Abstract
Disturbance is a key factor shaping ecological communities, but little is understood about how the effects of disturbance processes accumulate over time. When disturbance regimes change, historical processes may influence future community structure, for example, by altering invasibility compared to communities with stable regimes. Here, we use an annual plant model to investigate how the history of disturbance alters invasion success. In particular, we show how two communities can have different outcomes from species introduction, solely due to past differences in disturbance regimes that generated different biotic legacies. We demonstrate that historical differences can enhance or suppress the persistence of introduced species, and that biotic legacies generated by stable disturbance history decay over time, though legacies can persist for unexpectedly long durations. This establishes a formal theoretical foundation for disturbance legacies having profound effects on communities, and highlights the value of further research on the biotic legacies of disturbance.
Keywords: Biotic legacy, community ecology, community structure, disturbance history, disturbance regimes, invasion, reciprocal yield law, theoretical ecology
Disturbance has many effects on communities, and much is still not known about how these effects accumulate over time. We use an annual plant model to demonstrate how legacies of disturbance can affect future community composition, long after disturbance regimes have changed. In particular, we show that differences in disturbance history can alter invasion success even when present disturbance regimes are identical.

INTRODUCTION
How disturbance shapes communities has remained a central focus in ecological research for decades (Picket & White 1985; Walker, 2012), and interactions between disturbance properties (Box 1), species traits, and competition have all been investigated theoretically (Levin & Paine 1974; Lubchenco 1978; Connell 1978; Tilman 1994; Roxburgh et al. 2004; Shea et al. 2004; Turner 2010; Walker 2012; Newman 2019). For example, the complex nonlinear interactions between aspects of disturbance and species’ traits are known to have strong effects on diversity (e.g. Miller et al. 2011; Liao et al. 2016).
Box 1. Ontology of disturbance in ecology.
Disturbance is a general concept in ecology that encompasses many related phenomena, including specific destructive events as well as the recurring process of multiple such events. There are many definitions of disturbance in the literature, and we draw from them to clarify the interrelated concepts and terms associated with disturbance. Though there is inherent ambiguity in this broad topic, carefully parsing key aspects and properties of disturbance can improve clarity when studying how disturbance shapes ecosystems.
Disturbance event
A particular event at a specified place and time that disrupts ecosystem structure or the physical environment (following Pickett & White 1985). This disruption can occur at any level of organization: the ecosystem structure includes the communities, populations, and individuals, and the physical environment includes e.g. abiotic resources, substrate availability, and weather.
Disturbance type
The physical nature of a disturbance event. Different disturbances such as fires and floods may have similarities in their effects, but each type of disturbance has specific impacts on individuals. The type of the disturbance is closely related to its cause.
Disturbance regime
The distribution of disturbance events occurring over a given space and time. Empirically, a past collection of events has a joint statistical distribution of the quantified aspects of the disturbance events. Theoretically, regimes can be prescribed several ways, e.g. by defining a probability distribution of events, or a periodic or stochastic process. Dynamically generated disturbances can also be simulated. See Krebs et al. (2010) for detailed discussion of the term “fire regime.”
Aspects of disturbance
The distinct and conceptually independent properties of disturbance when considered as a complex, multifaceted concept (Roxburgh et al. 2004; Fraterrigo & Russak 2008). Both disturbance events and regimes have associated aspects. Events can have: intensity, the vigor of the disturbing force (e.g. Fraterrigo & Rusak 2008); duration, the temporal span of effects (e.g. Lake 2000); extent, the spatial span of effects (e.g. Miller et al. 2012a); and timing, the temporal placement measured with respect to season or life cycle (e.g. Miller et al. 2012b). Regimes have aspects that events cannot, such as autocorrelation, the statistical serial relation of events with themselves (e.g. Liao et al. 2016 for spatial autocorrelation, Garrison et al. 2012 for temporal). Some aspects of regimes have analogs in events. For example, frequency of a regime is the expected number of events per time period, and is analogous to the aspect of time since last disturbance associated with an event. Pace of change, the rate of onset of disturbance effects for an event. E.g. two floods could have the same extent and duration, but trigger different effects and responses depending on their speed of occurrence. For a regime, pace of change indicates that some aspect of the disturbance regime changes over time.
Press and pulse disturbance
A dichotomy distinguishing discrete and specific disturbance events (pulse) from disturbance that occurs uniformly and generally over time (press). This punctual vs. continual aspect can be treated as a distinction in duration of events. This allows the distinction between repeated acute disturbance and chronic disturbance (McCormick et al. 2015).
Disturbance cause, effect, response
Disturbance shapes ecosystems through a chain of impacts. The cause is the material impetus, the effect is its primary action, and the response is how species react. E.g. a fire could be caused by lightning, the effect is destruction of biomass, and the responses may include resprouting or increased germination.
Disturbance history
The record of disturbance experienced at a given location over a period of time. This can be theoretically represented by a procedure, or determined empirically as a set of events. This term relates to other concepts of the ecological past, e.g. “ecological memory” and “antecedent effects” (Ogle et al. 2015).
Disturbance refugia
Locations that are disturbed less intensely or less frequently than other areas within the surrounding landscape. These can act as important reservoirs of biotic and abiotic legacies (from Krawchuk et al. 2020).
Biotic legacy
The accumulated biological effects of a disturbance process acting on a community over time. “Material” legacies are the physical effects (e.g. biomass, species abundances, seed banks) and the “informational” legacies are the result of selection and filtering acting on populations subject to disturbance (following Franklin et al. 2000; Johnstone et al. 2016). Here, the persistent seedbank shaped by a stable regime over time constitutes the biotic legacy of historical disturbance.
Abiotic legacy
The accumulated material effects of a process acting over time that are not biological in nature. Examples include erosion, changes to stream beds, soil leaching, sediment deposition, etc.
Recently, interest in how the effects of disturbance regimes accumulate over time (Seabloom et al. 2020) and how changes in disturbance regimes influence invasion success has grown (Theoharides & Dukes 2007; Moles et al. 2012; Johnstone et al. 2016). Over time, disturbance regimes build a biotic legacy (Box 1) reflective of that environmental variation through cumulative effects; the history and its legacy may then influence present ecological processes. Importantly, when regimes change, there is a potential for mis‐match between a community shaped by one regime but experiencing another. This may alter niche opportunities, and historical legacies of disturbance may therefore affect both current and future dynamics as the impacts of changes to disturbance regimes unfold. History of disturbance may affect how new species integrate into communities in many ways, and we hypothesise that the persistence or extirpation of introduced species can be better understood if we explicitly account for the history of disturbance and changes in disturbance regimes.
Here, we develop a theoretical approach to this emerging research field, and address our basic hypotheses using a model of an annual plant community of two resident species and one invader. While previous work has shown how abiotic legacies can influence communities (Perring et al. 2016), our interest is the potential for disturbance history to create biotic legacies, and to understand how these legacies influence invasion success in the absence of abiotic legacies.
Although these concepts have been observed repeatedly – and increasingly – in empirical systems (Newman, 2019), there is relatively little theory developed on the topic. We ask two fundamental questions about changing disturbance regimes and species introductions in a model annual plant community. First (Q1): do historical changes in disturbance regimes alter present invasion success, compared to introductions taking place in a community operating continually under a stable disturbance regime? Because biotic legacies will vary across communities with different histories, we hypothesise that invaders may be more likely to succeed in a community subject to a recent change in disturbance regime compared to a community that has been operating under a single stable regime for a long time.
It is not clear how long effects of historical disturbance regimes may last. For example, recent disturbance events may influence the current abundances of species, but legacies of disturbance may interact with populations over longer timeframes. Thus, our second question is (Q2): how long does a given historical period of disturbance continue to affect community structure? The annual life cycle is rapid, and populations can change dramatically within a few years. Thus, we hypothesise that the effects of disturbance history will fade relatively rapidly after changing to a new regime.
Our findings demonstrate that overlooking disturbance history can lead to erroneous conclusions because two communities comprising the same species with the same disturbance regime can have different invasion outcomes due to differences in disturbance history. We also demonstrate that the effects of disturbance history may endure for remarkably long times. Our results provide a theoretical understanding to inform research examining disturbance and invasive species, and because they are based on exhaustively sampling all frequency‐intensity disturbance regime combinations, we capture all possible disturbance‐mediated dynamics that can be described within the present model context for a given community. Our prior work has shown that insights from this model can also explain features of microbial microcosm systems (Hall et al. 2012), and there is potential that our results will be more broadly applicable (Miller et al. 2011). We also use our findings to generate empirically testable hypotheses, for example, an increase in disturbance frequency or intensity can enable a species to successfully invade, whereas introduction under a regime with the higher value and a stable history would fail.
Materials and methods
We apply an annual plant model that represents a community of three species via the life‐history traits of germination rate, seedbank survival rate and seed yield. The model incorporates a disturbance regime, and allows frequency and intensity of disturbance events (Box 1) to be varied independently.
Model design
Each species j is defined by parameters specifying its maximum seed yield (Yj), germination rate (Gj), seedbank survival rate (sj) and how it competes (Fig. 1a). Competition is calculated via the ‘reciprocal yield law’ (Shinozaki & Kira 1956): each species’ seed yield is reduced in proportion to the reciprocal of the number of all other plants present that year, weighted by competitive effect (αkj). Note that αkj is the effect of species j on species k, and competition need not be symmetric. The reciprocal yield law is supported empirically (Harper 1977) and is expected to apply to wide ranges of plant species experiencing competition, provided that density is not high enough to directly cause mortality over the course of a single growth season (Ellner 1985).
Figure 1.
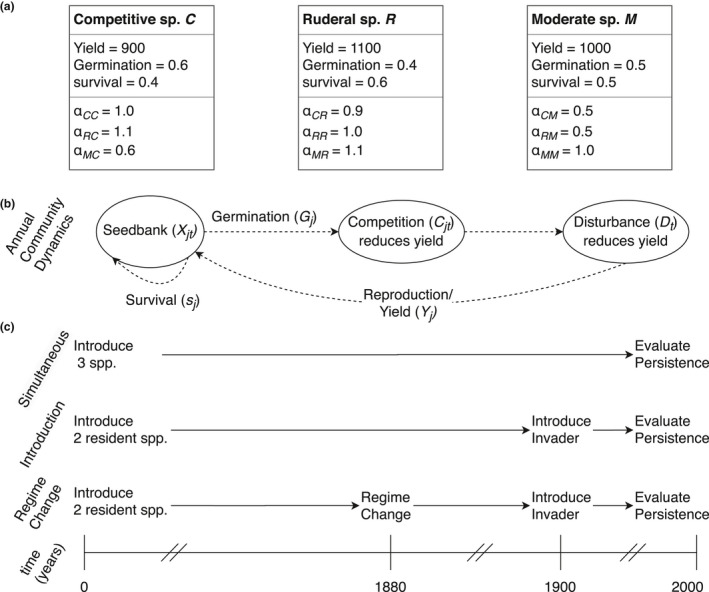
Species parameters, annual community dynamics, and experimental design. (a) species parameters. (b) The modeled annual life cycle. Each species germinates, grows, and reproduces under the effects of competition and disturbance, shown schematically here and formally in eq. 1. (c) Schematic of the three experiments performed to isolate the effects of disturbance history. In each experiment, community dynamics under disturbance are exhaustively sampled so that every possible regime of disturbance frequency and intensity is represented. ‘Simultaneous’ is a control, with all species arriving at the same time and with no change to disturbance regime. ‘Introduction’ introduces an invader species after two residents have stabilised. ‘Regime Change’ alters the disturbance regime before the invader is introduced.
Although our model can represent a wide variety of annual plant life histories, we focus our results on a test community chosen for its rich dynamics and comparability with prior work (Miller et al., 2011). We name the species by their life‐history strategies: the resident species employ opposing competitive (C) and ruderal (R) strategies (sensu Grime, 1977), and the invader has moderate traits (M). Species C has (YC, GC, sC) = (900, 0.6, 0.4), (αCC, αRC, αMC) = (1, 1.1, 0.6). Compared to species R, C has a higher germination rate, lower seedbank survival and stronger competition: its strategy performs best when disturbance is low. Species R has (YR, GR, sR) = (1100, 0.4, 0.6), (αCR, αRR , αMR) = (0.9, 1, 1.1). Compared to C it has a lower germination rate and higher seedbank survival, that is, R retains a larger seedbank and hedges against disturbance and is therefore ruderal. Species M has a moderate strategy, and its Y,G,s parameters are intermediate: (YM, GM, sM) = (1000, 0.5, 0.5), (αCM, αRM , αMM) = (0.5, 0.5, 1.0). It has a weak competitive effect on both resident species, though it experiences stronger competition from R than C. In the following species introduction analyses, species C and R always form the resident community, and M is always the introduced species (see Appendix S1 in Supporting Information for additional configurations). This describes a hypothetical set of species with Y, G, s parameters well within the range of those observed in real species. Our resident community displays classically opposed strategies, and using the moderate species as invader offers insight into how typical species may invade without invoking more extreme life‐history strategies commonly associated with invasive species. The resident species R, C, are unable to coexist in a constant environment with no disturbance; all coexistence between them is due to the disturbance process (Miller et al. 2011).
Disturbance process
Disturbance in our model reduces seed yield, that is, per capita fecundity. We denote the disturbance process by Dt, an independent, identically distributed stochastic process with independent parameters for disturbance frequency (F) and intensity (I). Intensity is reflected in the population as the proportion of seed yield destroyed by disturbance events, for example, grazing, mowing or seed predation (Box 1). We set Dt = 1−I, when disturbance occurs, and Dt = 1 otherwise. Disturbance occurs at each timestep with probability F so that mean return time for a disturbance event is 1/F. While many other aspects of disturbance have important consequences for community structure (Box 1), we focus here on (I, F) due to their salience in the theoretical and empirical disturbance literature. Frequency and intensity are quantifiable in a bounded range from zero to one. We therefore completely sample the regimes and visually display how changes to regimes alter communities. Each species achieves largest population size with no disturbance; that is, disturbance can only help a species via indirect effects through competition with other species. Likewise, there is no disturbance regime where I and F lie between 0 and 1 that will cause all species to go extinct.
Formal model specification
Let Xjt be the number of seeds of species j in the seedbank at time t. The model is a system of stochastic finite difference equations defined via the finite rate of increase λ. This is the multiplicative growth rate, such that Xj,t +1 = λjtXjt for each species j. Using the parameters for life‐history traits and the disturbance process, λ encodes contributions to the next year’s seedbank from seeds that did not germinate and survived, together with those that germinated and produced new seed yield, reduced by disturbance and competition (Fig. 1b):
| (1) |
where the competition denotes reduction of seeds via the reciprocal yield law:
| (2) |
Of note, the disturbance process interacts nonlinearly with species abundance through the competition term. This allows for complex dynamics resulting from the interplay of disturbance frequency with germination rates and survival, as well as disturbance intensity interacting with seed yields and survival.
Experimental design
To assess the effects of historical changes to regimes on persistence of introduced species, we configure the model to simulate species introductions in three experiments. Each involves simulating competition under disturbance for the same total period of time (ttot), then determining community composition and structure. We simulate 10 000 disturbance regimes for each experiment, sampling the full (I, F) space of all regimes possible in this model, using increments of 0.01 for I and F, each spanning the (0,1) interval. Each of the disturbance regimes sampled uses constant (I,F) for the duration of simulations, except when explicitly changed as part of the regime‐change experiments. We perform three types of experiment with differences in disturbance history, and how species are introduced (Fig. 1c).
In the first experiment, three species are introduced simultaneously (Simultaneous). Species are initialised with 1000 seeds at t = 0, and community dynamics are simulated for a total time of ttot = 2000 years. In the second experiment (Introduction), species R and C are initialised with 1000 seeds, then compete under disturbance for an equilibration period (tpre = 1900 years), to allow the resident community to reach a stable distribution. Then, species M is introduced with 10 seeds, and all three species interact for tpost = 100 years. For the third experiment, the disturbance regime is altered prior to the introduction of species M (Regime Change). Initially, residents equilibrate under a stable disturbance regime (I0, F0 ) for a period of tpre = 1880 years, then the regime is shifted to a new pattern (I1, F1 ). The residents compete under this new regime for an interim period of tint = 20 years, after which time species M is introduced with 10 seeds, and all three species compete for tp ost = 100 years. The disturbance regime is shifted by augmenting the intensity and frequency parameters, assuming toroidal boundaries on the (I,F) square. We use a shift of (0.2, 0.3), for example, (I0, F0) = (0.1, 0.1) → (I1, F1) = (0.3, 0.4) and (I0, F0)= (0.9, 0.8) →(I1, F1) = (0.1, 0.1). In a given plot of the (I,F) parameter space, some portion of communities will see an increase to Intensity and Frequency, while others will experience a decrease. All of our primary results use this same shift for the Regime Change, though we include examples of other shifts in the supporting information (Figs. S1, S13). We operationally define species persistence by requiring that a species’ mean seed number be over 25 for the last 5% of the total simulation period (100 years). Our results are qualitatively robust across a large range of seed numbers introduced and persistence thresholds (two orders of magnitude, Figs S14–S17).
The three experiments all use the same duration, species parameters and disturbance parameters, as well as identical realisations of the disturbance process, so that they have the exact same record of disturbance events and results are directly comparable (Fig. 1). Although each (I, F) regime uses one realisation of disturbance, results generated with different random initialisations show no notable difference from our primary results, indicating that it is sufficient to simulate once for each regime (Fig. S3).
Comparisons between the Introduction and Regime Change experiments isolate the effects of changing disturbance regimes on species invasion. For example, if an Introduction experiment under regime (I1, F1) has a different community of persisting species than a Regime Change experiment with final regime (I1, F1), then we conclude that this difference is due to the differences in disturbance history, because all other features of the two experiments are the same. If we instead compared the community under the new regime to a community that had stayed constantly in the old regime, the difference could be explained by the different current regimes, not by the different history. Likewise, comparison of Simultaneous and Introduction experiments can reveal priority effects (e.g. Fukami 2015; Delory et al. 2019), because the only difference between the experiments is the order of species arrival. Further results on order priority and identity of invader are provided in the supporting information (Figs S5–S12).
Because effects of historical regimes on community composition may be ephemeral, we change the value of tpost, and conduct a series of simulations where community composition is calculated after a range of times, from 100 to 4500 years after species introduction. This allows us to detect how long the effects of historical regime change persist. Further results for different time periods are shown in the supporting information (Figs S4 and S13).
RESULTS
Results for Simultaneous, Introduction and Regime Change experiments are plotted in Fig. 2 (a–c, respectively). The difference between Introduction and Regime Change communities (Fig. 2d) highlights the changes in composition that are specifically attributable to differences in disturbance history. The results of the Introduction experiment demonstrate differential success of invasion based on both disturbance frequency and intensity, and nonlinear interactions between different aspects of disturbance influence diversity (e.g. Fig. 2b, yellow). Fig. 2 also provides evidence of priority effects. For example, in large regions of low‐intensity regime space (I < 0.5, 0 < F < 1), C excludes R during the equilibration period of the Introduction experiment (Fig. 2b, green), because the competitive species performs better under lower disturbance. In contrast, three species persist when introduced at equal numbers to a system under a stable disturbance regime (Fig. 2a), because M suppresses C’s abundance, and M has less competitive effect on species R than C does. This demonstrates the ability of the disturbance regime to control invasion success. Note that both resident species (C, R) have lesser weight for interspecific competition experienced from the invader M (αCM = 0.5, αRM = 0.5) than they have for intraspecific competition (αCC = αRR =1.0), which helps M form part of a three species community under many regimes.
Figure 2.
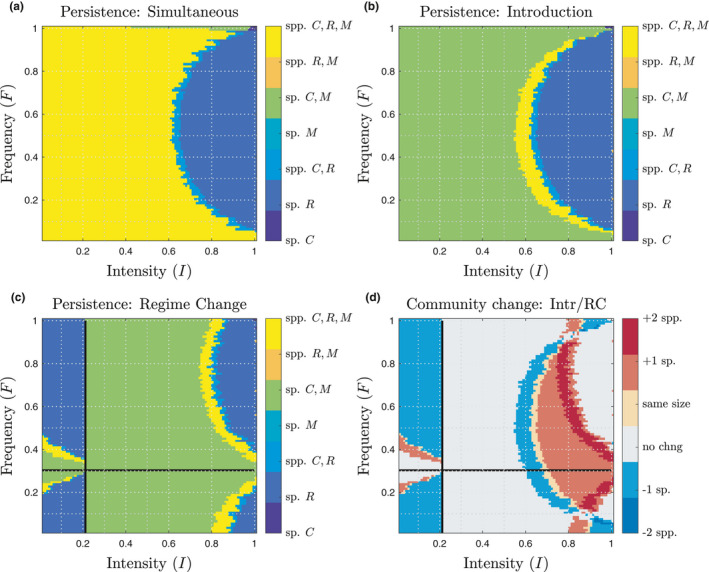
Species persistence (mean abundance >25 for the final 100 years), plotted across the unit square containing all possible disturbance regimes (I, F). (a) Simultaneous experiment: no history of disturbance, species introduced all together at t = 0. (b) Introduction experiment: resident equilibration period creates a biotic legacy for resident species C, R, then species M is introduced. (c) Regime Change: disturbance (I, F) shifted by (+0.2, +0.3). Outcomes plotted at final (I, F) values for the changed regime. Cross hairs in (c and d) indicate the shift in regime change. (d) Color code indicates the difference in species richness between communities under a given regime, comparing the Introduction and Regime Change experiments plotted in (b and c). Red indicates the Regime Change led to an increase in species richness, blue indicates Regime Change led to a decrease in species richness, grey indicates no change, and beige indicates the community size does not change even though its composition does. See SI Figs. S5, S6 for additional results when species C or R acts as invader.
Figure 2c shows species persistence in the Regime Change experiment, where each community experiences a shift in disturbance regime from an initial (I0, F0) to (I1, F1) = (I0 + 0.2, F0 + 0.3). To facilitate comparison, we show in Figure 2d a color‐coded panel that shows where the number of species persisting in the community differs between the Introduction and Regime Change experiments. We plot the species persistence results at the (I, F) coordinates of the final regime to completely isolate the effects of disturbance history: everything is the same in these two experiments at the time of species introduction, except that the Regime Change had a different regime in the past. There are many regimes (I1, F1) that share the same pattern of species persistence, regardless of whether the community had a history of only (I1, F1), or whether it first spent 1.8 k years in (I0, F0) (Fig. 2d, grey). However, roughly half of all disturbance regimes do show a difference in community composition due to regime change.
Our question (Q1) about whether a stable disturbance history and regime change can alter invasion success can be addressed with a simplified version of Fig. 2. Fig. 3 shows only the success and failure of the moderate species’ (M) invasion in the Introduction and Regime Change experiments. This result gives us clear evidence: there are many cases where changing regimes allows species M to invade where it could not if the community had a stable history (Fig. 3c, red). For example, under a stable regime of (I, F) = (0.8, 0.4), R excludes C, and also prevents M from invading. However, in the Regime Change experiment an initial period of (I0, F0) = (0.6, 0.1) leads to C excluding R, and when that regime is shifted to (I1, F1) = (0.8, 0.4) species M successfully invades. There are also many cases where the regime change serves to thwart invasion (Fig. 3c, blue). Interestingly, when Regime Change acts to increase the initial disturbance regime (Fig. 3c, top right of crosshairs), the change facilitates invasion for most regimes. When a shift decreases the initial regime (I 0, F 0) (Fig. 3c, bottom left of crosshairs), the Regime Change only hinders invasion. This is because large values of I, F lead to R excluding the others and maintaining a large seedbank. When shifted to a regime with less disturbance, this large seedbank prevents M from establishing, in cases where it could invade a much smaller population of C. Among all regimes, 16.4% changed invasion from unsuccessful to successful after regime change, 18.2% changed invasion from successful to unsuccessful, and the remaining 65.4% resulted in no change to invasion success.
Figure 3.
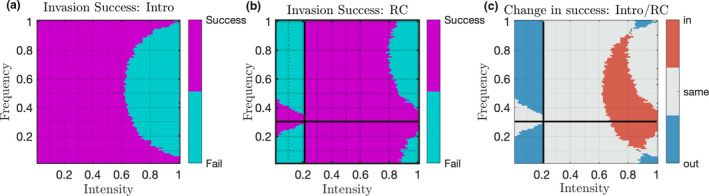
Illustration of how invasion success changes when disturbance regime is changed. (a) invasion success of species M in the introduction experiment. (b) success of invasion after regime change. (c) difference in success of invasion due to regime change—red indicates facilitation of invasion (‘in’), blue indicates invasion thwarted by regime change (‘out’), grey indicates no change in invasion success (‘same’). Note in this example, positive shifts (to larger frequency and intensity) mostly facilitate invasion while decreasing disturbance only prevents invasion.
In addition to invasion success or failure, we ask how the different histories of disturbance can alter the community composition. Using the same parameters as in Fig. 2, we examine species’ abundances over time in Fig. 4. Introduction experiments (Fig. 4a and b) show successful invasions by M under two different regimes, with differing final community composition in each case. The Regime Change experiments create different communities than the corresponding Introduction experiments (Fig. 4c and d). In the first example, a stable history of the same regime ((I, F) = (0.08, 0.4)) results in a two‐species community, with M having largest abundance (Fig. 4a). However, Regime Change (Fig. 4c) leads to three‐species coexistence, with C having the largest abundance. The higher intensity regime ((I,F) = (0.6,0.4)) shows a different effect of the Regime Change, wherein the stable regime produces a community of three species (Fig. 4b), and the Regime Change produces a community of two species (Fig. 4d). Thus, history of disturbance can alter community composition and species abundance, even when invasion success is unchanged.
Figure 4.
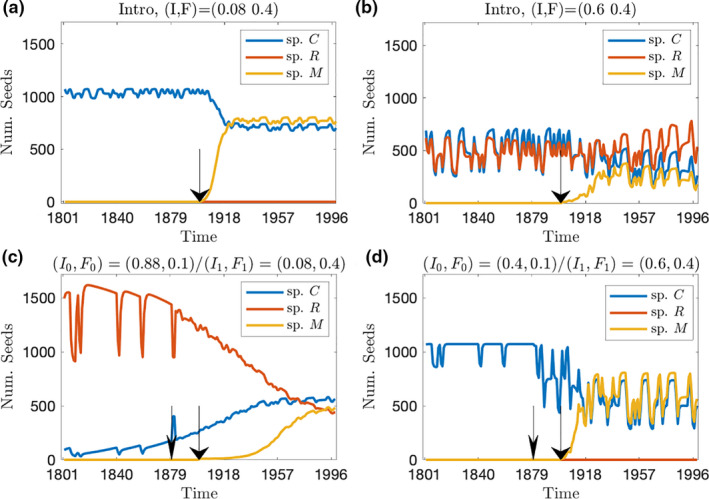
Seed abundance dynamics corresponding to four points from Fig. 2b and c illustrating how community composition after successful invasion differs between Introduction and Regime Change experiments. (a and b) Introduction experiment for two different regimes marked above the panels. (c and d) Regime change experiments with final regimes corresponding to the Introduction experiments shown directly above. Initial and final regimes are marked above the panels. Wide‐head arrow marks time of species introduction, narrow‐head arrow marks time of change for disturbance regime. The two‐year moving average is plotted to aid legibility.
To address how long the effects of historical disturbance can last (Q2), we analyze effects of changing t post, the time spent after the regime change and species introduction. Fig. 5 illustrates how the effects of disturbance history decay over time. The difference in communities between the Introduction and Regime Change experiments is plotted; each panel shows color‐coded differences in the communities due to the differences in disturbance history, with tpost ranging from 100 years to 4500 years. Matching intuition, legacy effects of disturbance history decay over time, and the proportion of disturbance space that shows no change in communities between the two experiments grows as t post increases (Fig. 5, grey). The biotic legacy can affect community composition 4500 years after exposure to a 1900 year disturbance regime, and this compositional difference can occur for both positive and negative shifts in disturbance frequency and intensity. While the speed of decay may depend on many factors, effects of a historical regime can last up to 10 000 years in some cases (Fig. S13), though in others there are only minimal effects after 100 years (Figs S7 and S8) .
Figure 5.
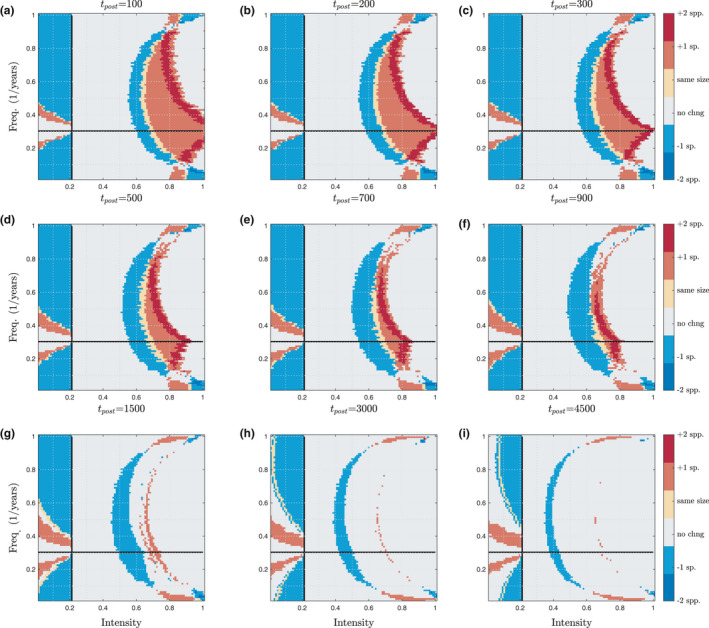
Persistence of disturbance legacy over time, plotted across disturbance frequency (F, units of 1/years) and disturbance intensity (dimensionless index). (a–i) Community differences between the Regime Change experiment and the simple Introduction experiment are plotted, using the same color coding scheme as Fig. 2d: red indicates that Regime Change increased species richness compared to the Introduction experiment, blue indicates a decrease in richness. All parameters are the same as Fig. 1, except for the manipulation of t post, the time period after species introduction when persistence is assessed. The effects of biotic legacy created by history of disturbance decay over time (more grey in the last panel than in the first), however, even 4500 years after a change to a historical regime, the effects of that legacy persist (e.g. blue region, (i)).
To explore how typical our results are, we analyze several other example communities in the online supporting information, including analyzing different shifts in Regime Change experiments, different species acting as invader, and different annual communities. We note here that the life‐history strategy of the invader is of crucial importance. In particular, when we analyze the same set of three species, but set C or R as the invader, the importance of history for invasion is minimal (Figs S5 and S6). Additional analysis of communities with different life‐history strategies shows that the strength of the effect of history on invasion often differs with different invaders (Figs S10 and S11), but in every community we have examined, history has a strong effect for at least one invader. We conclude that while these effects of past regimes on future invasions are not universal, our work suggests they are not rare. Determining a priori when to expect strong effects of past disturbance regimes on future communities and invasions will be a fascinating challenge for future research.
DISCUSSION
Disturbance regimes around the world are changing due to rapid global change (Vitousek et al., 1994; Vitousek et al., 1996; Hooper et al. 2012; Essl et al. 2015a). Moreover lags between anthropogenic changes and ecosystem effects are ubiquitous and worsen with time (Essl et al. 2015b; Komatsu et al. 2019). Our work suggests that historical regimes can influence future community invasibility, and that effects of past changes to disturbance regimes can linger due to long‐lasting biotic legacies. Importantly, we show that two systems with the same current disturbance regime may nevertheless have very different fates, solely due to differences in history. Thus, we raise awareness that analyzing community assembly or invasion based on stable processes whose distributions do not change over time may disregard important information about the past and how it shapes future dynamics.
We have shown that changing the history of disturbance can alter invasion success (Q1, Fig. 3c). Since the only difference between Regime Change and Introduction experiments is the disturbance history, this demonstrates that history of disturbance has clear and direct effects on community invasibility through creation of biotic legacies. The Simultaneous experiment displays a rich nonlinear response of diversity to disturbance (Figs. 2a, S10A), and regime changes and invasion result in extremely complex patterns of community change (Figs. 2d, S2D). While we cannot predict performance of a specific system, we now understand the types of behavior that may arise, and demonstrate how historical processes can accumulate to shape ecological communities.
In addition to changing invasion success and community structure, we have shown that the effects of disturbance history decay over time (Q2). Fig. 5I shows that 4500 years after a change to a historical regime, the effects of that biotic legacy can still register in community composition, for both positive and negative shifts in disturbance frequency and intensity. Though effects can decay relatively quickly, in some cases they can persist for millennia, and there seems to be no characteristic time scale for this decay. This is surprising, given that the effect of a historical period on the species composition can be seen after a time period more than double that of the original historical regime period. In longer‐lived organisms, there is precedent for even longer legacy effects of history, for example, the climate of 21 000 years ago was found to have a strong influence on patterns of tree species richness in Europe (Svenning & Skov 2007). Thus, our results illustrate that transient behavior is often more ecologically meaningful than asymptotic states (e.g. Hastings 2001; Hastings 2018; Fig. S13). Using sub‐models of the present model, we have previously established stability of coexistence and mechanisms thereof (Miller 2011), and demonstrated high richness of persisting species (Roxburgh 2004). However, for the timescale of a century after shifts in disturbance, species persistence is of primary interest, and stability results may be misleading (e.g. Fukami & Nakajima 2011, Yamamichi et al. 2014, Hastings 2018).
Observational studies have suggested the importance of disturbance history, and our work demonstrates a theoretical basis for strong effects of historical changes to disturbance regimes on community invasibility and community composition. We designed our experiments to rule out other possible explanations: all effects we describe must be due to the effects of disturbance history, and the biotic legacies left by the past processes. The historical aspects we consider are closely related to ‘antecedent effects’ and ‘memory’ of ecological systems, which have also been a growing topic of research, showing promising ability to better understand the future of ecosystems by considering the past as well as the present (Ogle et al. 2015). Thus, although it is commonly assumed that ecological processes can be well‐understood using stationary processes and current status, evidence is building that this assumption may be a poor one.
Our annual plant model is well‐suited as a tool to investigate our current and future questions, because the persistence of the seedbank allows for cumulative effects over time to be stored, and there is a rich record of theoretical work on annual plant systems (e.g. Ellner 1985; Roxburgh et al. 2004) that can inform our inquiry. Our results highlighting the importance of legacies also provide avenues for future research by integrating additional processes shown to be potentially important in determining how disturbance affects diversity. For example, disturbance only acts on seed yield in the present model. When disturbance alters other factors such as germination or mortality in this model, different mechanisms of diversity maintenance are engaged (Miller et al. 2012b). Thus, regime changes may alter invasion success and community structure via additional mechanisms. The regimes studied here are described entirely by two constant parameters (I, F). However, variation in intensity, I, and frequency, F, can alter community dynamics (Miller et al. 2019) and potentially interact with the formation of legacies. Likewise, we have studied abrupt change in disturbance regime, but gradual changes to disturbance regimes will be common in the coming decades of global change, and species may respond differently to gradual changes, compared to rapid shifts. Histories featuring regime change with an increase in disturbance tend to favor invasion in our model, though not always. In fact, when the ruderal species R is treated as an invader (Fig. S5), only minimal effects of disturbance history on invasion success are evident. More research will be necessary to understand the complex interactions between species’ traits and the importance of history, and these issues can be investigated within the present framework.
Other factors may affect the influence of disturbance history in annual plant systems; for example, productivity also interacts with disturbance to shape communities (Kondoh 2001), and this would enable yet another way for biotic legacies to accumulate. Additionally, regimes can be patchy in space, with local disturbance refugia acting as important sources of biotic and abiotic legacy (Box 1, Krachuk et al. 2020), and allowing for these would likely increase the effects of disturbance history. Moreover we expect that the interplay between ecological filtering and biotic legacies will allow for a variety of additional complex behaviours resulting from regime change and species introduction, because disturbance can act as a selective agent on a pool of potential resident species (e.g. Diaz et al. 1998; Grime 2006; Gompert et al. 2014). Interestingly, invasive species can often form their own legacies, such that effects of the invasion can persist even after the invader is removed (Corbin & D’Antonio 2012).
Our results on historical effects on invasions may apply to many different plant and non‐plant systems. Although our quantitative results are specific to a certain case, every three‐species community we have simulated has demonstrated effects of historical regimes change on invasion success for some invading species (Figs S5, S6, S9–14), indicating these effects are widespread and not dependent on specific model configurations. We have empirically tested qualitative predictions of our annual plant model on impacts of disturbance on diversity in laboratory microbial experiments (Hall et al. 2012), and these results can also help explain results of related studies in microcosms (Kassen et al. 2004; Benmayor et al. 2008).
Another system that is characterised by strong influence of disturbance and can be described by a similar model is the community of macroinvertebrates at hydrothermal vents (Miller et al. 2018), which are expected to experience rapid changes to disturbance regimes due to deep‐sea mining (Dunn et al. 2018). Our model is also structurally similar to those used to study insect populations that reproduce on an annual cycle and compete through shared parasitoids (Holt & Lawton, 1993), and this hints at the potential importance of biotic legacies in insect invasions as well. Finally, our techniques can be used to study effects of historical resource pulses (Yang et al. 2008) because when frequency is high, the disturbance regime can be interpreted as describing rare resource pulses that lead to increases in seed yield.
Our results show that historical changes to disturbance regimes can alter future community dynamics. They also generate rich areas for future theoretical (described above) and empirical research. Tests of hypotheses in laboratory and field systems will be required to fully assess how our findings apply to real‐world communities. Disturbance is known to play multiple roles in the establishment of exotic species (McIntyre & Lavorel 1994; Lake & Leishman 2004; Buckley et al. 2007; Lockwood et al. 2007) and biotic legacies of historical disturbance regimes are anticipated to affect productivity and carbon cycling (Volkova et al. 2018), diversity (Lunt & Spooner, 2005), nutrient cycling (Johnstone et al. 2010), and resilience (e.g. Johnstone et al. 2016). We propose that including history of disturbance and resultant biotic legacies in analyses will aid in developing better predictions of species invasions, community dynamics and ecosystem function.
Research at this forefront will increase our basic ecological understanding, and also inform applications to conservation biology, invasion ecology, restoration efforts and management. As our global ecosystems experience accelerating change, an understanding of these issues will allow us to build a foundation on which applied work can stand, so that each new problem does not need to be addressed de novo. As such, this research will then help to ameliorate and mitigate the ecological and economic damage that global change causes.
AUTHORSHIP
ADM and KS planned the research. ADM wrote and analyzed the model, and wrote the first draft of the paper. All authors contributed to interpreting the results, framing the manuscript, editing and approving the final manuscript draft.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/ele.13685.
Supporting information
Fig S1‐S17
ACKNOWLEDGEMENTS
This research was supported in part by NSF‐NERC award DEB‐1556444 to KS and AB.
Contributor Information
Adam D. Miller, Email: adm4@psu.edu.
Stephen H. Roxburgh, Email: stephen.roxburgh@csiro.au.
DATA AVAILABILITY STATEMENT
MATLAB code implementing the model is freely available under license (CC‐BY‐NC‐SA) at the Zenodo repository (https://doi.org/10.5281/zenodo.4429199).
References
- Benmayor, R. , Buckling, A. , Bonsall, M.B. , Brockhurst, M.A. & Hodgson, D.J. (2008). The interactive effects of parasites, disturbance, and productivity on experimental adaptive radiations. Evolution: Int. J. of Org. Evol., 62, 467–477. [DOI] [PubMed] [Google Scholar]
- Buckley, Y. , Bolker, B. & Rees, M. (2007). Disturbance, invasion and re‐invasion: managing the weed‐shaped hole in disturbed ecosystems. Ecol. Lett., 10, 809–817. [DOI] [PubMed] [Google Scholar]
- Connell, J. (1978). Diversity in tropical rain forests and coral reefs. Science, 199, 1302. [DOI] [PubMed] [Google Scholar]
- Corbin, J.D. & D’Antonio, C.M. (2012). Gone but not forgotten? invasive plants’ legacies on community and ecosystem properties. Invasive Plant Sci. Manage., 5, 117–124. [Google Scholar]
- Delory, B.M. , Weidlich, E.W. , von Gillhaussen, P. & Temperton, V.M. (2019). When history matters: The overlooked role of priority effects in grassland overyielding. Funct. Ecol., 33, 2369–2380. [Google Scholar]
- Diaz, S. , Cabido, M. & Casanoves, F. (1998). Plant functional traits and environmental filters at a regional scale. J. Veg. Sci., 9, 113–122. [Google Scholar]
- Dunn, D.C. , Van Dover, C.L. , Etter, R.J. , Smith, C.R. , Levin, L.A. , Morato, T. et al. (2018). A strategy for the conservation of biodiversity on mid‐ocean ridges from deep‐sea mining. Sci. Adv., 4, eaar4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner, S. (1985). ESS germination strategies in randomly varying environments. I. Logistic‐type models. Theor. Popul. Biol., 28, 50–79. [DOI] [PubMed] [Google Scholar]
- Essl, F. , Dullinger, S. , Rabitsch, W. , Hulme, P.E. , Pyšek, P. , Wilson, J.R. et al. (2015a). Delayed biodiversity change: no time to waste. Trends Ecol. Evol., 30, 375–378. [DOI] [PubMed] [Google Scholar]
- Essl, F. , Dullinger, S. , Rabitsch, W. , Hulme, P.E. , Pyšek, P. , Wilson, J.R. et al. (2015b). Historical legacies accumulate to shape future biodiversity in an era of rapid global change. Divers. Distrib., 21, 534–547. [Google Scholar]
- Franklin, J.F. , Lindenmayer, D. , MacMahon, J.A. , McKee, A. , Magnuson, J. , Perry, D.A. et al. (2000). Threads of continuity. Conserv. Biol. in Pract., 1, 8–16. [Google Scholar]
- Fraterrigo, J.M. & Rusak, J.A. (2008). Disturbance‐driven changes in the variability of ecological patterns and processes. Ecol. Lett., 11, 756–770. [DOI] [PubMed] [Google Scholar]
- Fukami, T. (2015). Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu. Rev. Ecol. Evol. Syst., 46, 1–23. [Google Scholar]
- Fukami, T. & Nakajima, M. (2011). Community assembly: alternative stable states or alternative transient states? Ecol. Lett., 14, 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison, A. , Miller, A. , Roxburgh, S.H. & Shea, K. (2012). More bang for the land manager’s buck: disturbance autocorrelation can be used to achieve management objectives at no additional cost. J. Appl. Ecol., 49, 1020–1027. [Google Scholar]
- Gompert, Z. , Comeault, A.A. , Farkas, T.E. , Feder, J.L. , Parchman, T.L. , Buerkle, C.A. et al. (2014). Experimental evidence for ecological selection on genome variation in the wild. Ecol. Lett., 17, 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime, J. (1977). Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat., 111, 1169. [Google Scholar]
- Grime, J.P. (2006). Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. J. Veg. Sci., 17, 255–260. [Google Scholar]
- Hall, A. , Miller, A. , Leggett, H. , Roxburgh, S. , Buckling, A. & Shea, K. (2012). Diversity–disturbance relationships: frequency and intensity interact. Biol. Lett., 8, 768–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, J.L. et al. (1977). Population Biology of Plants. Academic Press, New York, NY. [Google Scholar]
- Hastings, A. (2001). Transient dynamics and persistence of ecological systems. Ecol. Lett., 4, 215–220. [Google Scholar]
- Hastings, A. , Abbott, K.C. , Cuddington, K. , Francis, T. , Gellner, G. , Lai, Y.C. et al. (2018). Transient phenomena in ecology. Science, 361, eaat6412. [DOI] [PubMed] [Google Scholar]
- Holt, R.D. & Lawton, J.H. (1993). Apparent competition and enemy‐free space in insect host‐parasitoid communities. Am. Nat., 142, 623–645. [DOI] [PubMed] [Google Scholar]
- Hooper, D.U. , Adair, E.C. , Cardinale, B.J. , Byrnes, J.E. , Hungate, B.A. , Matulich, K.L. et al. (2012). A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature, 486, 105–108. [DOI] [PubMed] [Google Scholar]
- Johnstone, J.F. , Allen, C.D. , Franklin, J.F. , Frelich, L.E. , Harvey, B.J. , Higuera, P.E. et al. (2016). Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ., 14, 369–378. [Google Scholar]
- Johnstone, J.F. , Chapin, F.S. , Hollingsworth, T.N. , Mack, M.C. , Romanovsky, V. & Turetsky, M. (2010). Fire, climate change, and forest resilience in interior Alaska. Can. J. For. Res., 40, 1302–1312. [Google Scholar]
- Kassen, R. , Llewellyn, M. & Rainey, P.B. (2004). Ecological constraints on diversification in a model adaptive radiation. Nature, 431, 984–988. [DOI] [PubMed] [Google Scholar]
- Komatsu, K.J. , Avolio, M.L. , Lemoine, N.P. , Isbell, F. , Grman, E. , Houseman, G.R. et al. (2019). Global change effects on plant communities are magnified by time and the number of global change factors imposed. Proc. Natl. Acad. Sci. USA, 116, 17867–17873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh, M. (2001). Unifying the relationships of species richness to productivity and disturbance. Proc. R. Soc. B, 268, 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawchuk, M.A. , Meigs, G.W. , Cartwright, J.M. , Coop, J.D. , Davis, R. , Holz, A. et al. (2020). Disturbance refugia within mosaics of forest fire, drought, and insect outbreaks. Front. Ecol. Environ., 18, 235–244. [Google Scholar]
- Krebs, P. , Pezzatti, G.B. , Mazzoleni, S. , Talbot, L.M. & Conedera, M. (2010). Fire regime: history and definition of a key concept in disturbance ecology. Theor. in Biosci., 129, 53–69. [DOI] [PubMed] [Google Scholar]
- Lake, J.C. & Leishman, M.R. (2004). Invasion success of exotic plants in natural ecosystems: the role of disturbance, plant attributes and freedom from herbivores. Biol. Conserv., 117, 215–226. [Google Scholar]
- Lake, P. (2000). Disturbance, patchiness, and diversity in streams. J. North Am. Benth. Soc., 19, 573–592. [Google Scholar]
- Levin, S.A. & Paine, R.T. (1974). Disturbance, patch formation, and community structure. Proc. Natl. Acad. Sci. U.S.A., 71, 2744–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, J. , Ying, Z. , Woolnough, D.A. , Miller, A.D. , Li, Z. & Nijs, I. (2016). Coexistence of species with different dispersal across landscapes: a critical role of spatial correlation in disturbance. Proc. R. Soc. B, 283, 20160537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood, J. , Hoopes, M. & Marchetti, M. (2007). Invasion Ecology. Blackwell publishing ltd, Oxford, UK. [Google Scholar]
- Lubchenco, J. (1978). Plant species diversity in a marine intertidal community: importance of herbivore food preference and algal competitive abilities. Am. Nat, 112, 23–39. [Google Scholar]
- Lunt, I.D. & Spooner, P.G. (2005). Using historical ecology to understand patterns of biodiversity in fragmented agricultural landscapes. J. Biogeogr., 32, 1859–1873. [Google Scholar]
- McCormick, G.L. , Shea, K. & Langkilde, T. (2015). How do duration, frequency, and intensity of exogenous cort elevation affect immune outcomes of stress? Gen. Comp. Endocrinol., 222, 81–87. [DOI] [PubMed] [Google Scholar]
- McIntyre, S. & Lavorel, S. (1994). Predicting richness of native, rare, and exotic plants in response to habitat and disturbance variables across a variegated landscape. Conserv. Biol., 8, 521–531. [Google Scholar]
- Miller, A.D. , Hsing, P.Y. , Roxburgh, S.H. , Fisher, C.R. & Shea, K. (2018). Impacts of altered disturbance regimes on community structure and biodiversity mediated by fecundity–tolerance interactions. Nat. Resour. Model., 31, e12199. [Google Scholar]
- Miller, A. , Reilly, D. , Bauman, S. & Shea, K. (2012a). Interactions between frequency and size of disturbance affect competitive outcomes. Ecol. Res., 27, 783–791. [Google Scholar]
- Miller, A. , Roxburgh, S. & Shea, K. (2011). How frequency and intensity shape diversity–disturbance relationships. Proc. Natl. Acad. Sci. USA, 108, 5643–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A.D. , Roxburgh, S.H. & Shea, K. (2012b). Timing of disturbance alters competitive outcomes and mechanisms of coexistence in an annual plant model. Theor. Ecol., 5, 419–432. [Google Scholar]
- Miller, A.D. , Thompson, J.R. , Tepley, A.J. & Anderson‐Teixeira, K.J. (2019). Alternative stable equilibria and critical thresholds created by fire regimes and plant responses in a fire‐prone community. Ecography, 42, 55–66. [Google Scholar]
- Moles, A. , Flores‐Moreno, H. , Bonser, S. , Warton, D. , Helm, A. , Warman, L. et al. (2012). Invasions: the trail behind, the path ahead, and a test of a disturbing idea. J. Ecol., 100, 116–127. [Google Scholar]
- Newman, E.A. (2019). Disturbance ecology in the anthropocene. Front. Ecol. Evol., 7, 147. 10.3389/fevo. [DOI] [Google Scholar]
- Ogle, K. , Barber, J.J. , Barron‐Gafford, G.A. , Bentley, L.P. , Young, J.M. , Huxman, T.E. et al. (2015). Quantifying ecological memory in plant and ecosystem processes. Ecol. Lett., 18, 221–235. [DOI] [PubMed] [Google Scholar]
- Perring, M.P. , De Frenne, P. , Baeten, L. , Maes, S.L. , Depauw, L. , Blondeel, H. et al. (2016). Global environmental change effects on ecosystems: The importance of land‐use legacies. Global Change Biol., 22, 1361–1371. [DOI] [PubMed] [Google Scholar]
- Pickett, S. & White, P. (1985). The Ecology of Natural Disturbance and Patch Dynamics. Academic, New York, NY. [DOI] [PubMed] [Google Scholar]
- Roxburgh, S. , Shea, K. & Wilson, J. (2004). The intermediate disturbance hypothesis: Patch dynamics and mechanisms of species coexistence. Ecology, 85, 359–371. [Google Scholar]
- Seabloom, E.W. , Borer, E.T. & Tilman, D. (2020). Grassland ecosystem recovery after soil disturbance depends on nutrient supply rate. Ecol. Lett., 23, 1756–1765. 10.1111/ele.13591. [DOI] [PubMed] [Google Scholar]
- Shea, K. , Roxburgh, S. & Rauschert, E. (2004). Moving from pattern to process: coexistence mechanisms under intermediate disturbance regimes. Ecol. Lett., 7, 491–508. [Google Scholar]
- Shinozaki, K. & Kira, T. (1956). Intraspecific competition among higher plants. VII. Logistic theory of the CD effect. Journal of Institutes and Polytechnics, Osaka City University Series D, 7, 35–72. [Google Scholar]
- Svenning, J.C. & Skov, F. (2007). Ice age legacies in the geographical distribution of tree species richness in europe. Global Ecol. Biogeogr., 16, 234–245. [Google Scholar]
- Theoharides, K.A. & Dukes, J.S. (2007). Plant invasion across space and time: factors affecting nonindigenous species success during four stages of invasion. New Phytol., 176, 256–273. [DOI] [PubMed] [Google Scholar]
- Tilman, D. (1994). Competition and biodiversity in spatially structured habitats. Ecology, 75, 2–16. [Google Scholar]
- Turner, M.G. (2010). Disturbance and landscape dynamics in a changing world. Ecology, 91, 2833–2849. [DOI] [PubMed] [Google Scholar]
- Vitousek, P.M. (1994). Beyond global warming: ecology and global change. Ecology, 75, 1861–1876. [Google Scholar]
- Vitousek, P.M. , Antonio, C.M. , Loope, L.L. & Westbrooks, R. (1996). Biological invasions as global environmental change. Am. Sci., 84, 468. [Google Scholar]
- Volkova, L. , Roxburgh, S.H. , Weston, C.J. , Benyon, R.G. , Sullivan, A.L. & Polglase, P.J. (2018). Importance of disturbance history on net primary productivity in the world’s most productive forests and implications for the global carbon cycle. Global Change Biol., 24, 4293–4303. [DOI] [PubMed] [Google Scholar]
- Walker, L.R. (2012). The Biology of Disturbed Habitats. Oxford, UK: Oxford University Press. [Google Scholar]
- Yamamichi, M. , Yoshida, T. & Sasaki, A. (2014). Timing and propagule size of invasion determine its success by a time‐varying threshold of demographic regime shift. Ecology, 95, 2303–2315. [DOI] [PubMed] [Google Scholar]
- Yang, L.H. , Bastow, J.L. , Spence, K.O. & Wright, A.N. (2008). What can we learn from resource pulses. Ecology, 89, 621–634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S17
Data Availability Statement
MATLAB code implementing the model is freely available under license (CC‐BY‐NC‐SA) at the Zenodo repository (https://doi.org/10.5281/zenodo.4429199).


