Abstract
Background
The intestinal microbiota and its metabolites have been reported to play an important role in stroke. Gut microbiota–originating short‐chain fatty acids (SCFAs) modulate brain functions directly or indirectly through immune, endocrine, vagal, and other humoral pathways. However, relatively few investigations have evaluated the gut microbiome and SCFAs spectrum or their potential associations with stroke outcomes in acute ischemic stroke (AIS) patients with different stroke severities.
Methods
We used 16S rRNA gene sequencing and gas chromatography to compare the fecal microbial composition and SCFA spectrum between AIS patients (n = 140) and healthy controls (n = 92). Their associations with 90‐day poor functional outcomes were evaluated by logistic regression models.
Results
We found that the intestinal microbiota distinguished AIS patients from healthy controls. A lack of SCFAs‐producing bacteria and a low fecal SCFAs level defined dysbiosis in AIS patients, especially those with increased stroke severity. The SCFAs levels were negatively correlated with stroke severity and prognosis. Reduced SCFAs levels, especially acetate, were associated with an increased risk of 90‐day poor functional outcomes even after adjustments.
Conclusions
Dysbiosis of SCFAs‐producing bacteria and SCFAs in AIS patients increased the subsequent risk for poor functional outcomes, indicating that SCFAs could be potential prognostic markers and therapeutic targets for stroke.
Keywords: dysbiosis, functional outcome, gut microbiota, ischemic stroke, short‐chain fatty acids
Clinical Relevancy Statement
The intestinal microbiota and its metabolite short‐chain fatty acids (SCFAs) have been reported to play an important role in stroke. In this study, we identified a lack of SCFA‐producing bacteria and a low fecal SCFA level in acute ischemic stroke patients, especially those with increased stroke severity. Further analyses revealed negative correlations between SCFAs and stroke outcomes. These findings indicate that SCFAs could be potential prognostic markers and therapeutic targets for stroke.
Introduction
Ischemic stroke, caused by focal occlusion or arterial stenosis, seriously endangers human life in China. 1 Emerging evidence suggests that the gut‐brain axis plays an important role in stroke. 2 , 3 A previous study demonstrated that cerebral ischemia caused dysbiosis of the gut microbiota and disruption to the gut barrier; repairing the leaky gut was beneficial to the blood‐brain barrier. 4 Significant dysbiosis of the intestinal microbiota has also been found in ischemic stroke patients. 5 , 6 It has been reported that gut microbiota–originating short‐chain fatty acids (SCFAs) affect central nervous system diseases 7 , 8 ; modulate the brain functions directly or indirectly through immune, endocrine, vagal, and other humoral pathways 7 ; and regulate the permeability of both the gut and the blood‐brain barrier. 9 , 10 Therefore, the intestinal microbiota and its metabolite SCFAs might be new therapeutic targets for acute ischemic stroke (AIS). To date, the gut microbiome and fecal SCFAs spectrum, as well as their potential associations with stroke outcomes, have not yet been fully evaluated among AIS patients with different stroke severities.
Here, we designed a prospective observational study of 140 AIS patients and 92 healthy individuals in which fecal bacterial populations and SCFA levels were determined by 16S rRNA gene sequencing and gas chromatography, based on the hypothesis that differential microbial members and SCFA levels exist in AIS patients, and they correlate with stroke severity and prognosis. This study, therefore, provides new insights into the clinical treatment of AIS.
Methods
Study Design
This prospective observational study was conducted at the Department of Neurology, Nanfang Hospital, Southern Medical University (Guangzhou, China) and the Bureau of Agricultural Reclamation (Guangzhou, China) from June 2017 to January 2018. Patients diagnosed with AIS were consecutively recruited if they were aged >18 years and admitted within 48 hours of stroke onset. The detailed enrollment process is shown in Figure 1. Healthy controls who met the following criteria were recruited: (1) no prior history of myocardial infarction, stroke, or gut diseases; (2) no antibiotics/prebiotics/probiotics taken or gastrointestinal symptoms experienced in the past 3 months. Written informed consent was obtained from all participants or their legal representatives in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (NFEC‐2016‐148), and registered at http://www.chictr.org (ChiCTR‐ROC‐17011567).
Figure 1.
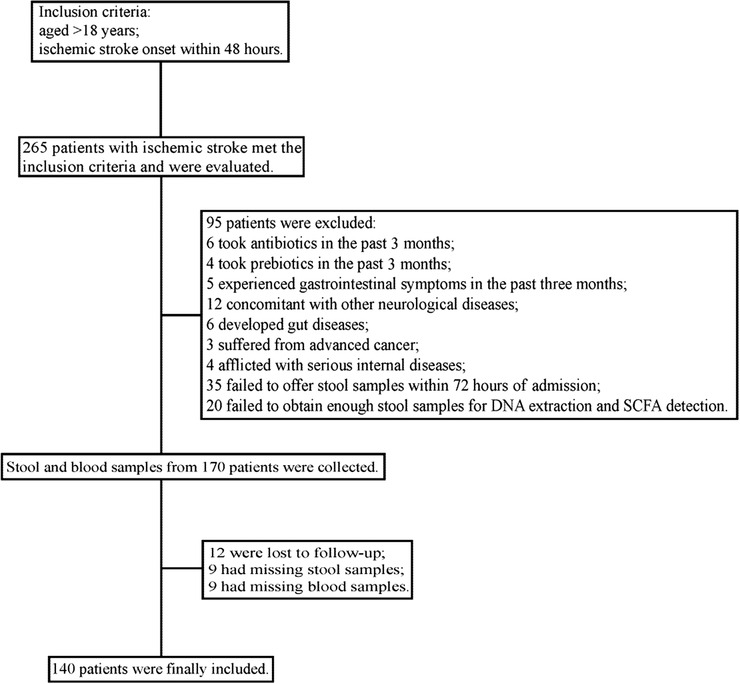
Enrollment process of the study. SCFA, short‐chain fatty acid.
Baseline characteristics were recorded at enrollment. The National Institutes of Health Stroke Scale (NIHSS) score was used to evaluate stroke severity, with scores < 5 points defined as mild stroke, those between 5 and 15 points defined as moderate stroke, and those > 15 points defined as severe stroke. The Essen score was utilized to assess stroke risk, with a high score indicating increased stroke risk. The modified Rankin Scale (mRS) score was assessed by 2 trained staff members blinded to the study design via structured follow‐up telephone interviews, to evaluate 90‐day poor functional outcomes (mRS score ≥ 3).
DNA Extraction, PCR Amplification, and Sequencing
Stool samples were stored at −80 °C within 3 hours after voiding, and 0.2 g of each sample was aliquoted for DNA extraction. Bacterial DNA was extracted with a PowerSoil DNA Extraction 88 Kit (Shenzhen Bioeasy Biotechnology Co, Ltd, Shanghai, China). The bar‐coded primers V4F (5′‐GTGTGYCAGCMGCCGCGGTAA‐3′) and V4R (5′‐CCGGACTACNVGGGTWTCTAAT‐3′) were used to amplify the V4 region of the bacterial 16S rRNA gene with a LightCycler 480 II real‐time fluorescence quantitative polymerase chain reaction (PCR) system (Roche Diagnostics, Ltd, Basel, Switzerland). Using GeneTools analysis software (version 4.03.05.0, SynGene) and an EZNA Gel Extraction Kit (Omega, USA), PCR products were mixed in equimolar ratios and purified. Finally, PCR amplicons were sequenced on an Illumina HiSeq 2500 platform.
Depending on the overlap between 2 paired‐end sequences, we used SeqPrep to merge the paired‐end sequences and assessed the quality of the results with the opensource software Quantitative Insights into Microbial Ecology (QIIME, version 1.9.1). Sequences >200 bp were trimmed to 200 bp, and those that were <200 bp were removed. Finally, the QIIME workflow script pick_closed_reference_otus.py was used to remove chimeras, cluster reference‐based operational taxonomic units and generate a BIOM file. All samples were normalized to the same level to avoid possible errors due to the use of different sequencing depths. To preserve as many sequences as possible, each sample was normalized to 3500 sequences in our study.
Fecal SCFA Detection
For SCFA analyses, 0.2 g of each fecal sample was separated. Four analytes, including total SCFAs, acetate, propionate, and butyrate, were targeted. Samples were first homogenized in 1.0 mL of ultrapure water that contained an internal standard of 2,2‐dimethylbutyric acid. The homogenate was then centrifuged at 12,000 rpm (4 °C, 10 minutes). The resulting supernatant was transferred to a new Eppendorf tube and mixed with 10 μL of 50% sulfuric acid, 0.5 g of sodium sulfate (Macklin, China), and 2 mL of analytically pure diethyl ether. The mixture was vortexed for 1 minute and then centrifuged at 5000 rpm (room temperature, 10 minutes). The ether layer was finally collected for gas chromatography with mass selective detection (5977B GC/MSD, Agilent Technologies, Santa Clara, CA, USA). An HP‐Free Fatty Acid Phase (HP‐FFAP) capillary column (30 m length, 0.25 mm internal diameter, 19091F‐433, Agilent Technologies) was used for chromatographic separation, with helium as the carrier gas. The oven temperature was increased by 15 °C/min, from 90 to 180 °C. Gas chromatography mass spectrometry (GC/MS) data were collected and analyzed with MassHunter Workstation Software (Agilent Technologies). Final concentrations were calculated based on internal standards and are expressed as micromoles per gram of wet feces (μmol/g).
Quantification of Serum FABP, d‐Lactate, LPS, and LBP
Serum was isolated by centrifugation at 3000 rpm for 10 minutes and stored at −80 °C until testing. The concentrations of fatty acid–binding protein (FABP), d‐lactate, lipopolysaccharide (LPS), and LPS‐binding protein (LBP) levels were determined using commercially available enzyme‐linked immunosorbent assay kits (Bioswamp, Myhalic Biotechnology Co, Ltd, Wuhan, China). All measurements were performed by 1 experienced staff member blinded to the study design. Standard curves were all within the expected range, indicating good dilution linearity and high precision.
Bioinformatics and Statistical Analyses
The 16S rRNA sequencing data were analyzed with QIIME 1.80. The β‐diversity (differences between microbial communities) was analyzed according to the unweighted UniFrac distance. Principal coordinates analysis (PCoA), a dimensionality reduction method that illustrates the relationships between samples based on a distance matrix, was performed to highlight the differences between different groups using the R package “ade4.” For microbial analysis, the Adonis test was performed. The relative abundance of dominant taxa is shown in stacked bar plots constructed using the R package “ggplot2.” To identify differentiated metagenomic biomarkers, a linear discriminant analysis (LDA) coupled with effect‐size measurement (LEfSe) was performed at http://huttenhower.sph.harvard.edu/galaxy. Significantly different bacteria with LDA scores of ≥2.5 were plotted on taxonomic bar plots. The relative abundance of microorganisms and concentrations of SCFAs are shown as box plots. The Pearson correlation coefficients were calculated and visualized by heat map in R using the “shiny” package.
The results are expressed as numbers (percentages, %) for categorical variables and medians (interquartile ranges) for continuous variables. Categorical variables were compared by the χ2 test or Fisher exact test, and continuous variables were compared by the Mann‐Whitney U test or Kruskal‐Wallis H test. The multivariate logistic regression models included all variables with statistical significance (P < .10) in the univariate model as well as those with clinical significance. All of these analyses were performed with IBM SPSS statistical software version 22.0 (SPSS, Inc, Chicago, IL, USA), and a 2‐tailed P‐value < .05 was considered statistically significant.
Results
Baseline Characteristics
Ultimately, 140 AIS patients and 92 healthy controls were recruited in this study. The median age of AIS patients was 59 years, and 67.9% were male. There was no significant difference in age, sex, or history of smoking between AIS patients and healthy controls. According to NIHSS scores, 78 mild stroke, 47 moderate stroke, and 15 severe stroke patients were included. The 3 AIS groups with different severities showed no significant differences in age, sex, or history of hypertension and diabetes; however, neutrophil (NEU) and N‐terminal B‐type natriuretic peptide (NT‐proBNP) levels increased with stroke severity. More details of the study participants are shown in Table 1. After the 90‐day follow‐up, 33 patients (23.6%) suffered poor functional outcomes. Patients who developed 90‐day poor functional outcomes were older; had a greater prevalence of atrial fibrillation; were more likely to exhibit severe stroke, cardioembolism, revascularization, dysphagia, and enteral nutrition; and had longer defecation time and higher NEU and NT‐proBNP levels than those who did not (Table 2).
Table 1.
Characteristics of the Study Participants
| Characteristics | Healthy controls | Stroke patients | P | Mild stroke | Moderate stroke | Severe stroke | P |
|---|---|---|---|---|---|---|---|
| No. of participants | 92 | 140 | 78 | 47 | 15 | ||
| Demographics | |||||||
| Male | 51 (55.4) | 95 (67.9) | .055 | 54 (69.2) | 33 (70.2) | 8 (53.3) | .44 |
| Age, y | 60 (13) | 59 (20) | .12 | 59 (19) | 59 (20) | 66 (28) | .31 |
| History of smoking | 24 (26.1) | 51 (36.4) | .099 | 30 (38.5) | 20 (42.6) | 1 (6.7) | .036 |
| Medical histories | |||||||
| History of hypertension | 39 (42.4) | 92 (65.7) | <.001 | 51 (65.4) | 30 (63.8) | 11 (73.3) | .97 |
| History of diabetes | 7 (7.6) | 37 (26.4) | <.001 | 23 (29.5) | 9 (19.1) | 5 (33.3) | .36 |
| Laboratory findings | |||||||
| NEU, ×109/L | 3.48 (1.54) | 5.64 (3.71) | <.001 | 4.98 (2.89) | 6.55 (3.25) | 8.14 (5.22) | <.001 |
| NT‐proBNP, pg/mL | 124.80 (423.92) | 77.46 (245.08) | 146.60 (377.41) | 1399.00 (1922.20) | .001 |
Data were presented as median (interquartile range) or number (%).
NEU, neutrophil; NT‐proBNP, N‐terminal B‐type natriuretic peptide.
Table 2.
Baseline Characteristics of Acute Ischemic Stroke Patients According to 90‐Day Functional Outcomes
| 90‐Day poor functional outcomes (mRS ≥ 3) | |||
|---|---|---|---|
| Characteristics | Yes (N = 33) | No (N = 107) | P |
| Demographics | |||
| Male | 20 (60.6) | 75 (70.1) | .31 |
| Age, y | 67 (23) | 59 (17) | .005 |
| Medical histories | |||
| History of hypertension | 24 (72.7) | 68 (63.6) | .33 |
| History of diabetes | 9 (27.3) | 28 (26.2) | .90 |
| History of AF | 11 (33.3) | 6 (5.6) | <.001 |
| History of CHD | 4 (12.1) | 5 (4.7) | .13 |
| History of stroke | 6 (18.2) | 16 (15.0) | .66 |
| Dietary habits | .067 | ||
| Vegetable‐based diet | 8 (24.2) | 25 (23.4) | |
| Mixed diet | 21 (63.6) | 48 (44.9) | |
| Meat‐based diet | 4 (12.1) | 34 (31.8) | |
| Stroke causes | <.001 | ||
| Thrombotic | 19 (57.6) | 44 (41.1) | |
| Lacunar | 0 | 37 (34.6) | |
| Embolic | 10 (30.3) | 11 (10.3) | |
| Other cause | 3 (9.1) | 4 (3.7) | |
| Unknown | 1 (3.0) | 11 (10.3) | |
| Antiplatelet agents | .011 | ||
| Aspirin | 13 (39.4) | 18 (16.8) | |
| Clopidogrel | 9 (27.3) | 26 (24.3) | |
| Aspirin+clopidogrel | 11 (33.3) | 63 (58.9) | |
| Clinical features | |||
| Dysphagia | 26 (78.8) | 15 (14.0) | <.001 |
| Enteral nutrition | 22 (66.7) | 10 (9.3) | <.001 |
| Revascularization | 14 (42.4) | 21 (19.6) | .008 |
| NIHSS score | 13 (10) | 3 (4) | <.001 |
| Defecation time | <.001 | ||
| No more than 48 h after stroke | 8 (24.2) | 70 (65.4) | |
| 48–120 h after stroke | 25 (75.8) | 37 (34.6) | |
| Laboratory findings | |||
| NEU, × 109/L | 6.14 (4.10) | 5.41 (3.42) | .037 |
| NT‐proBNP, pg/mL | 218.50 (1517.82) | 88.47 (257.68) | .007 |
Data were presented as median (interquartile range) or number (%).
AF, atrial fibrillation; CHD, coronary heart disease; mRS, modified Rankin Scale; NEU, neutrophil; NIHSS, National Institutes of Health Stroke Scale; NT‐proBNP, N‐terminal B‐type natriuretic peptide.
Intestinal Microbiota Distinguished AIS From Controls
To evaluate whether the AIS patients had specific intestinal microbial features, fecal samples obtained from each participant were analyzed. Based on the unweighted UniFrac distance, PCoA showed that the intestinal microbiota distinguished AIS patients from healthy controls (Figure 2A). AIS patients with higher stroke severity exhibited significantly greater distances from controls (Figure 2B). Taxonomic classification at the family level showed that most of the intestinal bacteria detected fell into Bacteroidaceae, Ruminococcaceae, Enterobacteriaceae, Prevotellaceae, Lachnospiraceae, Veillonellaceae, Porphyromonadaceae, Verrucomicrobiaceae, Rikenellaceae, Alcaligenaceae, Fusobacteriaceae, and Clostridiaceae, which occupied >80% of the total microbiota (Figure 2C). LEfSe was used to identify the differentially distributed microbiota between AIS patients and healthy controls. At the family level, Lactobacillaceae, Enterobacteriaceae, Porphyromonadaceae, etc, were found to be differently distributed (LDA score = 2.5, Figure 2D).
Figure 2.
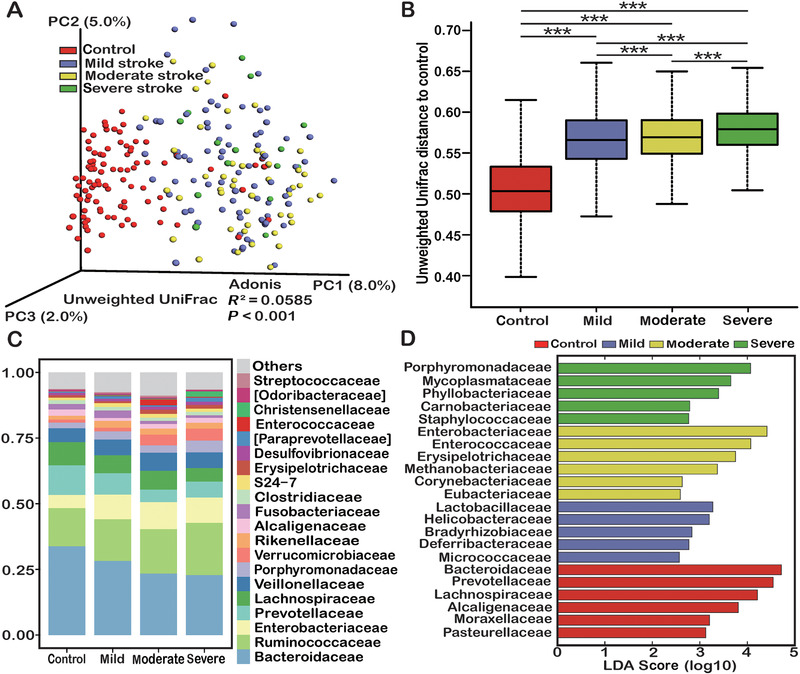
Comparisons of the gut microbiota between healthy controls and acute ischemic stroke patients. (A) Principal coordinates (PC) analysis based on unweighted UniFrac distances. (B) Unweighted UniFrac distances of each subgroup of acute ischemic stroke patients compared with controls. (C) Taxonomic summary of the gut microbiota of mild, moderate, and severe stroke patients and controls at the family level. (D) Discriminative taxa at the family level in mild, moderate, and severe stroke patients and controls based on the linear discriminant analysis (LDA = 2.5) and effect‐size pipeline. Statistical significance was considered at * P < .05, ** P < .01, and *** P < .001.
To further explore the gut microbial alterations between AIS and controls, we analyzed the abundance of selected bacteria according to taxonomic classification and LEfSe analysis. On the whole, a lack of SCFA‐producing bacteria (Roseburia, Bacteroides, Lachnospiraceae, Faecalibacterium, Blautia, and Anaerostipes, Figure 3A) and an overload of Lactobacillaceae, Akkermansia, Enterobacteriaceae, and Porphyromonadaceae (Figure 3B) defined dysbiosis in AIS patients. Besides, AIS patients with higher stroke severity seemed to have lower abundances of SCFA‐producing bacteria and higher abundances of Lactobacillaceae, Akkermansia, Enterobacteriaceae, and Porphyromonadaceae. The Firmicutes/Bacteroidetes (F/B) ratios were higher in AIS patients than in controls and higher in moderate stroke patients than in mild stroke patients (Figure 3B). However, because there were too few cases in the severe stroke group (n = 15), these analyses might not be precise or sufficiently generalizable.
Figure 3.
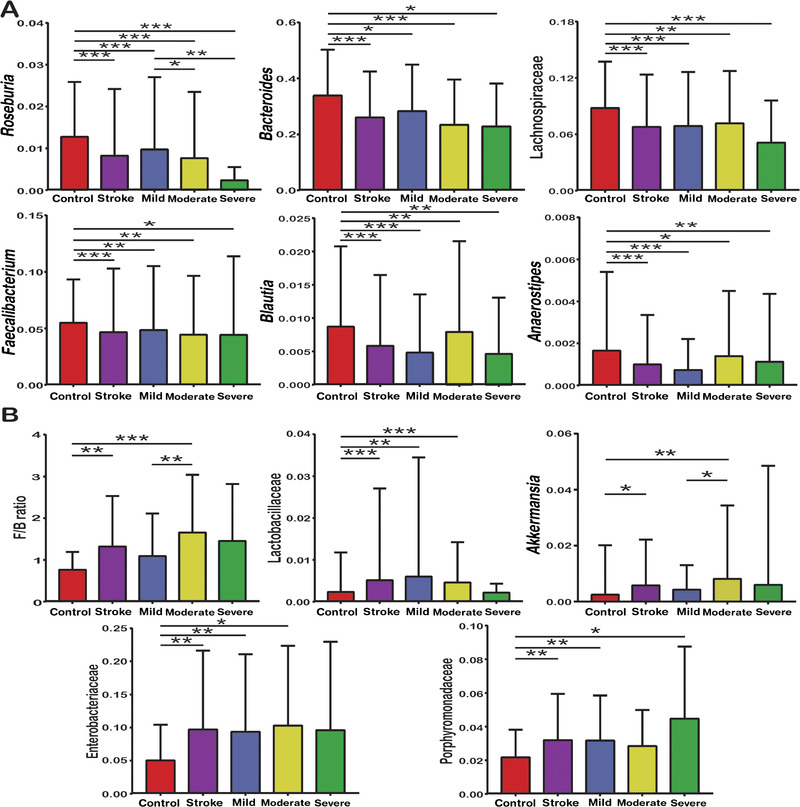
Reduced abundances of common short‐chain fatty acid–producing bacteria such as Roseburia, Bacteroides, Lachnospiraceae, Faecalibacterium, Blautia, and Anaerostipes (A); increased Firmicutes/Bacteroidetes (F/B) ratio (B); and overloads of Lactobacillaceae, Akkermansia, Enterobacteriaceae, and Porphyromonadaceae (B) defined dysbiosis of the gut microbiota in patients with acute ischemic stroke. Statistical significance was considered at * P < .05, ** P < .01, and *** P < .001.
Fecal SCFAs and Serum FABP, d‐Lactate, LPS, and LBP Levels in AIS and Controls
Because a lack of SCFA‐producing bacteria was observed in AIS patients, fecal total SCFAs, acetate, propionate, and butyrate levels were then analyzed. The median concentrations of total SCFAs, acetate, propionate, and butyrate in AIS were 111.03, 65.75, 20.46, and 10.15 μmol/g, whereas those in healthy controls were 130.11, 83.29, 24.29, and 14.04 μmol/g, respectively. A striking deficiency of fecal SCFAs was observed in AIS patients, especially those with increased stroke severity (Figure 4A). We also quantified serum FABP, d‐lactate, LPS, and LBP levels to explore the possible changes in the intestinal barrier. The results showed that FABP, d‐lactate, LPS, and LBP levels were much higher in AIS patients than in controls, but no significant difference was found among stroke patients with different severities (Figure 4B). Spearman correlations revealed that SCFA levels were negatively correlated with the F/B ratio; NIHSS, Essen, and 90‐day mRS scores; and white blood cell, serum urea nitrogen, and NT‐proBNP levels (Figure 4C). Interestingly, the abundance of Roseburia, one of the SCFA‐producing bacteria, also showed negative relations with NIHSS, Essen, and 90‐day mRS scores, whereas other SCFAs‐producing bacteria did not. Nevertheless, no relationship was found among SCFA‐producing bacteria, SCFAs, and FABP, d‐lactate, LPS, and LBP levels.
Figure 4.
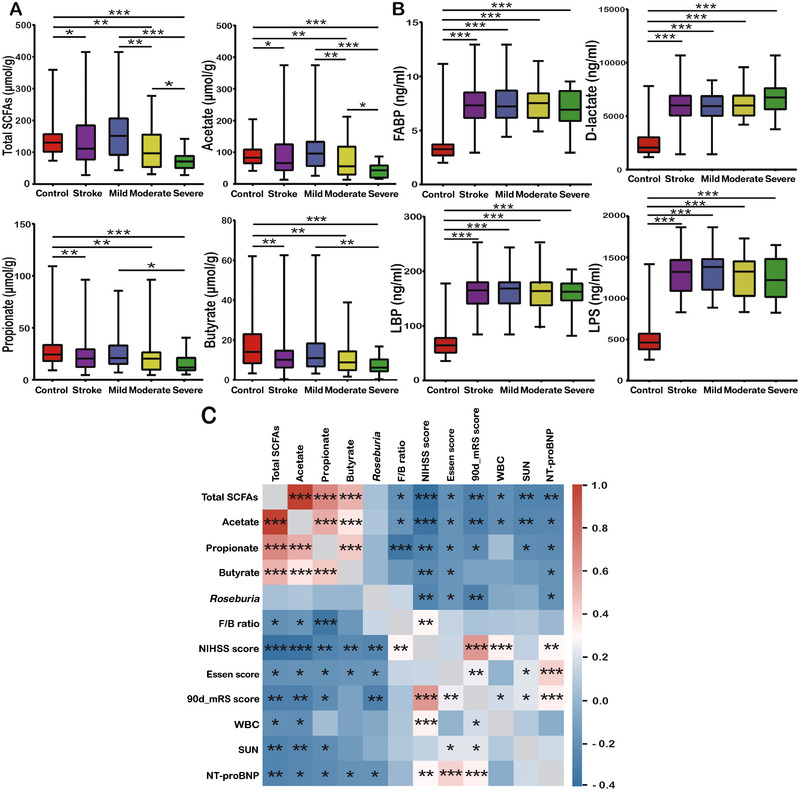
Compared with healthy controls, patients with acute ischemic stroke exhibited a lack of fecal SCFAs (total SCFAs, acetate, propionate, and butyrate) (A) and a disrupted intestinal barrier (B). The heat map (C) showed negative correlations between fecal SCFAs, Roseburia, and the F/B ratio; NIHSS, Essen, and 90‐day mRS scores; and WBC, SUN, and NT‐proBNP levels. Statistical significance was considered at * P < .05, ** P < .01, and *** P < .001. F/B, Firmicutes/Bacteroidetes; FABP, fatty acid–binding protein; LBP, LPS‐binding protein; LPS, lipopolysaccharide; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; NT‐proBNP, N‐terminal B‐type natriuretic peptide; SCFA, short‐chain fatty acid; SUN, serum urea nitrogen; WBC, white blood cell.
Associations Between SCFAs and 90‐Day Poor Functional Outcomes
The results above reveal that low SCFA levels seem to predict poor outcomes. We therefore sought to explore the associations between low log2‐transformed SCFA levels and 90‐day poor functional outcomes. Participants with 90‐day poor functional outcomes had significantly lower SCFA levels (except for butyrate) than those without (median, 88.24 vs 133.45 μmol/g, P = .001 for total SCFAs; median, 54.35 vs 80.11 μmol/g, P < .001 for acetate; median, 16.35 vs 20.75 μmol/g, P = .024 for propionate; and median, 9.58 vs 10.32 μmol/g, P = .57 for butyrate; Figure S1). The relationships between low log2‐transformed SCFA levels and 90‐day poor functional outcomes are shown in Table 3 and Tables S1–S4. Lower log2‐transformed total SCFA, acetate, propionate, and butyrate levels had odds ratios (ORs) of 2.39 (model 1; 95% CI, 1.44–3.98; P = .001), 2.33 (model 1; 95% CI, 1.48–3.69; P < .001), 1.72 (model 1, 95% CI, 1.09–2.70; P = .019), and 1.18 (model 1, 95% CI, 0.85–1.66; P = .33) for 90‐day poor functional outcomes.
Table 3.
Associations Between Low SCFAs and 90‐Day Poor Functional Outcomes
| Predictors | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR a (95% CI) | P | Adjusted OR a (95% CI) | P | Adjusted OR a (95% CI) | P | |
| Total SCFAs | 2.39 (1.44–3.98) | .001 | 2.37 (1.32–4.25) | .004 | NS | |
| Acetate | 2.33 (1.48–3.69) | <.001 | 2.49 (1.46–4.24) | .001 | 1.99 (1.11–3.55) | .021 |
| Propionate | 1.72 (1.09–2.70) | .019 | NS | NS | ||
| Butyrate | 1.18 (0.85–1.66) | .33 | NS | NS | ||
Model 1, unadjusted.
Model 2, adjusted for age; sex; history of hypertension, diabetes, atrial fibrillation, coronary heart disease and stroke; dietary habits; defecation time; NEU and NT‐proBNP levels.
Model 3, adjusted for the factors above, plus stroke etiology, antiplatelet agents, dysphagia, enteral nutrition, revascularization, and NIHSS score.
NEU, neutrophil; NIHSS, National Institutes of Health Stroke Scale; NS, nonsignificant; NT‐proBNP, N‐terminal B‐type natriuretic peptide; OR, odds ratio; SCFA, short‐chain fatty acid.
log2‐transformed.
Patients with reduced log2‐transformed SCFA levels had an increased risk of 90‐day poor functional outcomes (model 2; OR, 2.37; 95% CI, 1.32–4.25; P = .004 for total SCFAs; OR, 2.49; 95% CI, 1.46–4.24; P = .001 for acetate) after adjustments for age; sex; history of hypertension, diabetes, atrial fibrillation, coronary heart disease, and stroke; dietary habits; defecation time; and NEU and NT‐proBNP levels. This increase persisted even after further adjustments for stroke etiology, antiplatelet agents, dysphagia, enteral nutrition, revascularization, and NIHSS score (model 3; OR, 1.99; 95% CI, 1.11–3.55; P = .021 for acetate). Neither SCFA‐producing bacteria nor FABP, d‐lactate, LPS, and LBP levels were related to 90‐day poor functional outcomes.
Discussion
In this study, an in‐depth prospective observational investigation of the intestinal microbiota and fecal SCFAs spectrum was performed in Chinese AIS patients. Our results confirmed a striking imbalance of gut flora accompanied by a lack of SCFAs in AIS patients (especially those with increased stroke severity), which might trigger a leaky gut, though no significant relationship was found. Further analyses demonstrated that reduced SCFA levels, especially acetate, were significantly associated with an increased risk of 90‐day poor functional outcomes even after adjustments. These findings suggest that SCFAs could be potential prognostic markers and therapeutic targets for stroke.
With the widespread recognition of the “gut‐brain axis,” communications between the gut and the central nervous system through gut microbiota have been brought to attention. 2 , 3 , 11 Gut microbiota dysbiosis has been indicated to be a driving force for the development and progression of stroke. 12 , 13 Moreover, it has been confirmed that remodeling the gut microbiota offers an effective treatment for cerebral ischemic stroke. 14 We previouly showed via 16S rRNA sequencing that dysbiosis of the intestinal flora occurred in large‐artery atherosclerotic stroke patients 5 and a high Stroke Dysbiosis Index of the gut microbiome correlated with poor brain injury and prognosis. 13 Using quantitative reverse‐transcription PCR, Yamashiro et al also suggested that gut dysbiosis occurred in patients with ischemic stroke and correlated with host metabolism and inflammation. 6 In the present study, we further investigated the intestinal microbiota of AIS patients with different stroke severities and identified a lack of SCFA‐producing bacteria and an overgrowth of opportunistic pathogens (Enterobacteriaceae and Porphyromonadaceae) as well as Lactobacillaceae and Akkermansia.
SCFAs mostly include acetate, propionate, and butyrate and are produced mainly by gut microbial fermentation of dietary fiber and unabsorbed carbohydrates in the cecum and colon. SCFAs modulate the permeability of gut and blood‐brain barriers 9 , 10 and ameliorate hypertension, 15 diabetes, 16 multiple sclerosis (MS), 17 affective and cognitive dysfunction, 7 and cardiovascular diseases. 18 , 19 A case‐control study with a small sample size, conducted in Japan, found decreased acetic acid and increased valeric acid levels in patients with ischemic stroke. 6 In addition, increased Fusobacterium and deficiency of SCFAs were observed in poststroke cognitive impairment patients, and probiotic administration relieved depression and anxiety. 20 In this study, we found an increased F/B ratio, a lack of SCFA‐producing bacteria and SCFAs, and a disrupted intestinal barrier in AIS patients. High F/B ratios have also been shown in association with obesity, 21 the elderly, 22 and hypertension. 23 The reduced SCFAs levels observed in AIS patients might be attributed to dysbiosis of the gut microbiota, which possibly led to an impaired intestinal barrier. It has been suggested that transplanting fecal microbiota rich in SCFAs or administering butyrate to affect SCFAs levels, thus repairing the leaky gut, is an effective treatment for cerebral ischemic stroke. 14 Besides, modulating poststroke neuronal plasticity via circulating lymphocytes on microglial activation, SCFAs were identified as potential therapeutic targets for improving poststroke recovery. 24
A substantial body of literature links the supplementation of dietary fibers or microorganism‐derived SCFAs to improved body weight, glucose, and blood pressure control. 25 , 26 , 27 SCFAs have been implicated in many studies 10 , 28 , 29 as potential disease‐preventing or disease‐alleviating targets linked with risk factors, cardiovascular disease risk, and mortality. In this study, a sharp decrease was observed in fecal SCFAs of AIS patients, particularly those with increased stroke severity. Turbulent gut microbiota and low SCFAs levels, especially acetate, seemed to portend poor stroke outcomes. Recent studies have associated decrease of SCFAs with various diseases and adverse outcomes. For example, Yamada et al reported a rapid and sustained decrease of fecal SCFAs in critically ill patients with systemic inflammatory response syndrome. 30 Quantitative reductions in SCFAs, especially butyrate, were also shown to contribute to the progression of chronic kidney disease. 31 Furthermore, patients with MS exhibited significantly reduced propionic acid, whereas propionic acid intake increased T‐regulatory cells and reduced annual relapse and brain atrophy, suggesting that propionic acid might be a potent immunomodulatory supplement. 32 Changes in the SCFA spectrum vary among different disorders, and few SCFA‐intervened clinical trials have so far been conducted. Therefore, it remains uncertain which SCFAs are beneficial. The potential for SCFAs to be therapeutic targets would not been strongly supported until clinically intervened studies are conducted and replicated.
Enterobacteriaceae are harmful members of the human symbiotic microbial community that produce enteropathy and interfere with mucosal immunity as well as healthy gut microbes. 33 Our microbial analysis revealed that Enterobacteriaceae were strikingly enriched in AIS patients. Increased Enterobacteriaceae was shown to be strongly associated with disease severity and treatment effectiveness in patients with inflammatory bowel disease. 34 As previously described, Enterobacteriaceae were also one of the most detrimental pathogens for patients in the intensive care unit. 35 , 36 Bäumler et al have demonstrated that host‐derived nitrate boosts the growth of Escherichia coli in the inflamed gut. 37 Further studies are warranted to explore why Enterobacteriaceae are enriched in AIS patients and whether this enrichment affects stroke outcomes.
Lactobacillus and Akkermansia are mostly recognized as probiotics and beneficial microbes. 38 , 39 However, increased abundances of Lactobacillaceae and Akkermansia were observed among AIS patients in this study. Lactobacillus were also previously found to be enriched in patients with metabolic syndrome 40 and high stroke risk. 41 This discrepancy might be explained by strain and/or species specificity because some strains/species of Lactobacillus are opportunistic pathogens. Furthermore, we speculate that the enrichment of Lactobacillaceae and Akkermansia represents a compensatory response to the loss of other butyrate‐producing bacteria. Additional work is needed to determine the exact roles Lactobacillaceae and Akkermansia play in AIS patients.
Our study is the first to investigate the intestinal microbiota and fecal SCFA spectrum of Chinese AIS patients with different stroke severities, from which we observe a striking imbalance in the gut flora accompanied by a lack of SCFAs and a leaky gut. Further analyses identified negative correlations between SCFAs and prognosis. Thus, SCFAs might be therapeutic targets for improving stroke outcomes. However, this study has several limitations that should be considered. First, the prevalence of hypertension and diabetes was lower in the healthy controls than in the stroke patients in our study. Nevertheless, many stroke patients have risk factors such as hypertension or diabetes. Future studies should explore pre‐stroke and acute stroke to determine whether stroke itself influences the gut microbiota and its metabolites. Second, we collected the first fecal samples that were spontaneously defecated within 72 hours after admission, but the underlying changes might occur earlier. Though rectal swabs obtain samples immediately and provide similar efficacies as stool, 42 fecal samples were used in this study to avoid possible discomfort in patients. Moreover, samples were collected once and within 72 hours after admission to prevent the complicated effects of any antibiotics/prebiotics/probiotics on the gut microbiome. Our previous study indicated that the magnitude of gut microbiota dysbiosis increased during hospitalization in neurocritically ill patients. 43 However, no dynamic observations indicating either turbulence or recovery were available in this study. We would like to establish carefully designed longitudinal studies with large sample sizes in the future. Third, we could only recruit few severe stroke patients because of the defecation delay. The selection bias and limited sample size make the interpretation of the relationship between SCFAs and prognosis challenging. In addition, no interventions were involved in this investigation. It remains unclear whether SCFA supplements improve stroke outcomes.
Conclusions
In summary, our findings indicated that a lack of SCFA‐producing bacteria and a low fecal SCFA level defined dysbiosis in AIS patients. Reduced SCFA levels, especially acetate, were found to be associated with an increased risk of 90‐day poor functional outcomes, indicating that SCFAs could be useful biomarkers for identifying unfavorable prognoses. Dietary fiber supplements that increase SCFA levels, possibly SCFA‐producing bacteria as well, might be strongly recommended for AIS patients. High‐quality prospective studies with large sample sizes and, ideally, with clinically or experimentally dietary interventions are warranted to confirm our findings.
Statement of Authorship
J. Yin and Y. He equally contributed to the conception and design of the research; C. Tan and H. Zhou contributed to the design of the research; C. Tan, X. Gao, R. Xu, J. Zhu, and X. Zeng contributed to the acquisition and analysis of the data; C. Tan, Q. Wu, H. Wang, and Z. Cui contributed to the interpretation of the data; and C. Tan and Q. Wu drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Supporting information
Supplementary information
Financial disclosure: This study was funded by the Natural Science Foundation of China (81671171) and of the Guangdong Province (2017A030313821).
Conflicts of interest: None declared.
References
- 1. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Winek K, Dirnagl U, Meisel A. The gut microbiome as therapeutic target in central nervous system diseases: implications for stroke. Neurotherapeutics. 2016;13(4):762‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Durgan DJ, Lee J, McCullough LD, et al. Examining the role of the microbiota‐gut‐brain axis in stroke. Stroke. 2019;50(8):2270‐2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen R, Wu P, Cai Z, et al. Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain‐gut barriers. J Nutr Biochem. 2019;65:101‐114. [DOI] [PubMed] [Google Scholar]
- 5. Yin J, Liao SX, He Y, et al. Dysbiosis of gut microbiota with reduced trimethylamine‐N‐oxide level in patients with large‐artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4(11):e002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamashiro K, Tanaka R, Urabe T, et al. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One. 2017;12(2):e0171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dalile B, Van Oudenhove L, Vervliet B, et al. The role of short‐chain fatty acids in microbiota‐gut‐brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461‐478. [DOI] [PubMed] [Google Scholar]
- 8. Russo R, Cristiano C, Avagliano C, et al. Gut‐brain Axis: Role of Lipids in the Regulation of Inflammation, Pain and CNS Diseases. Curr Med Chem. 2018;25(32):3930‐3952. [DOI] [PubMed] [Google Scholar]
- 9. Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity‐related metabolic diseases? Obes Rev. 2013;14(12):950‐959. [DOI] [PubMed] [Google Scholar]
- 10. Koh A, De Vadder F, Kovatcheva‐Datchary P, et al. From dietary fiber to host physiology: short‐chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332‐1345. [DOI] [PubMed] [Google Scholar]
- 11. Cryan JF, O'Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179‐194. [DOI] [PubMed] [Google Scholar]
- 12. Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. 2016;22(5):516‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xia GH, You C, Gao XX, et al. Stroke Dysbiosis Index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front Neurol. 2019;10:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen R, Xu Y, Wu P, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. 2019;148:104403. [DOI] [PubMed] [Google Scholar]
- 15. Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5(2):202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tolhurst G, Heffron H, Lam YS, et al. Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein‐coupled receptor FFAR2. Diabetes. 2012;61(2):364‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haghikia A, Jorg S, Duscha A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43(4):817‐829. [DOI] [PubMed] [Google Scholar]
- 18. Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9(5):416‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16(3):137‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Kong C, Gong L, et al. The association of post‐stroke cognitive impairment and gut microbiota and its corresponding metabolites. J Alzheimers Dis. 2020;73(4):1455‐1466. [DOI] [PubMed] [Google Scholar]
- 21. Koliada A, Syzenko G, Moseiko V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spychala MS, Venna VR, Jandzinski M, et al. Age‐related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018;84(1):23‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadler R, Cramer JV, Heindl S, et al. Short‐chain fatty acids improve poststroke recovery via immunological mechanisms. J Neurosci. 2020;40(5):1162‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nicolucci AC, Hume MP, Martinez I, et al. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017;153(3):711‐722. [DOI] [PubMed] [Google Scholar]
- 26. Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151‐1156. [DOI] [PubMed] [Google Scholar]
- 27. Natarajan N, Hori D, Flavahan S, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein‐coupled receptor 41. Physiol Genomics. 2016;48(11):826‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Canfora EE, Jocken JW, Blaak EE: Short‐chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577‐591. [DOI] [PubMed] [Google Scholar]
- 29. Leung C, Rivera L, Furness JB, et al. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412‐425. [DOI] [PubMed] [Google Scholar]
- 30. Yamada T, Shimizu K, Ogura H, et al. Rapid and sustained long‐term decrease of fecal short‐chain fatty acids in critically ill patients with systemic inflammatory response syndrome. JPEN J Parenter Enteral Nutr. 2015;39(5):569‐577. [DOI] [PubMed] [Google Scholar]
- 31. Wang S, Lv D, Jiang S, et al. Quantitative reduction in short‐chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin Sci (Lond). 2019;133(17):1857‐1870. [DOI] [PubMed] [Google Scholar]
- 32. Duscha A, Gisevius B, Hirschberg S, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180(6):1067‐1080.e1016. [DOI] [PubMed] [Google Scholar]
- 33. Kau AL, Planer JD, Liu J, et al. Functional characterization of IgA‐targeted bacterial taxa from undernourished Malawian children that produce diet‐dependent enteropathy. Sci Transl Med. 2015;7(276):276ra224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou Y, Xu ZZ, He Y, et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems. 2018;3(1):e00188‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferrer M, Difrancesco LF, Liapikou A, et al. Polymicrobial intensive care unit‐acquired pneumonia: prevalence, microbiology and outcome. Crit Care. 2015;19(1):450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barbier F, Pommier C, Essaied W, et al. Colonization and infection with extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae in ICU patients: what impact on outcomes and carbapenem exposure? J Antimicrob Chemother. 2016;71(4):1088‐1097. [DOI] [PubMed] [Google Scholar]
- 37. Winter SE, Winter MG, Xavier MN, et al. Host‐derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339(6120):708‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dietrich CG, Kottmann T, Alavi M. Commercially available probiotic drinks containing Lactobacillus casei DN‐114001 reduce antibiotic‐associated diarrhea. World J Gastroenterol. 2014;20(42):15837‐15844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. 2017;106:171‐181. [DOI] [PubMed] [Google Scholar]
- 40. Lim MY, You HJ, Yoon HS, et al. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut. 2017;66(6):1031‐1038. [DOI] [PubMed] [Google Scholar]
- 41. Zeng X, Gao X, Peng Y, et al. Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate‐producing bacteria in the gut. Front Cell Infect Microbiol. 2019;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bansal S, Nguyen JP, Leligdowicz A, et al. Rectal and Naris Swabs: Practical and Informative Samples for Analyzing the Microbiota of Critically Ill Patients. mSphere. 2018;3(3):e00219‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu R, Tan C, Zhu J, et al. Dysbiosis of the intestinal microbiota in neurocritically ill patients and the risk for death. Crit Care. 2019;23(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


