Abstract
Bone morphogenetic protein (BMP) signaling is well known in bone homeostasis. However, the physiological effects of BMP signaling on mandibles are largely unknown, as the mandible has distinct functions and characteristics from other bones. In this study, we investigated the roles of BMP signaling in bone homeostasis of the mandibles by deleting BMP type I receptor Acvr1 in osteoblast lineage cells with Osterix‐Cre. We found mandibular bone loss in conditional knockout mice at the ages of postnatal day 21 and 42 in an age‐dependent manner. The decreased bone mass was related to compromised osteoblast differentiation together with enhanced osteoclastogenesis, which was secondary to the changes in osteoblasts in vivo. In vitro study revealed that deletion of Acvr1 in the mandibular bone marrow stromal cells (BMSCs) significantly compromised osteoblast differentiation. When wild type bone marrow macrophages were cocultured with BMSCs lacking Acvr1 both directly and indirectly, both proliferation and differentiation of osteoclasts were induced as evidenced by an increase of multinucleated cells, compared with cocultured with control BMSCs. Furthermore, we demonstrated that the increased osteoclastogenesis in vitro was at least partially due to the secretion of soluble receptor activator of nuclear factor‐κB ligand (sRANKL), which is probably the reason for the mandibular bone loss in vivo. Overall, our results proposed that ACVR1 played essential roles in maintaining mandibular bone homeostasis through osteoblast differentiation and osteoblast‐osteoclast communication via sRANKL.
Keywords: ACVR1, BMP signaling, osteoblast differentiation, osteoclast differentiation, sRANKL
ACVR1 promotes osteoblastic differentiation and inhibits osteoclastogenesis through osteoblast‐osteoclast communication via sRANKL. ACVR1 plays an essential role in the maintenance of mandibular bone homeostasis.
1. INTRODUCTION
The mandible is a bone with irregular shape and structure involved in respiration, pronunciation, and mastication. Inflammation, injury, bone diseases, and tumors related to jawbones are common in the clinic and often lead to bone loss. The same as other bones, mandibles also require bone remodeling to maintain bone homeostasis, which relies on a dynamic balance between bone formation and resorption. However, differently, it is well recognized that the jawbone is the only bone that is in contact with the external microenvironment (i.e., oral cavity), and it is remodeled much more actively than the other bones (Huja et al., 2006). Moreover, a plethora of notable differences have been described between the mandible and long bone, including development, compositions, and biological characteristics (Aghaloo et al., 2010; Huja et al., 2006; Omi & Mishina, 2020). For example, the mandible originates from the cranial neural crest, while other axial and appendicular bones arise from the mesoderm (Chai et al., 2000). The mandible is also distinct in its compositions compared with long bones, that is, the mandible has an abundance of immature collagen crosslinks, with a lower extent of lysine hydroxylation, which indicates that the mandibular collagen is more ready to undergo degradation and turnover (Matsuura et al., 2014). The cells responsible for bone remodeling show different characteristics in the mandibles from other bones. Mandibular BMSCs showed increases in alkaline phosphatase (ALP) activity, mineralization, osteogenic gene expressions, and induced bone formation in vitro and in vivo, compared with BMSCs from iliac and long bones (Aghaloo et al., 2010; Akintoye et al., 2006). Mandibular BMSCs respond differently to chemical drugs (Stefanik et al., 2008), stresses (Pravitharangul et al., 2018, 2019), as well as hormones (H. Liu et al., 2009; Vinel et al., 2018), compared with the same types of cells from other bones. As a result, mandibular bone is much less affected by osteoporosis than long bones (C. Lee et al., 2019). Therefore, the mandible is quite a signature structure. Most research on bone remodeling is focusing on long bones, while only a few were tested on mandibles (Y. P. Liu et al., 2010; Matsuura et al., 2014). As the mandible is so different from other bones, previous studies and conclusions on long bones may not be appropriately applicable to mandibles (Su et al., 2019). Therefore, it is necessary to explore whether some bone homeostasis related genes also play an important role in mandibular homeostasis.
Bone remodeling is regulated by a series of cytokines and growth factors, including bone morphogenetic proteins (BMPs). BMPs are members of the transforming growth factor‐β superfamily, and can induce the formation of bone and cartilage when implanted at ectopic sites (Urist, 1965). BMP signaling is transduced through serine/threonine kinase receptors known as BMP type I and type II receptors. Type I receptors are phosphorylated by type II receptors upon binding to BMP ligands, and then phosphorylate the cytoplasmic signaling molecules to initiate SMAD and non‐SMAD signaling cascades (Sanchez‐Duffhues et al., 2015).
ACVR1 is one of the BMP type I receptors. The recurrent mutation of ACVR1 (c.617 G > A, p.R206H), leading to the ligand‐independent ACVR1 activation (Groppe et al., 2007), has been detected in most cases of fibrodysplasia ossificans progressiva (FOP). FOP is a rare genetic disorder, characterized by skeletal malformations and progressive heterotopic ossification (Huning & Gillessen‐Kaesbach, 2014). However, the function of ACVR1 signaling on mandibular bone remodeling is largely unknown. A previous publication revealed that inactivation of Acvr1 in the neural crest resulted in craniofacial defects, including a hypotrophic mandible (Dudas et al., 2004). However, the detailed changes in bone remodeling in the mandible were not clear.
In this study, to comprehensively understand the roles of ACVR1 in mandibular bone remodeling, we ablated Acvr1 in an osteoblast‐specific manner using Osterix‐Cre. We identified that ACVR1 plays essential roles in maintaining mandibular bone homeostasis and mediating osteoblast‐osteoclast communication, and the functions exhibit anatomical site‐specificity.
2. MATERIALS AND METHODS
2.1. Mice
The generation of Acvr1 fx/fx; R26R/R26R mice, Acvr1 +/− mice and Osterix (Osx)‐Cre (+)/(−) mice has been reported (Kaartinen & Nagy, 2001; Mishina et al., 1999; Rodda & McMahon, 2006; Soriano, 1999). Acvr1 +/−; Osx‐Cre (+)/(−) were bred with Acvr1 fx/fx; R26R/R26R to generate the control (Cont.) (Acvr1 fx/+; Osx‐Cre (+)/(−); R26R/+) and the conditional knockout (cKO) (Acvr1 fx/−; Osx‐Cre (+)/(−); R26R/+) mice (Orvis et al., 2008). Homozygous mice for Acvr1 flox and R26R were bred with ubiquitin (Ubi)‐CreER™ (+)/(−) transgenic mice to generate the Ubi‐control (Ubi‐Cont.) (Acvr1 fx/+; Ubi‐CreER™ (+)/(−); R26R/+) and the mutant (Ubi‐Mut) (Acvr1 fx/−; Ubi‐CreER™ (+)/(−); R26R/+) mice (Shi et al., 2016). All experimental procedures were approved by the Institutional Animal Care and Use Committee at Jilin University.
2.2. Microcomputed tomography (micro‐CT) analyses
The mandibles from postnatal day 21 (PN21) and PN42 mice were scanned by micro‐CT (μCT 50; Scanco Medical AG). Briefly, the area of interest (AOI) was defined as the bone regions within the furcation area of the mandibular first molar. The mandibles were analyzed with a threshold of 180 mg HA/ccm. Bone parameters were analyzed using the manufacturer's software (Supplementary Information).
2.3. Histology, histomorphometry, and immunohistochemistry (IHC) staining
Mandibles from PN21 and PN42 mice were fixed, decalcified, and embedded in paraffin. The coronal sections were chosen (Supplementary Information and Figure S1). Hematoxylin and eosin (H&E) and tartrate‐resistant acid phosphatase (TRAP) staining were performed. Histomorphometry was performed using ImageJ (1.51j) (Supplementary Information).
For IHC staining, a standard protocol was used with rabbit antibodies against mouse osteoprotegerin (OPG; 1:200; Abcam) and receptor activator of nuclear factor‐κB ligand (RANKL; 1:100; Boster). Measurements of integrated optical density (IOD) were performed using Image‐Pro Plus software (4.5.0.29).
2.4. RNA extraction and quantitative reverse‐transcription polymerase chain reaction (RT‐qPCR)
After the mandibles were harvested, soft tissues, bone marrow, and the teeth were removed. TRIzol reagent was used to extract RNA. The primers were shown in Table 1. Quantitative RT‐PCR was performed as described (X. Zhang et al., 2019).
Table 1.
Sequences of primers
| Gene name | Sequences (5′ → 3′) |
|---|---|
| Gapdh | F: GGTTGTCTCCTGCGACTTCA |
| R: TGGTCCAGGGTTTCTTACTCC | |
| Runx2 | F: GCACAAACATGGCCAGATTCA |
| R: AAGCCATGGTGCCCGTTAG | |
| Osterix | F: AAGTTATGATGACGGGTCAGGTACA |
| R: AGAAATCTACGAGCAAGGTCTCCAC | |
| Alpl | F: CTCAACACCAATGTAGCCAAGAATG |
| R: GGCAGCGGTTACTGTGGAGA | |
| Bsp | F: AAGCACAGACTTTTGAGTTAGC |
| R: ACTTCTGCTTCTTCGTTCTCAT | |
| Col1α1 | F: CTGGCGGTTCAGGTCCAAT |
| R: TTCCAGGCAATCCACGAGC | |
| Opn | F: TACGACCATGAGATTGGCAGTGA |
| R: TATAGGATCTGGGTGCAGGCTGTAA | |
| Ocn | F: GCAGGAGGGCAATAAGGT |
| R: CGTAGATGCGTTTGTAGGC | |
| Ctsk | F: CACCCAGTGGGAGCTATGGAA |
| R: GCCTCCAGGTTATGGGCAGA | |
| Tracp | F: CAAGAACTTGCGACCATTGTTA |
| R: ATCCATAGTGAAACCGCAAGTA | |
| Mmp9 | F: GCCCTGGAACTCACACGACA |
| R: TTGGAAACTCACACGCCAGAAG | |
| Rankl | F: GCAGCATCGCTCTGTTCCTGTA |
| R: CCTGCAGGAGTCAGGTAGTGTGTC | |
| Opg | F: TCTTCAGGTTTGCTGTTCCTACC |
| R: TCTCTACACTCTCGGCATTCACTT |
2.5. Culture of mandibular BMSCs, and detection of cell viability and differentiation
Mandibular BMSCs were isolated from PN42 mice as reported (D. J. Lee et al., 2019). To disrupt Acvr1 in culture, Ubi‐CreER™ activity was induced by administering 100 ng/ml of (Z)‐4‐hydroxytamoxifen (TM) for 6 days (Kamiya, Ye, Kobayashi, Lucas, et al., 2008). To evaluate the Cre activity, β‐gal staining was performed.
For cell viability assay, a cell counting kit‐8 (CCK‐8) was used. For osteogenic differentiation, cells were induced with an osteogenic differentiation medium. After 7 and 14 days, ALP staining was performed. The positive area of ALP staining was measured using ImageJ. After 21 days, alizarin red (AR) staining was performed, and then 10% w/v cetylpyridinium chloride was used to dissolve bound AR and optical density was measured at 562 nm.
2.6. Bone marrow cells isolation and coculture systems
Bone marrow cells were flushed from bone marrows of femora and tibiae from PN42 mice (Shi et al., 2016). The cells were cultured for 24 h before nonadherent cells were harvested. The cells were then treated with 20 ng/ml M‐CSF (Peprotech) for 3 days to become M‐CSF dependent macrophages (MDMs).
To evaluate the effects of osteoblasts lacking Acvr1 on osteoclastogenesis, we set up both direct and indirect coculture systems (Supplementary Information). At the end of the culture, the cells were fixed and stained with TRAP. Osteoclasts were defined as TRAP‐positive multinucleated (≥3 nuclei) cells. The number of osteoclasts per well (N. Oc/well), as well as the number of osteoclasts with different numbers of nuclei, were counted, and the areas per osteoclast (Ar. Oc) were measured using Image‐Pro Plus software.
To further determine the role of sRANKL on osteoclastogenesis in vitro, the conditioned medium from BMSCs culture (Schulze et al., 2018) were treated with rabbit anti‐RANKL antibody (500 pg/ml) to neutralize sRANKL in the conditioned medium. Rabbit immunoglobulin G (IgG) was used as a negative control. MDMs were cultured with the treated conditioned medium for 11 days, and then the aforementioned TRAP staining and quantitative analyses were performed.
2.7. Western blot analysis
Protein samples were harvested, and the resulting lysates were run on 10% PAGE Gel (Tris‐Gly) and transferred to polyvinylidene fluoride membranes. The membranes were incubated with the antibodies at 4°C overnight. The antibodies used were as follows: rabbit anti‐RANKL (1:1000; Boster), rabbit anti‐OPG (1:1000; Abcam), and mouse anti‐glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (1:5000; Proteintech). Then, the membranes were incubated with horseradish peroxidase‐conjugated goat anti‐rabbit or goat anti‐mouse (Proteintech) secondary antibodies for 1 h at room temperature and exposed to HPR substrate ECL (Proteintech). The intensities of the bands were quantified using ImageJ.
2.8. Enzyme‐linked immunosorbent assay (ELISA)
Whole blood was collected from PN42 mice, and clotted for 1 h at room temperature followed by centrifugation at 6000 rpm for 30 min to obtain serum. The supernatant of BMSCs culture was collected after treated with 10 nM 1,25(OH)2D3 for 5 days. The concentrations of RANKL were measured using a mouse RANKL ELISA kit (R&D Systems).
2.9. Statistical analysis
Data were shown as the means ± standard deviations of triplicates, with all experiments repeated three times. For comparisons between two groups, unpaired Student's two‐tailed t‐tests were used. For comparisons among three groups, data differences were assessed using one‐way analysis of variance and Tukey's Honestly Significant Difference. Values of p < .05 were considered significant.
3. RESULTS
3.1. Acvr1 deletion using Osx‐Cre leads to decreased mandibular bone mass
To investigate the role of ACVR1 in mandibular bone remodeling, we generated Acvr1 cKO mice with inducible (Tet‐off) Osx‐Cre, which has recombinase activity in osteoprogenitor cells from embryonic stages on regular chow. The body weights of cKO mice were smaller at PN21 and PN42, compared with the Cont. mice at the corresponding time points (Figure S2).
To quantitatively assess the changes in mandibular bone mass, the mandibles were scanned by micro‐CT. In PN21 male mice, a 9% decrease in tissue mineral density (TMD) was found in cKO, compared with the controls (Figure 1a,b). In PN21 female mice, we found decreases in TMD, bone mineral density (BMD), and trabecular thickness (Tb. Th), compared with the female controls (Figure S3a,b).
Figure 1.
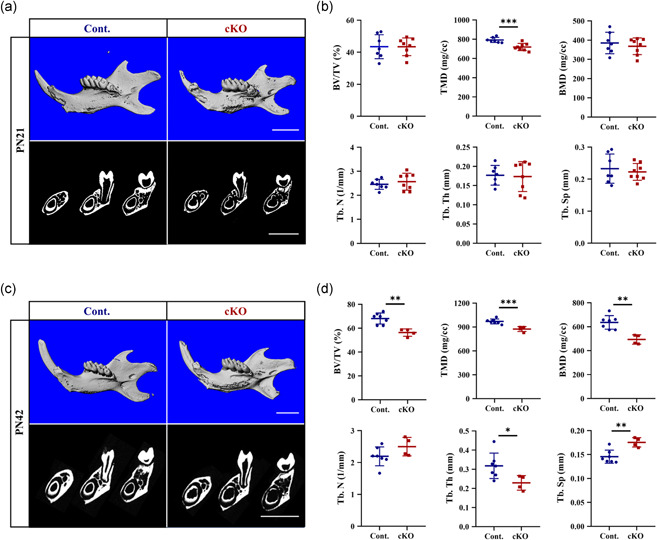
Micro‐computed tomography (CT) images of mandibles from (a) PN21 and (c) PN42 males. Bone parameters measured by micro‐CT at (b) PN21 and (d) PN42. n = 4–7. *p < .05; **p < .01; ***p < .001. Bar = 2 mm. cKO, conditional knockout
Consistent with the changes at PN21, these differences became more significant at PN42. In cKO male mice (Figure 1c,d), we found decreases in the bone volume fraction (BV/TV), TMD, BMD, and Tb. Th, and increase in trabecular separation (Tb. Sp), compared with the male controls. In female mice, the same changes were also found, except for an increased tendency of trabecular number (Tb. N) (p = .063) and Tb. Sp (p = .098) (Figure S3c,d).
These results demonstrated that ACVR1 positively regulated mandibular bone mass and mineralization in an age‐dependent manner.
3.2. Disruption of Acvr1 in osteoblasts results in a reduction of osteoblastic bone formation activity together with an increase of osteoclastic bone resorption activity in the mandible
Next, to evaluate how osteoblasts and osteoclasts participated in the bone homeostasis of the mandibles in Acvr1 cKO mice, we performed static histomorphometry. In PN21 cKO male mice, we identified decreases in osteoblast number per bone surface (N. Ob/BS) and osteoblast surface per bone surface (Ob. S/BS), compared with the controls (Figure 2b). Similar changes were also found in female mice at PN21 (Figure S4b–d).
Figure 2.
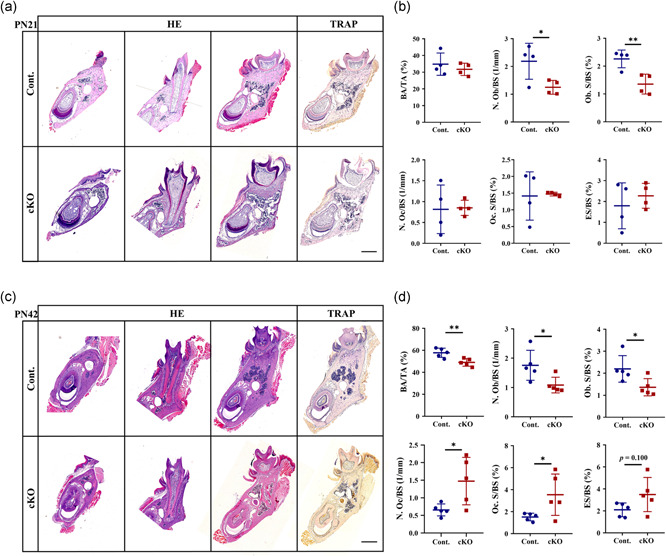
H&E and TRAP staining at (a) PN21 and (c) PN42 males. Histomorphometrical analyses of the mandibles at (b) PN21 and (d) PN42. n = 4–5. *p < .05; **p < .01. Bar = 500 μm. cKO, conditional knockout; H&E, hematoxylin and eosin; TRAP, tartrate‐resistant acid phosphatase
In PN42 male cKO mice, we found decreases in bone area per tissue area (BA/TA) (Figure 2d) and height of lingual plate (Figure S5a), demonstrating a decreased bone mass, which was consistent with the micro‐CT data (Figure 1c,d). Besides this, we also found decreases in N. Ob/BS and Ob. S/BS (Figure 2d). However, the osteoclast number per bone surface (N. Oc/BS) and osteoclast surface per bone surface (Oc. S/BS) were increased; and the eroded surface per bone surface (ES/BS) exhibited an increased tendency (Figure 2d, p = .100). Similar changes were also found in female mice at PN42 (Figure S5b–d).
Collectively, the mandibular bone loss in Acvr1 cKO mice was caused by a reduction of osteoblastic bone formation activity and an increase of osteoclastic bone resorption activity.
3.3. Deficiency of Acvr1 in osteoblasts leads to compromised osteoblast differentiation and secondary enhanced osteoclast differentiation through osteoblast‐osteoclast communication
To further determine the reason why Acvr1 cKO mice showed an osteopenic phenotype, we investigated the molecular changes for the differentiation of osteoblasts and osteoclasts. Quantitative RT‐PCR results showed that in cKO mandibles, the expressions of osteoblast differentiation markers Runx2, Col1a1, Alpl, Bsp, Opn, and Ocn were decreased (Figure 3a). The expression of Osx had a decreased tendency (Figure 3a, p = .052). The expression of osteoclast differentiation marker Ctsk was higher than the control group (Figure 3b). The expressions of Tracp and Mmp9 showed an increased tendency in cKO mice (Figure 3b, p = .081 and p = .131, respectively).
Figure 3.
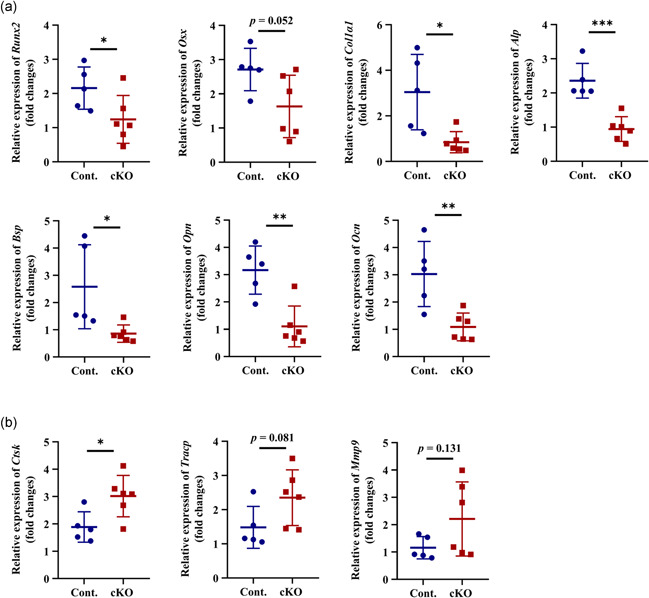
Quantitative RT‐PCR analyses of gene expressions for (a) osteoblast and (b) osteoclast markers in mandibles. n = 5–6. *p < .05; **p < .01; ***p < .001. cKO, conditional knockout; RT‐PCR, reverse‐transcription polymerase chain reaction
To determine the differentiation of Acvr1‐deficient osteoblasts in vitro, we cultured mandibular BMSCs from PN42 Ubi‐Cont. and Ubi‐Mut mice. After being cultured with TM for 6 days, LacZ positive cells could be seen, confirming the induction of Cre activity (Figure S6b). After 7 and 14 days of osteogenic induction, the areas of ALP positive staining were significantly lower in BMSCs lacking Acvr1, compared with the control cells (Figure 4a,b). AR staining showed that the mineralization in mutant BMSCs was significantly less (Figure 4a,b). To exclude the influence of cell number on the changes of ALP and AR staining, CCK‐8 assay showed that there was no difference in cell viability between Ubi‐Cont. and Ubi‐Mut BMSCs (Figure S6c). These results clearly manifested that deletion of Acvr1 compromised osteoblast differentiation but not proliferation.
Figure 4.
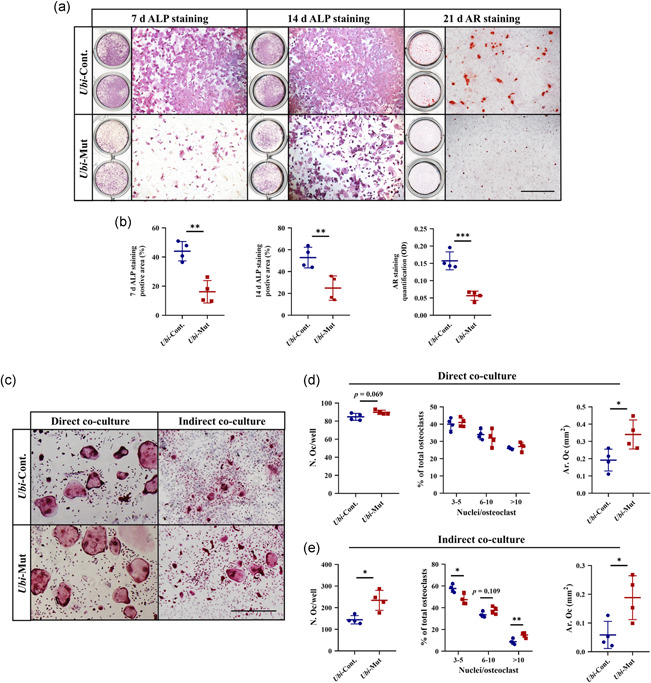
(a,b) ALP and AR staining and quantification. Bar = 1 mm. (c–e) TRAP staining and quantification. Bar = 500 μm. n = 4. *p < .05; **p < .01; ***p < .001. ALP, alkaline phosphatase; AR, alizarin red
To get further insights into how osteoclastogenesis was affected by Acvr1‐deficent osteoblasts, we set up a coculture system. After 11 days of direct coculture, the number of osteoclasts had a tendency to increase (p = .069), and the area of osteoclasts was increased in the Ubi‐Mut group, compared with Ubi‐Cont. group (Figure 4c,d). However for indirect coculture, when we compared osteoclastogenesis in the Ubi‐Mut group with that in the Ubi‐Cont. group, there were higher osteoclast numbers with much bigger size and more nuclei (Figures 4c and 4e). The results suggested that secreted molecules from osteoblasts probably played a more important role than direct contact between osteoblast‐osteoclast in terms of osteoclast formation.
Overall, our results indicated that deletion of Acvr1 in osteoblast lineage cells led to compromised osteoblast differentiation, which then significantly induced osteoclastogenesis through osteoblast‐osteoclast communication, mainly mediated by secreted molecules.
3.4. Increased osteoclastogenesis is mediated by increased secretion of soluble RANKL from osteoblast lineage cells
As osteoclastogenesis in cKO mice was enhanced, we hypothesized that there were changes in RANKL and/or OPG, which play critical roles in osteoclastogenesis. Quantitative RT‐PCR showed an increased tendency of Rankl expression (p = .058) and a decrease of Opg expression, leading to an increased ratio of Rankl/Opg (Figure 5a). IHC staining showed that RANKL was positive in the cytoplasm of osteoblast lineage cells in both Cont. and cKO mice (Figure 5b). Compared with the control group, the intensity of RANKL signals in the cKO group were stronger in osteoblast lineage cells (Figure 5b, red arrows, and 5c). OPG was also distributed in the cytoplasm of osteoblast lineage cells, however, there was no difference in the intensity between the two groups (Figure 5b, red arrows, and 5c). As a result, there was an increase in the ratio of RANKL/OPG (Figure 5c). However, the protein levels of RANKL and OPG within the mandible tissues detected by western blot showed no difference between the two groups (Figure 5d).
Figure 5.
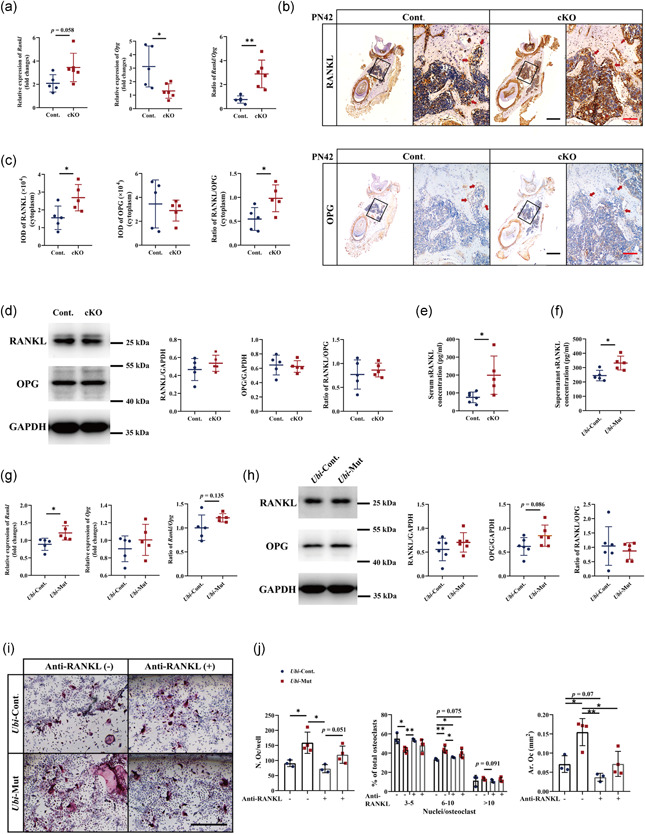
Levels of RANKL and OPG in vivo detected by (a) RT‐qPCR, (b,c) IHC staining (Bar = 500 and 100 μm for low and high magnifications, respectively), and (d) western blot. Levels of sRANKL detected by ELISA in the (e) serum and (f) cell culture supernatants. Levels of RANKL and OPG in vitro detected by (g) RT‐qPCR and (h) western blot. (i,j) TRAP staining and quantification. Bar = 500 μm. n = 4–6. *p < .05; **p < .01. cKO, conditional knockout; ELISA, enzyme‐linked immunosorbent assay; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; IHC, immunohistochemistry; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor‐κB ligand; RT‐qPCR, quantitative reverse‐transcription polymerase chain reaction; TRAP, tartrate‐resistant acid phosphatase
Next, to determine the levels of RANKL and OPG in vitro, we collected messenger RNA (mRNA) and protein samples from cultured Ubi‐Cont. and Ubi‐Mut BMSCs. Quantitative RT‐PCR showed an increase of Rankl expression and no difference in Opg expression in the Ubi‐Mut group, resulting in an increased tendency of Rankl/Opg ratio (p = .135) (Figure 5g). Western blot only showed an increased tendency of OPG in the Ubi‐Mut group (p = .086), while no difference in the protein levels of RANKL and RANKL/OPG ratio (Figure 5h).
Moreover, it is worth noting that there were more diffused and stronger positive signals of RANKL in the bone matrix and bone marrow in the cKO group, compared with the control group (Figure 5b). These RANKL‐positive signals implied the secreted form of RANKL (also known as soluble RANKL, sRANKL). As sRANKL also contributes to osteoclast differentiation, survival, and bone resorption activity, we examined the level of sRANKL in the serum of PN42 mice by ELISA. Consistent with IHC staining, an increased sRANKL was detected in the cKO mouse serum compared with the Cont. group (Figure 4e). Meanwhile, the level of sRANKL in the culture supernatant was significantly increased (Figure 5f), supporting the coculture results of enhanced osteoclastogenesis (Figure 4c–e).
To further dissect the underlying mechanism of osteoblast‐osteoclast communication, an anti‐RANKL antibody was added into the conditioned medium to block the sRANKL. Consistent with the indirect coculture system using transwell (Figures 4c and 4e), the indirect coculture using conditioned medium with antibody control also showed increased number of osteoclasts, which were much bigger and with more nuclei in the Ubi‐Mut group, compared with the Ubi‐Cont. group (Figure 5i,j). The addition of anti‐RANKL antibody in the Ubi‐Cont. group did not alter osteoclast formation (Figure 5i,j), suggesting that sRANKL played a modest role in osteoclastogenesis compared with mRANKL. Although relatively modest, the differences between the Ubi‐Cont. and Ubi‐Mut groups were eliminated by treating the conditioned medium with anti‐RANKL antibody (Figure 5i,j).
Taken together, these results suggested that osteoprogenitor cells lacking Acvr1 secreted more sRANKL, which was responsible for the enhanced osteoclastogenesis.
4. DISCUSSION
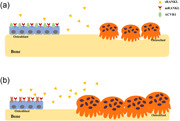
In this study, we demonstrated that ablation of Acvr1 in Osx‐expressing cells led to mandibular bone loss at PN21 and PN42. The osteopenic phenotype in cKO mice was due to the compromised osteoblast differentiation and enhanced osteoclast differentiation, which was probably mediated by increased secretion of sRANKL from osteoblast lineage cells. Moreover, in vitro study demonstrated that deletion of Acvr1 in BMSCs led to compromised osteoblast differentiation and increased osteoclastogenesis via secretion of sRANKL. We proposed a working model (Figure 6) to emphasize that ACVR1 played an essential role in the differentiation of osteoblasts, and mediates osteoblast‐osteoclast communication through sRANKL. Our findings suggested that ACVR1 played a significant role in maintaining mandibular bone homeostasis.
Figure 6.
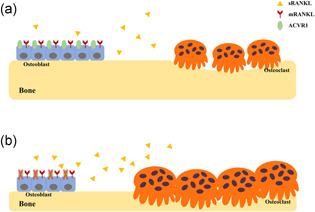
Schemes of the role of ACVR1 in maintaining mandibular bone homeostasis (a) When ACVR1 is present, osteoblast lineage cells can differentiate into osteoblasts, and regulate osteoclastogenesis via RANKL/OPG through both cell‐cell contact by membrane‐bound RANKL (mRANKL) as well as paracrine pathway by secreted form of soluble RANKL (sRANKL). (b) When ACVR1 is ablated specifically in osteoblast lineage cells, the differentiation of osteoblasts was compromised, and osteoclastogenesis was enhanced, which was mediated by the increased secretion of sRANKL from osteoblast lineage cells
Our current finding that ACVR1 positively regulated mandibular bone mass is supported by our previous study, demonstrating that ACVR1 is responsible for alveolar bone mass in the periodontium (X. Zhang et al., 2018). Moreover, our results also highlighted that ACVR1 played different roles in terms of bone mass among bones in different anatomical sites. In calvaria, ribs, and long bones, deletion of Acvr1 exhibited a higher bone mass (Kamiya et al., 2011; Shi et al., 2018). The different bone phenotypes between mandibles in our current study and other bones in the publications may be due to the distinct anatomical sites, or different Cre recombinases (i.e., 3.2‐kb Col1‐CreER and Osx‐Cre), or different time points upon observations (i.e., E18.5, PN21, PN42, and 3 months old). Another possible explanation is that Acvr1 is differentially expressed among different bones. In addition, the mandible is different from other bones in terms of embryonic origins, bone development, collagen compositions (Matsuura et al., 2014), cell autonomous characteristics (Akintoye et al., 2006; Azari et al., 2011), as well as mechanical loading (de Jong et al., 2010; Ehrlich & Lanyon, 2002). The site‐specificity of ACVR1 are supported by studies on another BMP receptor, BMPR1A, which also exhibits differential bone phenotypes depending on anatomical locations (H. Zhang et al., 2020).
Our results and other studies showed that BMP signaling mediated by BMP receptor in osteoblast lineage cells promoted osteoblast differentiation (Kamiya et al., 2010; Kamiya et al., 2011; Kamiya, Ye, Kobayashi, Lucas, et al., 2008; Kamiya, Ye, Kobayashi, Mochida, et al., 2008; Lim et al., 2016; Mishina et al., 2004; Shi et al., 2018; X. Zhang et al., 2018). Although the osteoclasts in these mouse models are wild type, osteoclastogenesis is reduced by the loss of BMP signaling in osteoblast lineage cells (Kamiya et al., 2010; Kamiya, Ye, Kobayashi, Lucas, et al., 2008; Kamiya, Ye, Kobayashi, Mochida, et al., 2008). However, in our cKO mandibles, we accidentally detected increased osteoclast activity in vivo (Figure 2) and enhanced osteoclastogenesis in vitro (Figure 4c–e). These results again highlighted the anatomical site specificity, revealing that the mandible lacking BMP signaling was distinct from other bones in terms of bone remodeling and osteoblast‐osteoclast communications.
RANKL and OPG are well‐known molecules expressed by osteoblast lineage cells, and the RANKL/OPG ratio determines osteoclast differentiation, survival, maturation, and function. Here, we detected increased osteoclasts in mandibles (Figures 2 and 4c–e), which was associated with an increased ratio of RANKL/OPG (Figure 5a–c). RANKL has three isoforms: two type II membrane proteins (mRANKL), and an sRANKL (Ikeda et al., 2001). Different from mRANKL which acts through cell‐cell contact, sRANKL is cleaved from the cell membrane by proteinases in the extracellular environment (O'Brien, 2010). Although, both mRANKL and sRANKL contribute to osteoclastogenesis, mRANKL is more potent than sRANKL in stimulating osteoclast formation, fusion, and survival (Lean et al., 2000; Nakashima et al., 2000). It is reported that the mRANKL is sufficient for osteoclastogenesis during skeletal development, while sRANKL does contribute to osteoclast formation and bone remodeling in adult mice (Xiong et al., 2018). In our current results, the level of RANKL detected by RT‐qPCR and IHC were increased (Figures 5a–c and 5g), indicating the upregulation of RANKL. However, the protein level of RANKL by western blot showed no difference (Figures 5d and 5h), which may be due to the processes of sample harvest (in vivo, mandibular bone marrows were removed; in vitro, supernatants were removed). Thus, we proposed that the elevated level of RANKL was likely caused by the upregulation of sRANKL but not mRANKL, which was confirmed by the ELISA analyses. However, we still could not exclude the possibility of the roles of RNA methylation participating in this process, as the methylation of RNA is a widespread regulatory mechanism, which can effectively regulate the translation of mRNA (Zaccara et al., 2019). To evaluate whether RNA methylation plays a role in our scenario is one of our future studies. Although sRANKL is not so potent in osteoclastogenesis, our experiments using anti‐RANKL antibody demonstrated that sRANKL does contribute to osteoblast‐osteoclast communication in the mandible in the scenario of osteoblast‐specific abruption of Acvr1.
As bone remodeling in vivo requires complex processes of osteoblast‐osteoclast communications, various coculture systems were established in vitro to imitate cell‐cell communications. Here, in our study, both direct and indirect coculture systems were used to determine the secondary changes of osteoclastogenesis. Our results demonstrated that osteoclastogenesis was significantly altered by secreted molecules, compared with direct contact (Figure 4c–e). However, we could not exclude the roles of direct contact between osteoblasts and osteoclasts, as the percentage of osteoclast with more nuclei increased in the indirect coculture, while it did not change in the direct coculture (Figure 4c–e). Moreover, when the anti‐RANKL antibody was used to neutralize the sRANKL in the conditioned medium, it only abolished the differences between the Ubi‐Cont. and Ubi‐Mut groups, without significantly altering osteoclastogenesis if compared with Ubi‐Mut groups treated with IgG control (Figure 5i,j). Thus, sRANKL partially, and was not the only mediator, participated in osteoblast‐osteoclast communication in the scenario of Acvr1 cKO cells.
In summary, our study identified the roles of BMP signaling mediated by ACVR1 in mandibular bone remodeling and osteoblast‐osteoclast communications. We demonstrated that ACVR1 promoted osteoblast differentiation and inhibited osteoclastogenesis through downregulation of sRANKL. However, further studies are still needed as we do not know how ACVR1 regulates the production of sRANKL; and how ACVR1 participates in bone remodeling under the microenvironments of mechanical loading and inflammation. This study provides novel insights into the regulation of mandibular bone remodeling and regeneration.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (81920108012, 81870741, and 81970903), the China Postdoctoral Science Foundation (2017M621219 and 2018T110258), the Jilin Provincial Department of Health (2019Q013), and the Jilin Provincial Department of Finance (JCSZ2019378‐6).
Hu Y, Hao X, Liu C, et al. Acvr1 deletion in osteoblasts impaired mandibular bone mass through compromised osteoblast differentiation and enhanced sRANKL‐induced osteoclastogenesis. J Cell Physiol. 2021;236:4580–4591. 10.1002/jcp.30183
Contributor Information
Ce Shi, Email: ceshi@jlu.edu.cn.
Hongchen Sun, Email: hcsun@jlu.edu.cn.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article. All materials are available from the corresponding author.
REFERENCES
- Aghaloo, T. L. , Chaichanasakul, T. , Bezouglaia, O. , Kang, B. , Franco, R. , Dry, S. M. , Atti, E. , & Tetradis, S. (2010). Osteogenic potential of mandibular vs. long‐bone marrow stromal cells. Journal of Dental Research, 89(11), 1293–1298. 10.1177/0022034510378427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akintoye, S. O. , Lam, T. , Shi, S. , Brahim, J. , Collins, M. T. , & Robey, P. G. (2006). Skeletal site‐specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone, 38(6), 758–768. 10.1016/j.bone.2005.10.027 [DOI] [PubMed] [Google Scholar]
- Azari, A. , Schoenmaker, T. , de Souza Faloni, A. P. , Everts, V. , & de Vries, T. J. (2011). Jaw and long bone marrow derived osteoclasts differ in shape and their response to bone and dentin. Biochemical and Biophysical Research Communications, 409(2), 205–210. 10.1016/j.bbrc.2011.04.120 [DOI] [PubMed] [Google Scholar]
- Chai, Y. , Jiang, X. , Ito, Y. , Bringas, P., Jr. , Han, J. , Rowitch, D. H. , & Sucov, H. M. (2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development, 127(8), 1671–1679. [DOI] [PubMed] [Google Scholar]
- de Jong, W. C. , Korfage, J. A. , & Langenbach, G. E. (2010). Variations in habitual bone strains in vivo: Long bone versus mandible. Journal of Structural Biology, 172(3), 311–318. 10.1016/j.jsb.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Dudas, M. , Sridurongrit, S. , Nagy, A. , Okazaki, K. , & Kaartinen, V. (2004). Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mechanisms of Development, 121(2), 173–182. 10.1016/j.mod.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Ehrlich, P. J. , & Lanyon, L. E. (2002). Mechanical strain and bone cell function: A review. Osteoporosis International (London), 13(9), 688–700. 10.1007/s001980200095 [DOI] [PubMed] [Google Scholar]
- Groppe, J. C. , Shore, E. M. , & Kaplan, F. S. (2007). Functional modeling of the ACVR1 (R206H) mutation in FOP. Clinical Orthopaedics and Related Research, 462, 87–92. 10.1097/BLO.0b013e318126c049 [DOI] [PubMed] [Google Scholar]
- Huja, S. S. , Fernandez, S. A. , Hill, K. J. , & Li, Y. (2006). Remodeling dynamics in the alveolar process in skeletally mature dogs. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology, 288(12), 1243–1249. 10.1002/ar.a.20396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huning, I. , & Gillessen‐Kaesbach, G. (2014). Fibrodysplasia ossificans progressiva: Clinical course, genetic mutations and genotype‐phenotype correlation. Molecular Syndromology, 5(5), 201–211. 10.1159/000365770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, T. , Kasai, M. , Utsuyama, M. , & Hirokawa, K. (2001). Determination of three isoforms of the receptor activator of nuclear factor‐kappaB ligand and their differential expression in bone and thymus. Endocrinology, 142(4), 1419–1426. 10.1210/endo.142.4.8070 [DOI] [PubMed] [Google Scholar]
- Kaartinen, V. , & Nagy, A. (2001). Removal of the floxed neo gene from a conditional knockout allele by the adenoviral Cre recombinase in vivo. Genesis, 31(3), 126–129. [DOI] [PubMed] [Google Scholar]
- Kamiya, N. , Kaartinen, V. M. , & Mishina, Y. (2011). Loss‐of‐function of ACVR1 in osteoblasts increases bone mass and activates canonical Wnt signaling through suppression of Wnt inhibitors SOST and DKK1. Biochemical and Biophysical Research Communications, 414(2), 326–330. 10.1016/j.bbrc.2011.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya, N. , Kobayashi, T. , Mochida, Y. , Yu, P. B. , Yamauchi, M. , Kronenberg, H. M. , & Mishina, Y. (2010). Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. Journal of Bone and Mineral Research, 25(2), 200–210. 10.1359/jbmr.090806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya, N. , Ye, L. , Kobayashi, T. , Mochida, Y. , Yamauchi, M. , Kronenberg, H. M. , Feng, J. Q. , & Mishina, Y. (2008). BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development, 135(22), 3801–3811. 10.1242/dev.025825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya, N. , Ye, L. , Kobayashi, T. , Lucas, D. J. , Mochida, Y. , Yamauchi, M. , Kronenberg, H. M. , Feng, J. Q. , & Mishina, Y. (2008). Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. Journal of Bone and Mineral Research, 23(12), 2007–2017. 10.1359/jbmr.080809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean, J. M. , Matsuo, K. , Fox, S. W. , Fuller, K. , Gibson, F. M. , Draycott, G. , Wani, M. R. , Bayley, K. E. , Wong, B. R. , Choi, Y. , Wagner, E. F. , & Chambers, T. J. (2000). Osteoclast lineage commitment of bone marrow precursors through expression of membrane‐bound TRANCE. Bone, 27(1), 29–40. 10.1016/s8756-3282(00)00306-9 [DOI] [PubMed] [Google Scholar]
- Lee, C. , Lee, J. H. , Han, S. S. , Kim, Y. H. , Choi, Y. J. , Jeon, K. J. , & Jung, H. I. (2019). Site‐specific and time‐course changes of postmenopausal osteoporosis in rat mandible: Comparative study with femur. Scientific Reports, 9(1), 14155. 10.1038/s41598-019-50554-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D. J. , Kwon, J. , Current, L. , Yoon, K. , Zalal, R. , Hu, X. , Xue, P. , & Ko, C. C. (2019). Osteogenic potential of mesenchymal stem cells from rat mandible to regenerate critical sized calvarial defect. Journal of Tissue Engineering, 10, 2041731419830427. 10.1177/2041731419830427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, J. , Shi, Y. , Karner, C. M. , Lee, S. Y. , Lee, W. C. , He, G. , & Long, F. (2016). Dual function of Bmpr1a signaling in restricting preosteoblast proliferation and stimulating osteoblast activity in mouse. Development, 143(2), 339–347. 10.1242/dev.126227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Guo, J. , Wang, L. , Chen, N. , Karaplis, A. , Goltzman, D. , & Miao, D. (2009). Distinctive anabolic roles of 1,25‐dihydroxyvitamin D(3) and parathyroid hormone in teeth and mandible versus long bones. Journal of Endocrinology, 203(2), 203–213. 10.1677/JOE-09-0247 [DOI] [PubMed] [Google Scholar]
- Liu, Y. P. , Behrents, R. G. , & Buschang, P. H. (2010). Mandibular growth, remodeling, and maturation during infancy and early childhood. Angle Orthodontist, 80(1), 97–105. 10.2319/020309-67.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura, T. , Tokutomi, K. , Sasaki, M. , Katafuchi, M. , Mizumachi, E. , & Sato, H. (2014). Distinct characteristics of mandibular bone collagen relative to long bone collagen: Relevance to clinical dentistry. BioMed Research International, 2014, 769414–769419. 10.1155/2014/769414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina, Y. , Crombie, R. , Bradley, A. , & Behringer, R. R. (1999). Multiple roles for activin‐like kinase‐2 signaling during mouse embryogenesis. Developmental Biology, 213(2), 314–326. 10.1006/dbio.1999.9378 [DOI] [PubMed] [Google Scholar]
- Mishina, Y. , Starbuck, M. W. , Gentile, M. A. , Fukuda, T. , Kasparcova, V. , Seedor, J. G. , Hanks, M. C. , Amling, M. , Pinero, G. J. , Harada, S. , & Behringer, R. R. (2004). Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. Journal of Biological Chemistry, 279(26), 27560–27566. 10.1074/jbc.M404222200 [DOI] [PubMed] [Google Scholar]
- Nakashima, T. , Kobayashi, Y. , Yamasaki, S. , Kawakami, A. , Eguchi, K. , Sasaki, H. , & Sakai, H. (2000). Protein expression and functional difference of membrane‐bound and soluble receptor activator of NF‐kappaB ligand: Modulation of the expression by osteotropic factors and cytokines. Biochemical and Biophysical Research Communications, 275(3), 768–775. 10.1006/bbrc.2000.3379 [DOI] [PubMed] [Google Scholar]
- O'Brien, C. A. (2010). Control of RANKL gene expression. Bone, 46(4), 911–919. 10.1016/j.bone.2009.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omi, M. , & Mishina, Y. (2020). Role of osteoclasts in oral homeostasis and jawbone diseases. Oral Science International. Advance online publication. 10.1002/osi2.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvis, G. D. , Jamin, S. P. , Kwan, K. M. , Mishina, Y. , Kaartinen, V. M. , Huang, S. , Roberts, A. B. , Umans, L. , Huylebroeck, D. , Zwijsen, A. , Wang, D. , Martin, J. F. , & Behringer, R. R. (2008). Functional redundancy of TGF‐beta family type I receptors and receptor‐Smads in mediating anti‐Mullerian hormone‐induced Mullerian duct regression in the mouse. Biology of Reproduction, 78(6), 994–1001. 10.1095/biolreprod.107.066605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravitharangul, A. , Suttapreyasri, S. , & Leethanakul, C. (2018). Iliac and mandible osteoblasts exhibit varied responses to LMHF vibration. Cell Biology International, 42(10), 1349–1357. 10.1002/cbin.11019 [DOI] [PubMed] [Google Scholar]
- Pravitharangul, A. , Suttapreyasri, S. , & Leethanakul, C. (2019). Mandible and iliac osteoblasts exhibit different Wnt signaling responses to LMHF vibration. Journal of Oral Biology and Craniofacial Research, 9(4), 355–359. 10.1016/j.jobcr.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda, S. J. , & McMahon, A. P. (2006). Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development, 133(16), 3231–3244. 10.1242/dev.02480 [DOI] [PubMed] [Google Scholar]
- Sanchez‐Duffhues, G. , Hiepen, C. , Knaus, P. , & Ten Dijke, P. (2015). Bone morphogenetic protein signaling in bone homeostasis. Bone, 80, 43–59. 10.1016/j.bone.2015.05.025 [DOI] [PubMed] [Google Scholar]
- Schulze, S. , Wehrum, D. , Dieter, P. , & Hempel, U. (2018). A supplement‐free osteoclast‐osteoblast co‐culture for pre‐clinical application. Journal of Cellular Physiology, 233(6), 4391–4400. 10.1002/jcp.26076 [DOI] [PubMed] [Google Scholar]
- Shi, C. , Iura, A. , Terajima, M. , Liu, F. , Lyons, K. , Pan, H. , Zhang, H. , Yamauchi, M. , Mishina, Y. , & Sun, H. (2016). Deletion of BMP receptor type IB decreased bone mass in association with compromised osteoblastic differentiation of bone marrow mesenchymal progenitors. Scientific Reports, 6, 24256. 10.1038/srep24256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, C. , Mandair, G. S. , Zhang, H. , Vanrenterghem, G. G. , Ridella, R. , Takahashi, A. , Zhang, Y. , Kohn, D. H. , Morris, M. D. , Mishina, Y. , & Sun, H. (2018). Bone morphogenetic protein signaling through ACVR1 and BMPR1A negatively regulates bone mass along with alterations in bone composition. Journal of Structural Biology, 201(3), 237–246. 10.1016/j.jsb.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano, P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics, 21(1), 70–71. 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Stefanik, D. , Sarin, J. , Lam, T. , Levin, L. , Leboy, P. S. , & Akintoye, S. O. (2008). Disparate osteogenic response of mandible and iliac crest bone marrow stromal cells to pamidronate. Oral Diseases, 14(5), 465–471. 10.1111/j.1601-0825.2007.01402.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, K. , Yuan, L. , Yang, J. , & Du, J. (2019). Numerical simulation of mandible bone remodeling under tooth loading: A parametric study. Scientific Reports, 9(1), 14887. 10.1038/s41598-019-51429-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist, M. R. (1965). Bone: Formation by autoinduction. Science, 150(3698), 893–899. 10.1126/science.150.3698.893 [DOI] [PubMed] [Google Scholar]
- Vinel, A. , Coudert, A. E. , Buscato, M. , Valera, M. C. , Ostertag, A. , Katzenellenbogen, J. A. , Katzenellenbogen, B. S. , Berdal, A. , Babajko, S. , Arnal, J. F. , & Fontaine, C. (2018). Respective role of membrane and nuclear estrogen receptor (ER) alpha in the mandible of growing mice: Implications for ERalpha modulation. Journal of Bone and Mineral Research, 33(8), 1520–1531. 10.1002/jbmr.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, J. , Cawley, K. , Piemontese, M. , Fujiwara, Y. , Zhao, H. , Goellner, J. J. , & O'Brien, C. A. (2018). Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy‐induced bone loss. Nature Communications, 9(1), 2909. 10.1038/s41467-018-05244-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccara, S. , Ries, R. J. , & Jaffrey, S. R. (2019). Reading, writing and erasing mRNA methylation. Nature Reviews Molecular Cell Biology, 20(10), 608–624. 10.1038/s41580-019-0168-5 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Zhang, Y. , Terajima, M. , Romanowicz, G. , Liu, Y. , Omi, M. , Bigelow, E. , Joiner, D. M. , Waldorff, E. I. , Zhu, P. , Raghavan, M. , Lynch, M. , Kamiya, N. , Zhang, R. , Jepsen, K. J. , Goldstein, S. , Morris, M. D. , Yamauchi, M. , Kohn, D. H. , & Mishina, Y. (2020). Loss of BMP signaling mediated by BMPR1A in osteoblasts leads to differential bone phenotypes in mice depending on anatomical location of the bones. Bone, 137, 115402. 10.1016/j.bone.2020.115402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Liu, Q. , Zhao, H. , Hu, Y. , Liu, C. , Yan, G. , Li, D. , Mishina, Y. , Shi, C. , & Sun, H. (2018). ACVR1 is essential for periodontium development and promotes alveolar bone formation. Archives of Oral Biology, 95, 108–117. 10.1016/j.archoralbio.2018.07.019 [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Shi, C. , Zhao, H. , Zhou, Y. , Hu, Y. , Yan, G. , Liu, C. , Li, D. , Hao, X. , Mishina, Y. , Liu, Q. , & Sun, H. (2019). Distinctive role of ACVR1 in dentin formation: Requirement for dentin thickness in molars and prevention of osteodentin formation in incisors of mice. Journal of Molecular Histology, 50(1), 43–61. 10.1007/s10735-018-9806-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. All materials are available from the corresponding author.


