Summary
Rice is a facultative short day (SD) plant. In addition to serving as a model plant for molecular genetic studies of monocots, rice is a staple crop for about half of the world’s population. Heading date is a critical agronomic trait, and many genes controlling heading date have been cloned over the last 2 decades. The mechanism of flowering in rice from recognition of day length by leaves to floral activation in the shoot apical meristem has been extensively studied. In this review, we summarise current progress on transcriptional and post‐transcriptional regulation of heading date in rice, with emphasis on post‐translational modifications of key regulators, including Heading date 1 (Hd1), Early heading date 1 (Ehd1), Grain number, plant height, and heading date7 (Ghd7). The contribution of heading date genes to heterosis and the expansion of rice cultivation areas from low‐latitude to high‐latitude regions are also discussed. To overcome the limitations of diverse genetic backgrounds used in heading date studies and to gain a clearer understanding of flowering in rice, we propose a systematic collection of genetic resources in a common genetic background. Strategies in breeding adapted cultivars by rational design are also discussed.
Keywords: expansion of rice areas, flowering, genetic pathways, heterosis, molecular breeding, Oryza sativa
Introduction
Following seed germination rice undergoes a vegetative growth phase before panicle initiation, at which there is a change in apical meristem identity from a vegetative meristem to a floral meristem as the plant enters the reproductive phase that leads to flowering. Flowering time (also known as heading date) is an important agronomic trait that determines seasonal and regional adaptation. If a cultivar flowers too early in a specific location there will be inadequate use of light and temperature resources, and consequent lower yield. By contrast, if a cultivar is too late in heading it cannot complete flowering and grain development before the onset of cold, also resulting in lower yield.
Heading date in rice is affected by exogenous factors such as photoperiod, temperature and nutrient availability (e.g. nitrogen levels), among which photoperiod is a key factor. Rice is a facultative short day (SD) plant; flowering is promoted under SD conditions and inhibited by long day (LD) conditions. However, in the juvenile growth stage of the vegetative growth phase, flowering cannot be promoted even under inductive daylength conditions. This stage is called the basic vegetative phase (BVP). Only after completion of the BVP can rice respond to photoperiodic stimuli for flowering, and this stage is called the photoperiod‐sensitive phase (Vergara & Chang, 1985; Nishida et al., 2001). The length of BVP and the sensitivity to exogenous factors is determined by endogenous genetic composition, a fundamental factor that determines differences in photoperiod response among rice genotypes and leads to extensive variation in heading date.
Genetic pathways controlling flowering in rice
The general process of photoperiod‐induced flowering is conserved in LD and SD plants. First, the leaves measure the photoperiod and generate a mobile flowering signal called a florigen under inductive daylength. The florigen is then transported from leaves to the shoot apex where the shoot apical meristem (SAM) perceives the florigen signal and activates downstream floral identity genes to trigger transition to flowering. The genetic pathway for photoperiod‐controlled flowering has been intensively studied in the model plant Arabidopsis, a facultative LD plant. In this plant, GIGANTEA (GI) protein promotes CONSTANS (CO) transcription in leaves, and CO protein directly activates transcription of the florigen FLOWERING LOCUS T (FT). However, there are multiple additional layers of transcriptional and post‐transcriptional modifications that regulate the photoperiodic flowering pathway to influence flowering time (Blumel et al., 2015).
Using forward and reverse genetics, as well as population genetic studies, many genes involved in photoperiodic flowering have been cloned into rice (Table 1). Most of these genes function upstream of the florigen genes. These genes can be roughly grouped to two signalling pathways with some crosstalk. The evolutionarily conserved OsGI–Hd1–Hd3a pathway (analogous to the Arabidopsis GI–CO–FT pathway) and Ehd1‐centred specific pathway in monocots involve Ehd1, Ghd7, Early heading date 2 (Ehd2, also known as RID1, or OsId1), Early heading date 3 (Ehd3), and Early heading date 4 (Ehd4), which has no counterpart in Arabidopsis (Fig. 1). Both pathways are finally integrated to florigen. Unlike Arabidopsis that has only one florigen gene, rice has two florigen genes, Heading date 3a (Hd3a) and RICE FLOWERING LOCUS T 1 (RFT1). Both genes encode a phosphatidylethanolamine binding protein that is a close homologue of Arabidopsis FT.
Table 1.
Cloned genes controlling heading date in rice.
| Gene symbol | Effect | Locus ID | Description | Reference |
|---|---|---|---|---|
| OsGI | SD/LD promotion | Os01g0182600 | Encodes an orthologue of Arabidopsis GIGANTEA, a plant‐specific nuclear protein that functions in diverse physiological processes | Hayama et al. (2002) |
| OsEF3 | SD/LD promotion | Os01g0566100 | Encodes an orthologue of Arabidopsis ELF3, a putative nematode responsive protein‐like protein | Fu et al. (2009) |
| OsLFL1 | LD repression | Os01g0713600 | Encodes a putative B3 DNA‐binding domain‐containing transcription factor | Peng et al. (2008) |
| OsWOX13 | LD promotion | Os01g0818400 | Encodes a WUSCHEL homeobox transcription factor | Minh‐Thu et al. (2018) |
| OsHESO1 | Maybe repression | Os01g0846450 | Encodes a homologue of Arabidopsis thaliana HEN1 suppressor 1 | Yano et al. (2016) |
| OsMADS51 | SD promotion | Os01g0922800 | Encodes a type I MADS‐box transcription factor | Kim et al. (2007) |
| SE13 | LD promotion | Os01g0949400 | Encodes phytochromobilin synthase, an orthologue of Arabidopsis HY2, a key enzyme in phytochrome chromophore biosynthesis | Saito et al. (2011) |
| LC2 | SD promotion | Os02g0152500 | Encodes a vernalisation insensitive 3‐like protein, a possible component of the PRC2 complex mediating H3K27me3 in target genes | Wang et al. (2013) |
| OsUbDKγ4 | LD repression | Os02g0290500 | Encodes ubiquitin‐like domain kinase γ4 | Song et al. (2017) |
| RCN2 | SD/LD repression | Os02g0531600 | Encodes rice TFL1‐like proteins that form a florigen repression complex (FRC) with 14‐3‐3 and OsFD1 | Nakagawa et al. (2002), Kaneko‐Suzuki et al. (2018) |
| SDG725 | SD/LD promotion | Os02g0554000 | Encodes histone H3 lysine 36‐specific methyltransferase | Sui et al. (2013) |
| OsCOL4 | SD/LD repression | Os02g0610500 | Encodes a member of the rice CONSTANS‐like (COL) family protein | Lee et al. (2010) |
| DTH2 | LD promotion | Os02g0724000 | Encodes a CONSTANS‐like protein that probably acts independently of Hd1 and Ehd1 | Wu et al. (2013) |
| HDR1 | LD repression | Os02g0793900 | Encodes a homologue of the ligand of the SNF1/AMPK/SnRK1 kinase PpSKI in moss | Sun et al. (2016) |
| Ehd4 | SD/LD promotion | Os03g0112700 | Encodes an Oryza‐genus‐specific CCCH‐type zinc finger protein | Gao et al. (2013) |
| Ef‐cd | SD/LD promotion | Os03g0122500 | A long noncoding RNA transcribed from the antisense strand of the DTH3 locus, and positively regulates expression of DTH3 | Fang et al. (2019) |
| DTH3 | SD/LD promotion | Os03g0122600 | Encodes a typical MIKC‐type MADS‐box protein OsMADS50, the putative SOC1/AGL20 orthologue in rice | Lee et al. (2004), Bian et al. (2011) |
| SE14 | LD repression | Os03g0151300 | Encodes a JmjC domain‐containing protein that functions in demethylation of H3K4me3 in RFT1 | Yokoo et al. (2014) |
| OsDof12 | LD promotion | Os03g0169600 | Encodes a DNA‐binding one finger (Dof) protein | Li et al. (2009) |
| OsNF‐YC2 | LD repression | Os03g0251350 | Encodes the HAP5 subunit of the heme activator protein (HAP) complex | Kim et al. (2016) |
| SDG718 | SD promotion | Os03g0307800 | Encodes enhancer of zeste 1, a key component of the PRC2 complex | Liu et al. (2014) |
| PHYB | LD repression | Os03g0309200 | Encodes photoreceptor phytochrome B | Takano et al. (2005) |
| OsCOL10 | SD/LD repression | Os03g0711100 | Encodes a member of rice CONSTANS‐like (COL) family proteins | Tan et al. (2016) |
| PHYA | LD repression | Os03g0719800 | Encodes photoreceptor phytochrome A | Takano et al. (2005) |
| OsWDR5 | SD/LD promotion | Os03g0725400 | Encodes WD repeat‐containing protein 5 | Jiang et al. (2018) |
| PHYC | LD repression | Os03g0752100 | Encodes photoreceptor phytochrome C | Takano et al. (2005) |
| Hd6 | LD repression | Os03g0762000 | Encodes the alpha subunit of protein kinase CK2 | Takahashi et al. (2001) |
| Hd16 | LD repression | Os03g0793500 | Encodes a casein kinase‐I protein | Dai & Xue (2010), Hori et al. (2013) |
| OsSFL1 | SD promotion | Os04g0166600 | Encodes a rice homologue of yeast SAP30, a component of histone deacetylation | Geng et al. (2020) |
| SDG708 | SD/LD promotion | Os04g0429100 | Encodes SET DOMAIN GROUP 708 protein, a histone H3 lysine 36‐specific methyltransferase | Liu et al. (2016) |
| OsRR1 | SD repression | Os04g0442300 | Encodes the type‐A response regulator that forms a heterodimer with Ehd1 | Cho et al. (2016) |
| OsCRY1b | SD/LD promotion | Os04g0452100 | Encodes cryptochrome, the blue/UV‐A light photoreceptor | Hirose et al. (2006) |
| RFL | promotion | Os04g0598300 | Encodes the rice homologue of transcription factor LFY | Rao et al. (2008) |
| HAF1 | SD/LD promotion | Os04g0648800 | Encodes a RING‐finger ubiquitin ligase | Yang et al. (2015) |
| OsHAPL1 | LD repression | Os05g0494100 | Encodes Heme activator protein like 1 | Zhu et al. (2017) |
| OsLF | SD/LD repression | Os05g0541400 | Encodes an atypical HLH protein | Zhao et al. (2011) |
| Hd17 | SD/LD promotion | Os06g0142600 | Encodes a homologue of Arabidopsis ELF3 protein | Matsubara et al. (2012), Saito et al. (2012) |
| RFT1 | LD promotion | Os06g0157500 | Encodes a rice florigen | Komiya et al. (2008) |
| Hd3a | SD promotion | Os06g0157700 | Encodes a florigen that is an orthologue of Arabidopsis FT | Kojima et al. (2002) |
| OsHAL3 | SD promotion | Os06g0199500 | Encodes a flavin mononucleotide‐binding protein | Sun et al. (2009) |
| Hd1 | SD promotion/LD repression | Os06g0275000 | Encodes the homologue of Arabidopsis CONSTANS | Yano et al. (2000) |
| SDG711 | LD repression | Os06g0275500 | Encodes enhancer of zeste 1, a key component of the PRC2 complex | Liu et al. (2014) |
| SE5 | SD/LD repression | Os06g0603000 | Encodes an enzyme implicated in phytochrome chromophore biosynthesis | Andrés et al. (2009) |
| OsFTIP1 | SD/LD promotion | Os06g0614000 | Encodes FT‐INTERACTING PROTEIN1 | Song et al. (2017) |
| OsNF‐YC4 | LD repression | Os06g0667100 | Encode the HAP5 subunit of the heme activator protein (HAP) complex | Kim et al. (2016) |
| OsMADS15 | promotion | Os07g0108900 | Encodes a MADS‐box transcription factor | Lu et al. (2012) |
| Ghd7 | LD repression | Os07g0261200 | Encodes a CCT domain protein | Xue et al. (2008) |
| OsCOL13 | SD/LD repression | Os07g0667300 | Encodes a CONSTANS‐like transcriptional activator | Sheng et al. (2016) |
| DTH7 | LD repression | Os07g0695100 | Encodes pseudoresponse regulator protein OsPRR37 | Koo et al. (2013), Yan et al. (2013), Gao et al. (2014) |
| Ehd3 | LD promotion | Os08g0105000 | Encodes a plant homeodomain finger‐containing protein | Matsubara et al. (2011) |
| Hd18 | SD/LD promotion | Os08g0143400 | Encodes a histone acetylase related to Arabidopsis FLOWERING LOCUS D | Shibaya et al. (2016) |
| DTH8 | SD promotion/LD repression | Os08g0174500 | Encodes the AP3 subunit of heme activator protein (HAP) complex | Wei et al. (2010), Yan et al. (2011) |
| SDG701 | SD/LD promotion | Os08g0180100 | Encodes the SET domain group protein | Liu et al. (2017) |
| GF14c | promotion | Os08g0430500 | Encodes a G‐box factor homologue of 14‐3‐3 | Taoka et al. (2011) |
| OsK4 | LD repression | Os08g0484600 | Encodes snf1‐related kinase interactor 2 that interacts with HDR1 and phosphorylates Hd1 | Sun et al. (2016) |
| OsNF‐YC6 | SD/LD repression | Os08g0496500 | Encodes a HAP5 subunit of the heme activator protein (HAP) complex | H. Zhang et al. (2019) |
| OsCOL15 | SD/LD repression | Os08g0536300 | Encodes a rice CONSTANS‐like protein | Wu et al. (2018) |
| SDG723 | LD promotion | Os09g0134500 | Encodes a trithorax group chromatin‐remodelling factor, an homologue of Arabidopsis Trithorax protein1 (ATX1) | Choi et al. (2014) |
| OsCO3 | SD repression | Os09g0240200 | Encodes a CONSTANS‐LIKE protein | Kim et al. (2008) |
| OsEMF2b | SD/LD promotion | Os09g0306800 | Encodes a polycomb‐group (PcG) protein, a homologue of Arabidopsis EMF2 | Yang et al. (2013), Xie et al. (2015) |
| SDG724 | SD/LD promotion | Os09g0307800 | Encodes histone methyltransferase | Sun et al. (2012) |
| OsMADS8 | SD/LD promotion | Os09g0507200 | Encodes a MADS‐box transcription factor | Kang et al. (1997) |
| OsFD1 | promotion | Os09g0540800 | Encoding a bZIP transcription factor | Taoka et al. (2011), Tsuji et al. (2013) |
| Ehd2 | SD/LD promotion | Os10g0419200 | Encodes the Cys2/His2‐type zinc finger transcription factor, an orthologue of maize INDETERMINATE1 | Matsubara et al. (2008), Wu et al. (2008) |
| Ehd1 | SD/LD promotion | Os10g0463400 | Encodes a B‐type response regulator | Doi et al. (2004) |
| OsMADS56 | LD repression | Os10g0536100 | Encodes a MADS‐box protein | Ryu et al. (2009) |
| RCN1 | SD/LD repression | Os11g0152500 | Encodes FL1‐like proteins that form a florigen repression complex (FRC) with 14‐3‐3 and OsFD1 | Nakagawa et al. (2002), Kaneko‐Suzuki et al. (2018) |
| Unnamed gene | Maybe repression | Os11g0187200 | Encodes a GATA zinc finger‐type transcription factor that was identified in a GWAS study | Yano et al. (2016) |
| OsFKF1 | SD/LD promotion | Os11g0547000 | Encodes a homologue of FLAVIN‐BINDING, KELCH REPEAT, F‐BOX 1 (FKF1) | Han et al. (2015) |
| DHD1 | SD/LD repression | Os11g0706200 | Encodes a GRAS family protein | H. Zhang et al. (2019) |
| OsVIL2 | SD/LD promotion | Os12g0533500 | Encodes a vernalisation insensitive 2‐like protein, a possible component of the PRC2 complex mediating H3K27me3 in target genes | Wang et al. (2013), Yang et al. (2013) |
Fig. 1.
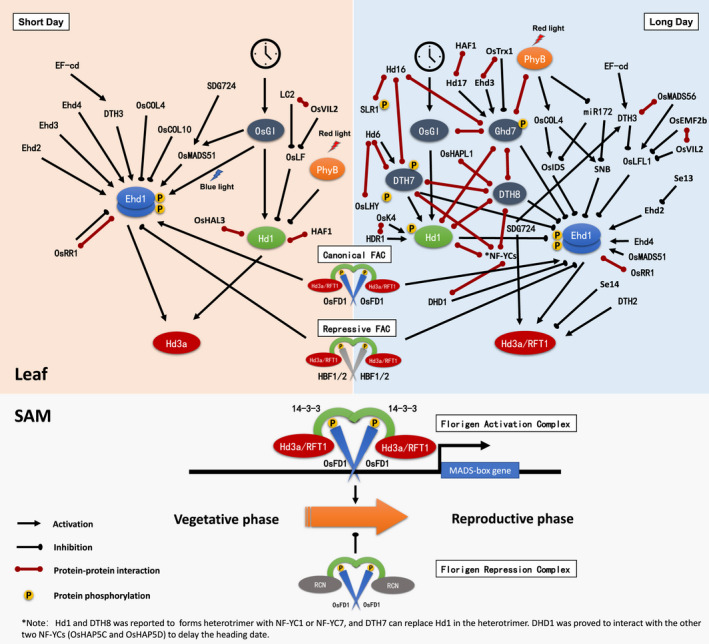
Genetic pathways controlling heading date in rice. Schematic representation of genetic pathways controlling flowering in rice. A circadian clock is shown at the top. Key heading date genes are indicated in oval backgrounds. Arrows indicate upregulation, and bars indicate downregulation. Rice has two florigen genes, Hd3a and RFT1, short day (SD) conditions accelerate heading by promoting the expression of Hd3a through Hd1 and Ehd1. Expression of Ehd1 can be upregulated or downregulated by many genes. Hd1 can be activated by OsGI under SD conditions. In long day (LD) conditions, Hd1 function reverses to flowering inhibition; this reversal is controlled by many other factors such as Ghd7 and DTH8 and is mediated by protein–protein interactions. The florigen proteins Hd3a and RFT1 are transported to the shoot apical meristem (SAM) after induction in the leaves. In the SAM, they form the florigen activation complex (FAC), and activate the expression of downstream targets, including members of MADS‐box transcription factor family genes that control phase transition of the SAM from vegetative to reproductive development. FAC and repressive FAC‐like complexes can also be formed in the SAM and leaves to fine tune the heading date.
Recognition of SDs through circadian clock and light signals
As a SD plant, flowering in rice is induced when the day length becomes less than a specific threshold, known as the critical day length. When the day length is shorter than 13 h, the florigen gene Hd3a is expressed in the morning even in rice seedlings, and when day length exceeds 13.5 h, expression of Hd3a decreases to less than one‐tenth of the 13 h d length level (Itoh et al., 2010). Based on physiological studies, it has been hypothesised that plants monitor day length changes by recognising light conditions at a specific time of day (Bünning, 1960). In rice, coincidence between a circadian clock controlled fluctuating internal signal and a periodic external signal can start the flowering process, and this mechanism is known as external coincidence (Song et al., 2015). Many heading date genes show rhythmic expression that is dependent on day length. OsGI has a rhythmic expression pattern that peaks at the end of the light period and activates expression of Hd1. Hd1 encodes the homologue of Arabidopsis CO and shows diurnal rhythmic expression that reaches a peak during the night both under SD and LD conditions but, under LD conditions, mRNA is also produced for several hours during the light phase (Izawa et al., 2002). Although Hd1 promotes flowering under SD, artificial overexpression of Hd1 during the light phase inhibits flowering under SD conditions (Fig. 2). This suggests that exposure of Hd1 to light converts it to a repressor of flowering (Ishikawa et al., 2011). Night‐break treatment under SD by exposure of rice plants to as little as 10 min of light in the middle of a long night results in delayed flowering and failure to induce Hd3a expression (Ishikawa et al., 2005).
Fig. 2.
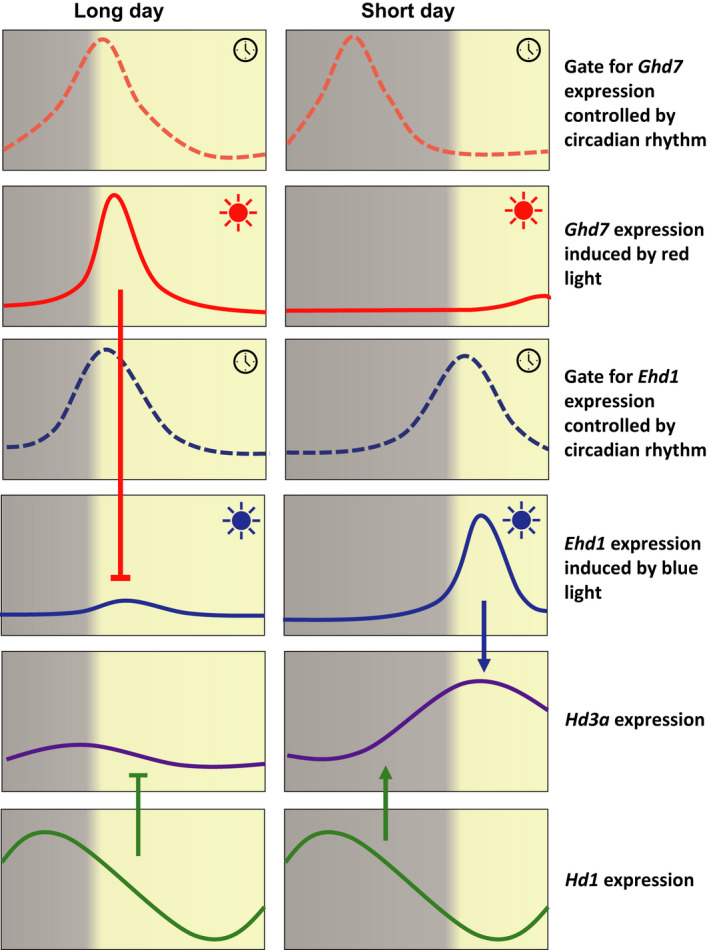
External coincidence model of rice heading regulation by circadian clock and day length. Diurnal expression of floral regulators Ghd7, Ehd1, Hd1 and Hd3a under long days (LD) and short days (SD). Arrows indicate induction, and bars indicate suppression of gene expression. Ghd7 expression has the highest red‐light inducibility around dawn under LD, whereas the inducibility peak shifts to midnight under SD conditions. Ehd1 expression has the highest blue‐light inducibility around dawn under both LD and SD conditions. The light‐sensitive phase set by the circadian clock is called a gate, which is indicated by dashed curves. Under LD, red light induces Ghd7 transcription around dawn, further suppressing the expression of Ehd1 and Hd3a. However, under SD, Ehd1 expresses around dawn and induces expression of Hd3a. Hd1 expression shows a diurnal rhythm and reaches a peak during the night under both SD and LD, but under LD, mRNA is also produced for several hours during the light phase. Dark phase‐expressed Hd1 promotes flowering under SD, whereas light phase‐expressed Hd1 inhibits flowering under LD.
Ghd7 encodes a CCT domain‐containing protein and is a key floral repressor in rice under LD conditions (Xue et al., 2008; Cai et al., 2019). Ghd7 is expressed in the morning under LD conditions. This day length‐dependent expression is achieved by a complex gating mechanism. A red‐light signal mediated by phytochromes induces Ghd7 expression only at a specific time of day when the gate (a light‐sensitive phase set by the circadian clock) for Ghd7 expression is open. Under LD conditions, the Ghd7 gate is set to open around dawn, and light induces Ghd7 expression. Under SD conditions it opens at midnight, therefore there is no light to induce Ghd7 expression (Itoh et al., 2010). By contrast with the Ghd7 gate for red light, the Ehd1 gate for blue light is not affected by day length. Ehd1 expression can be induced by blue‐light treatment in the morning in an OsGI‐dependent manner under both LD and SD conditions. Because Ehd1 expression is repressed by Ghd7, red light induces Ghd7 transcription, leading to suppression of Ehd1 and Hd3a expression in LD. Weak expression of Ghd7 in SDs allows induction of the Ehd1 gene, leading to activation of Hd3a expression (Fig. 2) (Itoh et al., 2010).
Photoperiod‐induced flowering through key integrators
SD conditions accelerate heading by promoting expression of Hd3a in a manner dependent on Hd1 and Ehd1. Expression of Ehd1 can be upregulated by Days to heading 3 (DTH3, also known as OsSOC1, OsMADS50) (Lee et al., 2004; Bian et al., 2011), Ehd2 (Matsubara et al., 2008; Wu et al., 2008), Ehd3 (Matsubara et al., 2011), Ehd4 (Gao et al., 2013), and OsMADS51 (Kim et al., 2007), and downregulated by Ghd7 (Xue et al., 2008), Days to heading 8 (DTH8, also known as Ghd8) (Wei et al., 2010; Yan et al., 2011), OsCONSTANS‐like 4 (OsCOL4) (Lee et al., 2010), OsCONSTANS‐like 10 (OsCOL10) (Tan et al., 2016), Heme activator protein like 1 (OsHAPL1) (Zhu et al., 2017), and Delayed heading date1 (DHD1) (Z. Y. Zhang et al., 2019). LD conditions suppress heading, and the expression level of Hd3a is much lower. Under LD conditions, RFT1 also functions as a florigen. Most LD heading date genes regulate florigen gene expression through Ehd1. For example, DTH3, OsMADS51, OsMADS56, Ehd2, Ehd4 and SIP1 (SDG723 Interaction Protein 1) induce Ehd1 expression, whereas Ghd7, DTH8, DTH7, OsCOL4, OsCOL10, OsLFL1 (Rice LEAFY COTYLEDON 2 and FUSCA 3‐LIKE 1), OsBBX14 (Rice B‐box 14), OsABF1 (ABA responsive element binding factor 1), HBF1 (Hd3a BINDING REPRESSOR FACTOR 1), RE1 (Regulator of Ehd1) and RIP1 (OsRE1‐INTERACTING PROTEIN 1) repress Ehd1 expression. Among them, proteins encoded by HBF1, SIP1 and RE1 can directly bind to the promoter of Ehd1 (Brambilla et al., 2017; Jiang et al., 2018, 2018,2018, 2018; Chai et al., 2020). However, transcriptional and genetic evidence revealed that DTH2 (Days to heading on chromosome 2) is likely to regulate florigen expression independently of Ehd1 (Wu et al., 2013; Yokoo et al., 2014).
Floral activation in the SAM by the florigen activation complex
The florigen proteins Hd3a and RFT1 are transported to the SAM after induction in leaves. In the SAM, they form the florigen activation complex (FAC) with bZIP transcription factor OsFD1 and 14‐3‐3 protein of the Gf14 family to induce expression of the primary targets, including members of MADS‐box transcription factor family genes that control the phase transition of SAM from vegetative to reproductive development (Taoka et al., 2011). Both yeast‐two‐hybrid assays and transgenic studies demonstrated that phosphorylation of OsFD1 is important for binding to 14‐3‐3 protein and is a rate‐limiting step for FAC formation (Taoka et al., 2011). FACs can also form in rice leaves, and these complexes are required to activate a positive feedback loop on Ehd1, Hd3a and RFT1 expression. In addition, two additional bZIPs, including Hd3a BINDING REPRESSOR FACTOR1 (HBF1) and HBF2, form alternative FACs with the florigens and reduce Hd3a and RFT1 expression to delay flowering. This situation suggests that Hd3a and RFT1 can regulate their own expression in leaves through forming antagonistic transcription factor complexes to fine tune photoperiod‐induced flowering responses (Brambilla et al., 2017). In addition, RICE CENTRORADIALIS (RCN) genes encoding PEBP‐like proteins are homologous to the Arabidopsis TERMINAL FLOWER 1 (TFL1) and have similarity to the florigens. RCN represses flowering through competition with Hd3a for 14‐3‐3 binding, and further combines with OsFD1 to form a FAC‐like hexameric complex named the florigen repression complex (FRC). The balance between FAC and FRC modulates florigen activity to optimise inflorescence development (Kaneko‐Suzuki et al., 2018). We recently identified a new CONSTANS‐like CCT domain protein called Delay heading date 4 (DHD4), which also represses flowering by affecting formation of the FAC complex in the SAM. By contrast with RCNs that interact with OsFD1 mediated by 14‐3‐3, DHD4 directly interacts with OsFD1 and affects the formation of FAC (Cai et al., 2020), and possibly represents another type of flowering regulation mechanism in SAM.
Transcriptional and post‐transcriptional regulation of key heading date regulators
Dual functional regulation of Hd1
Hd1 is the sole homologue of Arabidopsis CO. CO is generally known as a strong LD‐specific floral promoting factor in Arabidopsis (Putterill et al., 1995), although it may also play a role in repressing flowering under SD conditions (Luccioni et al., 2019). However, the function of Hd1 has diverged during evolution. It advances heading by activating transcription of the florigen genes under SDs, but delays heading under LD conditions (Yano et al., 2000). Post‐transcriptional regulation of Hd1 must also be involved in the functional conversion and other aspects of Hd1 function because Hd1 has a similar expression pattern in different photoperiods (Izawa et al., 2002; Ishikawa et al., 2011).
Whether Hd1 functions as an activator or suppressor of heading depends on the red‐light photoreceptor phytochrome B (PhyB). Mutation of PhyB or phytochrome chromophore synthesis gene photoperiod sensitivity 5 (Se5) attenuates the reversible function of Hd1 in which Hd1 works as an activator under all photoperiods in the absence of phytochromes. As Hd1 expression is not affected in the se5 mutant it is thought that phytochromes may affect Hd1 function post‐transcriptionally (Izawa et al., 2002).
Hd1 also loses LD heading suppression function and consistently promotes flowering under SD and LD in Ghd7‐ or DTH8‐deficient backgrounds (Nemoto et al., 2016; Du et al., 2017; Zhang et al., 2017; Zong et al., 2021). Transcriptional analyses revealed that Ghd7 and Hd1 do not regulate each other’s transcription levels, suggesting that the observed genetic interaction might occur post‐transcriptionally (Nemoto et al., 2016). Hd1 was shown to interact with flowering repressor Ghd7, and the Hd1–Ghd7 complex binds to specific sites in the Ehd1 promoter and suppresses expression of Ehd1 and florigen genes during daylight hours under LD conditions (Nemoto et al., 2016). As the interaction occurs between the transcription‐activating domain of Hd1 and CCT domain of Ghd7, it was hypothesised that the interaction might block or weaken the transcriptional activation activity of Hd1 and release the downstream gene transcriptional repression activity of Ghd7 (Zhang et al., 2017). Because the expression level of Ghd7 is significantly lower in SDs (Xue et al., 2008), less Hd1–Ghd7 complex forms and so Hd1 still promotes heading date on a Ghd7 background under SDs (Nemoto et al., 2016; Zhang et al., 2017).
DTH8 is another key floral repressor under LD conditions (Wei et al., 2010a,b; Yan et al., 2011; Dai et al., 2012). Like Ghd7, DTH8 interacts with Hd1 (Du et al., 2017; Zhu et al., 2017). The DTH8–Hd1 complex is necessary for the floral inhibition function of Hd1 in LDs. In dth8 background, Hd1 loses its alternative function and acts as an activator of Hd3a expression to promote flowering both in SD and LD conditions in the 93‐11 background. Another report suggested that DTH8 alone could not convert Hd1 function, but enhanced the effect of Ghd7 on the Hd1 functional conversion in the ZS97 background (Z. Y. Zhang et al., 2019). The exact mechanism by which DTH8 affects the Hd1 functional switch is not clear, but one possibility is that it acts by changing H3K27me3 levels in the Hd3a promoter (Du et al., 2017).
Several recent studies have revealed that both Ghd7 and DTH8 can interact with Hd1 and that DTH8 also interacts with Ghd7. Therefore, although Hd1 has the primary function for promoting expression of Hd3a/RFT1 and flowering regardless of day length, it can form a ternary repressive complex with DTH8 and Ghd7 to mediate photoperiodic heading (Cai et al., 2019; Zong et al., 2021). This view is supported by the observation that a functional Ghd7, DTH8 and Hd1 combination showed significantly stronger photoperiod sensitivity than other combinations in both near‐isogenic lines and natural populations (Zhang et al., 2015; Wang et al., 2019; Z.Y. Zhang et al., 2019; Zong et al., 2021), although there might be other types of transcriptional or post‐transcriptional regulation involving one or more of them (Wang et al., 2019). For example, Hd1, DTH8 and distinct NF‐YC subunits reported to form a trimeric complex that bound to the CO Response Element 2 (CORE2) in the Hd3a promoter, and Days to heading 7 (DTH7, also known as Hd2, OsPRR37 or Ghd7.1, another CCT domain‐containing protein strongly repressing flowering) can replace Hd1 in the complex (Goretti et al., 2017). Hd1 also interacts with DTH8 and OsHAPL1 and is likely to form a complex with general transcription factors to regulate the transcription of target genes to control heading date in rice (Zhu et al., 2017). These results indicated that Hd1, Ghd7, DTH8 and DTH7 do not act independently, but assemble into complexes to regulate the rice photoperiodic pathway.
Hd1 also interacts with Rice Halotolerance Protein 3 (OsHAL3), a flavin mononucleotide‐binding protein reported as a blue‐light sensor that does not contain a DNA‐binding domain. The interaction is inhibited by white or blue light. The OsHAL3–Hd1 complex promotes heading under SD, and activates Hd3a expression by direct binding to the Hd3a promoter. OsHAL3 expression exhibits a diurnal pattern that peaks at 4 h before light onset under SD, but peaks 4 h after light onset under LD conditions. It was proposed that the more OsHAL3 coincides with the midnight‐expressed Hd1 under SD, more OsHAL3–Hd1 complex is formed to activate Hd3a expression. The level of expression of OsHAL3 under LDs peaks during daytime, and light inhibits the formation of the OsHAL3–Hd1 complex. There is no obvious induction of Hd3a mRNA levels by OsHAL3 (Su et al., 2016).
Heading date associated factor 1 (HAF1), a C3HC4 RING domain‐containing E3 ubiquitin ligase, was identified as an Hd1 interacting protein in yeast‐two‐hybrid assays. HAF1 mediates ubiquitination and regulates circadian accumulation of Hd1 by targeting it for degradation via the 26S proteasome‐dependent pathway. The haf1 hd1 double mutant flowers as late as hd1 plants only in SD conditions, suggesting that HAF1 is essential for regulating heading by modulation of the diurnal rhythm of Hd1 protein in SDs (Yang et al., 2015). Additional research revealed that HAF1 also mediates ubiquitination and modulates the diurnal rhythm of OsELF3 (an orthologue of the Arabidopsis ELF3, also known as Hd17) under LDs (Zhu et al., 2018).
Protein level regulation of key floral integrator Ehd1
Ehd1 encodes a B‐type response regulator that functions upstream of florigen genes and promotes heading under both SD and LD conditions. Ehd1 integrates various upstream flowering signals and is recognised as key floral inducer in rice (Doi et al., 2004; Shrestha et al., 2014). In addition to regulation of the transcription level, post‐translational modifications also play critical roles in Ehd1 function. It has been reported that the activity of Ehd1 protein in promoting Hd3a and RFT1 expression is repressed by OsPhyA in a day length‐dependent manner (Osugi et al., 2011). Ehd1 activity can also be regulated by phosphorylation modification, as mutation of the key amino acid Asp‐63 to Glu to mimic a constitutive phosphorylated state greatly enhanced its function and caused extremely early heading. A separate study revealed that phosphorylation of Asp‐63 is required for formation of Ehd1 complexes, and dimerisation is important for normal Ehd1 function (Cho et al., 2016). Interestingly, OsRR1, a type‐A response regulator, can inhibit Ehd1 activity by direct interaction with Ehd1 to form an inactive heterodimer, and delays heading (Cho et al., 2016).
Post‐translational regulation of Ghd7 function
Previous studies have found that Ghd7 transcription was increased or unaltered in phyB mutants (Osugi et al., 2011; Weng et al., 2014). However, the level of Ghd7 protein was significantly decreased in a phyB mutant under LD conditions, suggesting that PhyB maintains the level of Ghd7 protein (Weng et al., 2014). More recently, one study found that constitutive expression of Ghd7 on a se5 background failed to accumulate Ghd7 protein or delay heading, suggesting that phytochromes are necessary for Ghd7 protein stability and normal function (Zheng et al., 2019). Further in vitro and in vivo analyses revealed that phytochromes (mainly OsPHYA and OsPHYB) directly interact with Ghd7 and stabilise Ghd7 protein. OsGI also interacts with Ghd7 and mediates its degradation in a 26S proteasome‐dependent manner. Interestingly, both OsPHYA and OsPHYB inhibit the interaction between OsGI and Ghd7, therefore blocking OsGI‐mediated Ghd7 degradation (Zheng et al., 2019). In addition to regulation of protein stability by phytochromes and OsGI, Ghd7 was also reported to be phosphorylated by Heading date 16 (Hd16), a casein kinase‐I protein (Hori et al., 2013). Hd16 delays heading by interaction with, and phosphorylation of, Ghd7, thereby enhancing the function of Ghd7, and suppressing expression of Ehd1 and downstream florigen genes (Hori et al., 2013).
Fine‐tuning photoperiod‐controlled flowering in rice by phosphorylation of floral regulators
In addition to the above‐mentioned phosphorylation and enhancement functions of Ghd7, Hd16 was earlier identified as EARLY FLOWERING 1 (EL1), which phosphorylates DELLA protein SLR1 (Slender rice 1, a key component in gibberellin signalling). Phosphorylation of SLR1 is important for maintaining its stability and negative regulation of gibberellin signalling (Dai & Xue, 2010). As phosphorylation of both Ghd7 and SLR1 results in suppression of heading under LD conditions, it is possible that Hd16 regulates both photoperiod and gibberellin responses by targeting different substrates in the rice flowering pathway. DTH7/OsPRR37/Ghd7.1/Hd2 is another substrate of Hd16. DTH7 directly interacts with, and is phosphorylated by, Hd16 in the middle and CCT domain‐containing C‐terminal regions (Choon‐Tak et al., 2015). DTH7 can also be phosphorylated in the middle region by casein kinase 2α (CK2α) encoded by the Heading date 6 (Hd6) gene (Choon‐Tak et al., 2015). Hd6, identified by QTL mapping, delays heading specifically under LD conditions. Other genetic studies have revealed that Hd6 delays heading only when Hd1 is functional. Extensive studies have revealed that Hd6 does not regulate Hd1 transcription, but represses expression of Hd3a and RFT1 in an Hd1‐dependent manner, suggesting that Hd6 modulates Hd1 function post‐translationally. However, Hd6 does not directly phosphorylate Hd1 protein, implying the presence of an unknown Hd6 target that might function with Hd1 (Ogiso et al., 2010). It has been reported that Hd1 acts genetically downstream of DTH7 to delay flowering time in LD conditions (Lin et al., 2000), and that DTH7 is necessary for LD heading repression function for conversion of Hd1 (Z. Y. Zhang et al., 2019). Given the direct phosphorylation of DTH7 by Hd6 (Choon‐Tak et al., 2015), DTH7 is likely to be a candidate protein that bridges the gap between Hd6 and Hd1. Further genetic and biochemical evidence is necessary to confirm this hypothesis.
Hd1 was also reported to be phosphorylated by OsK4, another protein kinase identified as Heading Date Repressor1 (HDR1) interaction protein. The HDR1–OsK4 complex acts to inhibit flowering in LDs by both transcriptional regulation of Hd1/Ehd1 expression, and probably by regulation of the phosphorylation state of Hd1 (Sun et al., 2016). Hd6 can phosphorylate OsLHY, an orthologue of the Arabidopsis circadian oscillator components LATE ELONGATED HYPOCOTYLN(LHY) (Ogiso et al., 2010), but mutation in either Hd6 or Hd16 does not affect the circadian clock (Nemoto et al., 2018). Natural variations in Hd6 and Hd16 are involved in fine tuning the critical day length in photoperiod‐controlled heading and may contribute to further northward expansion of rice cultivation areas (Nemoto et al., 2018).
Domestication and genetic improvement promote expansion of the rice growing region
The ancestral species of Asian cultivated rice (Oryza rufipogon Griff.) is SD sensitive and its distribution is limited to low‐latitude tropical and subtropical regions. After natural selection and artificial domestication, cultivated rice is now distributed across an extremely wide range of latitudes from 53°N to 40°S, and is grown under both LD and SD conditions (Koo et al., 2013). Weakened photoperiod sensitivity is a critical factor for adaptation of rice to high‐latitude regions. Allelic variants of several heading date genes have contribute to the northward advance of rice growing in Asia. Hd1, Ghd7, DTH8, Hd16, DTH7 and PhyB are flowering repressor genes under LDs. Combined Hd1, Ghd7 and DTH8 alleles produce strong photoperiod sensitivity, and are preferably distributed to low latitudes (Zong et al., 2021). Deletion or weakened alleles of these genes are associated with early flowering under natural day length and are preferably distributed to high latitudes (Xue et al., 2008; Takahashi et al., 2009; Wei et al., 2010; Huang et al., 2012; Koo et al., 2013; Gao et al., 2014; Kwon et al., 2014). Combinations of weak alleles of Ghd7, DTH8 and DTH7 act additively to reduce photoperiod sensitivity, and are likely to occur in high‐latitude areas such as the north‐eastern provinces of China (Gao et al., 2014). Further analysis revealed that genetic variations in DTH8, Ghd7, Hd1, DTH7, PhyB and OsCOL4 are correlated with differences in heading date, and a minimum combination of four weak alleles is required for the successful cultivation of rice at latitudes above 30°N (Zheng et al., 2016; Cui et al., 2020). Moreover, flowering activation genes, such as DTH2, Ehd4 and RFT1, have also undergone intensive selection and contributed to regional adaptation of rice to higher latitudes (Gao et al., 2013; Wu et al., 2013; Zhao et al., 2015).
Heading date genes contribute to heterosis in rice
Studies on natural variation and various genetic populations have revealed that several major heading date genes were also important heterosis genes, such as Hd3a, DTH8 and Ghd7 (Huang et al., 2016; Li et al., 2016). The heterozygous state of Hd3a is common in the most planted hybrid rice cultivars grown in China. Among 1063 three‐line hybrids studied, 98.5% of restorer lines had the ancestral type of homozygous Hd3a allele (Hd3a/Hd3a), whereas 76.7% of cytoplasmic male sterile lines were homozygous hd3a allele (hd3a/hd3a). The heterozygous state (Hd3a/hd3a) had higher grain numbers per panicle, seed setting rate and grain yield per plant than their parents (Huang et al., 2016). The heterozygous state of the tomato florigen SINGLE FLOWER TRUSS (orthologue of rice Hd3a) gene increased the yield in distinct genetic backgrounds and environments (Krieger et al., 2010). DTH8 is another major heading date gene that contributes to yield heterosis in hybrid rice cultivars. The heterozygous state, consisting of a strong allele and a nonfunctional allele of DTH8 (DTH8/dth8), is present in many Chinese hybrids, particularly in the two‐line hybrids (40.8%), including the well known superhybrid rice Liang‐you‐pei 9 (LYP9) (Li et al., 2016). More recently, studies have shown that the DTH8 locus has been divergently selected among rice subpopulations, and the genetic segment carrying japonica type DTH8 was introgressed into the male parent in modern indica hybrid cultivar breeding, and contributed to heterosis with overdominance effects (Huang et al., 2016; Lin et al., 2020). Similarly, Ghd7 has been divergently selected in the male and female parents of hybrid rice and that heterozygous state of Ghd7 consisting of a strong allele and a weak or nonfunctional allele was also found to be common in Chinese hybrid rice cultivars (Li et al., 2016; Lin et al., 2020).
The underlying mechanism of heterozygosity in heading date genes leading to heterosis is still not clear. One possible explanation is the pleiotropic effect of these heading date genes, for example Hd3a promotes lateral branching in addition to inducing flowering (Tsuji et al., 2015), and DTH8 and Ghd7 affect heading date, plant height and grain number per panicle simultaneously (Xue et al., 2008; Wei et al., 2010). We postulate that the heterozygous genotype may help to reach a balance on proper flowering time and ideal plant architecture. Whether other heading date genes also contribute to heterosis is an interesting subject, more studies are needed to reveal the underlying mechanism.
Conclusions and perspectives
Many genes controlling heading date in rice have been identified in recent decades. Our understanding of how these genes coordinately regulate heading date remain fragmentary. Gene expression can be regulated at the transcriptional and post‐transcriptional levels. In addition, there is evidence that epigenetic regulation also plays important roles in flowering time regulation. For example, many histone methylation‐related proteins (such as SDG701, SDG708, SDG724, SDG725, SDG711, SDG718, SDG723, LC2/OsVIL3, OsVIL2 and OsEMF2b) regulate flowering by methylation on the lysine residues in the N‐tails of histone H3 and cause either transcriptional silencing or activation of major flowering genes (Sun et al., 2012; Sui et al., 2013; Wang et al., 2013; Yang et al., 2013; Choi et al., 2014; Liu et al., 2014; Liu et al., 2016; Liu et al., 2017; Jiang et al., 2018; Liu et al., 2019). Se14 encodes a Jumonji C‐domain‐containing histone demethylase, HDT701 (also known as OsHDT1) and OsSFL1 encode components of histone deacetylase, which also strongly affect heading date (Li et al., 2011; Yokoo et al., 2014; Cho et al., 2018; Geng et al., 2020). Interested readers on this subject are referred to previous reviews (Shi et al., 2014; Sun et al., 2014).
Inspired by the well established flowering regulation pathways in the LD plant Arabidopsis, and through analysis of the expression of heading date genes in different rice genetic backgrounds, an evolutionary conserved OsGI–Hd1–Hd3a pathway and a rice‐specific Ehd1 pathway were proposed. Ehd1 was earlier proposed to function independently of Hd1, as it can effectively promote heading in a Hd1‐deficient background (Doi et al., 2004). However, some studies at the protein level have uncovered physical interactions between Hd1 and Ghd7, which form a complex that binds to the Ehd1 promoter to repress its expression (Nemoto et al., 2016), suggesting that day length responses in rice are controlled by integration of the two pathways into Ehd1 to repress heading under LD conditions.
Future perspective
Compared with the transcriptional regulation pathway in heading date, our knowledge on regulation of protein levels is still limited. Post‐transcriptional regulation of flowering has been extensively studied in Arabidopsis. Although there is conservation of the flowering mechanism between rice and Arabidopsis, rice is a SD plant and it is inappropriate to directly transfer knowledge from a LD plant such as Arabidopsis to rice. Further study at the level of protein regulation of heading date in rice will be helpful to distinguish the functions and evolutionary processes in photoperiod‐controlled growth of plants. Taking advantage of rapidly improving in planta protein–protein interaction detection methods, including traditional affinity purification‐mass spectrometry (AP‐MS) and newly developed proximity labelling methods such as TurboID‐mediated proximity labelling (Branon et al., 2018), large‐scale identification of interacting proteins with known heading date regulators is now possible. CRISPR (clustered regularly interspaced short palindromic repeats)‐based genomic editing will also facilitate the rapid unveiling of the functions of interacting proteins. Moreover, a recently proposed initiative to tag all rice proteins in locus using a CRISPR‐Cas‐based targeted insertion method (named the Rice Protein Tagging Project, RPTP) should greatly facilitate functional studies on heading date and other biological processes in rice (Lu et al., 2020).
Another important limitation of the study of heading date in rice is the diverse genetic backgrounds used in different studies, by contrast with the relatively centralised background in Arabidopsis (such as the Col‐0 ecotype). As functions of genes can vary in different backgrounds it is often difficult to integrate some conflicting results from multiple studies. We propose that the rice community systematically generate genetic resources in a common genetic background (such as Nipponbare for japonica and 93‐11 for indica) through genomic editing, transgenesis and traditional backcrossing. Integrating different genes and building a complete heading date pathway with the support of genetic evidence in a single background will advance our understanding of the plant flowering mechanism more rapidly, especially at the protein level.
Applications in rice breeding
The breeding of rice cultivars with optimum heading dates is crucial for high yield in specific cropping areas. In traditional breeding of rice (a predominantly self‐pollinated species) we select individuals with the desired heading date. However, this work becomes challenging in hybrid rice breeding, as testing heading date of every hybrid combination is labour and time consuming. This limitation is especially a problem in indica × japonica hybrids in which delayed heading is common. This bottleneck of unpredictable heading date limits the exploitation of substantial intersubspecies heterosis. Over past decades, many genes controlling flowering in rice have been cloned and functionally analysed, and their allelic distributions in different cropping areas have also been characterised. This should allow the rational design of cultivars with heading gene combinations best adapted to specific environmental conditions (Fig. 3). One approach is to pyramid heading date alleles in predetermined combinations by traditional cross‐breeding with the assistance of molecular markers, as attempted in a previous study (Wei et al., 2010). The second approach is to modulate the function or expression levels of heading date genes by genetic engineering, as many genes regulate rice heading date quantitatively. This can be achieved through the downregulation of genes by RNA interference or upregulation of genes by specific promoters (Hu et al., 2019). The third approach is to modify heading date genes using gene editing technology. CRISPR‐Cas genome editing enables targeted modification of specific heading date genes without affecting the genetic background, including targeted gene knockout, nucleotide base editing, insertion, deletion or replacement (Zhu et al., 2020). Moreover, multiple targets can be changed simultaneously, making breeding of new varieties with predetermined heading date gene combinations more efficient. Although exogenous T‐DNA is usually needed to deliver CRISPR‐Cas reagents into rice cells, these genetic elements can be easily removed in later generations to obtain transgene‐free edited lines. These advantages make the CRISPR‐Cas approach a fast, feasible and reliable technology in regulating heading date in rice.
Fig. 3.
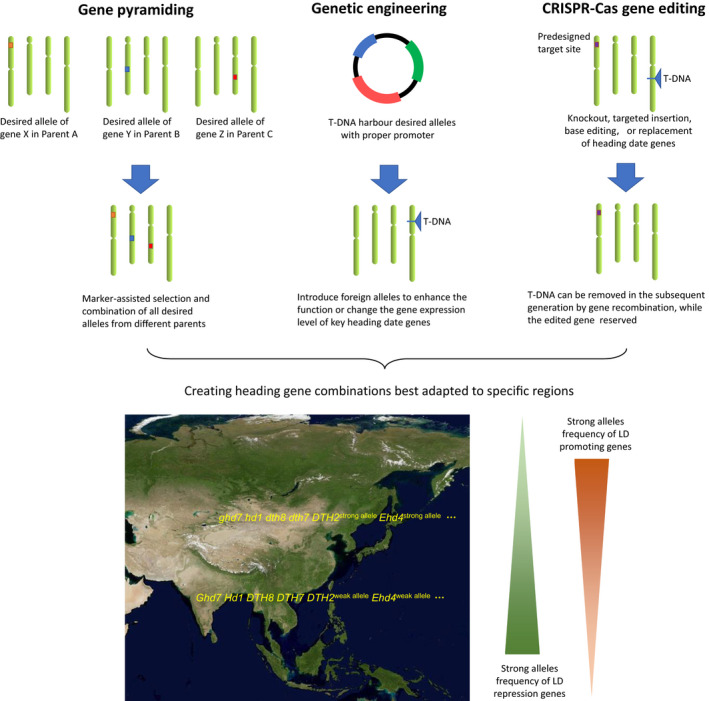
Strategies for breeding rice cultivars with optimum heading dates. Rational design of long day (LD) repression genes (such as Ghd7 and DTH8) and LD promoting genes (such as DTH2 and Ehd4) for rice cultivars adapting to different latitudes. Three strategies can be used, including pyramiding heading date alleles by traditional cross‐breeding with the assistance of molecular markers, modulating the function or expression level of heading date genes by genetic engineering, and modifying heading genes by CRISPR‐Cas gene editing technology.
Author contributions
JMW conceived the article. SRZ and SSZ contributed to the development of the ideas expressed within it. SRZ wrote the manuscript, with contributions from SSZ, SC, HGH, HQW, BYH, LC, ZX, LLL, LJ and HYW. SRZ and SSZ contributed equally to this work.
Acknowledgements
We apologise to colleagues whose work could not be included owing to space constraints. This work was supported by the Key Laboratory of Biology, Genetics and Breeding of Japonica Rice in Mid‐lower Yangtze River, Ministry of Agriculture and Rural Affairs, China; the Collaborative Innovation Center for Hybrid Rice in Yangtze River, China; and the Jiangsu Collaborative Innovation Center for Modern Crop Production, China; the National Key Research and Development Program of China (2020YFE0202300, 2016YFD0101801), the National Natural Science Foundation of China (31971910, 92035301) and Agricultural Science and Technology Innovation Fund project of Jiangsu Province (SCX(19)1079). The authors declare that they have no conflict of interest.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Andrés F, Galbraith DW, Talón M, Domingo C. 2009. Analysis of PHOTOPERIOD SENSITIVITY5 sheds light on the role of phytochromes in photoperiodic flowering in rice. Plant Physiology 151: 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian XF, Liu X, Zhao ZG, Jiang L, Gao H, Zhang YH, Zheng M, Chen LM, Liu SJ, Zhai HQ et al. 2011. Heading date gene, dth3 controlled late flowering in O. Glaberrima Steud. by down‐regulating Ehd1. Plant Cell Reports 30: 2243–2254. [DOI] [PubMed] [Google Scholar]
- Blumel M, Dally N, Jung C. 2015. Flowering time regulation in crops‐what did we learn from Arabidopsis? Current Opinion in Biotechnology 32: 121–129. [DOI] [PubMed] [Google Scholar]
- Brambilla V, Martignago D, Goretti D, Cerise M, Somssich M, de Rosa M, Galbiati F, Shrestha R, Lazzaro F, Simon R et al. 2017. Antagonistic Transcription Factor Complexes Modulate the Floral Transition in Rice. The Plant Cell 29: 2801–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branon TC, Bosch JA, Sanchez AD, Udeshi ND, Svinkina T, Carr SA, Feldman JL, Perrimon N, Ting AY. 2018. Efficient proximity labeling in living cells and organisms with TurboID. Nature Biotechnology 36: 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünning E. 1960. Circadian rhythms and the time measurement in photoperiodism. Cold Spring Harbor Symposia on Quantitative Biology 25: 249–256. [DOI] [PubMed] [Google Scholar]
- Cai M, Chen S, Wu M, Zheng T, Zhou L, Li C, Zhang H, Wang J, Xu X, Chai J et al. 2019. Early heading 7 interacts with DTH8, and regulates flowering time in rice. Plant Cell Reports 38: 521–532. [DOI] [PubMed] [Google Scholar]
- Cai M, Zhu S, Wu M, Zheng X, Wang J, Zhou L, Zheng T, Cui S, Zhou S, Li C et al. 2020. DHD4, A CONSTANS‐like family transcription factor, delays heading date through affecting the formation of FAC complex in rice. Molecular Plant. doi: 10.1016/j.molp.2020.11.013. [DOI] [PubMed] [Google Scholar]
- Chai J, Zhu S, Li C, Wang C, Cai M, Zheng X, Zhou L, Zhang H, Sheng P, Wu M et al. 2020. OsRE1 interacts with OsRIP1 to regulate rice heading date by finely modulating Ehd1 expression. Plant Biotechnology Journal. doi: 10.1111/pbi.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho L‐H, Yoon J, Pasriga R, An G. 2016. Homodimerization of Ehd1 is required to induce flowering in rice. Plant Physiology 170: 2159–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho LH, Yoon J, Wai AH, An G. 2018. Histone deacetylase 701 (HDT701) induces flowering in rice by modulating expression of OsIDS1. Molecular Cells 41: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SC, Lee S, Kim S‐R, Lee Y‐S, Liu C, Cao X, An G. 2014. Trithorax group protein Oryza sativa Trithorax1 controls flowering time in rice via interaction with early heading date3. Plant Physiology 164: 1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choon‐Tak K, Bon‐Hyuk K, Dami K, Soo‐Cheul Y, Nam‐Chon P. 2015. Casein kinases I and 2α phosphorylate Oryza sativa pseudo‐response regulator 37 (OsPRR37) in photoperiodic flowering in rice. Molecules and Cells 38: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Wang J, Feng L, Liu S, Li J, Qiao W, Song Y, Zhang Z, Cheng Y, Zhang L et al. 2020. A combination of long‐day suppressor genes contributes to the northward expansion of rice. Frontiers in Plant Science 11: 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Xue HW. 2010. Rice early flowering1, a CKI, phosphorylates DELLA protein SLR1 to negatively regulate gibberellin signalling. EMBO Journal 29: 1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Ding Y, Tan L, Fu Y, Liu F, Zhu Z, Sun X, Sun X, Gu P, Cai H et al. 2012. LHD1, an allele of DTH8/Ghd8, controls late heading date in common wild rice (Oryza rufipogon). Journal of Integrative Plant Biology 54: 790–799. [DOI] [PubMed] [Google Scholar]
- Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A. 2004. Ehd1, a B‐type response regulator in rice, confers short‐day promotion of flowering and controls FT‐Iike gene expression independently of Hd1. Genes & Development 18, 926–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A, Tian W, Wei M, Yan W, He H, Zhou D, Huang X, Li S, Ouyang X. 2017. The DTH8‐Hd1 module mediates day‐length‐dependent regulation of rice flowering. Molecular Plant 10: 948–961. [DOI] [PubMed] [Google Scholar]
- Fang J, Zhang F, Wang H, Wang W, Zhao F, Li Z, Sun C, Chen F, Xu F, Chang S et al. 2019. Ef‐cd locus shortens rice maturity duration without yield penalty. Proceedings of the National Academy of Sciences, USA 116: 18717–18722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C, Yang XO, Chen X, Chen W, Ma Y, Hu J, Li S. 2009. OsEF3, a homologous gene of Arabidopsis ELF3, has pleiotropic effects in rice. Plant Biology 11: 751–757. [DOI] [PubMed] [Google Scholar]
- Gao H, Jin M, Zheng XM, Chen J, Yuan D, Xin Y, Wang M, Huang D, Zhang Z, Zhou K et al. 2014. Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proceedings of the National Academy of Sciences, USA 111: 16337–16342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Zheng XM, Fei G, Chen J, Jin M, Ren Y, Wu W, Zhou K, Sheng P, Zhou F et al. 2013. Ehd4 encodes a novel and Oryza‐genus‐specific regulator of photoperiodic flowering in rice. PLoS Genetics 9: e1003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng YK, Zhang PX, Liu Q, Wei ZW, Riaz A, Chachar S, Gu XF. 2020. Rice homolog of Sin3‐associated polypeptide 30, OsSFL1, mediates histone deacetylation to regulate flowering time during short days. Plant Biotechnology Journal 18: 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goretti D, Martignago D, Landini M, Brambilla V, Gomez‐Ariza J, Gnesutta N, Galbiati F, Collani S, Takagi H, Terauchi R et al. 2017. Transcriptional and post‐transcriptional mechanisms limit heading date 1 (Hd1) function to adapt rice to high latitudes. PLoS Genetics 13: e1006530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SH, Yoo SC, Lee BD, An G, Paek NC. 2015. Rice FLAVIN‐BINDING, KELCH REPEAT, F‐BOX 1 (OsFKF1) promotes flowering independent of photoperiod. Plant, Cell & Environment 38: 2527–2540. [DOI] [PubMed] [Google Scholar]
- Hayama R, Izawa T, Shimamoto K. 2002. Isolation of rice genes possibly involved in the photoperiodic control of flowering by a fluorescent differential display method. Plant and Cell Physiology 43: 494–504. [DOI] [PubMed] [Google Scholar]
- Hirose F, Shinomura T, Tanabata T, Shimada H, Takano M. 2006. Involvement of rice cryptochromes in de‐etiolation responses and flowering. Plant and Cell Physiology 47: 915–925. [DOI] [PubMed] [Google Scholar]
- Hori K, Ogiso‐Tanaka E, Matsubara K, Yamanouchi U, Ebana K, Yano M. 2013. Hd16, a gene for casein kinase I, is involved in the control of rice flowering time by modulating the day‐length response. The Plant Journal 76: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li S, Xing Y. 2019. Lessons from natural variations: artificially induced heading date variations for improvement of regional adaptation in rice. Theoretical and Applied Genetics 132: 383–394. [DOI] [PubMed] [Google Scholar]
- Huang CL, Hung CY, Chiang YC, Hwang CC, Hsu TW, Huang CC, Hung KH, Tsai KC, Wang KH, Osada N et al. 2012. Footprints of natural and artificial selection for photoperiod pathway genes in Oryza . The Plant Journal 70: 769–782. [DOI] [PubMed] [Google Scholar]
- Huang X, Yang S, Gong J, Zhao Q, Feng Q, Zhan Q, Zhao Y, Li W, Cheng B, Xia J et al. 2016. Genomic architecture of heterosis for yield traits in rice. Nature 537: 629–633. [DOI] [PubMed] [Google Scholar]
- Ishikawa R, Aoki M, Kurotani K, Yokoi S, Shinomura T, Takano M, Shimamoto K. 2011. Phytochrome B regulates Heading date 1 (Hd1)‐mediated expression of rice florigen Hd3a and critical day length in rice. Molecular Genetics and Genomics 285: 461–470. [DOI] [PubMed] [Google Scholar]
- Ishikawa R, Tamaki S, Yokoi S, Inagaki N, Shinomura T, Takano M, Shimamoto K. 2005. Suppression of the floral activator Hd3a is the principal cause of the night break effect in rice. The Plant Cell 17: 3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Nonoue Y, Yano M, Izawa T. 2010. A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nature Genetics 42: 635–638. [DOI] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K. 2002. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes & Development 16: 2006–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Wang S, Zheng H, Li H, Zhang F, Su Y, Xu Z, Lin H, Qian Q, Ding Y. 2018. SIP1 participates in regulation of flowering time in rice by recruiting OsTrx1 to Ehd1. New Phytologist 219: 422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang PF, Wang SL, Jiang HY, Cheng BJ, Wu KQ, Ding Y. 2018. The COMPASS‐like complex promotes flowering and panicle branching in rice. Plant Physiology 176: 2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko‐Suzuki M, Kurihara‐Ishikawa R, Okushita‐Terakawa C, Kojima C, Nagano‐Fujiwara M, Ohki I, Tsuji H, Shimamoto K, Taoka KI. 2018. TFL1‐like proteins in rice antagonize rice FT‐like protein in inflorescence development by competition for complex formation with 14‐3‐3 and FD. Plant and Cell Physiology 59: 458–468. [DOI] [PubMed] [Google Scholar]
- Kang HG, Jang S, Chung JE, Cho YG, An G. 1997. Characterization of two rice MADS box genes that control flowering time. Molecules and Cells 7: 559–566. [PubMed] [Google Scholar]
- Kim SL, Lee SY, Kim HJ, Nam HG, An GH. 2007. OsMADS51 is a short‐day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3a . Plant Physiology 145: 1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Park HY, Jang YH, Lee KC, Chung YS, Lee JH, Kim JK. 2016. OsNF‐YC2 and OsNF‐YC4 proteins inhibit flowering under long‐day conditions in rice. Planta 243: 563–576. [DOI] [PubMed] [Google Scholar]
- Kim S‐K, Yun C‐H, Lee JH, Jang YH, Park H‐Y, Kim J‐K. 2008. OsCO3, a CONSTANS‐LIKE gene, controls flowering by negatively regulating the expression of FT‐like genes under SD conditions in rice. Planta 228: 355. [DOI] [PubMed] [Google Scholar]
- Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M. 2002. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short‐day conditions. Plant and Cell Physiology 43: 1096–1105. [DOI] [PubMed] [Google Scholar]
- Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K. 2008. Hd3a and RFT1 are essential for flowering in rice. Development 135: 767–774. [DOI] [PubMed] [Google Scholar]
- Koo BH, Yoo SC, Park JW, Kwon CT, Lee BD, An G, Zhang Z, Li J, Li Z, Paek NC. 2013. Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Molecularl Plant 6: 1877–1888. [DOI] [PubMed] [Google Scholar]
- Krieger U, Lippman ZB, Zamir D. 2010. The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nature Genetics 42: 459–463. [DOI] [PubMed] [Google Scholar]
- Kwon CT, Yoo SC, Koo BH, Cho SH, Park JW, Zhang Z, Li J, Li Z, Paek NC. 2014. Natural variation in Early flowering1 contributes to early flowering in japonica rice under long days. Plant, Cell & Environment 37: 101–112. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim J, Han J‐J, Han M‐J, An G. 2004. Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS‐LIKE 20 (SOC1/AGL20) ortholog in rice. The Plant Journal 38: 754–764. [DOI] [PubMed] [Google Scholar]
- Lee YS, Jeong DH, Lee DY, Yi J, Ryu CH, Kim SL, Jeong HJ, Choi SC, Jin P, Yang J et al. 2010. OsCOL4 is a constitutive flowering repressor upstream of Ehd1 and downstream of OsphyB . The Plant Journal 63: 18–30. [DOI] [PubMed] [Google Scholar]
- Li C, Huang L, Xu C, Zhao Y, Zhou DX. 2011. Altered levels of histone deacetylase OsHDT1 affect differential gene expression patterns in hybrid rice. PLoS ONE 6: e21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Huang Z, Song S, Xin Y, Mao D, Lv Q, Zhou M, Tian D, Tang M, Wu Q et al. 2016. Integrated analysis of phenome, genome, and transcriptome of hybrid rice uncovered multiple heterosis‐related loci for yield increase. Proceedings of the National Academy of Sciences, USA 113: E6026–E6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DJ, Yang CH, Li XB, Gan Q, Zhao XF, Zhu LH. 2009. Functional characterization of rice OsDof12. Planta 229: 1159–1169. [DOI] [PubMed] [Google Scholar]
- Lin HX, Yamamoto T, Sasaki T, Yano M. 2000. Characterization and detection of epistatic interactions of 3 QTLs, Hd1, Hd2, and Hd3, controlling heading date in rice using nearly isogenic lines. Theoretical and Applied Genetics 101: 1021–1028. [Google Scholar]
- Lin Z, Qin P, Zhang X, Fu C, Deng H, Fu X, Huang Z, Jiang S, Li C, Tang X et al. 2020. Divergent selection and genetic introgression shape the genome landscape of heterosis in hybrid rice. Proceedings of the National Academy of Sciences, USA 117: 4623–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Liu Y, Wang B, Luo Q, Shi J, Gan J, Shen WH, Yu Y, Dong A. 2019. The transcription factor OsSUF4 interacts with SDG725 in promoting H3K36me3 establishment. Nature Communications 10: 2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wei G, Shi JL, Jin J, Shen T, Ni T, Shen WH, Yu Y, Dong AW. 2016. SET DOMAIN GROUP 708, a histone H3 lysine 36‐specific methyltransferase, controls flowering time in rice (Oryza sativa). New Phytologist 210: 577–588. [DOI] [PubMed] [Google Scholar]
- Liu KP, Yu Y, Dong AW, Shen WH. 2017. SET DOMAIN GROUP701 encodes a H3K4‐methytransferase and regulates multiple key processes of rice plant development. New Phytologist 215: 609–623. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhou C, Zhao Y, Zhou S, Wang W, Zhou DX. 2014. The rice enhancer of zeste [E(z)] genes SDG711 and SDG718 are respectively involved in long day and short day signaling to mediate the accurate photoperiod control of flowering time. Frontiers in Plant Science 5: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SJ, Wei H, Wang Y, Wang HM, Yang RF, Zhang XB, Tu JM. 2012. Overexpression of a transcription factor OsMADS15 modifies plant architecture and flowering time in rice (Oryza sativa L.). Plant Molecular Biology Reporter 30, 1461–1469. [Google Scholar]
- Lu Y, Ronald PC, Han B, Li J, Zhu J‐K. 2020. Rice protein tagging project: a call for international collaborations on genome‐wide in‐locus tagging of rice proteins. Molecular Plant 13: 1663–1665. [DOI] [PubMed] [Google Scholar]
- Luccioni L, Krzymuski M, Sanchez‐Lamas M, Karayekov E, Cerdan PD, Casal JJ. 2019. CONSTANS delays Arabidopsis flowering under short days. The Plant Journal 97: 923–932. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Ogiso‐Tanaka E, Hori K, Ebana K, Ando T, Yano M. 2012. Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant and Cell Physiology 53: 709–716. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Yamanouchi U, Wang Z‐X, Minobe Y, Izawa T, Yano M. 2008. Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up‐regulating Ehd1 . Plant Physiology 148: 1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K, Yamanouchi U, Nonoue Y, Sugimoto K, Wang Z‐X, Minobe Y, Yano M. 2011. Ehd3, encoding a plant homeodomain finger‐containing protein, is a critical promoter of rice flowering. The Plant Journal 66: 603–612. [DOI] [PubMed] [Google Scholar]
- Minh‐Thu PT, Kim JS, Chae S, Jun KM, Lee GS, Kim DE, Cheong JJ, Song SI, Nahm BH, Kim YK. 2018. A WUSCHEL Homeobox transcription factor, OsWOX13, enhances drought tolerance and triggers early flowering in rice. Molecules and Cells 41: 781–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Shimamoto K, Kyozuka J. 2002. Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER 1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. The Plant Journal 29: 743–750. [DOI] [PubMed] [Google Scholar]
- Nemoto Y, Hori K, Izawa T. 2018. Fine‐tuning of the setting of critical day length by two casein kinases in rice photoperiodic flowering. Journal of Experimental Botany 69: 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto Y, Nonoue Y, Yano M, Izawa T. 2016. Hd1, a CONSTANS ortholog in rice, functions as an Ehd1 repressor through interaction with monocot‐specific CCT‐domain protein Ghd7. The Plant Journal 86: 221–233. [DOI] [PubMed] [Google Scholar]
- Nishida H, Okumoto Y, Nakagawa H, Ichitani K, Inoue H, Tanisaka T. 2001. Analysis of tester lines for rice (Oryza sativa L.) heading‐time genes using reciprocal photoperiodic transfer treatments. Annals of Botany 88, 527–536. [Google Scholar]
- Ogiso E, Takahashi Y, Sasaki T, Yano M, Izawa T. 2010. The role of casein kinase II in flowering time regulation has diversified during evolution. Plant Physiology 152: 808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osugi A, Itoh H, Ikeda‐Kawakatsu K, Takano M, Izawa T. 2011. Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice. Plant Physiology 157: 1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng LT, Shi ZY, Li L, Shen GZ, Zhang JL. 2008. Overexpression of transcription factor OsLFL1 delays flowering time in Oryza sativa . Journal of Plant Physiology 165: 876–885. [DOI] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. 1995. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80: 847–857. [DOI] [PubMed] [Google Scholar]
- Rao NN, Prasad K, Kumar PR, Vijayraghavan U. 2008. Distinct regulatory role for RFL, the rice LFY homolog, in determining flowering time and plant architecture. Proceedings of the National Academy of Sciences, USA 105: 3646–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu CH, Lee S, Cho LH, Kim SL, Lee YS, Choi SC, Jeong HJ, Yi J, Park SJ, Han CD et al. 2009. OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)‐dependent flowering in rice. Plant, Cell & Environment 32: 1412–1427. [DOI] [PubMed] [Google Scholar]
- Saito H, Ogiso‐Tanaka E, Okumoto Y, Yoshitake Y, Izumi H, Yokoo T, Matsubara K, Hori K, Yano M, Inoue H et al. 2012. Ef7 encodes an ELF3‐like protein and promotes rice flowering by negatively regulating the floral repressor gene Ghd7 under both short‐ and long‐day conditions. Plant and Cell Physiology 53: 717–728. [DOI] [PubMed] [Google Scholar]
- Saito H, Okumoto Y, Yoshitake Y, Inoue H, Yuan Q, Teraishi M, Tsukiyama T, Nishida H, Tanisaka T. 2011. Complete loss of photoperiodic response in the rice mutant line X61 is caused by deficiency of phytochrome chromophore biosynthesis gene. Theoretical and Applied Genetics 122: 109–118. [DOI] [PubMed] [Google Scholar]
- Sheng P, Wu F, Tan J, Zhang H, Ma W, Chen L, Wang J, Wang J, Zhu S, Guo X et al. 2016. A CONSTANS‐like transcriptional activator, OsCOL13, functions as a negative regulator of flowering downstream of OsphyB and upstream of Ehd1 in rice. Plant Molecular Biology 92: 209–222. [DOI] [PubMed] [Google Scholar]
- Shi J, Dong A, Shen WH. 2014. Epigenetic regulation of rice flowering and reproduction. Frontiers in Plant Science 5: 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibaya T, Hori K, Ogiso‐Tanaka E, Yamanouchi U, Shu K, Kitazawa N, Shomura A, Ando T, Ebana K, Wu J et al. 2016. Hd18, encoding histone acetylase related to Arabidopsis FLOWERING LOCUS D, is involved in the control of flowering time in rice. Plant and Cell Physiology 57: 1828–1838. [DOI] [PubMed] [Google Scholar]
- Shrestha R, Gomez‐Ariza J, Brambilla V, Fornara F. 2014. Molecular control of seasonal flowering in rice, Arabidopsis and temperate cereals. Annals of Botany 114: 1445–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Chen Y, Liu L, Wang Y, Bao S, Zhou X, Teo ZWN, Mao C, Gan Y, Yu H. 2017. OsFTIP1‐mediated regulation of florigen transport in rice is negatively regulated by the ubiquitin‐like domain kinase OsUbDKγ4. The Plant Cell 29: 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Shim JS, Kinmonth‐Schultz HA, Imaizumi T. 2015. Photoperiodic flowering: time measurement mechanisms in leaves. Annual Review of Plant Biology 66: 441–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Shan JX, Gao JP, Lin HX. 2016. OsHAL3, a blue light‐responsive protein, interacts with the floral regulator Hd1 to activate flowering in rice. Molecular Plant 9: 233–244. [DOI] [PubMed] [Google Scholar]
- Sui PF, Shi JL, Gao XY, Shen WH, Dong AW. 2013. H3K36 methylation is involved in promoting rice flowering. Molecular Plant 6: 975–977. [DOI] [PubMed] [Google Scholar]
- Sun C, Chen D, Fang J, Wang P, Deng X, Chu C. 2014. Understanding the genetic and epigenetic architecture in complex network of rice flowering pathways. Protein Cell 5: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Fang J, Zhao T, Xu B, Zhang F, Liu L, Tang J, Zhang G, Deng X, Chen F et al. 2012. The histone methyltransferase SDG724 mediates H3K36me2/3 deposition at MADS50 and RFT1 and promotes flowering in rice. The Plant Cell 24: 3235–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SY, Chao DY, Li XM, Shi M, Gao JP, Zhu MZ, Yang HQ, Luan S, Lin HX. 2009. OsHAL3 mediates a new pathway in the light‐regulated growth of rice. Nature Cell Biology 11: 845–U148. [DOI] [PubMed] [Google Scholar]
- Sun XH, Zhang ZG, Wu JX, Cui XA, Feng D, Wang K, Xu M, Zhou L, Han X, Gu XF et al. 2016. The Oryza sativa regulator HDR1 associates with the kinase OsK4 to control photoperiodic flowering. PLoS Genetics 12: e1005927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Shomura A, Sasaki T, Yano M. 2001. Hd6, a rice quantitative trait locus involved in photoperiod sensitivity, encodes the alpha subunit of protein kinase CK2. Proceedings of the National Academy of Sciences, USA 98: 7922–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Teshima KM, Yokoi S, Innan H, Shimamoto K. 2009. Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proceedings of the National Academy of Sciences, USA 106: 4555–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie XZ, Yuzurihara N, Hihara F, Ishizuka T, Yano M, Nishimura M, Miyao A, Hirochika H et al. 2005. Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. The Plant Cell 17: 3311–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Jin M, Wang J, Wu F, Sheng P, Cheng Z, Wang J, Zheng X, Chen L, Wang M et al. 2016. OsCOL10, a CONSTANS‐Like gene, functions as a flowering time repressor downstream of Ghd7 in rice. Plant and Cell Physiology 57: 798–812. [DOI] [PubMed] [Google Scholar]
- Taoka K‐i, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, Yamaguchi M, Nakashima C, Purwestri YA, Tamaki S et al. 2011. 14‐3‐3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476: 332. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Nakamura H, Taoka K, Shimamoto K. 2013. Functional diversification of FD transcription factors in rice, components of florigen activation complexes. Plant and Cell Physiology 54: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Tachibana C, Tamaki S, Taoka K, Kyozuka J, Shimamoto K. 2015. Hd3a promotes lateral branching in rice. The Plant Journal 82: 256–266. [DOI] [PubMed] [Google Scholar]
- Vergara BS, Chang TT. 1985. The flowering response of the rice plant to photoperiod: a review of the literature. Los Banos, Philippines: International Rice Research Institute. [Google Scholar]
- Wang J, Hu J, Qian Q, Xue HW. 2013. LC2 and OsVIL2 promote rice flowering by photoperoid‐induced epigenetic silencing of OsLF. Molecular Plant 6: 514–527. [DOI] [PubMed] [Google Scholar]
- Wang P, Gong R, Yang Y, Yu SB. 2019. Ghd8 controls rice photoperiod sensitivity by forming a complex that interacts with Ghd7. BMC Plant Biology 19: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Liu L, Xu J, Jiang L, Zhang W, Wang J, Zhai H, Wan J. 2010. Breeding strategies for optimum heading date using genotypic information in rice. Molecular Breeding 25: 287–298. [Google Scholar]
- Wei X, Xu J, Guo H, Jiang L, Chen S, Yu C, Zhou Z, Hu P, Zhai H, Wan J. 2010. DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiology 153: 1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng X, Wang L, Wang J, Hu Y, Du H, Xu C, Xing Y, Li X, Xiao J, Zhang Q. 2014. Ghd7 is a central regulator for growth, development, adaptation and responses to biotic and abiotic stresses. Plant Physiology 164: 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, You CJ, Li CS, Long T, Chen GX, Byrne ME, Zhang QF. 2008. RID1, encoding a Cys2/His2‐type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proceedings of the National Academy of Sciences, USA 105: 12915–12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Zhang Y, Zhang M, Zhan X, Shen X, Yu P, Chen D, Liu Q, Sinumporn S, Hussain K et al. 2018. The rice CONSTANS‐like protein OsCOL15 suppresses flowering by promoting Ghd7 and repressing RID1 . Biochemical and Biophysical Research Communications 495: 1349–1355. [DOI] [PubMed] [Google Scholar]
- Wu W, Zheng XM, Lu G, Zhong Z, Gao H, Chen L, Wu C, Wang HJ, Wang Q, Zhou K et al. 2013. Association of functional nucleotide polymorphisms at DTH2 with the northward expansion of rice cultivation in Asia. Proceedings of the National Academy of Sciences, USA 110: 2775–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie SY, Chen M, Pei R, Ouyang YD, Yao JL. 2015. OsEMF2b acts as a regulator of flowering transition and floral organ identity by mediating H3K27me3 deposition at OsLFL1 and OsMADS4 in rice. Plant Molecular Biology Reporter 33: 121–132. [Google Scholar]
- Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X et al. 2008. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nature Genetics 40: 761–767. [DOI] [PubMed] [Google Scholar]
- Yan W, Liu H, Zhou X, Li Q, Zhang J, Lu L, Liu T, Liu H, Zhang C, Zhang Z et al. 2013. Natural variation in Ghd7.1 plays an important role in grain yield and adaptation in rice. Cell Research 23, 969–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan WH, Wang P, Chen HX, Zhou HJ, Li QP, Wang CR, Ding ZH, Zhang YS, Yu SB, Xing YZ et al. 2011. A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Molecular Plant 4: 319–330. [DOI] [PubMed] [Google Scholar]
- Yang J, Lee S, Hang RL, Kim SR, Lee YS, Cao XF, Amasino R, An G. 2013. OsVIL2 functions with PRC2 to induce flowering by repressing OsLFL1 in rice. The Plant Journal 73: 566–578. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fu D, Zhu C, He Y, Zhang H, Liu T, Li X, Wu C. 2015. The RING‐finger ubiquitin ligase HAF1 mediates heading date 1 degradation during photoperiodic flowering in rice. The Plant Cell 27: 2455–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Yamamoto E, Aya K, Takeuchi H, Lo PC, Hu L, Yamasaki M, Yoshida S, Kitano H, Hirano K et al. 2016. Genome‐wide association study using whole‐genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nature Genetics 48: 927–934. [DOI] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y et al. 2000. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS . The Plant Cell 12: 2473–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo T, Saito H, Yoshitake Y, Xu Q, Asami T, Tsukiyama T, Teraishi M, Okumoto Y, Tanisaka T. 2014. Se14, encoding a JmjC domain‐containing protein, plays key roles in long‐day suppression of rice flowering through the demethylation of H3K4me3 of RFT1 . PLoS ONE 9: e96064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhu S, Liu T, Wang C, Cheng Z, Zhang X, Chen L, Sheng P, Cai M, Li C et al. 2019. DELAYED HEADING DATE1 interacts with OsHAP5C/D, delays flowering time and enhances yield in rice. Plant Biotechnology Journal 17: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhou X, Yan W, Zhang Z, Lu L, Han Z, Zhao H, Liu H, Song P, Hu Y et al. 2015. Combinations of the Ghd7, Ghd8 and Hd1 genes largely define the ecogeographical adaptation and yield potential of cultivated rice. New Phytologist 208: 1056–1066. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hu W, Shen G, Liu H, Hu Y, Zhou X, Liu T, Xing Y. 2017. Alternative functions of Hd1 in repressing or promoting heading are determined by Ghd7 status under long‐day conditions. Scientific Report 7: 5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Zhang B, Qi FX, Wu H, Li ZX, Xing YZ. 2019. Hd1 function conversion in regulating heading is dependent on gene combinations of Ghd7, Ghd8, and Ghd7.1 under long‐day conditions in rice. Molecular Breeding 39: 92. [Google Scholar]
- Zhao J, Chen H, Ren D, Tang H, Qiu R, Feng J, Long Y, Niu B, Chen D, Zhong T et al. 2015. Genetic interactions between diverged alleles of Early heading date 1 (Ehd1) and Heading date 3a (Hd3a)/ RICE FLOWERING LOCUS T1 (RFT1) control differential heading and contribute to regional adaptation in rice (Oryza sativa). New Phytologist 208: 936–948. [DOI] [PubMed] [Google Scholar]
- Zhao XL, Shi ZY, Peng LT, Shen GZ, Zhang JL. 2011. An atypical HLH protein OsLF in rice regulates flowering time and interacts with OsPIL13 and OsPIL15. New Biotechnology 28: 788–797. [DOI] [PubMed] [Google Scholar]
- Zheng T, Sun J, Zhou S, Chen S, Lu J, Cui S, Tian Y, Zhang H, Cai M, Zhu S et al. 2019. Post‐transcriptional regulation of Ghd7 protein stability by phytochrome and OsGI in photoperiodic control of flowering in rice. New Phytologist 224: 306–320. [DOI] [PubMed] [Google Scholar]
- Zheng XM, Feng L, Wang J, Qiao W, Zhang L, Cheng Y, Yang Q. 2016. Nonfunctional alleles of long‐day suppressor genes independently regulate flowering time. Journal of Integrative Plant Biology 58: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Peng Q, Fu D, Zhuang D, Yu Y, Duan M, Xie W, Cai Y, Ouyang Y, Lian X et al. 2018. HAF1 modulates circadian accumulation of OsELF3 controlling heading date under long‐day conditions in rice. The Plant Cell 30: 2352–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Li C, Gao C. 2020. Applications of CRISPR‐Cas in agriculture and plant biotechnology. Nature Reviews: Molecular Cell Biology 21: 661–677. [DOI] [PubMed] [Google Scholar]
- Zhu S, Wang J, Cai M, Zhang H, Wu F, Xu Y, Li C, Cheng Z, Zhang X, Guo X et al. 2017. The OsHAPL1‐DTH8‐Hd1 complex functions as the transcription regulator to repress heading date in rice. Journal of Experimental Botany 68: 553–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong W, Ren D, Huang M, Sun K, Feng J, Zhao J, Xiao D, Xie W, Liu S, Zhang H et al. 2021. Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading. New Phytologist 229: 1635–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


