Abstract
Aim
To undertake a cost‐effectiveness analysis of dapagliflozin in treating high‐risk patients with type 2 diabetes mellitus (T2DM), using both directly observed events in the DECLARE‐TIMI 58 trial and surrogate risk factors to predict endpoints not captured within the trial.
Methods
An established T2DM model was adapted to integrate survival curves derived from the DECLARE‐TIMI 58 trial, and extrapolated over a lifetime for all‐cause mortality, hospitalization for heart failure, stroke, myocardial infarction, hospitalization for unstable angina, and end‐stage kidney disease. The economic analysis considered the overall DECLARE trial population, as well as reported patient subgroups. Total and incremental costs, life‐years and quality‐adjusted life‐years associated with dapagliflozin versus placebo were estimated from the perspective of the UK healthcare payer.
Results
In the UK setting, treatment with dapagliflozin compared to placebo was estimated to be dominant, with an expected increase in quality‐adjusted life‐years from 10.43 to 10.48 (+0.06) and a reduction in lifetime total costs from £39 451 to £36 899 (−£2552). Across all patient subgroups, dapagliflozin was estimated to be dominant, with the greatest absolute benefit in the prior heart failure subgroup (incremental lifetime costs −£4150 and quality‐adjusted life‐years +0.11).
Conclusions
The results of this study demonstrate that dapagliflozin compared to placebo appears to be cost‐effective, when considering evidence reported from the DECLARE‐TIMI 58 trial, at established UK willingness‐to‐pay thresholds. The findings highlight the potential of dapagliflozin to have a meaningful impact in reducing the economic burden of T2DM and its associated complications across a broad T2DM population.
Keywords: cost‐effectiveness, dapagliflozin, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) is increasing, driven by increased incidence, ageing and population growth. 1 T2DM and its associated complications, including macrovascular (coronary artery disease, peripheral arterial disease and stroke) and microvascular complications (diabetic kidney disease, neuropathy and retinopathy), are a significant economic burden to healthcare providers, 2 , 3 with the management of people with T2DM and vascular complications costing up to 2.5 times more than that of those without such complications. 4
Metformin is generally recommended as the first‐line treatment of T2DM, with sodium‐glucose co‐transporter‐2 (SGTL2) inhibitors often recommended as second line treatment in individuals who have been unable to achieve glycaemic control with metformin (or as first‐line treatment in cases of metformin intolerance). 5 , 6 , 7 , 8 SGLT2 inhibitors have demonstrated efficacy in reducing glycated haemoglobin (HbA1c) levels and body weight in people with T2DM without increasing hypoglycaemia. 9 , 10 , 11 , 12 , 13 In addition, they have demonstrated cardiovascular (CV) benefits 14 , 15 , 16 , 17 and kidney function improvements. 16 , 18 , 19 , 20 , 21 , 22
DECLARE‐TIMI 58 was the largest SGLT2 inhibitor CV outcome trial (CVOT; n = 17 160) and applied broad eligibility criteria (age ≥40 years, HbA1c 48‐107 mmol/mol, creatinine clearance ≥60 mL/min), including people with established CV disease (defined as clinically evident ischaemic heart disease, ischaemic cerebrovascular disease or peripheral artery disease) or with multiple CV risk factors (≥55‐year‐old men and ≥60‐year‐old women with at least one of the following: dyslipidaemia, hypertension or current smoking). The trial demonstrated that dapagliflozin reduced the risk of hospitalization for heart failure (HHF) or CV death compared with placebo, as well as reducing kidney disease progression, in individuals with or without established CV disease. The findings of a reduction in CV disease with dapagliflozin in both high‐ and low‐CV‐risk populations has also been suggested in a number of real‐world studies. 23 , 24 , 25 , 26 , 27 While the safety and clinical efficacy results of the DECLARE‐TIMI 58 trial are well described, the impact of the observed beneficial effects of dapagliflozin on its cost‐effectiveness is not known.
Previous economic evaluations have provided evidence supporting the cost‐effectiveness of dapagliflozin as a monotherapy, dual therapy or triple therapy compared to other antidiabetes therapies for managing T2DM. For example, on a background insulin regime, dapagliflozin was cost‐effective compared to insulin alone, 28 on a background of metformin, dapagliflozin was cost‐effective when compared to sulphonylureas, 29 , 30 , 31 , 32 , 33 thiazolidinediones 29 and dipeptidyl peptidase‐4 (DPP‐4) inhibitors, 33 , 34 and on a background of metformin and sulphonylureas, dapagliflozin was cost‐effective compared to a dipeptidyl peptidase‐4 inhibitor. 35 Dapagliflozin monotherapy was cost‐effective compared to glimepiride, acarbose and DPP‐4 inhibitor monotherapy in individuals with T2DM receiving no other antidiabetes therapy. 36 , 37 , 38 Furthermore, a recent systematic review demonstrated SGLT2 inhibitors to be the most cost‐effective option, compared to other treatments for T2DM, 39 with much of their benefit driven by improved quality of life related to weight loss and low rates of hypoglycaemia, with estimates of long‐term CV benefit being derived from changes in established risk factors using published risk equations. 40 , 41 However, data from DECLARE‐TIMI 58 and other CVOTs suggest that SGLT2 inhibitors are associated with CV benefits above and beyond those predicted via the modification of traditional risk factors included in these risk equations, and not considering event rates driven by these additional factors is likely to underestimate the cost‐effectiveness of dapagliflozin.
The Cardiff T2DM Model is an economic model, developed using equations from the UK Prospective Diabetes Study (UKPDS), 40 , 41 , 42 that provides cost‐effectiveness of the long‐term health and economic impact of managing T2DM in terms of cost per quality‐adjusted life‐year (QALY) gained, and has been utilised in several economic evaluations of T2DM treatments. 31 , 34 , 43 , 44 , 45 , 46 , 47 , 48 , 49 An adaptation of this model to incorporate survival curves derived from CVOTs and real‐world studies of SGLT2 inhibitors was recently employed to evaluate the cost‐effectiveness of SGLT2 inhibitors as a drug class for treating T2DM. 50 In the present study, the Cardiff T2DM Model was adapted to integrate survival curves derived specifically from the DECLARE‐TIMI 58 trial, and was used to evaluate the cost‐effectiveness of dapagliflozin across population subgroups included in DECLARE‐TIMI 58. This analysis is the first to apply extrapolated survival curves from DECLARE‐TIMI 58 to a full cost‐effectiveness analysis, using directly observed events in the trial and incorporating relevant surrogate factors to predict cost‐effectiveness in a T2DM population. It aims to provide economic analysis of dapagliflozin that is highly relevant to decision making in clinical practice in diabetes, where the reduction in preventable complications should be given considerable weight not only for individual patients, but also in healthcare systems running at, or close to, capacity. As DECLARE‐TIMI 58 is the longest, largest and broadest SGLT2 inhibitor CVOT to date, including both a high‐risk secondary prevention group with established CV disease as well as a large primary prevention group, the results of this economic analysis are likely to be broadly applicable to the majority of the T2DM population.
2. METHODS
2.1. Model overview
The analysis used an adaptation of the Cardiff T2DM cost‐effectiveness model, 51 a patient‐level fixed‐time increment (6‐monthly) Monte Carlo simulation, which includes a kidney module to track progression through chronic kidney disease (CKD) stages. The kidney module allows an annual decline in estimated glomerular filtration rate (eGFR) to consequently map declining eGFR to CKD stage, allowing costs and disutility per CKD stage to be captured within the model. This version of the model was adapted to include survival curves derived from DECLARE‐TIMI 58, extrapolated over a lifetime, with endpoints including all‐cause mortality, initial and secondary HHF, initial and secondary stroke, initial and secondary myocardial infarction, hospitalization for unstable angina pectoris, and end‐stage kidney disease (ESKD). The equations used for each of these endpoints are summarized in Supplementary Table S1. Incidence of blindness and lower limb ulceration due to diabetes were estimated via the UKPDS 82 risk equations. 41 Due to the reliance of the UKPDS equations on surrogate risk factors (eg, HbA1c, systolic blood pressure [SBP] and weight), the effects of treatment were applied to these modifiable risk factors in the first year and their progression tracked over the remaining horizon.
Adherence to treatment and escalation of background T2DM therapies were implicitly assumed to be captured within the extrapolation of survival curves. Treatment with dapagliflozin was assumed to be discontinued when eGFR declined below 45 mL/min/1.73 m2, and an additional annual probability of dapagliflozin discontinuation was also modelled in line with observations from DECLARE‐TIMI 58. 16 Once individuals had discontinued dapagliflozin, they were assumed to be treated with placebo, and subject to the placebo complication risks for the remainder of the modelled time horizon.
2.2. Model inputs
For each modelled CV outcome, survival curves were fitted to Kaplan‐Meier data collected over the DECLARE‐TIMI 58 trial for the overall DECLARE population, and reported patient subgroups (established cardiovascular disease [eCVD] vs. multiple risk factors [MRF], and prior heart failure [HF] vs. no prior HF). Survival was predicated over a lifetime by extrapolating 4‐year survival curves. Where there was no significant difference reported between trial arms, a single survival curve was fitted to pooled data from both arms of the trial.
To account for long‐term increases in mortality risk associated with ageing, country‐specific life tables 52 were applied if the age‐ and sex‐specific probability of death in the general population exceeded the predicted probability of death from the survival curves.
Model inputs, such as treatment effects on modifiable risk factors (Supplementary Table S2), baseline patient characteristics (Table 1) and incidence of adverse events and discontinuation of dapagliflozin (Supplementary Table S2), were sourced from DECLARE‐TIMI 58 publications, 16 , 53 , 54 supplemented with UKPDS data 42 where published data from DECLARE‐TIMI 58 were unavailable. Following the application of treatment effects to modifiable risk factors in Year 1 of the simulation, HbA1c and SBP were assumed to progress in line with UKPDS data 40 over the remaining time horizon. Following the application of an initial weight loss of −2.42 kg and −0.63 kg to the dapagliflozin and placebo arms, respectively, the dapagliflozin treatment effect on weight was assumed to be lost over a period of 1 year. Following this, weight was assumed to decline at an annual rate of −0.40 and −0.35 kg in the dapagliflozin and placebo arms, respectively, based on observations from DECLARE‐TIMI 58. 16
TABLE 1.
Mean baseline characteristics for the overall population and by patient subgroup
| Variable | Overall | eCVD | MRF | Prior HF | No prior HF | Source |
|---|---|---|---|---|---|---|
| Age, years | 63.80 | 62.50 | 64.70 | 64.32 | 64.00 | Raz et al 2018, 53 Kato et al 2019 27 |
| Proportion female | 0.37 | 0.28 | 0.44 | 0.34 | 0.38 | Wiviott et al 2019, 16 Kato et al 2019 27 |
| Duration diabetes, years | 10.50 | 12.00 | 11.70 | 10.00 | 11.00 | Wiviott et al 2019, 16 Kato et al 2019 27 |
| Height, m | 1.68 | 1.68 | 1.68 | 1.68 | 1.68 | UKPDS 33 42 |
| HbA1c, mmol/mol | 67.2 | 67.5 | 66.8 | 65.8 | 63.9 | Raz et al 2018, 53 Kato et al 2019 27 |
| SBP, mmHg | 135.00 | 134.00 | 135.60 | 135.00 a | 135.00 a | Raz et al 2018 53 |
| Weight, kg | 90.55 | 90.55 | 90.27 | 91.94 | 87.73 | Calculation |
| eGFR, mL/min/1.73 m2 | 85.20 | 84.90 | 87.00 | 84.99 | 89.00 | Raz et al 2018, 53 Kato et al 2019 27 |
| Heart rate | 73.00 | 71.50 | 74.10 | 73.00 a | 73.00 a | Raz et al 2018 53 |
Note: Where data required by the UK Prospective Diabetes Study (UKPDS) 82 risk equations were unavailable, UKPDS values were applied.
Abbreviations: eCVD, established cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HF, heart failure; MI, myocardial infarction; MRF, multiple risk factors; SBP, systolic blood pressure.
Not reported for subgroup, assumed equal to overall population.
Within the model, kidney disease progression is modelled with treatment‐specific linear declines in eGFR, which are mapped to CKD stages. Additionally, a constant ESKD rate is applied per treatment arm to capture those individuals progressing to this health state. Specific values for the eGFR slope and ESKD rates were derived from DECLARE‐TIMI 58 data. 54
Utility decrements associated with T2DM complications, CKD health states and weight changes were sourced from published literature and applied additively (Supplementary Table S3). Published costs of T2DM complications and CKD health states were inflated to 2019 prices where necessary. The cost of dapagliflozin was sourced from UK price lists 55 and applied to the treatment arm (Supplementary Table S3).
2.3. Validation
To ensure that predicted CV outcomes over the modelled time horizon were plausible, modelled output was validated to UKPDS survival curves (Supplementary Figures S1‐S5). To further validate the model, predictions over a time horizon of 4.2 years were validated to reported outcomes from DECLARE‐TIMI 58 (Supplementary Figure S2).
2.4. Cost‐utility analysis
The economic analysis considered adults with T2DM at increased risk of CV disease as represented by DECLARE‐TIMI 58. Total and incremental costs, life‐years and QALYs associated with dapagliflozin versus placebo were estimated over a lifetime for a cohort of 1000 patients, simulated 1000 times. Costs and health benefits were applied from the perspective of the UK healthcare payer, and discounted at 3.5% annually. A willingness‐to‐pay threshold of £20 000 56 was applied in the derivation of net monetary benefit (ie, the total monetary value of costs and QALYs) and to assess cost‐effectiveness.
2.5. Sensitivity analysis
Sensitivity analysis was conducted to assess the impact of alternative modelling assumptions on predicted outcomes. Within this study, there was uncertainty surrounding the CKD health state costs, which were defined as CKD care costs and included inpatient stays, nephrology outpatient visits, antihypertensive drugs and general practice visits. Due to the potential for double counting of some complication‐related costs, a scenario was conducted whereby the CKD stage costs were removed from the analysis.
Sensitivity analysis was undertaken in the overall population, and analyses compared results for the following scenarios: exclusion of CKD costs prior to ESKD; no natural weight change assumed; dapagliflozin treatment effects on weight assumed not to be lost after 1 year; and no annual discontinuation of SGLT2 inhibitor treatment.
3. RESULTS
3.1. Base‐case analysis
The predicted incidence of events over a lifetime for the overall population and per population subgroup are presented in Figure 1. Across all populations, HHF was the event that demonstrated the greatest reduction in incidence with dapagliflozin treatment. In the overall population, dapagliflozin was predicted to prevent 30 HHF events per 1000 patients. A greater number of events were predicted to be prevented in high‐risk subgroups: 46 and 50 per 1000 patients in the eCVD and prior HF subgroups, respectively. Treatment with dapagliflozin was also associated with fewer ESKD events compared to the control arm: four and 13 fewer events per 1000 patients were predicted in the full population and prior HF subgroup, respectively. A greater number of events were predicted per life‐year in higher‐risk patient subgroups (eCVD vs. MRF; prior HF vs. no prior HF) and consequently the estimated avoidance of future events with the use of dapagliflozin was greater in these individuals (Figure 2).
FIGURE 1.
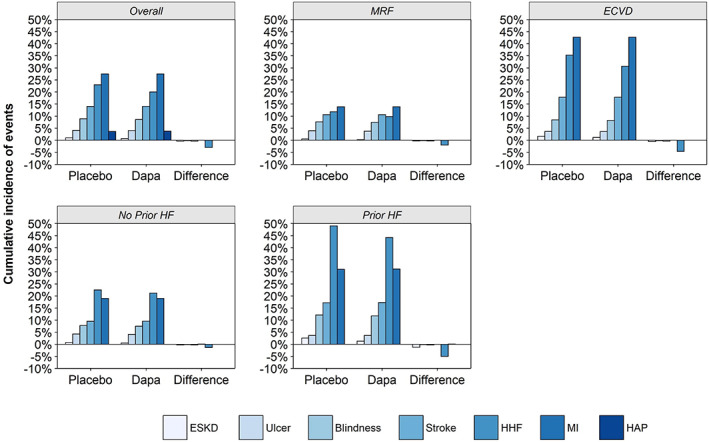
Cumulative incidence of events (%) predicted over a lifetime for the overall population and population subgroups. Dapa, dapagliflozin; ECVD, established cardiovascular disease; ESKD, end‐stage kidney disease; HAP, hospitalization for unstable angina pectoris; HF, heart failure; HHF, hospitalization for heart failure; MI, myocardial infarction; MRF, multiple risk factors
FIGURE 2.
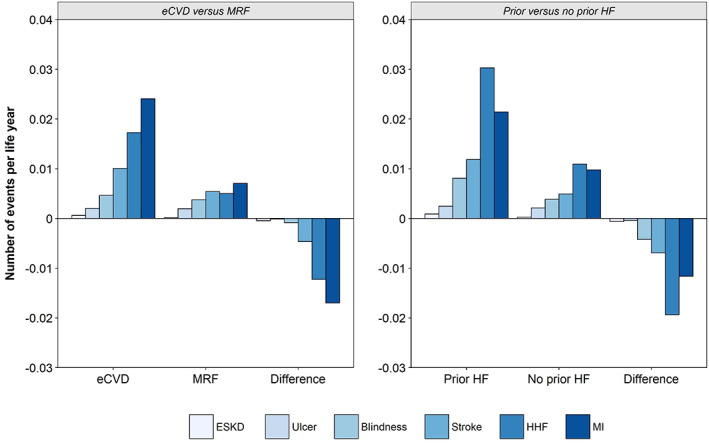
Number of events predicted per life‐year in higher‐risk patient subgroups. eCVD, established cardiovascular disease; ESKD, end‐stage kidney disease; HF, heart failure; HHF, hospitalization for heart failure; MI, myocardial infarction; MRF, multiple risk factors
Figure 3 presents the breakdown of estimated costs by treatment and category of complications per population. In the overall population, and across low‐risk subpopulations, total estimated costs were driven by kidney costs. Despite high costs associated with an ESKD event, as a consequence of limited predicted numbers of these events in a population with relatively good baseline kidney function, the kidney costs are largely driven by CKD costs. In the full population, costs associated with CKD contribute 86% of kidney costs in the placebo arm, and 90% of kidney costs in the dapagliflozin arm. In high‐risk subgroups, however, higher costs are accrued from macrovascular events, due to an increased risk of experiencing both an initial and subsequent event in these subgroups. In the prior HF subgroup, for instance, almost 30% of the cohort is expected to experience an initial HHF event, with nearly 70% of these leading to a secondary HHF event within the modelled time horizon. Due to the high cost burden of HHF, costs in this population are driven by CV events. Likewise, in the eCVD population, more than 20% of the cohort experience an initial HHF event in the placebo arm, compared to less than 10% in the MRF subgroup. In addition to this, patients with eCVD are expected to experience a greater number of myocardial infarction events compared to the MRF subgroup (30% compared to 11% of the cohort). A higher incidence of initial events in the eCVD subgroup also leads to a greater number of secondary events, therefore making CV costs comparable to costs accrued from CKD in the model. Across subgroups, decreases in kidney and complication costs associated with dapagliflozin offset the additional costs associated with treatment.
FIGURE 3.
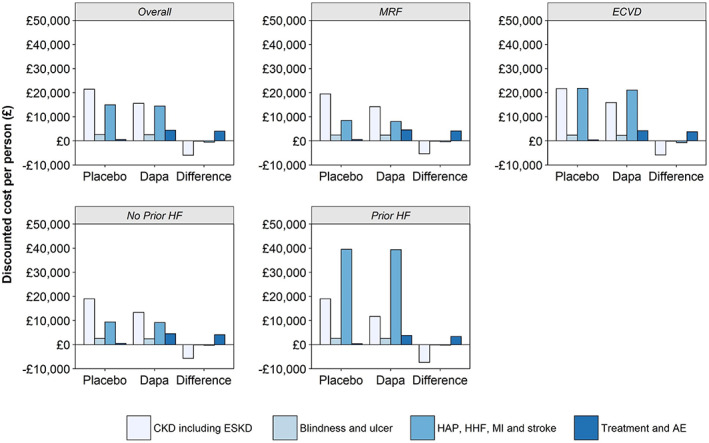
Total discounted costs predicted per person over a lifetime for the overall population and population subgroups. AE, adverse event; Dapa, dapagliflozin; ECVD, established cardiovascular disease; ESKD, end‐stage kidney disease; HAP, hospitalization for unstable angina pectoris; HF, heart failure; HHF, hospitalization for heart failure; MI, myocardial infarction; MRF, multiple risk factors
Table 2 summarizes the lifetime discounted cost, QALY, life‐year, and cost‐effectiveness estimates at the patient level. QALY gains of between 0.06 and 0.10 were predicted for individuals treated with dapagliflozin across all patient populations, driven by the slower kidney disease progression associated with dapagliflozin, and fewer ESKD events predicted in this arm. For the overall population, most of the QALY gain estimated related to differences in kidney disease progression and ESKD (~64%), however, a large proportion was attributable to macro‐ and microvascular complications (~33%). The remaining difference in QALYs relates to differences in weight and adverse events (~4%). Patterns in estimated QALY gains and cost‐effectiveness across patient subgroups reflected the pattern in events avoided; consequently, while dapagliflozin was associated with cost‐savings across all subgroups, it was estimated to be most cost‐effective in higher‐risk subgroups.
TABLE 2.
Lifetime discounted cost‐effectiveness results, by population subgroup
| Scenario | Outcome | Control | Dapagliflozin | Difference |
|---|---|---|---|---|
| Overall | Life‐years | 13.53 | 13.54 | 0.00 |
| QALYs | 10.43 | 10.48 | 0.06 | |
| Costs | £39 451 | £36 899 | −£2552 | |
| NMB | £169 071 | £172 730 | £3660 | |
| ICER | Dominant | |||
| MRF | Life‐years | 13.81 | 13.81 | 0.00 |
| QALYs | 10.53 | 10.60 | 0.07 | |
| Costs | £30 837 | £29 086 | −£1752 | |
| NMB | £179 776 | £183 002 | £3226 | |
| ICER | Dominant | |||
| eCVD | Life‐years | 12.63 | 12.63 | 0.00 |
| QALYs | 8.96 | 9.06 | 0.09 | |
| Costs | £46 293 | £43 462 | −£2831 | |
| NMB | £132 946 | £137 641 | £4694 | |
| ICER | Dominant | |||
| No prior HF | Life‐years | 13.69 | 13.70 | 0.00 |
| QALYs | 10.38 | 10.45 | 0.07 | |
| Costs | £31 505 | £29 486 | −£2018 | |
| NMB | £176 105 | £179 490 | £3385 | |
| ICER | Dominant | |||
| Prior HF | Life‐years | 10.80 | 10.81 | 0.00 |
| QALYs | 6.70 | 6.80 | 0.11 | |
| Costs | £61 568 | £57 419 | −£4150 | |
| NMB | £72 420 | £78 486 | £6067 | |
| ICER | Dominant |
Abbreviations: CVOT, cardiovascular outcome trial; eCVD, established cardiovascular disease; HF, heart failure; ICER, incremental cost‐effectiveness ratio; MRF, multiple risk factors; NMB, net monetary benefit; QALY, quality‐adjusted life‐year.
3.2. Sensitivity analysis
Dapagliflozin remained cost‐effective across the majority of scenarios modelled in the sensitivity analysis. Assuming no annual discontinuation of dapagliflozin resulted in the highest increase in cost‐savings, with estimated savings of £3768 with dapagliflozin compared to £2552 in the base‐case analysis. This scenario also resulted in an increase in incremental QALYs, with dapagliflozin being associated with an increase of 0.12 QALYS in comparison to 0.06 in the base case. These results are driven by the fact that patients remain on dapagliflozin treatment for longer, and so receive the continued benefit associated with treatment.
The greatest QALY gains associated with dapagliflozin were observed when the weight benefit associated with dapagliflozin was not removed after 1 year of treatment. This resulted in an increase of 0.26 QALYs compared to placebo, as patients maintained their weight loss throughout the modelled time horizon. This scenario was also associated with large cost savings of £2609 per patient. Assuming that there is no natural weight gain of patients resulted in minimal differences to the base‐case scenario. QALY gains were marginally lower (0.07 in the scenario analysis vs. 0.06 in the base‐case analysis), as well as cost savings being slightly lower in this scenario (£2548 compared to £2552 in the base‐case analysis).
In contrast to the above scenarios, removing costs associated with CKD stages resulted in treatment with dapagliflozin no longer being cost‐effective at a willingness‐to‐pay threshold of £20 000 per QALY. This is due to the modest differences between macrovascular events experienced between treatment arms in the model, resulting in the kidney disease progression costs being a big driver of the cost‐effectiveness. When CKD costs prior to ESKD were excluded from the analysis, the additional costs of dapagliflozin were not offset by any large costs associated with placebo, thereby making the analysis less cost‐effective. It should be noted, however, that CKD stages are associated with a cost burden, and so the results of this scenario are unlikely, but are illustrative to show the impact on modelled outcomes in the most extreme case of no CKD stage costs.
3.3. UK health economic impact
The potential value associated with dapagliflozin at the population level is considerable, due principally to the size of the relevant T2DM population. Supplementary Table S4 presents results for the overall DECLARE‐TIMI 58 population scaled to the UK population based on estimates of T2DM prevalence 1 , 57 and the proportion of the general T2DM population represented by the trial. 58 , 59
4. DISCUSSION
In this study we undertook an economic evaluation using data from the DECLARE‐TIMI 58 trial and demonstrated that dapagliflozin appears to be a cost‐effective treatment for people with T2DM and with established CV disease or with multiple risk factors for CV disease, and for people with T2DM with and without prior HF. The cost‐effectiveness of dapagliflozin across the patient subgroups reflected the pattern in events avoided; dapagliflozin was estimated to be most cost‐effective in higher‐risk subgroups in which dapagliflozin was associated with the greatest reduction in kidney disease progression and number of ESKD events.
There have been two previous economic analyses using SGLT2 inhibitor CVOTs, both with EMPA‐REG. 60 , 61 However, EMPA‐REG recruited people with T2DM with eCVD only, thereby limiting the generalizability of these cost‐effectiveness results to a wider T2DM population. In addition, a recent study compared the cost of preventing HF events in people with T2DM treated with empagliflozin, canagliflozin or dapagliflozin. The results demonstrated empagliflozin to be the most cost‐saving treatment. However, the analysis did not take into account differences in trial design; EMPA‐REG included only patients with eCVD, 14 CANVAS included 66% with eCVD, 15 whereas only 41% in DECLARE‐TIMI 58 had eCVD. 16 DECLARE‐TIMI 58 may be considered to be more representative of the general T2DM population than EMPA‐REG and CANVAS as it included a broader population. The results of DECLARE‐TIMI 58 demonstrated that the HF and kidney outcome benefits of SGLT2 inhibitors observed in patients with established disease also extend to lower‐risk patients. Of particular relevance to delivering value within the context of a healthcare system is the prevention of primary events. In this regard, the large proportion of individuals in DECLARE‐TIMI 58 without established comorbidities is both noteworthy and in contrast to evidence for other SGLT2 inhibitors. Given the high costs associated with treating people with comorbidities, the value of delaying disease progression and the development of HF and CKD presents a huge opportunity to avoid the cascade of costs associated with treating these comorbidities. Of note is a recent analysis utilizing synthesized evidence from three CVOTs and observational data (EMPA‐REG, CANVAS, DECLARE‐TIMI 58 and CVD‐REAL), which demonstrated that SGLT2 inhibitors as a class are cost‐effective and often cost‐saving when compared to placebo. 50 The present economic analysis used data from DECLARE‐TIMI 58 in isolation, which considers the full range of patients with the potential to benefit from treatment with dapagliflozin.
The approach of using survival curves to model event incidence in a T2DM population did not explicitly capture disease progression in such a way as comprehensive risk equations may have done. This becomes particularly relevant during the extrapolation of survival curves when the incidence of clinical events is likely to depend on a patient's history of events and is a limitation of this study. However, directly modelling event rate reduction without the need to use surrogate risk markers, at least for kidney and CV endpoints, is a strength afforded by the design of DECLARE‐TIMI 58.
A further limitation of this study is that the HbA1c difference observed in DECLARE‐TIMI 58 underestimates the glycaemic benefits of dapagliflozin due to the protocol encouraging the add‐on of other antihyperglycaemic treatments to reach HbA1c targets according to accepted guidelines and local best practices, and the number of add‐on treatments was greatest in the placebo group. In addition, the results of this study may be considered only relevant to patients who meet the eligibility criteria of DECLARE‐TIMI 58, and to UK patients (as costs were specific to the UK). This study examined the costs associated with treating high‐risk patients with T2DM with dapagliflozin. However, the availability of resources and the capacity of the healthcare system has not been considered and warrants investigation.
In summary, this study highlights the cost‐benefit of treating people with T2DM with dapagliflozin, and demonstrates the reduction in clinical events, particularly HHF and kidney events. As future projections of T2DM prevalence in the UK continue to rise, so will the proportion of people with T2DM affected by HF and/or kidney disease. The results of this study demonstrate that avoiding these adverse events with dapagliflozin treatment appears to be cost‐effective at cost‐effectiveness thresholds conventionally applied in the UK. In conclusion, the results of this study highlight the potential of dapagliflozin to have a meaningful impact in reducing the economic burden of T2DM and its associated complications.
CONFLICT OF INTEREST
P.M., A.R.M. and R.B. are employees of Health Economics and Outcomes Research Ltd. Health Economics and Outcomes Research Ltd received fees from AstraZeneca in relation to this study. K.B., I.G.‐N. and P.A.J. are employees of AstraZeneca. L.A.L. has received research funding from, has provided CME on behalf of, and/or has acted as an advisor to AstraZeneca, Boehringer Ingelheim, Eli Lilly, GSK, Janssen, Lexicon, Merck, Novo Nordisk, Sanofi, and Servier. A.C. reports grants and personal fees from AstraZeneca and Novo Nordisk and personal fees from Abbott, Eli Lilly, Sanofi, Boehringer Ingelheim, Merck Sharp & Dohme, Medial Early‐Sign and GlucoMe. J.W. has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Mundipharma, Napp, Novo Nordisk, Sanofi and Takeda, and research support from AstraZeneca and Novo Nordisk. DLB discloses the following relationships ‐ Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, MyoKardia, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co‐Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda.
AUTHOR CONTRIBUTIONS
P.M., K.B., I.G.‐N. conceptualized and designed the study. P.M. and R.B. were responsible for data analysis. P.M., A.R.M., R.B., K.B., I.G.‐N., D.B., L.A.L., P.A.J., O.M., A.C. and J.W. contributed to interpretation of the results, preparation and review of the manuscript, approved the final manuscript for publication, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGMENTS
This work was funded by AstraZeneca, who provided support for data analysis and medical writing for this study.
McEwan P, Morgan AR, Boyce R, et al. The cost‐effectiveness of dapagliflozin in treating high‐risk patients with type 2 diabetes mellitus: An economic evaluation using data from the DECLARE‐TIMI 58 trial. Diabetes Obes Metab. 2021;23:1020–1029. 10.1111/dom.14308
Funding information This work was funded by AstraZeneca who provided support for data analysis and medical writing for this study.
DATA AVAILABILITY STATEMENT
The datasets analysed during the current study were sourced from and are available in the original publications referenced.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas ‐ 8th Edition. 2017. https://diabetesatlas.org/resources/2017-atlas.html [PubMed]
- 2. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77‐82. [Google Scholar]
- 3. Baxter M, Hudson R, Mahon J, et al. Estimating the impact of better management of glycaemic control in adults with type 1 and type 2 diabetes on the number of clinical complications and the associated financial benefit. Diabetes Med. 2016;33(11):1575‐1581. [DOI] [PubMed] [Google Scholar]
- 4. van Dieren S, Beulens JW, van der Schouw YT, et al. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(Suppl 1):S3‐S8. [DOI] [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence . Clinical guideline [NG28]: type 2 diabetes in adults: management. 2017. https://www.nice.org.uk/guidance/ng28. (Accessed 25 August 2020).
- 6. Scottish Intercollegiate Guideline Network . SIGN 154: pharmacological management of glycaemic control in people with type 2 diabetes. 2017.
- 7. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255‐323. [DOI] [PubMed] [Google Scholar]
- 9. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose Cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose Cotransporter‐2 inhibitors). Circulation. 2017;136(3):249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diabetes Vasc Dis Res. 2016;13(2):119‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020‐1031. [DOI] [PubMed] [Google Scholar]
- 12. Fitchett D, Butler J, van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA‐REG OUTCOME(R) trial. Eur Heart J. 2018;39(5):363‐370. [DOI] [PubMed] [Google Scholar]
- 13. Verma S, Mazer CD, Al‐Omran M, et al. Cardiovascular outcomes and safety of Empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA‐REG OUTCOME. Circulation. 2018;137(4):405‐407. [DOI] [PubMed] [Google Scholar]
- 14. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 15. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 16. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 17. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995‐2008. [DOI] [PubMed] [Google Scholar]
- 18. Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691‐704. [DOI] [PubMed] [Google Scholar]
- 19. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 20. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. [DOI] [PubMed] [Google Scholar]
- 21. Heerspink HJL, Stefansson BV, Chertow GM, et al. Rationale and protocol of the Dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA‐CKD) randomized controlled trial. Nephrol Dial Transplant. 2020;35(2):274‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. AJMC . Dapagliflozin Meets All Targets in Patients with Chronic Kidney Disease, With and Without Diabetes. 2020. https://www.ajmc.com/view/dapagliflozin-meets-all-targets-in-patients-with-chronic-kidney-disease-with-and-without-diabetes
- 23. Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709‐717. [DOI] [PubMed] [Google Scholar]
- 24. Norhammar A, Bodegard J, Nystrom T, et al. Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE‐TIMI 58 trial: a nationwide observational study. Diabetes Obes Metab. 2019;21(5):1136‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Persson F, Nystrom T, Jorgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all‐cause mortality in people with type 2 diabetes (CVD‐REAL Nordic) when compared with dipeptidyl peptidase‐4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2018;20(2):344‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139(22):2516‐2527. [DOI] [PubMed] [Google Scholar]
- 27. Kato ET, Silverman MG, Mosenzon O, et al. Effect of Dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139(22):2528‐2536. [DOI] [PubMed] [Google Scholar]
- 28. Sánchez‐Covisa J, Capel M, Schmidt R, Charokopou M, Verheggen BG. The cost‐effectiveness of Dapagliflozin in combination with insulin for the treatment of type 2 diabetes mellitus (T2dm) in Spain. Value Health. 2014;17(7):A350. [DOI] [PubMed] [Google Scholar]
- 29. Chakravarty A, Rastogi M, Dhankhar P, Bell KF. Comparison of costs and outcomes of dapagliflozin with other glucose‐lowering therapy classes added to metformin using a short‐term cost‐effectiveness model in the US setting. J Med Econ. 2018;21(5):497‐509. [DOI] [PubMed] [Google Scholar]
- 30. Charokopou M, Vioix H, Verheggen BG, Dillon S, Franks D. Dapagliflozin (Forxiga®) versus Glipizide as add‐on therapies in type 2 diabetes mellitus (T2dm); an update of the cost‐effectiveness based on long‐term clinical evidence from UKNhs perspective. Value Health. 2014;17(7):A343. [DOI] [PubMed] [Google Scholar]
- 31. Charokopou M, McEwan P, Lister S, et al. The cost‐effectiveness of dapagliflozin versus sulfonylurea as an add‐on to metformin in the treatment of type 2 diabetes mellitus. Diabet Med. 2015;32(7):890‐898. [DOI] [PubMed] [Google Scholar]
- 32. Sabale U, Ekman M, Granström O, Bergenheim K, McEwan P. Cost‐effectiveness of dapagliflozin (Forxiga®) added to metformin compared with sulfonylurea added to metformin in type 2 diabetes in the Nordic countries. Prim Care Diabetes. 2015;9(1):39‐47. [DOI] [PubMed] [Google Scholar]
- 33. Tzanetakos C, Tentolouris N, Kourlaba G, Maniadakis N. Cost‐effectiveness of Dapagliflozin as add‐on to metformin for the treatment of type 2 diabetes mellitus in Greece. Clin Drug Investig. 2016;36(8):649‐659. [DOI] [PubMed] [Google Scholar]
- 34. Charokopou M, McEwan P, Lister S, et al. Cost‐effectiveness of dapagliflozin versus DPP‐4 inhibitors as an add‐on to metformin in the treatment of type 2 diabetes mellitus from a UK healthcare system perspective. BMC Health Serv Res. 2015;15:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Charokopou M, Vioix H, Eddowes LA, et al. Cost‐effectiveness of Dapagliflozin compared to Dpp‐4 inhibitors as triple therapy in combination with metformin and a Sulphonylurea in the treatment of type 2 diabetes mellitus from a UK health care perspective. Value Health. 2014;17(7):A347. [DOI] [PubMed] [Google Scholar]
- 36. Shao H, Zhai S, Zou D, et al. Cost‐effectiveness analysis of dapagliflozin versus glimepiride as monotherapy in a Chinese population with type 2 diabetes mellitus. Curr Med Res Opin. 2017;33(2):359‐369. [DOI] [PubMed] [Google Scholar]
- 37. Gu S, Mu Y, Zhai S, Zeng Y, Zhen X, Dong H. Cost‐effectiveness of dapagliflozin versus Acarbose as a Monotherapy in type 2 diabetes in China. PLoS One. 2016;11(11):e0165629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Charokopou M, Vioix H, Verheggen B, et al. Cost‐effectiveness of dapagliflozin versus Dpp‐4 inhibitors as monotherapy in the treatment of type 2 diabetes mellitus from a UK health care perspective. Value Health. 2014;17(7):A347. [DOI] [PubMed] [Google Scholar]
- 39. Rahman W, Solinsky PJ, Munir KM, Lamos EM. Pharmacoeconomic evaluation of sodium‐glucose transporter‐2 (SGLT2) inhibitors for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2019;20(2):151‐161. [DOI] [PubMed] [Google Scholar]
- 40. Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom prospective diabetes study (UKPDS) outcomes model (UKPDS no. 68). Diabetologia. 2004;47(10):1747‐1759. [DOI] [PubMed] [Google Scholar]
- 41. Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom prospective diabetes study: UKPDS 82. Diabetologia. 2013;56(9):1925‐1933. [DOI] [PubMed] [Google Scholar]
- 42. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837‐853. [PubMed] [Google Scholar]
- 43. Sabale U, Ekman M, Granstrom O, et al. Cost‐effectiveness of dapagliflozin (Forxiga(R)) added to metformin compared with sulfonylurea added to metformin in type 2 diabetes in the Nordic countries. Prim Care Diabetes. 2015;9(1):39‐47. [DOI] [PubMed] [Google Scholar]
- 44. van Haalen HG, Pompen M, Bergenheim K, et al. Cost effectiveness of adding dapagliflozin to insulin for the treatment of type 2 diabetes mellitus in The Netherlands. Clin Drug Investig. 2014;34(2):135‐146. [DOI] [PubMed] [Google Scholar]
- 45. Elgart JF, Caporale JE, Gonzalez L, Aiello E, Waschbusch M, Gagliardino JJ. Treatment of type 2 diabetes with saxagliptin: a pharmacoeconomic evaluation in Argentina. Health Econ Rev. 2013;3(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bergenheim K, Williams SA, Bergeson JG, et al. US cost‐effectiveness of saxagliptin in type 2 diabetes mellitus. Am J Pharm Benefits. 2012;4(1):20‐28. [Google Scholar]
- 47. Grzeszczak W, Czupryniak L, Kolasa K, Sciborski C, Lomon ID, McEwan P. The cost‐effectiveness of saxagliptin versus NPH insulin when used in combination with other oral antidiabetes agents in the treatment of type 2 diabetes mellitus in Poland. Diabetes Technol Ther. 2012;14(1):65‐73. [DOI] [PubMed] [Google Scholar]
- 48. Granstrom O, Bergenheim K, McEwan P, et al. Cost‐effectiveness of saxagliptin (Onglyza(R)) in type 2 diabetes in Sweden. Prim Care Diabetes. 2012;6(2):127‐136. [DOI] [PubMed] [Google Scholar]
- 49. Erhardt W, Bergenheim K, Duprat‐Lomon I, McEwan P. Cost effectiveness of saxagliptin and metformin versus sulfonylurea and metformin in the treatment of type 2 diabetes mellitus in Germany: a Cardiff diabetes model analysis. Clin Drug Investig. 2012;32(3):189‐202. [DOI] [PubMed] [Google Scholar]
- 50. McEwan P, Bennett H, Khunti K, et al. Assessing the cost‐effectiveness of SGLT2i in type 2 diabetes mellitus: a comprehensive economic evaluation using clinical trial and real‐world evidence. Diabetes Obes Metabol. 2020;22(12):2364‐2374. [DOI] [PubMed] [Google Scholar]
- 51. McEwan P, Ward T, Bennett H, Bergenheim K. Validation of the UKPDS 82 risk equations within the Cardiff diabetes model. Cost Effect Resour Allocat. 2015;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Office for National Statistics . National life tables: UK. 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables
- 53. Raz I, Mosenzon O, Bonaca MP, et al. DECLARE‐TIMI 58: participants' baseline characteristics. Diabetes Obes Metab. 2018;20(5):1102‐1110. [DOI] [PubMed] [Google Scholar]
- 54. Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE‐TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606‐617. [DOI] [PubMed] [Google Scholar]
- 55.Haymarket Media Group Ltd. Monthly Index of Medical Specialities. https://www.mims.co.uk/
- 56. National Institute for Health and Care Excellence (NICE) . Guide to the methods of technology appraisal. 2013. https://www.nice.org.uk/process/pmg9/resources/guide‐to‐the‐methods‐of‐technology‐appraisal‐2013‐pdf‐2007975843781. (Accessed 25 August 2020). [PubMed]
- 57. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2017;14:88. [DOI] [PubMed] [Google Scholar]
- 58. Birkeland KI, Bodegard J, Norhammar A, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2019;21(4):968‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wittbrodt ET, Eudicone JM, Bell KF, Enhoffer DM, Latham K, Green JB. Eligibility varies among the 4 sodium‐glucose cotransporter‐2 inhibitor cardiovascular outcomes trials: implications for the general type 2 diabetes US population. Am J Manag Care. 2018;24(8 Suppl):S138‐S145. [PubMed] [Google Scholar]
- 60. Gourzoulidis G, Tzanetakos C, Ioannidis I, et al. Cost‐effectiveness of Empagliflozin for the treatment of patients with type 2 diabetes mellitus at increased cardiovascular risk in Greece. Clin Drug Investig. 2018;38(5):417‐426. [DOI] [PubMed] [Google Scholar]
- 61. Nguyen E, Coleman CI, Nair S, Weeda ER. Cost‐utility of empagliflozin in patients with type 2 diabetes at high cardiovascular risk. J Diabetes Complications. 2018;32(2):210‐215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
The datasets analysed during the current study were sourced from and are available in the original publications referenced.


