Abstract
In the global KEYNOTE‐042 study (Clinicaltrials.gov, NCT02220894), pembrolizumab significantly improved overall survival (OS) vs chemotherapy in patients with previously untreated programmed death ligand 1 (PD‐L1)‐positive locally advanced/metastatic non–small‐cell lung cancer (NSCLC) without EGFR/ALK alterations. We present results from patients in KEYNOTE‐042 enrolled from China in the global or extension study (NCT03850444; protocol identical to global study). Patients were randomized 1:1 (stratified by ECOG performance status 0 vs 1, squamous vs nonsquamous histology and PD‐L1 tumor proportion score [TPS] ≥50% vs 1%‐49%) to 35 cycles of pembrolizumab 200 mg every 3 weeks (Q3W) or investigator's choice of 4 to 6 cycles of carboplatin plus paclitaxel or pemetrexed Q3W with optional pemetrexed maintenance for nonsquamous tumors. Primary endpoints were OS in patients with PD‐L1 TPS ≥50%, ≥20% or ≥1%. Two hundred sixty‐two patients (pembrolizumab, n = 128; chemotherapy, n = 134) were enrolled from China. At data cutoff (February 21, 2020; median follow‐up, 33.0 [range, 25.6‐41.9] months), pembrolizumab was shown to improve OS vs chemotherapy in patients with PD‐L1 TPS ≥50% (hazard ratio [95% CI], 0.63 [0.43‐0.94]), TPS ≥20% (0.66 [0.47‐0.92]) and TPS ≥1% (0.67 [0.50‐0.89]). Grade 3 to 5 treatment‐related adverse events occurred less frequently with pembrolizumab vs chemotherapy (19.5% vs 68.8%). In 22 patients who completed 35 cycles of pembrolizumab, objective response rate was 77.3% and median duration of response was 27.6 months. Consistent with the global KEYNOTE‐042 study, pembrolizumab improved OS vs chemotherapy in this study of Chinese patients with locally advanced/metastatic NSCLC and PD‐L1 TPS ≥1%, supporting first‐line pembrolizumab monotherapy for PD‐L1‐positive advanced/metastatic NSCLC in China.
Keywords: chemotherapy, non–small‐cell lung cancer, pembrolizumab, programmed death ligand 1
Short abstract
What's new?
The global KEYNOTE‐042 clinical study helped establish single‐agent pembrolizumab, an anti‐PD‐1 monoclonal antibody, as a standard first‐line treatment option in patients with previously untreated PD‐L1‐positive locally advanced/metastatic non–small‐cell lung cancer (NSCLC) without EGFR/ALK aberrations. Because disease characteristics and responses to anticancer therapy may differ between Asian and other populations, there is a need to evaluate these treatments specifically in Asian patients. Here, the authors show that pembrolizumab also improved overall survival and was better tolerated than chemotherapy among Chinese patients with PD‐L1 TPS ≥1% in the KEYNOTE‐042 study. These data support first‐line pembrolizumab monotherapy for PD‐L1‐positive advanced/metastatic NSCLC in China.
Abbreviations
- AE
adverse event
- DOR
duration of response
- ECOG
Eastern Cooperative Oncology Group
- HR
hazard ratio
- NSCLC
non–small‐cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PD‐L1
programmed death ligand 1
- PFS
progression‐free survival
- Q3W
every 3 weeks
- TPS
tumor proportion score
1. INTRODUCTION
Monoclonal antibodies targeting programmed death 1 and programmed death ligand 1 (PD‐[L]1) have demonstrated significant improvements in clinical outcomes relative to standard chemotherapy in patients with advanced non–small‐cell lung cancer (NSCLC). 1 , 2 Because Asian patients are often underrepresented in international randomized clinical trials of anti‐PD‐(L)1 therapies, 3 and disease characteristics (eg, EGFR mutation prevalence) 4 and responses to anticancer therapy may differ between Asian and Caucasian populations, 5 there is a need to evaluate these treatments specifically in Asian patients. At present, CheckMate‐078 is the only Phase 3 study that has evaluated anti‐PD‐1 therapy in a predominantly Chinese patient population with previously treated NSCLC. In this prior study, the PD‐1 inhibitor nivolumab improved overall survival (OS) compared with docetaxel. 6
Here, we present results from a Phase 3 clinical trial evaluating first‐line anti‐PD‐1 therapy in Chinese patients with advanced PD‐L1‐positive NSCLC. As previously reported, the anti‐PD‐1 antibody pembrolizumab significantly prolonged OS compared with platinum‐based chemotherapy in patients with previously untreated metastatic NSCLC without sensitizing EGFR/ALK alterations and PD‐L1 tumor proportion score (TPS) ≥50% in the KEYNOTE‐024 study, 7 , 8 and in patients with TPS ≥1% in the global KEYNOTE‐042 study (data cutoff, February 26, 2018; N = 1274). 9
The current report provides results from the KEYNOTE‐042 China study, which includes patients enrolled from China in the global or China extension study of KEYNOTE‐042 (data cutoff, February 21, 2020). The objective of our study was to evaluate the efficacy and safety of pembrolizumab vs platinum‐based chemotherapy in Chinese patients with PD‐L1‐positive, locally advanced/metastatic NSCLC without targetable EGFR/ALK alterations.
2. MATERIALS AND METHODS
2.1. Study design and patients
The global, prospective, randomized, open‐label, Phase 3 KEYNOTE‐042 study (Clinicaltrials.gov, NCT02220894; MK‐3475‐042) was previously published with the full protocol. 9 The KEYNOTE‐042 China extension study (NCT03850444) is an extension to the KEYNOTE‐042 global study and included only patients enrolled from mainland China after global enrollment was completed.
Eligible patients were randomized 1:1 (stratified by Eastern Cooperative Oncology Group performance score 0 vs 1, squamous vs nonsquamous histology, and PD‐L1 TPS ≥50% vs 1%‐49%) to pembrolizumab 200 mg every 3 weeks (Q3W) for 35 cycles or carboplatin AUC 5 or 6 plus investigator's choice of paclitaxel 200 mg/m2 or pemetrexed 500 mg/m2 Q3W for 4‐6 cycles (optional pemetrexed maintenance encouraged for nonsquamous tumors). Clinically stable patients with disease progression could continue pembrolizumab at the investigator's discretion upon consultation with the sponsor.
2.2. Outcomes
The primary endpoint was OS in the PD‐L1 TPS ≥50%, ≥20% and ≥1% groups. Secondary endpoints were progression‐free survival (PFS) and objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors version 1.1 by blinded independent central review and safety. Duration of response (DOR) and PFS‐2 (time from randomization to objective tumor progression on next‐line treatment, including subsequent anti‐PD‐[L]1 therapy, or death from any cause, whichever occurs first) 10 were exploratory endpoints. PFS‐2 events were characterized as follows: time of investigator‐assessed disease progression resulting in cessation of second‐line therapy; start of third‐line therapy following no disease progression on second‐line therapy; and time of death after either stopping second‐line therapy with no disease progression and no third‐line therapy, or after not receiving second‐line therapy. Patients were censored for PFS‐2 at the time of last known survival if they were alive and either had not received second‐line therapy or had stopped second‐line therapy without disease progression and had not initiated third‐line therapy.
2.3. Statistical analysis
Data for randomized patients from mainland China were analyzed by assigned treatment for efficacy (intention‐to‐treat population [ITT]) and by treatment received for safety. Efficacy endpoints were also analyzed in patients who completed 35 cycles or 2 years of pembrolizumab therapy. To support regulatory approval in China, a protocol amendment allowed for continued enrollment to a total of ~140 patients from China with PD‐L1 TPS ≥50% after the global study stopped enrolling, with analysis planned after ~60 total OS events occurred among Chinese patients with PD‐L1 TPS ≥50%. No multiplicity adjustments were applied and no alpha was allocated, as this extension study, which was intended to assess outcomes in a Chinese population, was not powered for statistical analysis given the smaller sample size. OS, PFS and PFS‐2 were estimated using the Kaplan‐Meier method. Between‐group treatment differences were assessed with the stratified log‐rank test and hazard ratios (HRs) were estimated with a stratified Cox proportional‐hazards model, as previously described. 9
3. RESULTS
3.1. Patients and treatment
Of 745 patients screened at 27 sites in China, 262 eligible patients (global study, n = 92; extension study [ie, after global study enrollment ended], n = 170) were randomized to pembrolizumab (n = 128) or chemotherapy (n = 134) between August 26, 2016, and January 4, 2018 (Figure S1). Baseline characteristics were similar between treatment arms (Table 1). All patients allocated to pembrolizumab and 125 allocated to chemotherapy received planned study treatment (Figure S1). Median (range) follow‐up duration, defined as the time from randomization to the database cutoff date, was 33.0 (25.6‐41.9) months. Median (range) treatment duration was 6.4 (1 day‐25.4) months with pembrolizumab and 3.0 (1 day‐24.9) months with chemotherapy.
TABLE 1.
Patient demographic and disease characteristics at baseline
| Characteristic | Pembrolizumab (n = 128) | Chemotherapy (n = 134) | Completed 35 cycles of pembrolizumab (n = 22) |
|---|---|---|---|
| Age, median (range), years | 62.0 (22‐78) | 62.0 (32‐82) | 61.5 (52‐72) |
| Sex | |||
| Male | 105 (82.0) | 119 (88.8) | 19 (86.4) |
| Female | 23 (18.0) | 15 (11.2) | 3 (13.6) |
| ECOG PS score | |||
| 0 | 31 (24.2) | 29 (21.6) | 7 (31.8) |
| 1 | 97 (75.8) | 105 (78.4) | 15 (68.2) |
| Histology | |||
| Squamous | 71 (55.5) | 76 (56.7) | 12 (54.5) |
| Nonsquamous | 57 (44.5) | 58 (43.3) | 10 (45.5) |
| PD‐L1 TPS | |||
| TPS ≥50% | 72 (56.3) | 74 (55.2) | 18 (81.8) |
| TPS ≥20% | 101 (78.9) | 103 (76.9) | 20 (90.9) |
| TPS ≥1% | 128 (100) | 134 (100) | 22 (100) |
| Smoking status | |||
| Current | 28 (21.9) | 29 (21.6) | 2 (9.1) |
| Former | 69 (53.9) | 77 (57.5) | 17 (77.3) |
| Never | 31 (24.2) | 28 (20.9) | 3 (13.6) |
| Treated brain metastases | 2 (1.6) | 2 (1.5) | 1 (4.5) |
| Prior therapy | |||
| Neoadjuvant | 3 (2.3) | 0 (0) | 0 (0) |
| Adjuvant | 6 (4.7) | 2 (1.5) | 0 (0) |
| Radiotherapy | 6 (4.7) | 4 (3.0) | 2 (9.1) |
Note: Data are No. (%), unless otherwise noted.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; PD‐L1, programmed death ligand 1; TPS, tumor proportion score.
3.2. Efficacy outcomes in the ITT population
OS improved with pembrolizumab vs chemotherapy across all prespecified PD‐L1 TPS cut‐points (HRs: TPS ≥50%, 0.63 [95% CI, 0.43‐0.94]; TPS ≥20%, 0.66 [95% CI, 0.47‐0.92]; TPS ≥1%, 0.67 [95% CI, 0.50‐0.89]; Figure 1A‐C) and in the TPS 1% to 49% group (HR, 0.71 [95% CI, 0.46‐1.09]; exploratory analysis, Figure 1D). HRs for OS also favored pembrolizumab across most patient subgroups (Figure 1E).
FIGURE 1.
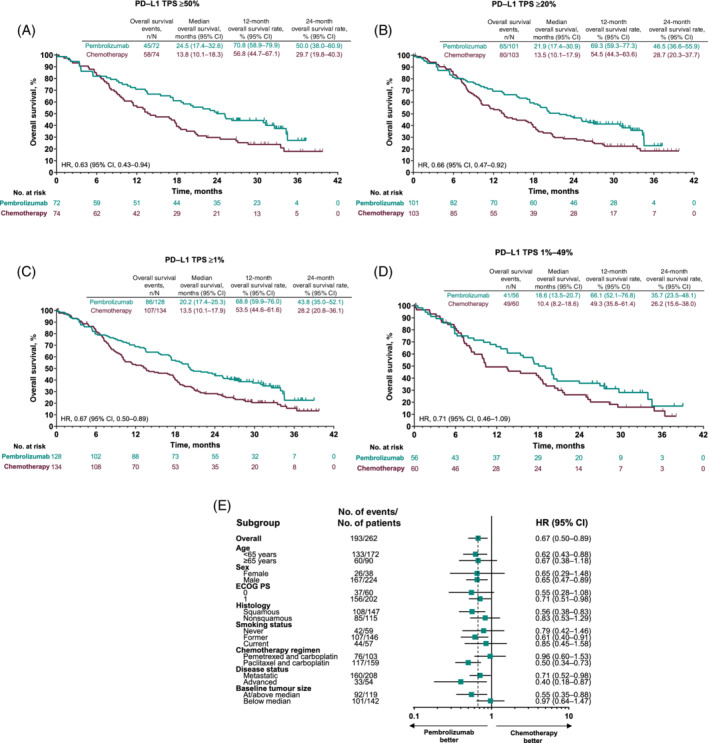
Overall survival (OS) analyses in the intention‐to‐treat population. Kaplan‐Meier estimates of OS among patients with (A) PD‐L1 TPS ≥50%; (B) PD‐L1 TPS ≥20%; and (C) PD‐L1 TPS ≥1%. (D) PD‐L1 TPS 1%‐49%; (E) OS analysis in key subgroups in patients with PD‐L1 TPS ≥1%. Vertical dotted line in subgroup analysis represents HR in the overall population. The intention‐to‐treat population comprised all patients who were randomized to treatment, according to the treatment assigned. OS was defined as time from randomization to death from any cause. ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NR, not reached; PD‐L1, programmed death ligand 1; TPS, tumor proportion score [Color figure can be viewed at wileyonlinelibrary.com]
Pembrolizumab improved PFS vs chemotherapy only in the TPS ≥50% group (HR, 0.84 [95% CI, 0.58‐1.21]; Table S1). PFS was similar to pembrolizumab vs chemotherapy in the PD‐L1 TPS ≥20% (HR, 0.95 [95% CI, 0.69‐1.29]) and PD‐L1 TPS ≥1% groups (HR, 1.00 [95% CI, 0.76‐1.31]; Table S1, Figure S2).
ORR was higher with pembrolizumab vs chemotherapy across all TPS groups (ORRs: PD‐L1 TPS ≥50%, 40.3% vs 24.3%; PD‐L1 TPS ≥20%, 33.7% vs 24.3%; PD‐L1 TPS ≥1%, 31.3% vs 24.6% for pembrolizumab vs chemotherapy, respectively; Table S1). Median DOR was 16.5 months with pembrolizumab and 11.7 months with chemotherapy in the TPS ≥50% group and ~15 to 16 months vs 11 months, respectively, in the TPS ≥20% and ≥1% groups. The time to response with pembrolizumab and chemotherapy was similar across all TPS groups (~2.1‐2.2 months; Table S1).
Median (95% CI) PFS‐2 was 14.3 (10.8‐17.0) months in the pembrolizumab group and 8.9 (7.8‐10.7) months in the chemotherapy group (HR, 0.57; 95% CI, 0.43‐0.75). Pembrolizumab was associated with a PFS‐2 benefit irrespective of PD‐L1 expression (Table S1).
3.3. Safety in the ITT population
Treatment‐related adverse events (AEs) occurred less frequently with pembrolizumab (82.0%) vs chemotherapy (92.0%), including Grade 3 to 5 events (19.5% vs 68.8%; Table 2). As anticipated with immunotherapy, immune‐mediated AEs and infusion reactions, irrespective of treatment attribution by the investigator, occurred more frequently in pembrolizumab‐treated (26.6%) vs chemotherapy‐treated (5.6%) patients; Grade 3 to 5 events occurred in 7.0% vs 3.2%. Eight vs three patients, respectively, had Grade 3 immune‐mediated AEs and infusion reactions. No Grade 4 events occurred in the pembrolizumab arm; one Grade 4 AE (infusion reaction) occurred in the chemotherapy arm. One Grade 5 event (pneumonitis) occurred in the pembrolizumab arm; no Grade 5 AE occurred in the chemotherapy arm (Table 2). Overall, four chemotherapy‐treated patients discontinued treatment at Cycle 1, three of them due to Grade 3 to 4 infusion reactions (Figure S1).
TABLE 2.
Adverse events in the as‐treated population a
| Pembrolizumab (n = 128) | Chemotherapy (n = 125) | |||
|---|---|---|---|---|
| Adverse event, No. (%) | Any grade | Grades 3 to 5 | Any grade | Grades 3 to 5 |
| Treatment‐related adverse events | ||||
| Any | 105 (82.0) | 25 (19.5) | 115 (92.0) | 86 (68.8) |
| Led to death | 7 (5.5) | 7 (5.5) b | 4 (3.2) | 4 (3.2) c |
| Occurring in ≥10% of patients in either group | ||||
| Alanine aminotransferase increased | 22 (17.2) | 2 (1.6) | 27 (21.6) | 1 (0.8) |
| Aspartate aminotransferase increased | 22 (17.2) | 1 (0.8) | 23 (18.4) | 1 (0.8) |
| Rash | 17 (13.3) | 1 (0.8) | 5 (4.0) | 0 (0) |
| Pyrexia | 15 (11.7) | 1 (0.8) | 10 (8.0) | 0 (0) |
| Hypothyroidism | 15 (11.7) | 0 (0) | 0 (0) | 0 (0) |
| Pruritus | 14 (10.9) | 0 (0) | 2 (1.6) | 0 (0) |
| Anemia | 11 (8.6) | 1 (0.8) | 64 (51.2) | 21 (16.8) |
| Fatigue | 8 (6.3) | 0 (0) | 20 (16.0) | 1 (0.8) |
| Decreased appetite | 7 (5.5) | 0 (0) | 36 (28.8) | 3 (2.4) |
| Platelet count decreased | 4 (3.1) | 0 (0) | 28 (22.4) | 7 (5.6) |
| Neutrophil count decreased | 3 (2.3) | 0 (0) | 70 (56.0) | 45 (36.0) |
| Nausea | 2 (1.6) | 0 (0) | 23 (18.4) | 1 (0.8) |
| Constipation | 2 (1.6) | 0 (0) | 17 (13.6) | 0 (0) |
| Leukopenia | 3 (2.3) | 0 (0) | 16 (12.8) | 8 (6.4) |
| White blood cell count decreased | 2 (1.6) | 0 (0) | 64 (51.2) | 22 (17.6) |
| Alopecia | 1 (0.8) | 0 (0) | 32 (25.6) | 1 (0.8) |
| Vomiting | 1 (0.8) | 0 (0) | 16 (12.8) | 1 (0.8) |
| Neutropenia | 0 (0) | 0 (0) | 14 (11.2) | 6 (4.8) |
| Immune‐mediated adverse events and infusion reactions | ||||
| Any | 34 (26.6) | 9 (7.0) | 7 (5.6) | 4 (3.2) |
| Hypothyroidism | 15 (11.7) | 0 (0) | 0 (0) | 0 (0) |
| Pneumonitis | 10 (7.8) | 3 (2.3) | 0 (0) | 0 (0) |
| Hyperthyroidism | 7 (5.5) | 1 (0.8) | 0 (0) | 0 (0) |
| Infusion reactions | 4 (3.1) | 1 (0.8) | 7 (5.6) | 4 (3.2) |
| Hepatitis | 2 (1.6) | 2 (1.6) | 0 (0) | 0 (0) |
| Severe skin reactions | 2 (1.6) | 1 (0.8) | 0 (0) | 0 (0) |
| Thyroiditis | 2 (1.6) | 0 (0) | 0 (0) | 0 (0) |
| Colitis | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) |
| Pancreatitis | 1 (0.8) | 1 (0.8) | 0 (0) | 0 (0) |
Note: Adverse events were graded using NCI CTCAE version 4.0.
The as‐treated population comprised all randomized patients who received at least 1 dose of study treatment, according to the treatment received.
Seven patients in the pembrolizumab arm had Grade 5 treatment‐related AEs (acute left ventricular failure, myocardial infarction, multiple organ dysfunction syndrome, pneumonia, malignant neoplasm progression, interstitial lung disease and pulmonary embolism [n = 1 each].
Four patients in the chemotherapy arm had Grade 5 treatment‐related AEs (septic shock [n = 2], ketoacidosis [n = 1] and pulmonary embolism [n = 1]).
3.4. Patients who completed 35 cycles of pembrolizumab
As of the February 21, 2020 data cutoff date, 22 patients had completed 35 cycles of pembrolizumab therapy. Of these, 1 patient had discontinued therapy due to progressive disease. Baseline disease characteristics were generally similar between the patients who completed 35 cycles of pembrolizumab therapy and the ITT population with the exception of a higher proportion of patients with PD‐L1 TPS ≥50% (82% vs 56%) and former smokers (77% vs 54%), and a lower proportion of current smokers (9% vs 22%) in patients who completed 35 cycles of pembrolizumab therapy; however, some of the patient numbers were very small (Table 1).
As of the data cutoff date, 20 of 22 patients (91%) who completed 35 cycles (~2 years) of pembrolizumab remained alive. ORR among patients who completed 35 cycles (~2 years) of pembrolizumab was 77% and median (range) DOR was 27.6 (6.1‐33.2+) months (+ indicates that response was ongoing at the last disease assessment before the data cutoff).
4. DISCUSSION
These results from the KEYNOTE‐042 China extension study provide the first report of Phase 3 data evaluating anti‐PD‐1 therapy in Chinese patients with previously untreated locally advanced/metastatic NSCLC. Pembrolizumab improved OS over chemotherapy among patients with PD‐L1 TPS ≥50%, ≥20% and ≥1%. The HR for OS more strongly favored pembrolizumab in the KEYNOTE‐042 China study than in the global study population; this may have been driven in part by the moderate proportion of patients from China with squamous disease (56% vs 39% of patients, respectively) and with PD‐L1 TPS ≥50% (56% vs 47%, respectively) and PD‐L1 TPS ≥20% (78% vs 64%). 9 As observed in the global KEYNOTE‐042 study, 9 the HR for PFS favored pembrolizumab over chemotherapy in the TPS ≥50% group. ORR was higher with pembrolizumab vs chemotherapy across all TPS groups evaluated (TPS ≥50%, ≥20% and ≥1), and estimated median DOR was >15 months with pembrolizumab. We additionally found that PFS‐2 was improved with pembrolizumab vs chemotherapy across all TPS groups evaluated. Safety findings in the ITT population of this KEYNOTE‐042 China study were consistent with the known safety profile of pembrolizumab in advanced NSCLC. 7 , 9 Pembrolizumab was better tolerated than chemotherapy, with 3.5‐fold lower incidence of Grade 3 to 5 treatment‐related AEs (19.5% vs 68.8%, respectively). Additionally, durable responses were observed among the patients who completed 35 cycles of pembrolizumab in this study, and 91% remained alive at data cutoff, further demonstrating the benefit of a 2‐year course of first‐line pembrolizumab treatment in Chinese patients with previously untreated locally advanced or metastatic PD‐L1‐positive NSCLC.
Tumor PD‐L1 expression is an established biomarker for patient selection with pembrolizumab monotherapy in patients with advanced NSCLC with PD‐L1 TPS ≥1%. 9 , 11 The OS benefit of pembrolizumab over chemotherapy in Chinese patients with PD‐L1 TPS ≥50% in the current study (HR, 0.63 [95% CI, 0.43‐0.94]) and the global KEYNOTE‐042 study (HR, 0.69; P = .0003) confirms similar findings among patients with previously untreated metastatic NSCLC and PD‐L1 TPS ≥50% in the international phase 3 KEYNOTE‐024 study 7 (HR, 0.60; P = .005). Our results and those of the global study also extend findings from KEYNOTE‐024, showing OS benefit among patients with PD‐L1 TPS ≥1% (HRs: China cohort, 0.67 [95% CI, 0.50‐0.89]; global study, 0.81 [P = .0018]), including among those with PD‐L1 TPS 1%‐49% in our KEYNOTE‐042 China study (HR, 0.71 [95% CI, 0.46‐1.09]). 9 The ORR findings with pembrolizumab monotherapy among patients with PD‐L1 TPS ≥50% were also similar between the current study (ORR, 40.3%), the global KEYNOTE‐042 study (ORR, 39%) 9 and the KEYNOTE‐024 study (ORR, 44.8%). 7 Furthermore, as previously shown in KEYNOTE‐024, 12 we found that PFS‐2 improved with pembrolizumab vs chemotherapy, demonstrating that the benefit of first‐line treatment with pembrolizumab was maintained through the next line of therapy. Overall, our findings support the preferential use of pembrolizumab in the first‐line setting among Chinese patients with previously untreated locally advanced or metastatic PD‐L1‐positive NSCLC.
This study had certain limitations. The KEYNOTE‐042 China study included fewer patients than the global study and was not powered for formal statistical analysis. Nonetheless, estimated HRs in this study are similar to those in the global study, suggesting that there is also substantial OS benefit with pembrolizumab vs chemotherapy as first‐line treatment in Chinese patients with PD‐L1‐positive (TPS ≥1%) advanced NSCLC.
In conclusion, consistent with the global KEYNOTE‐042 study, pembrolizumab improved OS and was better tolerated than standard chemotherapy in this longer‐term follow‐up of approximately 3 years in Chinese patients with locally advanced or metastatic NSCLC without EGFR mutations or ALK alterations, across each PD‐L1 TPS group evaluated (TPS ≥50%, ≥20%, and ≥1%). We additionally found that patients who completed 2 years of pembrolizumab treatment had durable responses. Together with outcomes in the global study, these results support first‐line use of pembrolizumab monotherapy for PD‐L1‐positive advanced NSCLC in patients from China, and the recent approval of pembrolizumab monotherapy in China by the National Medical Products Administration (NMPA) for the first‐line treatment of patients with locally advanced or metastatic NSCLC with TPS ≥1% and no EGFR or ALK genomic tumor aberrations.
CONFLICT OF INTEREST
Y‐L Wu has received speaker fees from AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, Hengrui, MSD, Roche, Pfizer and Sanofi; and research funding to the institution from AstraZeneca, BMS and Pfizer. L Zhang (Guangzhou) has received research grants from Hengrui, BMS and Innovent Biologics. Q Zhou has received honoraria from AstraZeneca and Roche. CC Zhou has received honoraria from Boehringer Ingelheim, Eli Lilly, Hengrui, MSD, Sanofi, F. Hoffmann‐La Roche Ltd. and Qilu. T Dang and S Sadowski are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. DA Kush is an employee of and stockholder in Merck & Co., Inc., Kenilworth, NJ, USA. Y Zhou and B Li are employees of MSD China. T Mok has received grants or research support from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Clovis Oncology, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Novartis, Pfizer, Roche/Genentech, SFJ Pharmaceuticals, Taiho and XCovery; honoraria and/or speakers' fees from Amoy Diagnostics Co. Ltd., AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, InMed Medical Communication, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Merck Serono, Novartis, Pfizer, PRIME Oncology, Roche/Genentech, SFJ Pharmaceuticals, Taiho, Virtus Medical Group, and Takeda Oncology; is a stockholder in Biolidics Ltd., Hutchison ChiMed, Loxo‐Oncology, OrigiMed. and Sanomics Ltd.; and has served as an advisory board member and/or consultant for ACEA Biosciences Inc., Alpha Biopharma Co., Ltd., AstraZeneca, Bayer, Daiichi Sankyo, Incyte Corporation, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Cirina, CStone Pharmaceuticals, Eli Lilly, Fishawack Facilitate Ltd., geneDecode Co. Ltd. Hengrui Therapeutics, Ignyta Inc., IQVIA, Janssen, Loxo‐Oncology, Merck Serono, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, MoreHealth, Novartis, OncoGenex Technologies Inc., OrigiMed, Pfizer, Roche/Genentech, Sanofi‐Aventis, SFJ Pharmaceuticals, Takeda, Vertex Pharmaceuticals, Virtus Medical Group, Biolidics Ltd. and Yuhan Corp; and has leadership positions with ASCO, CSCO and IASLC.
L Zhang (Beijing), Y Fan, JY Zhou, W Li, CP Hu, GY Chen and X Zhang have nothing to disclose.
ETHICS STATEMENT
The KEYNOTE‐042 global study is registered at Clinicaltrials.gov, NCT02220894 (https://clinicaltrials.gov/ct2/show/NCT02220894), and the KEYNOTE‐042 China Extension study is registered at Clinicaltrials.gov, NCT03850444 (https://clinicaltrials.gov/ct2/show/NCT03850444). The study protocol was approved by institutional review boards/independent ethics committees at each site. All patients provided written informed consent.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGEMENTS
Medical writing assistance was provided by Sheri Arndt, PharmD, of ICON plc (North Wales, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Fabricio Souza, MD, and Jianxin Lin, MS, of Merck & Co., Inc., Kenilworth, NJ, USA, and JingWen Wang of MSD China, Shanghai, China, provided additional support for the analysis and interpretation of the data.
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
The funder of the study participated in study design, data collection, data analysis, data interpretation and writing of the report. The corresponding author had access to all the data in the study and had final responsibility for the decision to submit for publication.
Wu Y‐L, Zhang L, Fan Y, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD‐L1‐positive locally advanced or metastatic non–small‐cell lung cancer: KEYNOTE‐042 China Study. Int. J. Cancer. 2021;148:2313–2320. 10.1002/ijc.33399
Yi‐Long Wu and Li Zhang are co‐primary authors.
DATA AVAILABILITY STATEMENT
Data will be available according to Merck Sharp & Dohme's data sharing policy, which, including restrictions, is available at http://engagezone.merck.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
REFERENCES
- 1. Raju S, Joseph R, Sehgal S. Review of checkpoint immunotherapy for the management of non‐small cell lung cancer. Immunotargets Ther. 2018;7:63‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Visconti R, Morra F, Guggino G, Celetti A. The between now and then of lung cancer chemotherapy and immunotherapy. Int J Mol Sci. 2017;18:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peng L, Wu YL. Immunotherapy in the Asiatic population: any differences from Caucasian population? J Thorac Dis. 2018;10:S1482‐S1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257‐262. [DOI] [PubMed] [Google Scholar]
- 5. Soo RA, Loh M, Mok TS, et al. Ethnic differences in survival outcome in patients with advanced stage non‐small cell lung cancer: results of a meta‐analysis of randomized controlled trials. J Thorac Oncol. 2011;6:1030‐1038. [DOI] [PubMed] [Google Scholar]
- 6. Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14:867‐875. [DOI] [PubMed] [Google Scholar]
- 7. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non–small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 8. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE‐024: pembrolizumab versus platinum‐based chemotherapy for advanced non‐small‐cell lung cancer with PD‐L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537‐546. [DOI] [PubMed] [Google Scholar]
- 9. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393:1819‐1830. [DOI] [PubMed] [Google Scholar]
- 10. European Medicines Agency . Guideline on the evaluation of anticancer medicinal products in man. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/11/WC500238764.pdf. Accessed August 26, 2020.
- 11. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small‐cell lung cancer. N Engl J Med. 2015;372:2018‐2028. [DOI] [PubMed] [Google Scholar]
- 12. Brahmer JR, Rodriguez‐Abreu D, Robinson AG, et al. Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PD‐L1 tumor proportion score (TPS) ≥50% enrolled in KEYNOTE‐024. J Clin Oncol. 2017;35(15 suppl):9000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
Data will be available according to Merck Sharp & Dohme's data sharing policy, which, including restrictions, is available at http://engagezone.merck.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.


