Abstract
Introduction
Advancing age is a known risk factor for developing atrial fibrillation (AF), yet it is unknown which electrophysiological changes contribute to this increased susceptibility. The goal of this study is to investigate conduction disturbances and unipolar voltages (UV) related to aging.
Methods
We included 216 patients (182 male, age: 36–83 years) without a history of AF undergoing elective coronary artery bypass surgery. Five seconds of sinus rhythm were recorded intraoperatively at the right atrium (RA), Bachmann's bundle (BB), the left atrium and the pulmonary vein area (PVA). Conduction delay (CD), ‐block (CB), ‐velocity (CV), length of longest CB lines and UV were assessed in all regions.
Results
With aging, increasing conduction disturbances were found, particularly at RA and BB (RA: longest CB line r s = .158, p = .021; BB: CB prevalence r s = .206, p = .003; CV r s = −.239, p < .0005). Prevalence of low UV areas (UV <5th percentile) increased with aging at the BB and PVA (BB: r s = .237, p < .0005 and PVA: r s = .228, p = .001).
Conclusions
Aging is accompanied by an increase in conduction disturbances during sinus rhythm and a higher prevalence of low UV areas, particularly at BB and in the RA. These electrophysiological alterations could in part explain the increasing susceptibility to AF development associated with aging.
Keywords: aging, atrial fibrillation, electrophysiological study, mapping
1. INTRODUCTION
Advanced age is a known risk factor for developing atrial fibrillation (AF). 1 , 2 Prevalence of AF is low before the age of 60 but rises steeply afterwards. 3 Even though the relation between age and AF is clear, there is limited data on the electrophysiological changes associated with aging that might underlie this relation. 4 , 5 , 6
Electrogram morphology in relation to age has been investigated by endocardial mapping of the right atrium (RA) during sinus rhythm in 111 patients (mean age: 57.0 ± 14.1 years) with paroxysmal AF. Patients ≥60 years had more abnormal electrograms, defined as electrograms with a duration of ≥100 ms and/or containing eight or more negative deflections. 4 In 23 patients (age: 17–75 years) with left‐sided accessory pathways, Kojodjojo et al. 6 found that endocardial wave propagation velocity in both the left (left atrium [LA]) and RA declines with increasing age. These previous studies were limited to either the RA or LA and included a relatively low number of recording sites or examined only one electrophysiological parameter.
Additionally, interatrial block becomes more prevalent with age, which is associated with the development of AF. Bachmann's bundle (BB) is likely to be involved in the pathophysiology of interatrial block and therefore, this could indicate that BB also might be involved in the pathophysiology underlying the higher prevalence of AF with advancing age. 7 However, so far no mapping studies have evaluated if and to what extend BB undergoes electrophysiological changes with aging.
The goal of this study was to examine conduction disturbances and unipolar voltages in in a large cohort of patients with a wide age range using a high‐resolution epicardial mapping approach of the RA, LA, and BB to identify electropathology associated with aging.
2. METHODS
2.1. Study population
Patients (age ≥18 years) without a history of AF undergoing elective open chest cardiac surgery for coronary bypass grafting were included. Echocardiographic images were used to identify left atrial dilatation (LA diameter >45 mm). This study was approved by the Medical Ethical Committee in the Erasmus Medical Center (MEC 2010‐054 and MEC 2014‐393) and follows the declaration of Helsinki principles. Written informed consent was obtained from all patients.
2.2. Mapping procedure
Before commencement of extracorporeal circulation, high‐resolution epicardial mapping was performed as described previously. 8 A bipolar wire was attached to the crista terminalis, serving as a reference electrode, and a steel wire was fixed to subcutaneous tissue in the thorax and used as an indifferent electrode. Recordings were made during sinus rhythm using either a 128‐unipolar electrode (electrode diameter: 0.65 mm) or a 192‐unipolar electrode (electrode diameter: 0.45 mm) array. Interelectrode distances of both arrays were 2 mm.
The mapping array was shifted over predefined sites to cover both atria (Figure 1). Five seconds of sinus rhythm were recorded at each mapping site. This recording included a bipolar reference electrograms, all unipolar electrograms, a surface electrocardiogram and a calibration signal with an amplitude of 2 mV and a duration of 1000 ms. Sample rate was 1 kHz and the signals were amplified (gain 1000), filtered (bandwidth: 0.5–400 Hz), analogue‐to‐digital converted (16‐bits) and stored on a hard disk.
Figure 1.
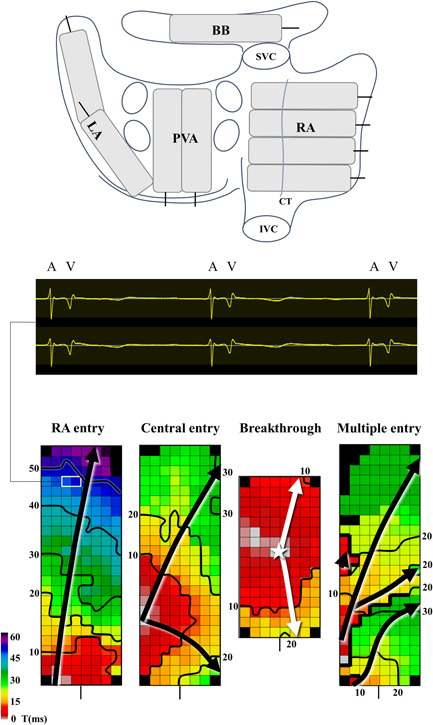
The upper panel depicts an overview of the mapping locations on a posterior view of the heart. The middle panel shows three sinus beats on two electrograms with atrial potentials followed by a ventricular far‐field signal. The lower panel illustrates the various wavefront entry sites on BB, which are explained further in the text. Activation maps are depicted with 10 ms isochrones, arrows show the main direction of wavefront propagation and thick black lines indicate CB area. A, atrial signal; BB, Bachmann's bundle; CT, crista terminalis; IVC, inferior vena cava; LA, left atrium; PVA, pulmonary vein area; RA, right atrium; SVC, superior vena cava; V, ventricular far‐field signal
2.3. Analysis of mapping data
Electrograms were visualized and annotated using dedicated mapping software. The steepest negative atrial deflection was marked if the amplitude exceeded the noise level of the electrogram at least twice. For every sinus beat, local activation times (LATs) were used to construct activation maps. Electrogram files were excluded when primary deflections were marked in less than 40% of the mapping array due to poor quality recordings. Atrial extra systolic beats were excluded. If more than one location (RA, BB, LA, pulmonary vein area (PVA)) of a patient was unavailable for any reason, the patient was excluded.
2.4. Analysis of conduction abnormalities and electrogram amplitude
Peak‐to‐peak amplitude of each primary deflection was measured to create voltage maps. As the cutoff point for low voltage, the 5th percentile of all unipolar peak‐to‐peak amplitudes was taken. Continuous areas of low voltages were defined as connecting electrodes from which peak‐to‐peak amplitudes below the 5th percentile were recorded.
Similar to previous studies, conduction delay (CD) was determined as LAT differences (conduction times (CTs) of 7–11 ms between two adjacent electrodes and conduction block (CB) as LAT differences of ≥12 ms between two electrodes. 9 If a CD area was connected to a CB area it was labeled as a continuous CDCB area (cCDCB). The combination of CD and CB together was abbreviated as CDCB.
Conduction velocity (CV) was computed by a method of velocity vectors estimated between neighboring electrodes (longitudinal, transversal, and diagonal) based on the technique using finite differences described and developed by Salama et al. 10 To assess heterogeneity in CV, we determined the coefficient of variation (CoV‐CV = CVσ/CVµ) using the calculated velocity vectors from each electrode.
All locations (except BB when using the 192‐array) had multiple mapping sites (Figure 1). All voltage and conduction parameters were calculated for each mapping site separately and expressed as either an average or summation for each location or for all locations together (biatrial). Additionally, we identified sites with the highest degree of conduction disturbances defined as the maximum percentage CB or CDCB lowest CV. As the main direction of myocardial fibers at BB is known, 11 we used this location to investigate differences between transverse and longitudinal conduction slowing. For this purpose, we included only CTs ≥4 ms; CTs of 1–3 ms were considered “normal” conduction. We calculated the ratio of the prevalence of CTs ≥4 ms in the transverse direction to the longitudinal direction (TL4 ratio) in patients in whom BB was activated from the RA to the LA, as in this case wavefront propagation is approximately parallel to the fiber direction.
2.5. Wave entry sites at BB
A prior study demonstrated that patients with a history of AF more often had a wavefront entering in the middle of BB. 9 Therefore, at BB, the site of wavefront entry was examined for all patients as depicted in Figure1. Entry sites were classified as right atrial entry (RA entry), central entry, breakthrough or multiple entry sites. RA entry site was defined as a wavefront entering BB from the right atrial side. A wavefront entering from the lateral side of the array and propagating radially to the left and right was defined as a central entry. A wavefront entering in the middle of the array was defined as breakthrough. When more than one wavefront activated BB simultaneously, it was labeled as multiple entry.
2.6. Statistical analysis
Normally distributed data is described as mean ± SD, skewed data as median [interquartile range] and categorical data as absolute number (percentage). When a β coefficient value from multiple linear regression is described, it is depicted as β [95% confidence interval]. The measure of correlation between age and baseline characteristics and electrophysiological parameters was determined by Spearman's rank correlation. Continuous data were tested with an independent samples t test or analysis of variance; a Mann–Whitney U or Kruskal–Wallis test was used when data were skewed. Categorical data was tested using χ 2 test or a Fisher's exact test where appropriate. (Multiple) linear regression was performed to determine associations between electrophysiological parameters and age, 10log transformation was performed for TL‐ratio, continuous area of low voltage (RA/BB) and prevalence of low voltage (RA/BB/PVA) as the criteria for lineair regression were violated. We included various cardiovascular risk factors and electrode array set‐up (128‐ or 192‐ electrode array) in the regression model as independent variables to eliminate a possible confounding effect. All statistical analyses were performed with IBM SPSS statistics for Windows, version 25 (IBM Corp.).
3. RESULTS
3.1. Baseline characteristics
A total of 216 patients (182 [84.3%] male) aged between 36 and 83 years (median: 66.7 [58.9–72.5] years) were included. The relative frequency distribution of age in our population is demonstrated in the histogram in the upper panel of Figure 2. Detailed baseline characteristics are depicted in Table 1. Except for BMI, (r s = −.23, p = .001), none of the baseline characteristics were associated with age. Mapping data consisted of a total of 413,606 electrograms (1882.5 [1701–2125] electrograms per patient). Sinus rhythm cycle length (CL) was 901.8 [766.8–1013.3] ms and was positively correlated to age (r s = .239, p < .0005).
Figure 2.
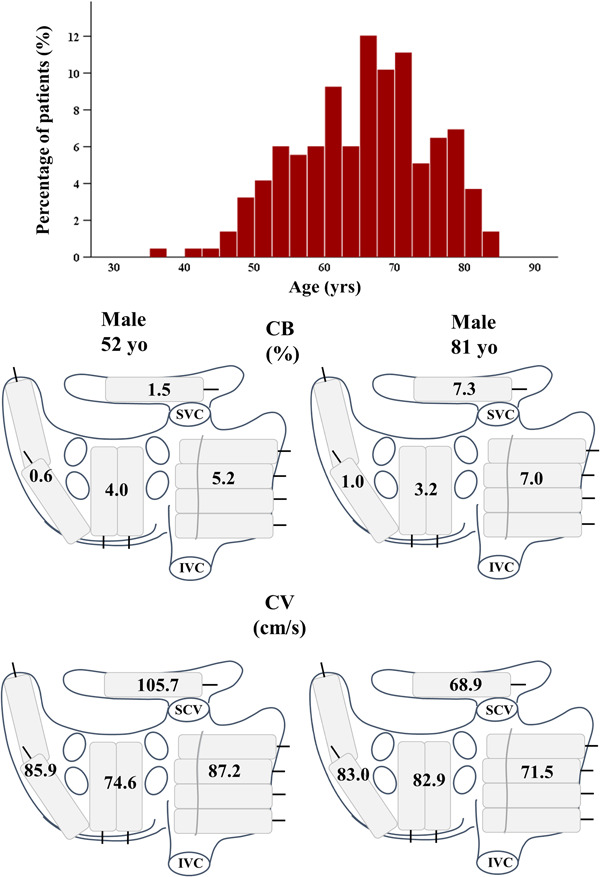
The upper panel shows a histogram depicting the relative distribution of age in the study population. The lower panel demonstrates the distribution of CB prevalence and CV of a typical younger (left) and older (right) patient. See text for further description. CB, conduction block; CV, conduction velocity; IVC, inferior vena cava; SVC, superior vena cava
Table 1.
Baseline characteristics
| N = 216 | |
|---|---|
| Age, median [IQR] | 66.7 [58.9–72.5] years |
| Gender female, n (%) | 34 (15.7) |
| BMI, mean (SD)a | 28.4 (4.1) kg/m2 |
| Hypertension, n (%) | 139 (64.4) |
| Diabetes mellitus, n (%) | 75 (34.7) |
| Dyslipidemia, n (%) | 98 (45.4) |
| Thyroid disease, n (%) | 9 (4.2) |
| Myocardial infarction, n (%) | 105 (48.6) |
| Left ventricular EF: | |
| Normal | 162 (75.0) |
| Mild impairment | 47 (21.8) |
| Moderate impairment | 6 (2.8) |
| Severe impairment | 1 (0.5) |
| Left atrial enlargementb, n/n (%) | 25/123 (20.3) |
| Missing | 93 |
| LA diameter, median [IQR] | 39.0 mm [37–43] |
| Missing | 125 |
Abbreviations: BMI, body mass index, EF, ejection fraction.
Indicates a significant correlation with age.
Left atrial enlargement was defined as a left atrial diameter more than 45 mm.
3.2. Relation between conduction disturbances and aging
The middle and lower panel of Figure 2 demonstrate examples of regional differences in the prevalence of CB and local CV in a 52‐ and a 81‐year‐old patient. CB prevalence was higher in the older patient and differences between the two patients were most pronounced at BB and the RA. Similarly, CV was substantially lower in these locations in the older patient.
With aging, the prevalence of biatrial CB increased (r s = .158, p = .020). Maximum prevalence of CB and CDCB (r s = .218, p = .001; r s = .143, p = .035), regardless of location, also increased with advancing age. The scatterplot in the upper panel of Figure 3 demonstrates the relation between age and maximum prevalence of CB. Maximum prevalence of CB and CDCB was found at the RA in most patients; in respectively 132 (61.1%) and 122 (56.5%) patients. Age of patients did not differ among locations (RA, BB, PVA, or LA) of CB or CDCB maximum prevalence (p = .529 and p = .146, respectively).
Figure 3.
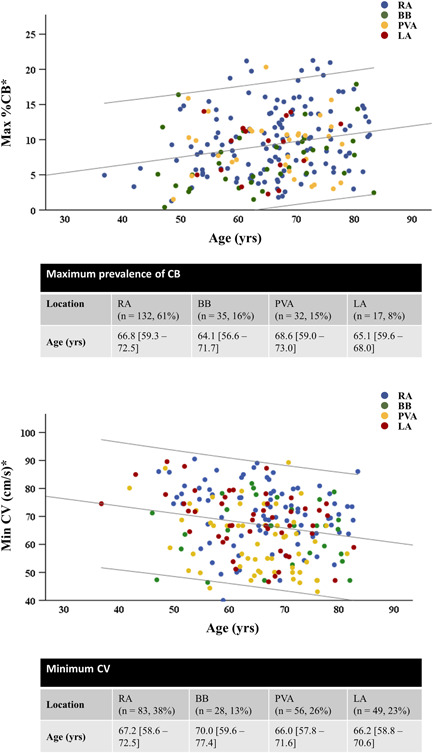
The correlation between age and maximum CB prevalence (upper panel) and age and lowest CV (lower panel). Colors indicate the location of maximum CB prevalence and lowest CV. Lines indicate 95% confidence intervals. *Max %CB: p = .001/Min CV: p = .004. CB, conduction block, CV, conduction velocity
Significant associations between conduction parameters and age were found at BB and RA; outcomes are summarized in Table S1. At BB, prevalence of CD, CB, and cCDCB as well as several properties of conduction disorders increased with aging. At the RA, longest CB line (r s = .158, p = .021) and maximum CB prevalence significantly increased with age (r s = .196, p = .004). Interestingly, at the RA, only the amount of CB was affected, whereas at BB the amount of CD also increased with aging.
In the entire group, biatrial CV was 86.9 [81.8–91.8] cm/s. Lowest CV decreased with aging (r s = −.210, p = .002) (Figure 3), which in most patients was found at the RA (n = 83, 38%). Age of patients among the locations (RA, BB, PVA, or LA) of lowest CV did not differ (p = .240). In both the RA and at BB a decline in CV with aging was seen (RA min CV: r s = −.231, p = .001 and BB CV: r s = −.239, p < .0005). Additionally, CoV‐CV correlated with age biatrially and locally in the RA and at BB (biatrial max CoV‐CVrs: 0.142, p = .037; RA CoV‐CV: 0.163, p = .017; RA max CoV‐CV: 0.198, p = .004; BB CoV: 0.215, p = .002).
After multivariate analysis for correction of a possible confounding effect of cardiovascular risk factors, all but three conduction parameters were still related to aging as can be seen in Table S2.
3.3. Sinus rhythm wavefront entry site at BB
Age did not differ among patients with different wave entry sites (RA entry: 64.7 [57.0–71.5] years, central entry: 67.2 [60.8–72.3] years, breakthrough: 69.2 [66.6–74.7] years, multiple entry: 68.4 [61.1–73.7] years, p = .249). Additionally, patients with a “regular” wave entry (RA entry) at BB were not significantly younger than patients with another entry site, although a trend towards significance was observed (64.7 [57.0–71.5] versus 67.7 [61.8–72.7] years, p = .062).
3.4. Differences between transverse and longitudinal conduction slowing
Examples of TL4 ratios in a 51‐ and a 70‐year‐old patient are depicted in the upper panel of Figure 4. Both patients had a similar prevalence of longitudinal CTs ≥4 ms (both 6.1%). However, the older patient had a higher prevalence of transversal CTs ≥4 ms (6.9% vs. 2.9%) resulting in a higher TL4 ratio (1.14 vs. 0.46).
Figure 4.
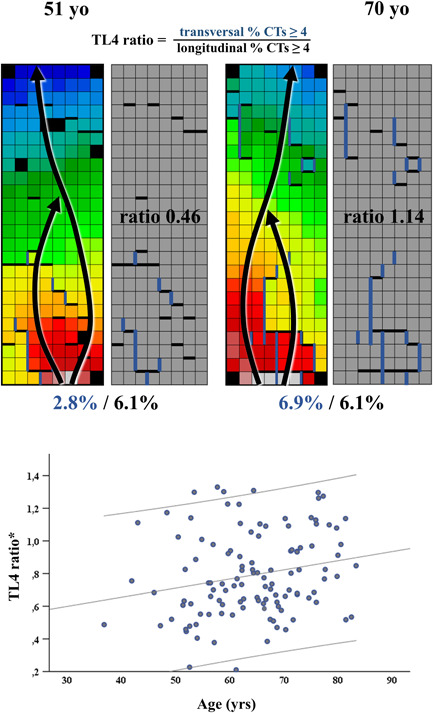
The upper panel shows examples of a low TL4 ratio in a young patient (left) and a high TL4 ratio in an old patient (right). Arrows show the main direction of wavefront propagation, thick blue and black lines show CT's ≥4 ms, respectively, in transversal and longitudinal direction. The lower panel depicts the correlation between age and TL4 ratio in patients with a RA entry site (n = 116). Lines indicate 95% confidence intervals. *p = .022. CT, conduction time
In the 116 patients with a RA entry site, aging was associated with an increased TL4 ratio (β = .004 [0.001–0.006])/year, p = .011 and r s = .221, p = .017), indicating slowing of transverse conduction to a greater extent than longitudinal conduction with advancing age (lower panel of Figure 4). After correcting for possible influence of BB sinus rhythm rate, which was 839.3 [751.4–1011.3] ms in this group of patients, age remained an independent predictor for a higher TL4 ratio (β = .006 [0.001–0.011], p = .028). Also, after correcting for cardiovascular risk factors, age remained an independent predictor for a higher TL4 ratio (β = .003 [0.000–0.006]/year, p = .046).
3.5. Low voltage zones and aging
Figure 5 depicts scatterplots of median amplitudes per location and age. The cutoff for low voltage was based on the 5th percentile of the amplitudes of all atrial deflections and was 0.7339 mV. With advancing age, in the RA and BB median amplitude was lower (RA: r s = −.291, p < .0005; BB: r s = −.328, p < .0005), the largest continuous area of low voltage increased (RA: r s = .175, p = .010; BB: r s = .136, p = .045) and in BB the prevalence of low voltage areas was higher (BB: r s = .237, p < .0005). At the PVA, the prevalence of low voltage area, but not the size of continuous areas, increased with advancing age (r s = .228, p = .001). Tables S3 and S4 summarize outcomes of the voltage parameters.
Figure 5.
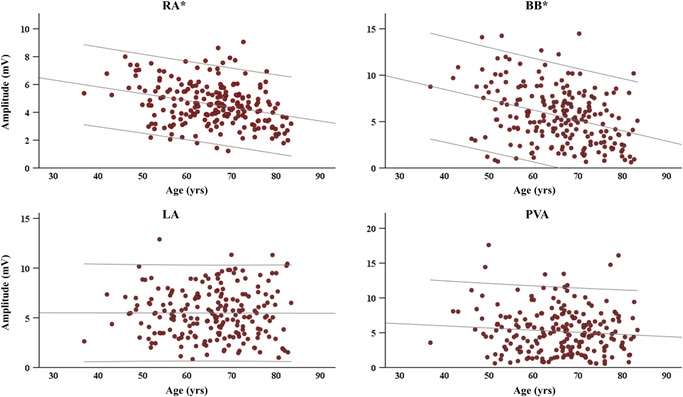
Plots demonstrating the relation between voltage and age for each location separately. Lines indicate 95% confidence intervals. *RA: p < .0005, BB: p < .0005. BB, Bachmann's bundle; LA, left atrium; PVA, pulmonary vein area; RA, right atrium
After multivariate testing to correct for a confounding effect of various cardiovascular risk factors, all abovementioned parameters remained significantly related to age.
4. DISCUSSION
4.1. Key findings
Using a high‐resolution epicardial mapping approach, we found that advancing age is associated with an increasing amount of conduction disturbances and decreasing unipolar voltages. With aging, throughout the atria, CB occurred more frequently, and conduction slowed. Specifically, at BB and RA conduction abnormalities were more prevalent in older patients. Furthermore, this is the first study suggesting enhanced lateral uncoupling in the intact aging human heart. Low voltage areas were more pronounced at BB, RA, and PVA in older patients.
4.2. Electrophysiological changes related to aging
Several endocardial mapping studies have also found a relation between age and slowing of conduction and low voltage areas. In these studies, no correction for cardiovascular risk factors was performed. To the best of our knowledge, our study was the first to perform this correction, which we were able to do due to the large size of our study population.
A decrease in wavefront propagation velocity in the RA and LA with aging was found in an electro‐anatomic mapping study by Kodjojo et al. 6 in 23 patients. Additionally, two studies focusing on electrophysiological parameters in relation to age in the RA divided patients into three age groups (≤30, 31–59, and ≥60 years). They found an increase in low voltage areas and fractionated electrograms along the crista terminalis with aging. 5 , 12 Kistler et al. 5 found conduction slowing in the RA by calculating CV in six locations throughout the RA. 5 Interestingly, in our study, the average CV for the entire RA area did not change with aging. Yet, the lowest CV for the RA area was lower in older patients, indicating regional differences in CV. The differences found in the abovementioned studies are in relatively small populations and patients are divided into groups based on age. Our study uses age as a continuous parameter to find true correlations between electrophysiological parameters and age. Large interindividual variability is to be expected as sinus‐node and consequently sinus rhythm activation pathways differ from patient to patient. 13 , 14 Additionally, the included patients did not have a history of AF. The moment of observation in relation to possible AF‐onset increases interindividual variability in electropathology as well. These reasons are part of the explanation for the lack of strong correlations in our study. A certain degree of increasing electropathology with age during sinus rhythm is still observed. However, our outcomes of only weak‐moderate correlations suggest that age is not the most important factor for development of AF. Possibly, the observed electropathology is mostly a result of underlying cardiovascular disease and associated risk factors.
4.3. Structural changes related to aging
Various studies have investigated structural changes in the atria occurring during aging. Compensatory cellular hypertrophy due to loss of cardiomyocytes by apoptosis and necrosis in advancing age is seen as well as increased fibrosis content which impairs cell‐to‐cell interactions. 15 , 16 The relation between age and atrial fibrosis has been extensively studied. Almost all studies found a positive relation between fibrosis and aging in patients with and without a history of AF. 17 , 18 However, Platonov et al. 19 found that age‐related changes are unlikely to be the sole cause of advanced fibrosis. This could be another reason for interindividual variability in electropathology which we observed. Nevertheless, structural remodeling that does accompany aging could explain the increase in low voltage areas, conduction disturbances including (local) conduction slowing seen in our study and is likely to contribute to development of AF.
In 1988, Spach et al. 20 found progressive electrical uncoupling of side‐to‐side connections with increasing age and development of collagenous septa between fibers impairing conduction even more. This uncoupling resulted in a slower transverse propagation at a long (800 ms) CL in older atrial muscle bundles. 20 Similarly, Koura et al. 21 found connexin modification in the RA free wall of canine atria with aging. In infant dogs the gap junction Cx43 was distributed over the entire cell surface, while in older dogs Cx43 was more localized to the cell termini causing lateral uncoupling. Consequentially, wavefront curvature differed in the older dogs already at a CL of 500 ms. In younger dogs, an elliptical pattern of propagation was seen, while older dogs with slower transverse propagation showed a more “square” wavefront by crowding of transverse isochrones. This side‐to‐side uncoupling gives rise to micro re‐entry circuits, facilitating AF domestication. 20 , 22 Interestingly, a study in mice by Jansen et al., 23 found that reduced ventricular connexin43 (Cx43) expression results in excessive collagen deposition. In our study, we measured a transverse to longitudinal CTs ≥4 ms prevalence ratio in patients with a similar conduction direction. Of course, we measure at a more macroscopic level than Spach et al. 20 in their study, and we can only determine the effective CTs between measured areas of tissue. Furthermore, the fiber direction in relation to our mapping array might vary from patient to patient resulting in a slight variation in direction of CDs. Still, in 116 patients we found that with increasing age transverse conduction deteriorates relatively more than longitudinal conduction, implying lateral uncoupling with aging.
4.4. Sinus rhythm wave entry at BB
In previous studies, it was shown that different activation patterns at BB have a possible arrhythmogenic connotation. Teuwen et al. 9 found that patients with a history of AF more often had a wavefront entering in the middle of BB compared to patient without a history of AF. Mouws et al. 14 found that patients with (ischemic and) valvular heart disease more often had a wavefront entering in the middle of BB compared to patients with only ischemic heart disease. In an anatomical study by Knol et al. 24 in 19 postmortem dissected hearts, the relation of the interatrial septum to BB was shown which could be the origin of breakthrough waves. Furthermore, a posterosuperior bundle was found in all hearts that coincides with a central entry site at BB. As all patients have this posterosuperior bundle, it is interesting that differences were found in the previous studies by Teuwen et al. and Mouws et al. 14 Hypothetically, development of conduction disturbances could lead to a change in preferential activation route from a “normal” RA entry site to an entry site in the middle of BB using the posterosuperior bundle or the interatrial septum, which would make wave entry site at BB an indirect indicator for these disturbances. This is supported by the findings in our study, where patients with a “normal” RA entry were younger compared to other entry sites, although this did not reach significance. However, as the the exact location of the mapping array as well as anatomical structures might shift patient to patient, an entry site might sometimes be misclassified.
4.5. Limitations
As mentioned above, due to large interindividual variability in the electrophysiological parameters no strong correlations were found. Even though age is a major risk factor for AF and plays a role in the development of electropathology, the weak correlations suggest that age is not the most contributing risk factor. However, not all electropathology may be manifest during sinus rhythm measurements. Furthermore, as we only included patients without a history of AF, the older patients in our group represent patients with a less severe arrhythmogenic substrate as a result of aging than patients of the same age who have already developed AF which would also diminish correlations.
5. CONCLUSION
Aging is accompanied by an increase in conduction abnormalities, general conduction slowing and low voltage areas, particularly at BB and RA. Additionally, lateral uncoupling at BB increases with advancing age. These changes could be a depiction of the arrhythmogenic substrate and may in part account for the rising prevalence of AF with aging.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The authors would like to kindly thank J. A. Bekkers, W. J. van Leeuwen, F. B. S. Oei, F. R. N. van Schaagen, P. C. van de Woestijne, A. Yaksh, C. P. Teuwen, E. A. H. Lanters, R. Starreveld, L. N. van Staveren, L. J. M. E. van der Does, C. S. Serban, R. K. Kharbanda, M. S. van Schie, and M. C. Roos for their contribution to this work, as well as I. Kardys, for advice on statistical analysis. Natasja M. S. de Groot is supported by grants from the Investigator‐Initiated Study Program of Biosense Webster, Inc. (ICD 783454), the CVON (grant number: 914728) and NWO‐Vidi (grant number: 91717339) and Medical Delta.
van der Does WFB, Houck CA, Heida A, et al. Atrial electrophysiological characteristics of aging. J Cardiovasc Electrophysiol. 2021;32:903–912. 10.1111/jce.14978
Disclosures: None.
DATA AVAILABILITY STATEMENT
Data supporting the findings of this study are available on reasonable request from the corresponding author.
REFERENCES
- 1. Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949‐953. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840‐844. [PubMed] [Google Scholar]
- 3. Wilke T, Groth A, Mueller S, et al. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15:486‐493. [DOI] [PubMed] [Google Scholar]
- 4. Centurion OA, Isomoto S, Shimizu A, et al. The effects of aging on atrial endocardial electrograms in patients with paroxysmal atrial fibrillation. Clin Cardiol. 2003;26:435‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kistler PM, Sanders P, Fynn SP, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109‐116. [DOI] [PubMed] [Google Scholar]
- 6. Kojodjojo P, Kanagaratnam P, Markides V, Davies DW, Peters N. Age‐related changes in human left and right atrial conduction. J Cardiovasc Electrophysiol. 2006;17:120‐127. [DOI] [PubMed] [Google Scholar]
- 7. Martínez‐Sellés M, Massó‐Van roessel A, Álvarez‐García J, et al. Interatrial block and atrial arrhythmias in centenarians: prevalence, associations, and clinical implications. Heart Rhythm. 2016;13:645‐651. [DOI] [PubMed] [Google Scholar]
- 8. van der Does LJ, Yaksh A, Kik C, et al. QUest for the arrhythmogenic substrate of atrial fibrillation in patients undergoing cardiac surgery (QUASAR Study): Rationale and Design. J Cardiovasc Transl Res. 2016;9:194‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teuwen CP, Yaksh A, Lanters EAH, et al. Relevance of conduction disorders in Bachmann's bundle during sinus rhythm in humans. Circ Arrhythm Electrophysiol. 2016;9:e003972. [DOI] [PubMed] [Google Scholar]
- 10. Salama G, Kanai A, Efimov IR. Subthreshold stimulation of Purkinje fibers interrupts ventricular tachycardia in intact hearts. Experimental study with voltage‐sensitive dyes and imaging techniques. Circ Res. 1994;74:604‐619. [DOI] [PubMed] [Google Scholar]
- 11. van Campenhout MJ, Yaksh A, Kik C, et al. Bachmann's bundle: a key player in the development of atrial fibrillation? Circ Arrhythm Electrophysiol. 2013;6:1041‐1046. [DOI] [PubMed] [Google Scholar]
- 12. Roberts‐Thomson KC, Kistler PM, Sanders P, et al. Fractionated atrial electrograms during sinus rhythm: relationship to age, voltage, and conduction velocity. Heart Rhythm. 2009;6:587‐591. [DOI] [PubMed] [Google Scholar]
- 13. Boineau JP, Canavan TE, Schuessler RB, Cain ME, Corr PB, Cox JL. Demonstration of a widely distributed atrial pacemaker complex in the human heart. Circulation. 1988;77:1221‐1237. [DOI] [PubMed] [Google Scholar]
- 14. Mouws E, Lanters EAH, Teuwen CP, et al. Impact of ischemic and valvular heart disease on atrial excitation: a high‐resolution epicardial mapping study. J Am Heart Assoc. 2018;(6):7 e008331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagibin V, Egan Benova T, Viczenczova C, et al. Ageing related down‐regulation of myocardial connexin‐43 and up‐regulation of MMP‐2 may predict propensity to atrial fibrillation in experimental animals. Physiol Res. 19 2016;65(Suppl 1):S91‐S100. [DOI] [PubMed] [Google Scholar]
- 16. Xu GJ, Gan TY, Tang BP, et al. Alterations in the expression of atrial calpains in electrical and structural remodeling during aging and atrial fibrillation. Mol Med Rep. 2013;8:1343‐1352. [DOI] [PubMed] [Google Scholar]
- 17. Gramley F, Lorenzen J, Knackstedt C, et al. Age‐related atrial fibrosis. Age. Dordr). 2009;31:27‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akoum N, Mahnkopf C, Kholmovski EG, Brachmann J, Marrouche NF. Age and sex differences in atrial fibrosis among patients with atrial fibrillation. Europace. 2018;20:1086‐1092. [DOI] [PubMed] [Google Scholar]
- 19. Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58:2225‐2232. [DOI] [PubMed] [Google Scholar]
- 20. Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res. 1988;62:811‐832. [DOI] [PubMed] [Google Scholar]
- 21. Koura T, Hara M, Takeuchi S, et al. Anisotropic conduction properties in canine atria analyzed by high‐resolution optical mapping: preferential direction of conduction block changes from longitudinal to transverse with increasing age. Circulation. 2002;105:2092‐2098. [DOI] [PubMed] [Google Scholar]
- 22. Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side‐to‐side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397‐413. [DOI] [PubMed] [Google Scholar]
- 23. Jansen JA, van Veen TAB, de Jong S, et al. Reduced Cx43 expression triggers increased fibrosis due to enhanced fibroblast activity. Circ Arrhythm Electrophysiol. 2012;5:380‐390. [DOI] [PubMed] [Google Scholar]
- 24. Knol WG, Teuwen CP, Kleinrensink GJ, Bogers A, de Groot NMS, Taverne Y. The Bachmann bundle and interatrial conduction: comparing atrial morphology to electrical activity. Heart Rhythm. 2019;16:606‐614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
Data supporting the findings of this study are available on reasonable request from the corresponding author.


