom were likely noninfectious at the time of delivery. The highest rate of positive neonatal tests was in infants born to symptomatic mothers who had first positive tests within one week of delivery and were presumably more infectious. There was no difference in the rate of positive tests between infants who were separated from their mothers (1.9%) and infants who roomed-in (1.6%).
Table 1.
Rates of positive infant SARS-CoV-2 testing
| Time from positive test to delivery (days) | All mothers (n) | Infants with positive PCR testN (%) | Asymptomatic mothers (n) | Infants with positive PCR testN (%) | Symptomatic mothers (n) | Infants with positive PCR testN (%) |
| ≥8 | 642 | 3 (0.5) | 387 | 2 (0.5) | 252 | 1 (0.4) |
| 4 to 7 | 374 | 12 (3.2) | 197 | 4 (2.0) | 173 | 7 (4.1) |
| 3 to –3 | 3258 | 46 (1.4) | 2725 | 36 (1.3) | 517 | 10 (1.9) |
| 4 to 7 | 11 | 2 (18.2) | 7 | 0 (0.0) | 4 | 2 (50) |
| All | 4285 | 63 (1.5) | 3316 | 42 (1.3) | 946 | 20 (2.1) |
Neonatal outcomes
To date, studies that have assessed neonatal outcomes after hospital discharge have been reassuring. No infant tracked in the Spanish Registry who tested positive in-hospital was readmitted due to possible SARS-CoV-2 infection. Similarly, a New York City follow-up study by Dumitriu et al. did not identify clinical illness or hospital readmission related to SARS-CoV-2 infection in a cohort of 101 infants [26].
Tables 2 and 3 compare maternal and infant characteristics between the 63 infants in the AAP Registry who tested positive for SARS-CoV-2 and the 3621 infants who had negative tests. Clinical signs possibly consistent with COVID-19 infection were more common in the infants who tested positive (25.4% vs 13.9%), but this could be explained by a greater rate of immaturity (36.8 vs 38.1 weeks). Figure 1 depicts the rate of observed clinical signs among all infants and shows that respiratory signs predominated, likely associate with prematurity. No in-hospital infant death was attributable to COVID-19 infection.
Table 2.
Maternal characteristics of neonates per neonatal SARS-CoV-2 testing result
| Maternal Characteristic | Infants with positive PCR tests (n = 63) | Infants with negative PCR tests (n = 3621) |
| Non-white (%) | 66.7 | 62.2 |
| Hispanic (%) | 52.4 | 49.2 |
| LOS (days) | 4.8 | 3.1 |
| Died (%) | 0 | 0.2 |
| Sick at home/hospitalized for COVID-19 (%) | 31.7 | 22.1 |
| C-section delivery ($) | 47.6 | 36.9 |
PCR, polymerase chain reaction.
Table 3.
Characteristics of infants per neonatal SARS-CoV-2 testing result
| Infant characteristic | Infants with positive PCR tests (n = 63) | Infants with negative PCR tests (n = 3621) |
| Mean birth weight (g) | 2873 | 3099 |
| Mean gestational age (wk) | 36.8 | 38.1 |
| Sex (% male) | 50.8 | 52.2 |
| Apgar at 5 minutes | 8.5 | 8.8 |
| LOS (days) | 10.3 | 5.1 |
| Signs of COVID-19 (%) | 25.4 | 13.9 |
PCR, polymerase chain reaction.
FIGURE 1.
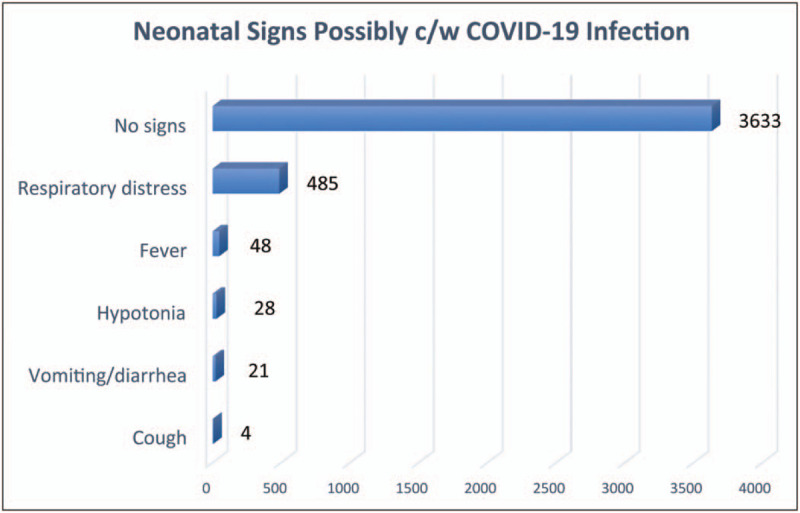
Number of infants in the AAP Registry with clinical signs of illness.
CONCLUSION
Existing data support the importance of strategies to shield pregnant women from acquiring COVID-19 infection. ACOG recommends vaccination of this vulnerable group. Infants born to mothers who have tested positive for SARS-CoV-2 appear to be at low risk of testing positive. Modes of mother-to-infant transmission require rigorous case definitions and further investigation. If mother and other caregivers adhere to recommended infection control practices, infants can room in with their mothers so long as the mother is well enough to care for her infant.
Acknowledgements
With great appreciation to the hundreds of individuals who together comprise the National Perinatal COVID-19 Registry Investigator Study Group and who voluntarily abstracted, vetted, and entered data on mother-infant dyads during a time of high stress.
Financial support and sponsorship
This work was partially supported by Centers for Disease Control and Prevention grant BAA 75D301-20-R-67897: Perinatal COVID-19 in the United States: Surveillance and Epidemiology.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Barrero-Castillero A, Beam KS, Bernardini LB, et al. COVID-19: neonatal-perinatal perspectives. J Perinatol 2020; 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a comprehensive and current review of aspects of perinatal COVID-19 beyond the scope of this article
- 2▪.Sutton D, Fuchs K, D’Alton M, Goffman DN. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020; 382:2163–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article was one of the first to present information about the likelihood of SARS-CoV-2 infection in pregnant women.
- 3.Miller ES, Grobman WA, Sakowicz A, et al. Clinical implications of universal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) testing in pregnancy. Obstet Gynecol 2020; 136:232–234. [DOI] [PubMed] [Google Scholar]
- 4.Naqvi M, Burwick RM, Ozimek JA, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) universal testing experience on a Los Angeles labor and delivery unit. Obstet Gynecol 2020; 136:235–236. [DOI] [PubMed] [Google Scholar]
- 5▪.Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA 2020; 324:390–392. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides insight into reasons minority populations are disproportionately affected by SARS-CoV-2.
- 6▪▪.Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]; This large population-based study demonstrates that SARS-CoV-2 causes more severe disease in pregnant vs nonpregnant women aged 15-44 years.
- 7▪.Jering KS, Claggett BL, Cunningham JW, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med 2021; e209241.[online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study replicates the findings of Zambrano et al. using a different data base.
- 8▪▪.Woodworth KR, Olsen EO, Neelam V, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in Pregnancy- SET-NET, 16 Jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides important clinical information about birth and infant outcomes following maternal SARS-CoV-2 infection.
- 9.Khalil A, von Dadelszen P, Draycott T, et al. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA 2020; 324:705–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical presentation of Coronavirus Disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol 2020; 136:1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad AF, Chappell L, Saade GR, Pacheco LD. Corticosteroids in the management of pregnant patients with Coronavirus Disease (COVID-19). Obstet Gynecol 2020; 136:823–826. [DOI] [PubMed] [Google Scholar]
- 12▪.Shah PS, Diambomba Y, Acharya G, et al. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand 2020; 99:565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides insight into the difficulty of creating and applying a rigorous case definition for the different modalities of transmission of SARS-CoV-2 to newborn infants.
- 13▪.Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 2020; 323:1848–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]; This and reference 14 were among the first case reports to suggest in utero transmission.
- 14▪.Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020; 323:1846–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]; This and reference 13 were among the first case reports to suggest in utero transmission.
- 15▪.Kimberlin DW, Stagno S. Can SARS-CoV-2 Infection be acquired in utero? More definitive evidence is needed. JAMA 2020; 323:1788–1789. [DOI] [PubMed] [Google Scholar]; This editorial provided perspective on the difficulty of being certain about transplacental SARS-CoV-2 transmission.
- 16.Wong YP, Khong TY, Tan GC. The effects of COVID-19 on placenta and pregnancy: what do we know so far? Diagnostics 2021; 11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020; 11:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of a few studies that provides more rigorous evidence for transplacental SARS-CoV-2 transmission.
- 18▪.Shende P, Gaikwad P, Gandhewar M, et al. Persistence of SARS-CoV-2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Hum Reprod 2020; deaa367. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of a few studies that provides more rigorous evidence for transplacental SARS-CoV-2 transmission.
- 19▪.Von Kohorn I, Stein SR, Shikani BT, et al. In utero severe acute respiratory syndrome coronavirus 2 infection. J Pediatric Infect Dis Soc 2020; 9:769–771. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of a few studies that provides more rigorous evidence for transplacental SARS-CoV-2 transmission.
- 20▪▪.Flannery DD, Gouma S, Dhudasia MB, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr 2021; e210038. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent study that correlates maternal and infant antibody testing and makes the case for a low risk of transplacental SARS-CoV-2 transmission.
- 21.Edlow AG, Li JZ, Collier AY, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open 2020; 3:e2030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪.Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ 2020; 369:m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]; The initial population based study of maternal SARS-CoV-2 infection.
- 23.Gale C, Quigley MA, Placzek A, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health 2020; 5:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Luna M, Fernández Colomer B, de Alba Romero C, et al. Neonates born to mothers with COVID-19: Data from the Spanish Society of Neonatology Registry. Pediatrics 2021; 147:e2020015065. [DOI] [PubMed] [Google Scholar]
- 25▪.AAP National Perinatal COVID-19 Registry, accessed at: https://services.aap.org/en/community/aap-sections/sonpm/in-the-spotlight/, February 4, 2021. [Google Scholar]; The largest Registry of demographics and in-hospital outcomes of mother-infant dyads in which the mother has tested positive for SARS-CoV-2 in the window from 14 days before to 3 days after delivery.
- 26.Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr 2021; 175:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]


