Abstract
Aims
Sodium–glucose co‐transporter 2 (SGLT2) inhibitors improve clinical outcome in patients with heart failure (HF), but the mechanisms behind their beneficial effects are not yet fully understood. We examined the effects of empagliflozin on renal sodium and glucose handling in patients with acute HF.
Methods and results
This study was a pre‐defined sub‐study of a double‐blind, randomized, placebo‐controlled, multicentre study (EMPA‐RESPONSE‐AHF). Patients were allocated within 24 h of an acute HF admission to either empagliflozin 10 mg/day (n = 40) or placebo (n = 39) for 30 days. Markers of glucose and sodium handling were measured daily during the first 96 h and at day 30. Patients were 76 (range 38–89) years old and 33% had diabetes. The use of loop diuretics during the first 96 h was similar in both groups. Empagliflozin increased fractional glucose excretion with a peak after 24 h (21.8% vs. 0.1%; P < 0.001), without affecting plasma glucose concentration, while fractional sodium and chloride excretion and urinary osmolality remained unchanged (P >0.3 for all). However, empagliflozin increased plasma osmolality (delta osmolality at 72 h: 5 ± 8 vs. 2 ± 5 mOsm/kg; P = 0.049). Finally, there was an early decline in estimated glomerular filtration rate with empagliflozin vs. placebo (−10 ± 12 vs. −2 ± 12 mL/min/1.73 m2; P = 0.009), which recovered within 30 days.
Conclusion
In patients with acute HF, empagliflozin increased fractional glucose excretion and plasma osmolality, without affecting fractional sodium excretion or urine osmolality and caused a temporary decline in estimated glomerular filtration rate. This suggests that empagliflozin stimulates osmotic diuresis through increased glycosuria rather than natriuresis in patients with acute HF.
Keywords: Acute heart failure, Sodium–glucose co‐transporter 2 inhibitors, Empagliflozin, Diuresis, Kidney
Graphical representation of changes in urinary and plasma volume and osmolality. As more glucose is excreted as a result of sodium‐glucose co‐transporter 2 (SGLT2) inhibition, more water is drawn to the urine keeping osmolality constant. As a result of increased electrolyte free water excretion, plasma osmolality is moderately increased and total volume of plasma and interstitial fluid is decreased.
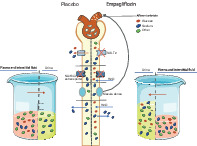
Introduction
In patients with diabetes and/or chronic kidney disease, sodium–glucose co‐transporter 2 (SGLT2) inhibitors consistently showed beneficial effects on cardiovascular outcomes and particularly on heart failure (HF) hospitalizations. 1 , 2 , 3 , 4 Recently, one larger randomized clinical trial demonstrated that dapagliflozin reduced cardiovascular death and HF hospitalizations in patients with established chronic HF with reduced ejection fraction (HFrEF) with and without diabetes. 5 A post‐hoc analysis showed that treatment with dapagliflozin was safe and effective regardless of diuretic use or dose. 6 The beneficial effects of SGLT2 inhibitors on HF outcomes have been attributed to cardiometabolic and renal protective qualities, as well as to its diuretic properties. These were even described in chronic ‘stable’ euvolaemic HF. 6 , 7 , 8 In acute HF, we recently showed that early addition of empagliflozin to standard diuretic treatment increased cumulative diuresis after 4 days with a possible reduction in HF‐related events. 9 However, the mechanisms behind increased diuresis of SGLT2 inhibitors in acute HF are unknown. 10 , 11 In the present mechanistic study, we investigated the effect of SGLT2 inhibition on renal function, and urinary sodium, chloride and glucose excretion in acute HF patients randomized to either empagliflozin or placebo.
Methods
Patients
The present study is a pre‐defined analysis of the EMPA‐RESPONSE‐AHF trial of which the rationale and main results have been published recently. 9 In short, EMPA‐RESPONSE‐AHF was a double‐blind, placebo‐controlled multicentre pilot study enrolling 79 patients in five centres in the Netherlands, on the safety and efficacy of empagliflozin in patients with acute HF. Within 24 h of hospital admission, patients were randomized 1:1 to either empagliflozin 10 mg (for 30 days) (n = 40) or matching placebo (n = 39). The trial was approved by the ethics committee at each study centre and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients participating in the trial provided written informed consent.
Biomarkers
Spot urine and plasma samples were collected at baseline, daily during the first 96 h of hospitalization and after 30 days. Serum sodium, glucose, and creatinine and spot urinary creatinine and sodium were measured as part of safety monitoring and were analysed according to procedures of the local laboratories of each participating hospital. Urinary glucose, chloride and osmolality were measured at a central laboratory in the University Medical Center Groningen (UMCG) in frozen samples. All samples were stored at –80°C within 2 h of collection and thawed before analysis. Urinary glucose was measured only after database lock to ensure maintenance of the double‐blind nature of the trial. Urinary chloride and potassium were measured using ISE indirect reagents for COBAS C, the measuring range for potassium is 3–100 mmol/L with an analytical variation of <5%, the measuring range for chloride is 20–250 mmol/L, with an analytical variation of <5%. Urinary glucose was measured using the GLUC3 pack for COBAS C, the measuring range for urinary glucose is 0.11–249.6 mmol/L (normal range: 0.06–0.83 mmol/L), with a variation of ∼1%. Urinary osmolality, the total number of solute particles (or osmoles) per kilogram of fluid, was both measured and calculated in order to gain insight in the constituents of urine osmolality. Urine osmolality was measured using an automatic freezing point depression osmometer (Osmo Station OM‐6050, ARKRAY), with a measuring range of 0–2000 mOsm/kg and an analytical variation of <1%. Between measurements tubes were capped to prevent evaporation.
Aldosterone concentrations were measured using the Aldosterone RIA kit by MT Diagnostics. Renin concentrations were measured using the Renin Kit III by Cis Bio International. Aldosterone and renin measurements were performed in the Pharmacology Laboratory of the Erasmus Medical Center Rotterdam.
Calculation of urinary osmolality was done according to the following formula: osmolality = 2 × [Na+] + 2 × [K+] + [Glucose] + [Urea], with all concentrations being concentrations of molecules in urine. The same formula was used to calculate plasma osmolality. All fractional excretion percentages were calculated by a standard formula: 100% × FEx = (Ux × Pcreat)/(Px × Ucreat), in which Ux is the urinary concentration of the analyte and Px represents the plasma concentration of the analyte. Ucreat and Pcreat represent urinary and plasma concentrations of creatinine, respectively. In sensitivity analysis, estimated 24 h urinary sodium excretion was calculated using the earlier defined formula by the International Cooperative Study on Salt, Other Factors, and Blood Pressure (INTERSALT) investigators. 12
Statistical analysis
Baseline characteristics were explored using a t‐test for normally distributed variables and a Mann–Whitney U test for non‐normally distributed variables. For further analysis, variables were normalized by logarithmic transformation where necessary. The effect of empagliflozin use on changes in clinical outcomes [i.e. estimated glomerular filtration rate (eGFR); systolic blood pressure; plasma and urinary osmolality; fractional excretion of sodium (FeNa), chloride (FeCl) and glucose (FeGlu); renin; aldosterone] during 96 h and 30 days were analysed with repeated measures linear mixed‐effect (LME) models, which account for individual variations in changes and intercepts by estimating the random effects per individual. To correct for their highly skewed nature, renin and aldosterone were log‐transformed before being analysed in the linear mixed models. For each clinical variable, change from baseline was calculated and used as an outcome in the LME model. We performed a nested model adjusted for baseline values and time, whereas for each outcome a second model was performed including baseline values, time, treatment arm and the treatment x time interaction term. Further, we compared the two models without and with the treatment interaction term using analysis of variance (ANOVA), where a P‐value <0.05 was considered significant for a treatment effect during the full treatment period (either 96 h or 30 days depending on the variable). The effects of empagliflozin on changes in outcome at specific time point were considered significant for interaction terms P < 0.05. LME models were conducted using the lme function in the ‘nlme’ package. All analyses were performed in R studio, version 1.3.959. 13
Results
Baseline characteristics of the study population have been published elsewhere. 9 In brief, patients were 76 (range 38–89) years old, 33% were female and median N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) was 5236 [interquartile range (IQR) 3482–8276] pg/mL. Background medical treatment at baseline was similar between the groups and there were no differences in loop diuretic doses, vasodilator or inotrope use or guideline‐recommended HF medication. Baseline eGFR was 54 ± 17 mL/min/1.73 m2, median plasma glucose was 7.8 (IQR 6.2–8.9) mmol/L and 33% had a history of type 2 diabetes mellitus. In urine, glucose concentrations at baseline were 0.1 (IQR 0.1–0.2) mmol/L, with low FeGlu [0.1 (IQR 0.1–0.1)%]. In urine, baseline osmolality was 335 (IQR 322–380) mOsm/kg, whereas median plasma osmolality was 305 (IQR 302–311) mOsm/kg. Before start of randomized treatment but within 24 h of admission and after initiation of loop diuretic therapy, spot urinary sodium was 99 (IQR 67–111) mmol/L, with a FeNa of 2.2 (IQR 0.9–4.3)%. No between group differences were observed between patients treated with empagliflozin or placebo for any of these baseline variables (Table 1 ).
Table 1.
Baseline characteristics
| Empagliflozin (n = 40) | Placebo (n = 39) | P‐value | |
|---|---|---|---|
| Age (years) | 79 (73–83) | 73 (61–83) | 0.141 |
| Female sex | 16 (40) | 10 (26) | 0.263 |
| Systolic blood pressure (mmHg) | 128 ± 22 | 121 ± 25 | 0.253 |
| eGFR (mL/min/1.73 m2) | 53 ± 18 | 54 ± 16 | 0.824 |
| Plasma osmolality (mOsm/kg) | 305 (302–309) | 305 (302–312) | 0.691 |
| Urine osmolality (mOsm/kg) | 330 (322–379) | 341 (324–384) | 0.530 |
| Plasma levels of: | |||
| Creatinine (mg/dL) | 1.3 ± 0.4 | 1.3 ± 0.4 | 0.723 |
| Urea (mmol/L) | 11.0 (7.5–12.8) | 9.0 (7.3–13.1) | 0.916 |
| Sodium (mmol/L) | 140 (137–142) | 140 (138–142) | 0.806 |
| Potassium (mmol/L) | 3.9 (3.5–4.2) | 3.9 (3.5–4.4) | 0.406 |
| Glucose (mmol/L) | 7.9 (6.2–9.6) | 7.7 (6.3–8.8) | 0.323 |
| Renin (pg/mL) | 12.4 (4.8–63.4) | 80.1 (14.0–179.3) | 0.011 |
| Aldosterone (pg/mL) | 182.3 (130.5–292.0) | 192.7 (114.6–344.3) | 0.653 |
| Urinary levels of: | |||
| Creatinine (mmol/L) | 3.5 (1.9–5.5) | 3.4 (2.0–5.1) | 0.944 |
| Urea (mmol/L) | 111 (60–142) | 98 (78–140) | 0.976 |
| Sodium (mmol/L) | 100 (68–110) | 92 (69–112) | 0.734 |
| Potassium (mmol/L) | 27 (21–33) | 29 (20–41) | 0.399 |
| Glucose (mmol/L) | 0.2 (0.1–0.3) | 0.1 (0.1–0.2) | 0.264 |
| Fractional excretion of (%): | |||
| Sodium | 2.1 (0.9–4.1) | 2.2 (0.9–4.4) | 0.964 |
| Glucose | 0.1 (0.1–0.1) | 0.1 (0.0–0.1) | 0.890 |
Categorical variables are depicted as n (%), normally distributed variables are depicted as mean ± standard deviation, non‐parametric variables are depicted as median (interquartile range).
eGFR, estimated glomerular filtration rate.
Table 2 shows plasma and spot urinary electrolytes over time, stratified by treatment arm. Empagliflozin significantly decreased spot urinary sodium concentration as compared with placebo. The most pronounced effect was seen after 48 h (56.2 vs. 79.0 mmol/L, P = 0.011). In contrast, treatment with empagliflozin did not change FeNa at any time point as compared with placebo (Table 2 , Figure 1A, P = 0.956 for ANOVA difference between models with and without treatment interaction), indicating that while net urinary sodium concentration decreases, a similar amount of glomerularly filtered sodium is reabsorbed in the renal tubuli compared with placebo. In sensitivity analysis, median calculated 24 h sodium excretion after 24 h also did not show differences between patients treated with empagliflozin compared with placebo (P = 0.235). Moreover, no differences in the occurrence of hyponatraemia were seen between both treatment arms (P > 0.2); delta serum sodium from baseline to 96 h also did not change between the treatment arms (−0.17 vs. −0.18 mmol/L, P = 0.99), nor did empagliflozin change serum sodium at any time point (P = 0.302 for ANOVA difference between models with and without treatment interaction). Likewise, urinary chloride excretion was lower in patients treated with empagliflozin, but FeCl was unaltered by empagliflozin use (Figure 1B, P = 0.922 for ANOVA difference between models with and without treatment interaction).
Table 2.
Urinary parameters over the course of treatment
| Empagliflozin (n = 40) | Placebo (n = 39) | P‐value | |
|---|---|---|---|
| Spot urinary sodium (mmol/L) | |||
| Baseline | 90 ± 31 | 87 ± 35 | 0.706 |
| 24 h | 69 ± 28 | 85 ± 37 | 0.040 |
| 48 h | 56 ± 29 | 79 ± 44 | 0.011 |
| 72 h | 63 ± 41 | 70 ± 27 | 0.400 |
| 96 h | 56 ± 29 | 69 ± 32 | 0.089 |
| 30 days | 60 ± 30 | 56 ± 28 | 0.683 |
| Fractional excretion of sodium (%) | |||
| Baseline | 2.1 (0.9–4.1) | 2.2 (0.9–4.4) | 0.964 |
| 24 h | 1.4 (0.9–2.4) | 1.5 (0.6–3.2) | 0.874 |
| 48 h | 1.0 (0.3–1.7) | 1.4 (0.7–2.1) | 0.256 |
| 72 h | 1.4 (0.8–1.7) | 1.0 (0.6–2.3) | 0.840 |
| 96 h | 0.8 (0.4–1.9) | 1.0 (0.7–1.7) | 0.476 |
| 30 days | 0.7 (0.3–1.7) | 0.7 (0.4–2.0) | 0.388 |
| Spot urinary glucose (mmol/L) | |||
| Baseline | 0.2 (0.1–0.3) | 0.1 (0.1–0.2) | 0.264 |
| 24 h | 50.4 (17.1–94.8) | 0.2 (0.1–0.3) | <0.001 |
| 48 h | 41.3 (17.2–79.1) | 0.2 (0.1–0.3) | <0.001 |
| 72 h | 35.2 (16.2–96.2) | 0.2 (0.1–0.3) | <0.001 |
| 96 h | 30.3 (9.9–75.2) | 0.2 (0.1–0.4) | <0.001 |
| 30 days | 13.1 (1.6–58.9) | 0.2 (0.1–0.3) | <0.001 |
| Fractional excretion of glucose (%) | |||
| Baseline | 0.1 (0.1–0.1) | 0.1 (0.0–0.1) | 0.890 |
| 24 h | 21.8 (10.1–29.8) | 0.1 (0.1–0.1) | <0.001 |
| 48 h | 13.6 (5.4–24.0) | 0.1 (0.0–0.1) | <0.001 |
| 72 h | 16.0 (4.2–24.4) | 0.1 (0.0–0.1) | <0.001 |
| 96 h | 6.0 (2.5–21.8) | 0.1 (0.0–0.1) | <0.001 |
| Spot urinary urea (mmol/L) | |||
| Baseline | 108.0 (60.8–142.1) | 97.5 (77.6–139.5) | 0.881 |
| 24 h | 136.0 (93.3–172.7) | 124.0 (91.3–191.6) | 0.935 |
| 48 h | 160.2 (116.0–194.0) | 159.3 (111.3–105.7) | 0.840 |
| 72 h | 154.5 (122.9–185.0) | 157.5 (128.5–207.5) | 0.426 |
| 96 h | 179.6 (121.0–256.7) | 172.2 (144.9–233.0) | 0.427 |
| 30 days | 214.0 (141.0–272.6) | 166.6 (85.2–294.5) | 0.333 |
| Fractional excretion of urea (%) | |||
| Baseline | 37.9 (30.3–45.0) | 37.6 (28.0–48.5) | 0.984 |
| 24 h | 33.1 (28.6–41.9) | 35.3 (21.4–43.0) | 0.664 |
| 48 h | 29.6 (22.2–39.3) | 30.5 (25.7–39.7) | 0.572 |
| 72 h | 31.1 (26.3–37.1) | 32.0 (24.9–39.2) | 0.952 |
| 96 h | 32.4 (24.2–38.0) | 33.5 (29.5–39.0) | 0.346 |
Figure 1.
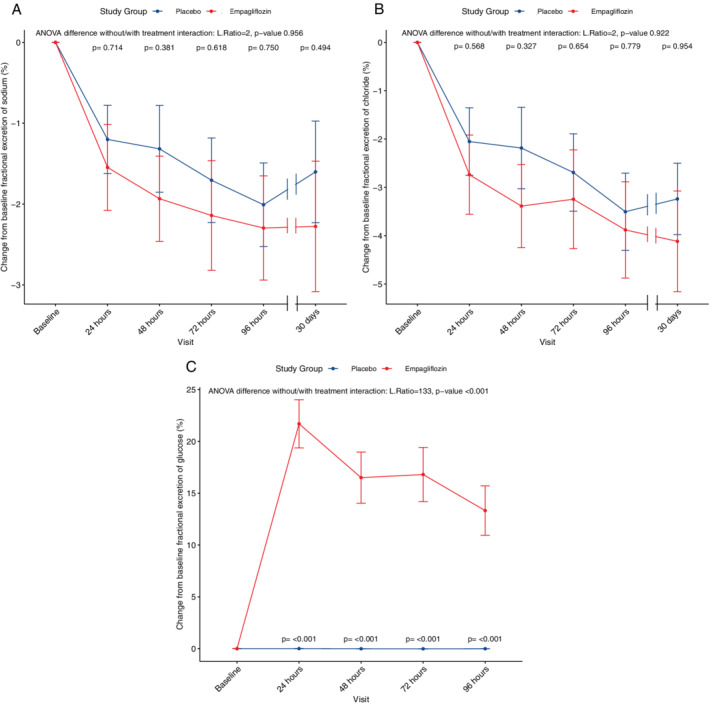
Progression of fractional excretion of sodium (A), chloride (B) and glucose (C) over the course of treatment. For each clinical variable, changes from baseline were calculated and used as outcomes in linear mixed‐effect models. Two models were performed, one adjusted for baseline values, the second model adjusted for baseline values and the interaction term between treatment and time. In each panel, the results for the ANOVA tests between the two models is depicted (likelihood ratio and P‐value). For placebo and empagliflozin, mean values are shown with dots, the bars represent standard error. A P‐value for interaction between each time point and treatment is shown.
Empagliflozin significantly increased both urinary glucose concentration and FeGlu, with a peak in FeGlu after 24 h (median 21.8% vs. 0.1%, P < 0.001; Table 2 , Figure 1C ). Although FeGlu decreased over the course of treatment, it was still significantly higher in patients treated with empagliflozin compared with placebo after 96 h (median 6.0% vs. 0.1%, P < 0.001). A similar pattern was seen for urinary glucose concentration up to 30 days of treatment (Table 2 ). Plasma glucose levels, however, were not changed by empagliflozin use (P = 0.763 for ANOVA difference between models with and without treatment interaction). FeGlu was similar in patients with and without diabetes in the empagliflozin arm (P = 0.182 for interaction, online supplementary Figure S2 ). Plasma urea was additionally increased in patients treated with empagliflozin, while fractional excretion of urea and urinary urea remained unaffected (P = 0.036, 0.99 and 0.782 for ANOVA difference between models with and without treatment respectively).
During the first 72 h, empagliflozin caused a significant decrease in eGFR (−10 ± 12 vs. −2 ± 12 mL/min/1.73 m2) compared with placebo (P = 0.009), as shown in Table 3 and Figure 2A . This significant decline in eGFR was attenuated at 96 h and 30 days (P = 0.133 and 0.681 respectively). In addition, empagliflozin significantly increased urinary output (cumulative urinary output after 48 h: 6084 ± 2480 mL vs. 4222 ± 1911 mL, P = 0.010; n = 41), which resulted in a greater negative fluid balance [cumulative fluid balance after 48 h: −3050 (IQR −1280 to −4753) mL vs. −1200 mL (IQR −710 to −2425), P = 0.010; n = 38].
Table 3.
Estimated glomerular filtration rate over the course of treatment
| Empagliflozin (n = 40) | Placebo (n = 39) | P‐value | |
|---|---|---|---|
| eGFR (mL/min/1.73 m2) | |||
| Baseline | 53 ± 18 | 54 ± 16 | 0.824 |
| 24 h | 44 ± 14 | 52 ± 19 | 0.022 |
| 48 h | 43 ± 16 | 53 ± 19 | 0.013 |
| 72 h | 42 ± 16 | 54 ± 18 | 0.006 |
| 96 h | 45 ± 18 | 53 ± 20 | 0.101 |
| 30 days | 50 ± 21 | 54 ± 19 | 0.511 |
| Change in eGFR from baseline (mL/min/1.73 m2) | |||
| 24 h | −9 ± 9 | −2 ± 8 | 0.002 |
| 48 h | −10 ± 12 | −2 ± 10 | 0.004 |
| 72 h | −10 ± 12 | −2 ± 12 | 0.009 |
| 96 h | −7 ± 11 | −3 ± 15 | 0.133 |
| Day 30 | −6 ± 15 | −4 ± 16 | 0.681 |
eGFR, estimated glomerular filtration rate.
Figure 2.
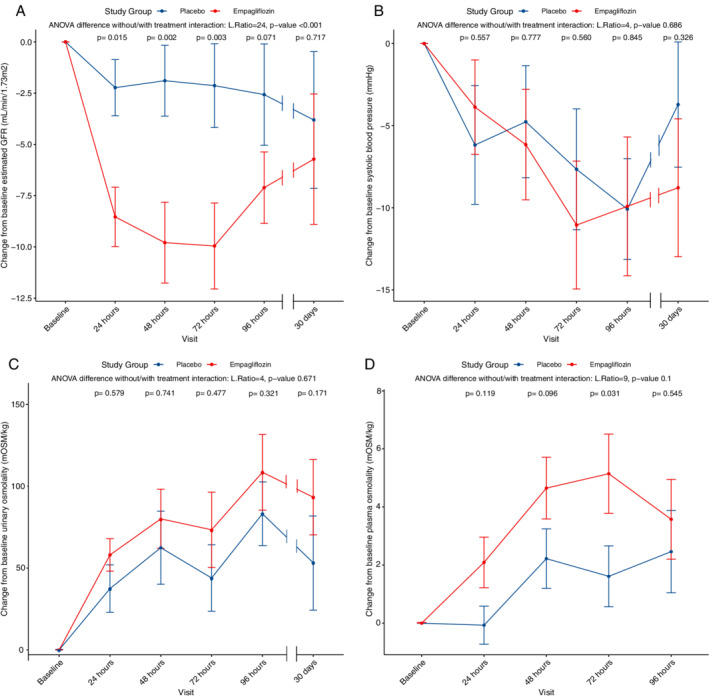
Delta estimated glomerular filtration rate (GFR) (A), delta systolic blood pressure progression (B), delta urine osmolality (C) and delta plasma osmolality (D) over the course of treatment. For each clinical variable, changes from baseline were calculated and used as outcomes in linear mixed‐effect models. Two models were performed, one adjusted for baseline values, the second model adjusted for baseline values and the interaction term between treatment and time. In each panel, the results for the ANOVA tests between the two models is depicted, (likelihood ratio and P‐value). For placebo and empagliflozin, mean values are shown with dots, the bars represent standard error. A P‐value for interaction between each time point and treatment is shown.
Although urinary volumes significantly increased after initiation of empagliflozin, no impact on urinary osmolality was seen (Figure 2C ). In other words, the number of particles per kilogram of urine did not change despite a larger urinary volume. However, a significant shift was seen in the constituents making up urine osmolality, with glucose making up a larger proportion of the total urinary particles compared with placebo (Figure 3 and Graphical Abstract). Measured and calculated urine osmolality showed a strong correlation (r of log‐transformed variables 0.91–0.99) (online supplementary Table S1 ).
Figure 3.
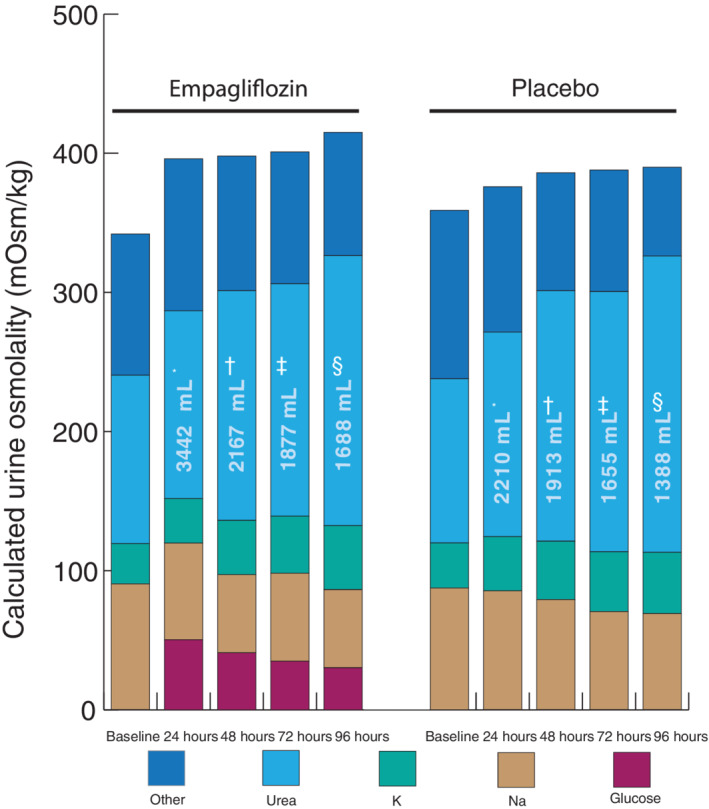
Composites of urinary molecules making up osmolality. Osmolality per time point for both empagliflozin and placebo. Volumes depicted on the bars represent total urinary volume per 24 h, y axis represents spot urinary osmolality. *n = 58; † n = 47; ‡ n = 44; § n = 35.
At baseline, plasma osmolality was similar in both groups. During the first 72 h, empagliflozin modestly increased plasma osmolality (delta plasma osmolality 5 ± 8 mOsm/kg vs. 2 ± 5 mOsm/kg; P = 0.049). Moreover, we found a significant interaction for plasma osmolality between time at 72 h and treatment effect (P = 0.031, Figure 2D ).
Median baseline renin was 28.8 (1.0–1820) pg/mL, median baseline aldosterone was 185.9 (31.0–1810) pg/mL. Median plasma renin concentration at baseline was higher in the placebo group than in the empagliflozin group (12.4 pg/mL vs. 80.1 pg/mL, P = 0.011). Empagliflozin significantly increased renin with a peak after 72 h, after which a plateau was reached and significance was lost (P = 0.016 for ANOVA between models with and without treatment effect) (Figure 4A ). Aldosterone was not altered by empagliflozin use (P = 0.217 for ANOVA between models with and without treatment effect) (Figure 4B ).
Figure 4.
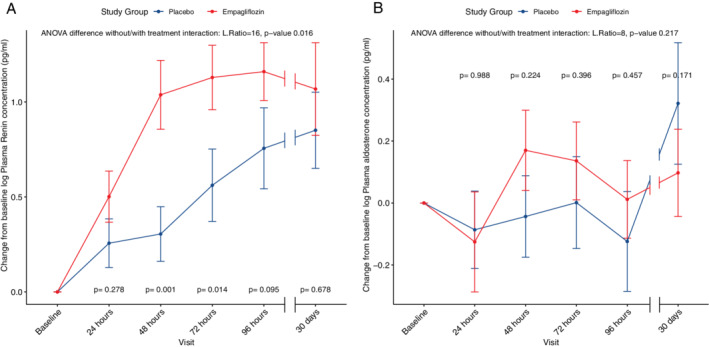
Delta renin (A) and aldosterone (B) over the course of treatment. For renin and aldosterone (both log‐transformed), changes from baseline were calculated and used as outcomes in linear mixed‐effect models. Two models were performed, one adjusted for baseline values, the second model adjusted for baseline values and the interaction term between treatment and time. In each panel, the results for the ANOVA tests between the two models is depicted (likelihood ratio and P‐value). For placebo and empagliflozin, mean values are shown with dots, the bars represent standard error. A P‐value for interaction between each time point and treatment is shown.
No significant correlations, as analysed with linear regression, could be seen for log FeGlu and log FeNa, for log FeGlu and fluid balance or for log FeNa and fluid balance (online supplementary Figure S1 ).
Discussion
In this pre‐defined post‐hoc analysis of the EMPA‐RESPONSE‐AHF trial, we found that patients with acute HF receiving empagliflozin had a higher urinary output and a more negative fluid balance. Interestingly, FeNa did not increase and urinary osmolality remained similar between both groups. Nonetheless, FeGlu significantly increased after initiation of empagliflozin. Moreover, empagliflozin temporarily reduced renal function in the first days after an acute HF hospital admission.
The increase in FeGlu with SGLT2 inhibition by empagliflozin was expected, as blocking the receptor responsible for tubular reabsorption of glucose would intuitively lead to increased excretion of filtered glucose. Importantly, FeGlu was similar in patients with and without diabetes and plasma glucose levels remained unchanged, suggesting that pre‐existing diabetes and/or plasma glucose supply to the glomerulus do not play an important role. Our finding that FeNa was not increased with empagliflozin was somewhat unexpected, since blockage of the SGLT2 receptor in the proximal tubule prevents reabsorption of both glucose and sodium. However, it is well known that in contrast to glucose, sodium can be reabsorbed throughout the entire tubule. Therefore, our findings suggest that sodium reabsorption might have been blocked by empagliflozin in the proximal tubule, and that this was compensated by an increased reabsorption of sodium in the rest of the tubule and collecting duct. 14
Similar to a post‐hoc analysis of the EMPA‐REG OUTCOME trial, a transient decline in eGFR was seen after initiating treatment with empagliflozin. 15 This course in eGFR over time was seen in the CANVAS Program as well. 16 This has been postulated to be related to the juxtaglomerular feedback mechanism and a correction in glomerular hyperfiltration. 17 , 18 Loss of chloride is sensed by the macula densa in the distal convoluted tubule and leads to release of adenosine, causing afferent vasoconstriction and a decreased renal blood flow, in order to spare salt. If we assume that the established drop in glomerular filtration rate is indeed the result of activation of the macula densa, there should still be high levels of sodium and chloride present in the distal convoluted tubule in order for juxtaglomerular feedback mechanism to be activated. 19 This would mean that although we do not find increased concentrations of sodium in the urine, proximal tubular sodium resorption is indeed diminished. This notion is further supported by the fact that renin levels were increased compared with placebo for the first 72 h, following a similar pattern compared to drop in eGFR. The increased osmotic diuresis resulting from glycosuria probably leads to an increase in renin levels. Of course, these results need to be interpreted in light of background therapy and one should be wary to draw conclusions based on these data alone. Consequently, sodium is likely reabsorbed distally from the macula densa, e.g. in the collecting duct. In the collecting ducts, multiple mediators regulate urine dilution and sodium reabsorption, one of which is vasopressin. 20 Yet, since urinary osmolality remained unchanged and vasopressin only plays a minor role in sodium reabsorption, it is unlikely that an increase in vasopressin is truly responsible for this increase in sodium reabsorption in the collecting duct. We did not find an increase in aldosterone after initiation of empagliflozin, making aldosterone an unlikely cause for distal tubular or collecting duct sodium reabsorption. Other drivers of sodium reabsorption include insulin and insulin‐like growth factor‐1, while endothelin‐1 and nitric oxide decrease sodium reabsorption. 21 , 22 , 23 The precise contribution of each of these factors on sodium reabsorption in the collecting duct after blocking the SGLT2 receptor and treatment with loop diuretics should be assessed in future studies.
Interestingly, our data show a modest increase in plasma osmolality. These effects were even more pronounced in patients with lower serum sodium and lower plasma osmolality. In our data no effect on serum sodium could be found, which might be explained by the fact that only a small proportion of patients presented with hyponatraemia and the lowest measured sodium upon admission was 129 mmol/L. Moreover, this might position SGLT2 inhibitors as a treatment for tissue congestion or residual congestion as increased plasma osmolality attracts fluid from the interstitial space into the blood stream. 24 This notion is supported by earlier findings that the SGLT2 inhibitor dapagliflozin has been calculated to reduce interstitial fluid volume three times more than it reduces blood volume, compared to an interstitial fluid reduction of only 66% of the reduction in blood volume by bumetanide. 25 The fact that empagliflozin did not lower blood pressure in these severely diseased patients despite larger negative fluid balance also supports the theory of a more stable refill rate from interstitial fluid to plasma as a result of increased plasma osmolality (Figure 2B ). However, we should account for the small study group here and the fact that other larger trials did find a reduction of 5–10 mmHg in systolic blood pressure after initiation of a SGLT2 inhibitor. 26 , 27 Still, this increase in plasma osmolality might also be expected since distal diluting segments receive higher tubular flow as a result of empagliflozin. The kidneys perceive this as a state of hypervolaemia which, through stimulation of the countercurrent system of the vasa recta, leads to more distal sodium reabsorption and less free water clearance. 28
As described earlier, the majority of HF patients are discharged with residual congestion with consequent impaired prognosis. 29 Our analyses might provide insights into new possibilities to overcome residual congestion due to significantly improved net fluid loss (negative fluid balance) and increased plasma osmolality. Naturally, our data need to be validated in other (larger) clinical cohorts.
Taken together, our findings that empagliflozin increased FeGlu, while both FeNa and urinary osmolality remained unchanged, suggest that empagliflozin most prominently stimulates osmotic diuresis as a result of increased FeGlu instead of natriuresis. Our results are in line with earlier studies that reported that SGLT2 inhibitors increased urinary volume without an increase in FeNa. 8 , 30 In another study comprising of patients with type 2 diabetes and stable chronic HF (median NT‐proBNP: 399 pg/mL), an early increase in urinary sodium excretion was seen. However, in this study fluid administration was high in these patients, sodium intake was not standardized per protocol, and intermittent urinary measurements were only obtained throughout the first 6 h. Therefore, the circadian rhythm in sodium excretion could still have affected these findings. Since we included acute HF patients with more signs and symptoms of fluid congestion (median NT‐proBNP: 5236 pg/mL), differences might further be explained by our sicker cohort with likely even higher neurohumoral activation potentially leading to increased sodium reabsorption and eventually maintaining FeNa constant. This is in contrast to a study in patients with euvolaemic, stable HF, where empagliflozin increased both FeGlu and FeNa, and even exhibited a small synergistic effect of concomitant treatment with intravenous bumetanide. 7
Limitations
Several potential limitations of the present study can be identified. The first limitation of this study is the relatively small sample size. Second, we collected spot urine samples and not 24 h measurements. Consequently, we do not know the total sodium output over the course of hospitalization. Conceivably, increased volumes of urine, even with a lower concentration of sodium, will eventually lead to an increased absolute total of urinary sodium. As per protocol, treating physicians were blinded to the treatment arm and urinary excretion of glucose. However, urinary levels of sodium excretion were measured in the local labs and could therefore be perceived in the electronic medical records. Third, plasma levels of glucose were not measured at the 30‐day follow‐up visit, as this was not incorporated in the study protocol. Therefore, no FeGlu could be calculated at day 30. Fourth, the timing of loop diuretic administration was not standardized. This was left to the discretion of the treating physician. Therefore, some patients were on continuous loop diuretic infusion, while others were on intravenous bolus loop diuretic treatment. So, the natriuretic effect of a recent loop diuretic bolus, rather than that of empagliflozin cannot be ruled out. Still, considering the randomized nature of this trial, these treatment differences should be equal in both arms. Moreover, patients in both treatment arms were on equal total doses of intravenous and oral loop diuretics, and the number of patients on either oral or intravenous loop (bolus vs. continuous) diuretics was similar as well (online supplementary Table S2 ). Additionally, calculating fractional excretion levels relies on measured plasma creatinine levels. These values changed throughout follow‐up, which might have affected calculated values of fractional excretion.
Conclusion
In patients hospitalized with acute HF, empagliflozin caused an increase in urinary output, FeGlu and plasma osmolality, without affecting FeNa or urine osmolality. Additionally, a significant, temporary decline in eGFR was seen. These results suggest that empagliflozin primarily stimulates osmotic diuresis in patients with acute HF through increased glycosuria rather than through natriuresis.
Funding
EMPA‐RESPONSE‐AHF was funded by Boehringer Ingelheim through an investigator‐initiated study grant.
Conflict of interest: A.H.J.D. received a research grant from AstraZeneca. P.v.d.M. received consultancy and/or research grants from Vifor Pharma, AstraZeneca, Servier, Novartis, Pfizer, Ionis. H.J.L.H. received consultancy fees and/or research grants from AbbVie, AstraZeneca, Boehringer Ingelheim, CSL Pharma, Fresenius, Gilead, Janssen, Merck, Mitsubishi Tanabe, and Mundipharma. K.D. received consultancy fees from Abbott. A.A.V. received consultancy fees and/or research grants from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Myokardia, Novartis, Roche Diagnostics, Servier. All other authors have nothing to disclose.
Supporting information
Figure S1. Correlation, as analysed using linear regression, between FeGlu, FeNa and fluid balance.
Figure S2. Interaction between history of diabetes mellitus and fractional excretion of glucose.
Table S1. Correlation between measured and calculated urine osmolality per time point.
Table S2. Median cumulative dose of loop diuretics per patient during the first 96 h of hospitalization.
References
- 1. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 2. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, Von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA‐REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334. [DOI] [PubMed] [Google Scholar]
- 3. Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 4. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JP, Ruff CT, Gause‐Nilsson IA, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CE, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 6. Jackson A, Dewan P, Anand I, Bělohlávek J, Bengtsson O, de Boer R, Böhm M, Boulton D, Chopra V, Demets D, Docherty K, Dukát A, Greasley P, Howlett J, Inzucchi S, Katova T, Køber L, Kosiborod M, Langkilde AM, Lindholm D, Ljungman C, Martinez F, O'Meara E, Sabatine M, Sjöstrand M, Solomon S, Tereshchenko S, Verma S, Jhund P, McMurray J. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA‐HF. Circulation 2020;142:1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Griffin M, Rao VS, Ivey‐Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, Riello R, Bellumkonda L, Cox Z, Collins S, Jeon S, Turner JM, Wilson FP, Butler J, Inzucchi SE, Testani JM. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 2020;142:1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE‐CHF trial. Circulation 2020;142:1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, van Eck JW, Heerspink HJ, Voors AA. Randomized, double‐blind, placebo‐controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA‐RESPONSE‐AHF). Eur J Heart Fail 2020;22:713–722. [DOI] [PubMed] [Google Scholar]
- 10. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016;134:752–772. [DOI] [PubMed] [Google Scholar]
- 11. Bjornstad P, Laffel L, Tamborlane WV, Simons G, Hantel S, Von Eynatten M, George J, Marquard J, Cherney DZ. Acute effect of empagliflozin on fractional excretion of sodium and eGFR in youth with type 2 diabetes. Diabetes Care 2018;41:e129–e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, Stamler J, Elliott P; INTERSALT Co‐Operative Research Group . Estimating 24‐hour urinary sodium excretion from casual urinary sodium concentrations in western populations: the INTERSALT study. Am J Epidemiol 2013;177:1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RStudio | Open source & professional software for data science teams ‐ RStudio. https://rstudio.com/ (13 August 2020).
- 14. Ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure – pathophysiology, evaluation, and therapy. Nat Rev Cardiol 2015;12:184–192. [DOI] [PubMed] [Google Scholar]
- 15. Wanner C, Heerspink HJ, Zinman B, Inzucchi SE, Koitka‐Weber A, Mattheus M, Hantel S, Woerle HJ, Broedl UC, Von Eynatten M, Groop PH; EMPA‐REG OUTCOME Investigators . Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA‐REG OUTCOME trial. J Am Soc Nephrol 2018;29:2755–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neuen BL, Ohkuma T, Neal B, Matthews DR, De Zeeuw D, Mahaffey KW, Fulcher G, Li Q, Jardine M, Oh R, Heerspink HL, Perkovic V. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS Program. J Am Soc Nephrol 2019;30:2229–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilcox CS. Antihypertensive and renal mechanisms of SGLT2 (sodium‐glucose linked transporter 2) inhibitors. Hypertension 2020;75:894–901. [DOI] [PubMed] [Google Scholar]
- 18. Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–597. [DOI] [PubMed] [Google Scholar]
- 19. Eickhoff MK, Dekkers CC, Kramers BJ, Laverman GD, Frimodt‐Møller M, Jørgensen NR, Faber J, Danser AH, Gansevoort RT, Rossing P, Persson F, Heerspink HJ. Effects of dapagliflozin on volume status when added to renin‐angiotensin system inhibitors. J Clin Med 2019;8:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baylis PH. Osmoregulation and control of vasopressin secretion in healthy humans. Am J Physiol 1987;253(5 Pt 2):R671–R678. [DOI] [PubMed] [Google Scholar]
- 21. Weber KT. Aldosterone in congestive heart failure. N Engl J Med 2001;345:1689–1697. [DOI] [PubMed] [Google Scholar]
- 22. Zaika O, Palygin O, Tomilin V, Mamenko M, Staruschenko A, Pochynyuk O. Insulin and IGF‐1 activate Kir4.1/5.1 channels in cortical collecting duct principal cells to control basolateral membrane voltage. Am J Physiol Renal Physiol 2016;310:F311–F321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garvin JL, Herrera M, Ortiz PA. Regulation of renal NaCl transport by nitric oxide, endothelin, and ATP: clinical implications. Annu Rev Physiol 2011;73:359–376. [DOI] [PubMed] [Google Scholar]
- 24. Boorsma EM, ter Maaten JM, Damman K, Dinh W, Gustafsson F, Goldsmith S, Burkhoff D, Zannad F, Udelson JE, Voors AA. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol 2020;17:641–655. [DOI] [PubMed] [Google Scholar]
- 25. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJ, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 2018;20:479–487. [DOI] [PubMed] [Google Scholar]
- 26. Serenelli M, Böhm M, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, DeMets DL, Bengtsson O, Sjöstrand M, Langkilde AM, Anand IS, Chiang CE, Chopra VK, de Boer RA, Diez M, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CE, Verma S, Docherty KF, Jhund PS, McMurray JJ. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA‐HF). Eur Heart J 2020;41:3402–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 28. Gottschalk CW, Mylle M. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am J Physiol 1959;196:927–936. [DOI] [PubMed] [Google Scholar]
- 29. Rubio‐Gracia J, Demissei BG, ter Maaten JM, Cleland JG, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison BA, Givertz MM, Bloomfield DM, Dittrich H, Damman K, Pérez‐Calvo JI, Voors AA. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol 2018;258:185–191. [DOI] [PubMed] [Google Scholar]
- 30. Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC. Interaction between the sodium‐glucose‐linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc 2018;7:e007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Correlation, as analysed using linear regression, between FeGlu, FeNa and fluid balance.
Figure S2. Interaction between history of diabetes mellitus and fractional excretion of glucose.
Table S1. Correlation between measured and calculated urine osmolality per time point.
Table S2. Median cumulative dose of loop diuretics per patient during the first 96 h of hospitalization.


