Abstract
We examined the geospatial dimension of delays to diagnosis of breast cancer in a prospective study of 1541 women newly diagnosed in the African Breast Cancer—Disparities in Outcomes (ABC‐DO) Study. Women were recruited at cancer treatment facilities in Namibia, Nigeria, Uganda and Zambia. The baseline interview included information used to generate the geospatial features: urban/rural residence, travel mode to treatment facility and straight‐line distances from home to first‐care provider and to diagnostic/treatment facility, categorized into country/ethnicity (population)‐specific quartiles. These factors were investigated in relation to delay in diagnosis (≥3 months since first symptom) and late stage at diagnosis (TNM: III, IV) using logistic regression, adjusted for population group and sociodemographic characteristics. The median (interquartile range) distances to first provider and diagnostic and treatment facilities were 5 (1‐37), 17 (3‐105) and 62 (5‐289) km, respectively. The majority had a delay in diagnosis (74%) and diagnosis at late stage (64%). Distance to first provider was not associated with delay in diagnosis or late stage at diagnosis. Rural residence was associated with delay, but the association did not persist after adjustment for sociodemographic characteristics. Distance to the diagnostic/treatment facility was associated with delay (highest vs lowest quartile: odds ratio (OR) = 1.56, 95% confidence interval (CI) = 1.08‐2.27) and late stage (overall: OR = 1.47, CI = 1.05‐2.06; without Nigerian hospitals where mostly local residents were treated: OR = 1.73, CI = 1.18‐2.54). These findings underscore the need for measures addressing the geospatial barriers to early diagnosis in sub‐Saharan African settings, including providing transport or travel allowance and decentralizing diagnostic services.
Keywords: breast cancer, cohort study, early diagnosis, geospatial barrier, sub‐Saharan Africa
Short abstract
What's new?
Survival from breast cancer is poor in sub‐Saharan Africa, due largely to the high proportion of women who are diagnosed at advanced stages. In this study, the authors examined geospatial information to assess women's prediagnostic journey to breast cancer diagnosis, with special attention to delays in diagnosis, in the regions of Namibia, Nigeria, Uganda, and Zambia. Geospatial factors particularly long travel distances to diagnostic and treatment facilities were identified as major barriers to early diagnosis. The findings underscore the need for policies to address these barriers to ensure breast cancer diagnosis at a curable stage.
Abbreviations
- ABC‐DO
African Breast Cancer—Disparities in Outcomes
- BHGI
Breast Health Global Initiative
- CI
confidence interval
- ISCP
International Society of Clinical Pathologists
- OR
odds ratio
- SEP
socioeconomic position
- SSA
sub‐Saharan Africa
1. INTRODUCTION
Breast cancer is the second most cause of cancer death in women in sub‐Saharan Africa (SSA) 1 and is expected to increase by more than 100% by 2040. 2 Although breast cancer has excellent prognosis in high‐income settings owing to significant improvements in early diagnosis and treatment, survival from this disease in SSA remains poor. 3 , 4 Explicitly, 5‐year survival is >85% in high‐resource settings including North America, Sweden, France, and Australia vs about 59% in SSA settings. 3 , 5 To reduce this gap and curtail the predicted increase in breast cancer deaths in SSA, efforts are needed to improve access to quality breast cancer care including diagnostics and treatment.
The poorer survival in SSA is largely attributable to advanced disease at presentation, 3 , 4 which has been found to be associated with both patient‐level barriers (eg, low level of education, a lack of breast cancer knowledge, poor health‐seeking behaviors) and system‐level barriers (eg, referral pathways). 6 , 7 , 8 , 9 , 10 , 11 Compounding educational, cultural, socioeconomic and health systems' barriers to reaching cancer diagnostic and treatment services is the geospatial dimension of healthcare access in SSA settings. Notably, many African countries have only a few, or even a single, cancer treatment facility, which, coupled with their immense size (ie, 23 of Africa's 54 countries are larger than 500 000 m2, the area of France), means that patients need to travel large distances to reach cancer diagnostic and treatment facilities, incurring financial and logistic challenges.
A systematic review of 27 studies (of which only one is from SSA, 12 namely from South Africa) on distance as a barrier to cancer diagnosis and treatment concluded that patients who live far from hospitals and need to travel more than 50 miles/80 km (1 hour driving) or more are more likely to have an advanced disease at diagnosis, inappropriate treatment, a worse prognosis and poorer quality of life compared to those living closer to hospitals. 13 However, data on geospatial barriers to cancer diagnosis and treatment are scarce in regions of SSA other than South Africa although a similar phenomenon has been observed between large physical distance to healthcare services and underutilization of obstetrics care 14 as well as unmet surgical needs. 15
Given the utility of geospatial information in identifying gaps in healthcare access to inform public health planning including the development of cancer control measures, comprehensive geospatial studies on cancer care and outcomes are needed in SSA. We, therefore, aimed to characterize the geospatial dimensions of a woman's pathway to breast cancer diagnosis and examine their associations with time from symptom recognition to diagnosis and disease stage at diagnosis within the wide‐ranging multicountry prospective African Breast Cancer—Disparities in Outcomes (ABC‐DO) study.
2. METHODS
The ABC‐DO study was approved by the ethics committees of all involved institutions: International Agency for Research on Cancer (IEC 13‐19, IEC15‐18), London School of Hygiene and Tropical Medicine (6459), Federal Medical Centre Owerri, Abia State University Teaching Hospital, University of Zambia Biomedical Research Ethics Committee (004‐08‐15), Uganda National Council for Science and Technology (HS 1588) and the Ministry of Health and Social Services of Namibia (17/3/3).
2.1. Study population
The ABC‐DO study protocol has been described in detail elsewhere. 16 Briefly, ABC‐DO is a prospective study of outcomes after breast cancer diagnosis among patients consecutively recruited in five SSA countries. One country, South Africa, was not included in the present analysis due to a different data collection system, and the geospatial influence on stage at diagnosis has already been investigated at that site. 12 The following recruitment centers are included: the Windhoek Central Hospital in Namibia; the Cancer Diseases Hospital and University Teaching Hospital in Lusaka, and the Kabwe General Hospital in Kabwe, Zambia; the Mulago Hospital and the Uganda Cancer Institute in Kampala, Uganda; and the Abia State University Teaching Hospital and the Maranatha private clinic, both in Aba, and the Federal Medical Centre in Owerri, Nigeria. The participating hospitals vary in terms of their catchment populations (ie, national referral hospitals in Namibia, Uganda and Zambia; state‐wide for the Nigerian hospitals; regional for the Kabwe General Hospital, Zambia).
In all countries, women aged ≥18 years who were newly admitted with histologically confirmed or suspected breast cancer at the participating hospitals were invited to participate in the study. Recruitment was conducted from September‐December 2014 to April 2017 in Namibia, Uganda and Nigeria and from May 2016 to September 2017 in Zambia. Women were included irrespective of current address (including foreign residents to capture the reality in this setting) and irrespective of ability to pay for or intention to undergo further treatment. Out of 1637 potentially eligible women, 10 refused to participate, 2 were too ill, 2 passed away prior to being enrolled and 82 (mostly in Nigeria) had nonmalignant disease, leaving 1541 eligible consenting women with histopathological/cytological (90%) or clinical diagnosis of breast cancer. All 1541 women provided a written informed consent or, if illiterate, a fingerprint. Five distinct population groups were defined for the present analysis according to country of recruitment and, for Namibia only, ethnicity: Namibian‐black, Namibian‐non‐black, Nigerian, Ugandan and Zambian women.
2.2. Data collection
At the time of recruitment, each woman completed an interview with a study research assistant. Women were asked about demographics, socioeconomic status (ie, possession of nine specific items, such as home ownership, indoor water, flush toilet, electricity, vehicle, used to create a socioeconomic position [SEP] score) and awareness about breast cancer (five questions used to create a breast cancer awareness score as the total number of correct answers). Women were also asked about their prediagnostic journey (ie, from first symptom recognition through to diagnosis) including date of first symptom recognition, location of residential home, urbanization of the area of residence, location of each healthcare provider visited and type of providers she visited prior to reaching the recruitment hospital (ie, formal: private doctor, private hospital, community clinic, public hospital; informal: traditional healer, spiritual healer, home or community care provider, pharmacists, other), outcome of each visit (eg, being referred to another provider/facility, being reassured and told not to worry, being told she has breast cancer), transport and time taken to travel from home to the recruitment hospital, and self‐perceived barriers to reaching the hospital (eg, transport, distance, fear of dying).
Information on TNM breast cancer stage at diagnosis was extracted from clinical records, using a standardized study proforma. The date of diagnosis was defined according to the European Network of Cancer Registries guidelines, 17 where date of biopsy/cytology is prioritized over date of hospital presentation.
2.3. Data analysis
The levels of detail of free‐text home and provider addresses varied from woman to woman, with some including street‐level information and others including only names of cities, regions, states or districts. We geocoded these addresses, as well as addresses of diagnostic and treatment locations, by searching for the locations in the National Geospatial‐Intelligence Agency GEOnet Names Server database and assigning the corresponding geocoded longitude and latitude http://geonames.nga.mil/gns/html/index.html. Where needed, search engines, such as Google Maps, were used to aid geocoding. We presented the residential homes and the recruitment hospitals on a world map (World Health Organization) using QGIS 3.4.14 (QGIS Development Team, 2018). We then calculated straight‐line distances in kilometers from the residential home to the first healthcare provider visited and from home to the diagnostic and treatment facilities (“geodist” command in Stata 14, TX). In Namibia, the vast majority of diagnoses were conducted via a network of pathological laboratories, allowing tumor specimens to be obtained locally and couriered for histological review by the Namibian Institute of Pathology in Windhoek. Only after histological confirmation did the woman travel to the treatment facility (where she was recruited and treated). Thus, for Namibian women, the nearest pathological laboratory was geocoded and used to calculate distance to diagnosis, unless they went directly to the treatment facility. For the other settings, the locations of the laboratories where diagnostic analytics were performed were used as the location of diagnostic facility unless the location was in the proximity of recruitment hospital. Distances were categorized into population‐specific quartiles. Predefined distance cut‐off points were also used for population‐specific analyses.
Distance to the diagnostic facility was used for all delay analyses and distance to the treatment facility, where staging was performed, was used for all stage analyses. Travel time was not assessed in these analyses because of a very low correlation between travel time and distance, which likely resulted from women reporting their travel time on the day of the visit rather than the travel time for the whole journey, which can be more than 1 day for some women. Logistic regression models were used to examine associations between each one of the geospatial characteristics and each one of the two outcomes: delay in diagnosis (a prediagnostic journey of 3 months or more, calculated as date of diagnosis minus date of first symptom recognition) and late stage (Stages III‐IV vs Stages 0‐II). For distances, a test of linear trend was performed by fitting the continuous variables. Three sets of odds ratios (ORs), and their corresponding 95% confidence intervals (CIs), were estimated. First, each geospatial variable was examined separately adjusting for population group only—this variable was taken as an a priori confounder in all models except for the population‐specific analyses. Second, in addition to adjustment for population group, geospatial variables were mutually adjusted. Third, distance variable and rural residence variable were included in the same model simultaneously while adjusting for population group and other potential confounders, which were identified based on the associations observed in relation to distance to treatment facility. Patient type was not included as a confounder because it is highly dependent on population group. Outcome of first visit was also not included as a confounder because the group of women who went directly to the treatment facility were more likely to be living nearer to the facility and overadjustment was concerned. Self‐reported barriers contributing to delays were not included because they were considered as intermediate or subsequent to the outcome of interest.
Finally, we explored heterogeneity of the effects of distance with diagnostic delay and with stage by population group, degree of urbanization (urban vs rural) and SEP. For Namibian women, we also compared the odds of delay in diagnosis and late stage at diagnosis according to residential location in Windhoek, or outside Windhoek and within, or beyond, 50 km from the nearest pathological laboratory.
3. RESULTS
3.1. Cohort description
Out of 1541 women recruited to the study, 23 women (1.5%) were missing information on residential address, leaving 1518 women in the analytical sample. Of these, 33% were recruited from Windhoek, Namibia, 27% from Kampala, Uganda, 13% from Kabwe and Lusaka, Zambia, and 26% from Aba and Owerri, Nigeria. Nearly 4% traveled from other neighboring countries. The mean age at breast cancer diagnosis was 50 years (SD: 13, range: 19‐97 years), being the lowest in Nigerian and Ugandan women (48 years) and the highest in Namibian non‐black women (57 years) (Table 1). The overall mean SEP score was 4.4 (out of a possible maximum of 9), being the highest in Namibian non‐black women (7.8) and the lowest in Ugandan women (2.6). The proportion who attended a technical school or university was the highest for Namibian non‐black (40%) and Nigerian (35%) women (Table 1).
TABLE 1.
Characteristics of ABC‐DO women with a geocoded residential address and their prediagnostic journey to breast cancer diagnosis
| Population | Total | Namibia black | Namibia non‐black | Nigeria | Uganda | Zambia |
|---|---|---|---|---|---|---|
| Size of the catchment area (km2) N = national; S = state | — | 825 419 (N) | 825 419 (N) | 5530 (Imo S) 6320 (Aba S) | 241 037 (N) | 752 618 (N) 1547 (Kabwe) |
| No. of ABC‐DO women (%) | 1518 (100) | 397 (26) | 104 (7) | 398 (26) | 416 (27) | 203 (13) |
| Sociodemographic characteristics | ||||||
| Age at breast cancer diagnosis, mean (SD) | 50.3 (13.7) | 52.9 (15.1) | 57.3 (12.5) | 48.6 (12.2) | 48.1 (12.7) | 49.6 (14.7) |
| Educational level | ||||||
| Primary school or less | 681 (45) | 209 (53) | 13 (13) | 110 (28) | 242 (58) | 107 (53) |
| Secondary/high school | 509 (34) | 136 (34) | 49 (47) | 147 (37) | 123 (30) | 54 (27) |
| Technical/university | 328 (22) | 52 (13) | 42 (40) | 141 (35) | 51 (12) | 42 (21) |
| Socioeconomic position score, mean (SD) a | 4.4 (2.3) | 5.0 (2.6) | 7.8 (1.2) | 4.9 (1.5) | 2.6 (1.3) | 4.0 (2.1) |
| Patient type | ||||||
| Public | 935 (62) | 286 (72) | 41 (39) | 2 (1) | 403 (97) | 203 (100) |
| Private without insurance | 420 (28) | 32 (8) | 0 (0) | 375 (94) | 13 (3) | 0 (0) |
| Private with insurance | 163 (11) | 79 (20) | 63 (61) | 21 (5) | 0 (0) | 0 (0) |
| Breast cancer knowledge score, mean (SD) b | 3.5 (0.8) | 3.5 (0.9) | 4.1 (0.8) | 3.5 (0.8) | 3.4 (0.8) | 3.3 (0.7) |
| Geospatial and other characteristics of prediagnostic journey | ||||||
| Urbanization of area of residence | ||||||
| Urban (town or city) | 814 (54) | 224 (56) | 93 (89) | 254 (64) | 111 (27) | 132 (65) |
| Rural (rural or village) | 704 (46) | 173 (44) | 11 (11) | 144 (36) | 305 (73) | 71 (35) |
| Type of first HCP | ||||||
| Formal | 1429 (94) | 394 (99) | 103 (99) | 369 (93) | 361 (87) | 202 (100) |
| Informal | 89 (6) | 3 (1) | 1 (1) | 29 (7) | 55 (13) | 1 (0) |
| Outcome of visit to the first HCP c | ||||||
| Breast cancer not suspected/tests done but no results | 493 (32) | 122 (31) | 17 (16) | 81 (20) | 211 (51) | 62 (31) |
| Breast cancer suspected/referral | 741 (49) | 273 (69) | 87 (84) | 102 (26) | 144 (35) | 135 (67) |
| Went directly to the treatment facility (ie, place of recruitment) | 284 (19) | 2 (1) | 0 (0) | 215 (54) | 61 (15) | 6 (3) |
| No. of HCP contacts before reaching the recruitment hospital, mean (SD) | 2.0 (1.8) | 2.8 (1.4) | 2.6 (1.2) | 0.6 (0.7) | 2.9 (2.2) | 1.5 (0.8) |
| Means of transport to the first HCP | ||||||
| Car | 302 (20) | 144 (36) | 78 (75) | 32 (8) | 10 (2) | 38 (19) |
| Public transport | 861 (57) | 132 (33) | 6 (6) | 358 (90) | 272 (65) | 93 (46) |
| Walk | 292 (19) | 114 (29) | 18 (17) | 8 (2) | 87 (21) | 65 (32) |
| Other, missing | 63 (4) | 7 (2) | 2 (2) | 0 (0) | 47 (11) | 7 (3) |
| Means of transport to the treatment facility | ||||||
| Car | 240 (16) | 85 (21) | 75 (72) | 35 (9) | 12 (3) | 33 (16) |
| Public transport, foot | 920 (61) | 82 (21) | 12 (12) | 363 (91) | 303 (73) | 160 (79) |
| Transport provided by hospital | 232 (15) | 213 (54) | 15 (14) | 0 (0) | 1 (0) | 3 (1) |
| Other, missing | 126 (8) | 17 (4) | 2 (2) | 0 (0) | 100 (24) | 7 (3) |
| Distance in kilometer (median, IQR) from home to: | ||||||
| First HCP | 5 (1, 37) | 1 (1, 44) | 1 (1, 1) | 7 (1, 29) | 14 (4, 62) | 4 (1, 62) |
| Diagnostic facility d | 17 (3, 105) | 5 (1, 62) | 1 (1, 12) | 8.5 (2, 30) | 76 (15, 191) | 110 (5, 374) |
| Treatment facility | 62 (5, 289) | 457 (195, 583) | 238 (1, 292) | 6 (2, 28) | 80.5 (15, 196) | 156 (8, 374) |
| Travel time in hours (median, IQR) on the day of visit to: | ||||||
| First HCP | 0.7 (0.3, 1.5) | 0.5 (0.3, 1.1) | 0.2 (0.2, 0.3) | 0.8 (0.5, 1.3) | 1.0 (0.5, 2) | 0.6 (0.3, 1.0) |
| Treatment facility e | 1.0 (0.7, 4.0) | 5.1 (0.5, 10.6) | 2.5 (0.3, 5.3) | 1.0 (0.5, 1.0) | 1.5 (1.0, 3.0) | 1.0 (0.8, 1.8) |
| Self‐perceived barriers to diagnostic delay (yes) f | ||||||
| Transport | 192 (13) | 31 (8) | 0 (0) | 20 (5) | 98 (24) | 43 (21) |
| Hospital too far | 125 (8) | 6 (2) | 1 (1) | 40 (10) | 55 (13) | 23 (11) |
| Other obligations/no permission from family member | 33 (2) | 2 (1) | 0 (0) | 18 (5) | 9 (2) | 4 (2) |
| Embarrassment | 34 (2) | 1 (0) | 0 (0) | 21 (5) | 2 (0) | 10 (5) |
| Pain or discomfort | 89 (6) | 0 (0) | 0 (0) | 26 (7) | 42 (10) | 21 (10) |
| Fear of dying/treatment | 89 (6) | 2 (1) | 0 (0) | 40 (10) | 8 (2) | 39 (19) |
| No trust in medicine/prefer traditional healer | 56 (4) | 3 (1) | 1 (1) | 36 (9) | 12 (3) | 4 (2) |
| Difficulty with making an appointment or reaching a doctor | 78 (5) | 14 (4) | 1 (1) | 47 (12) | 10 (2) | 6 (3) |
| Cost of diagnostic tests/treatment | 108 (7) | 2 (1) | 0 (0) | 36 (9) | 68 (16) | 2 (1) |
| Any barrier | 419 (28) | 61 (15) | 7 (7) | 138 (35) | 134 (32) | 79 (39) |
| Early diagnosis of breast cancer outcomes to be examined | ||||||
| Months between first symptom recognition and diagnosis, median (IQR) f | 7 (2.8, 16) | 6.5 (2.4, 15.2) | 2.0 (0.5, 5.5) | 5.5 (2.2, 12) | 11 (5.6, 20.7) | 7.8 (2.9, 16.4) |
| Categories | ||||||
| <3 months | 373 (26) | 106 (28) | 60 (61) | 113 (31) | 45 (12) | 49 (25) |
| 3 months or more | 1044 (74) | 267 (72) | 38 (39) | 255 (69) | 340 (88) | 144 (75) |
| Missing | 101 | 24 | 6 | 30 | 31 | 10 |
| Stage at breast cancer diagnosis | ||||||
| 0‐II | 513 (36) | 142 (36) | 78 (75) | 90 (24) | 136 (36) | 67 (41) |
| III or IV | 902 (64) | 255 (64) | 26 (25) | 279 (76) | 247 (64) | 95 (59) |
| Missing | 103 | 0 | 0 | 29 | 33 | 41 |
Abbreviations: ABC‐DO, the African Breast Cancer—Disparities in Outcomes; HCP, healthcare provider; IQR, interquartile range; km, kilometer; N, national; No, number; S, state.
Socioeconomic position score was constructed based on the total number of specific items possessed (eg, home ownership, indoor water, flush toilet, electricity, vehicle).
Breast cancer knowledge score was constructed based on the total number of correct answers given to five questions about breast cancer.
“Breast cancer not suspected/tests done but no results” includes the women who reported having been told not to worry or that they had something else, or undergone tests but never received the results. “Breast cancer suspected/referral” includes those who reported having been told that they had breast cancer or been referred to a provider/facility outside the recruitment hospitals. “Went directly to the treatment facility” are those who went directly to one of the recruitment hospitals.
Distance to the nearest laboratory within the network of the National Institute of Pathology that provides diagnostic services was used for Namibian women who did not go directly to the Windhoek Central Hospital. Distance to the Windhoek Central Hospital was used if they went directly to the hospital.
The question asked about the travel time on the day of the visit, which might not capture the entire journey from home to the treatment facility.
If time since first symptom recognition to diagnosis was 5 years or longer, the value was set to missing as it was assumed that the self‐reported symptom(s) were related to a previous condition.
3.2. Descriptives of prediagnostic journey
The majority of the women reported living in a town or city (urban) except in Uganda where 73% reported living in a village or rural area (rural) (Table 1). The median straight‐line distance to first healthcare provider was 5 km. At the first visit to a healthcare provider, 49% of the women were referred to another provider/facility (31%) or told that they had breast cancer (17%), whereas 32% were not suspected of having breast cancer (28%) or underwent a test but never received the results (5%). In Uganda and Nigeria, more women (13% and 7%, respectively) visited an informal provider as their first point of contact during the prediagnostic journey than in other populations (<1%). The median straight‐line distance to the treatment facility was 62 km and was closest in Nigeria (6 km) and furthest in Namibian black women (457 km). To reach the hospitals, women traveled by public transport/foot (61%), car (16%) or other means. Maps of residential homes and country‐specific distribution of the straight‐line distance from home to the recruitment hospital are displayed in Figure 1.
FIGURE 1.
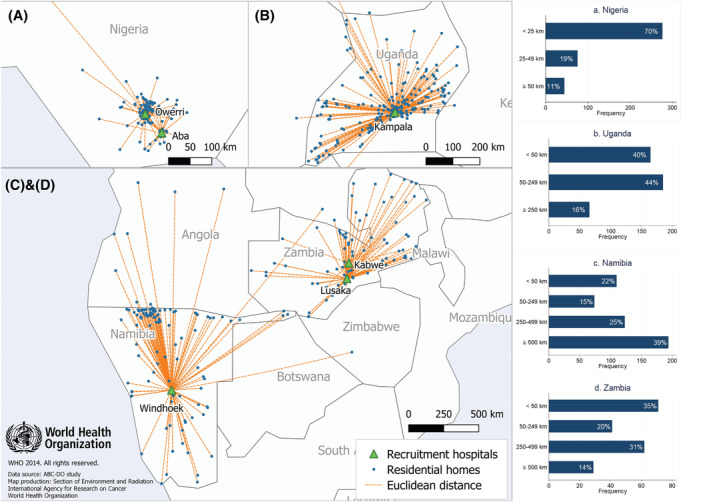
Residential locations of participants and hospitals where they were recruited and received cancer care. Each blue dot represents a location where at least one participant resided. The bar charts show the distribution of straight‐line distance (Euclidean distance) to the recruitment hospitals. The Euclidean distance from home to recruitment hospital was categorized as follows: A, <25, 25‐49, ≥50 km for Nigeria, B, <50, 50‐249, ≥250 km for Uganda, and C,D, <50, 50‐249, 250‐499, ≥500 km for Namibia and Zambia. Disclaimer: The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries
Overall, 28% of the women reported experiencing some kind of barrier, which they considered to have contributed to delay in reaching the hospital. The distributions of perceived barriers varied across the populations (Table 1). For example, the proportion of women reporting distance to the hospital as a barrier was higher in Uganda (13%), Zambia (11%) and Nigeria (10%) than in Namibia (black: 2%, non‐black: 1%) despite the longer distances observed in Namibia. A transport‐related barrier was more commonly reported in Uganda (24%) and Zambia (21%) than in the other countries. About 12% of Nigerian women reported difficulty with making doctor's appointments, whereas only 2% to 4% did in the other populations.
Table 2 shows that population‐specific quartiles of distance to treatment facility were associated with certain sociodemographic characteristics (ie, age at breast cancer diagnosis, educational level, SEP [country‐specific tertiles], patient type), outcome of first visit and, as expected, with other geospatial variables (ie, degree of urbanization of area of residence, distance to first healthcare provider, modes of transport used to reach the first healthcare provider and the treatment facility, and travel time to first healthcare provider and the treatment facility). Breast cancer knowledge score, type of first provider and the number of provider contacts did not differ significantly by distance. Of the self‐reported barriers, transport, distance and difficulty with making appointment/getting a hold of a health professional were more commonly reported by women living farther away from the hospital.
TABLE 2.
Characteristics of women by population‐specific quartile of distance to the cancer treatment facility (recruitment hospital)
| Population‐specific quartile of distance to the cancer treatment facility a | Quartile 1 (shortest) | Quartile 2 | Quartile 3 | Quartile 4 (longest) | |
|---|---|---|---|---|---|
| Total N = 1518 | N (%) | N (%) | N (%) | N (%) | P value b |
| Sociodemographic factors | |||||
| Age at breast cancer diagnosis, mean (SD) | 48.4 (12.6) | 51.0 (14.1) | 51.6 (14.7) | 50.5 (13.4) | .006 |
| Ethnicity | |||||
| Non‐black | 39 (38) | 15 (14) | 25 (24) | 25 (24) | |
| Black | 385 (27) | 324 (23) | 361 (26) | 344 (24) | .08 |
| Educational level | |||||
| Primary school or less | 146 (21) | 166 (24) | 194 (28) | 175 (26) | |
| Secondary/high school | 164 (32) | 105 (21) | 128 (25) | 112 (22) | |
| Technical/university | 114 (35) | 68 (21) | 64 (20) | 82 (25) | <.001 |
| Country‐specific tertiles of socioeconomic position score c | |||||
| Tertile 1 (lowest) | 120 (18) | 143 (22) | 230 (35) | 170 (26) | |
| Tertile 2 | 166 (32) | 128 (25) | 101 (20) | 116 (23) | |
| Tertile 3 (highest) | 138 (40) | 68 (20) | 55 (16) | 83 (24) | <.001 |
| Patient type | |||||
| Public | 244 (26) | 215 (23) | 258 (28) | 218 (23) | |
| Private without insurance | 112 (27) | 78 (19) | 101 (24) | 129 (31) | |
| Private with insurance | 68 (42) | 46 (28) | 27 (17) | 22 (13) | <.001 |
| Breast cancer knowledge score, mean (SD) | 3.5 (0.9) | 3.5 (0.8) | 3.5 (0.8) | 3.4 (0.8) | .32 |
| Geospatial and other characteristics of prediagnostic journey | |||||
| Urbanization of area of residence | |||||
| Urban (town or city) | 347 (43) | 193 (24) | 117 (14) | 157 (19) | |
| Rural (rural or village) | 77 (11) | 146 (21) | 269 (38) | 212 (30) | <.001 |
| Type of first HCP | |||||
| Formal | 404 (28) | 318 (22) | 362 (25) | 345 (24) | |
| Informal | 20 (22) | 21 (24) | 24 (27) | 24 (27) | .70 |
| Outcome of visit to the first HCP | |||||
| Breast cancer not suspected/tests done but no results | 118 (24) | 117 (24) | 124 (25) | 134 (27) | |
| Breast cancer suspected/referral | 210 (28) | 150 (20) | 197 (27) | 184 (25) | |
| Went directly to the treatment facility (ie, place of recruitment) | 96 (34) | 72 (25) | 65 (23) | 51 (18) | .009 |
| No. of HCP contacts before reaching the recruitment hospital, mean (SD) | 1.9 (1.7) | 2.0 (1.8) | 2.1 (1.7) | 2.2 (1.7) | .26 |
| Means of transport to the first HCP | |||||
| Car | 99 (33) | 75 (25) | 53 (18) | 75 (25) | |
| Public transport | 250 (29) | 174 (20) | 236 (27) | 201 (23) | |
| Walk | 65 (22) | 75 (26) | 82 (28) | 70 (24) | |
| Other, missing | 10 (16) | 15 (24) | 15 (24) | 23 (37) | .001 |
| Means of transport to the treatment facility | |||||
| Car | 103 (43) | 51 (21) | 39 (16) | 47 (20) | |
| Public transport, foot | 274 (30) | 197 (21) | 226 (25) | 223 (24) | |
| Transport provided by cancer association | 18 (8) | 65 (28) | 85 (37) | 64 (28) | |
| Other, missing | 29 (23) | 26 (21) | 36 (29) | 35 (28) | <.001 |
| Distance in kilometer (median, IQR) from home to: | |||||
| First HCP | 1 (1, 6) | 3 (1, 31) | 12 (1, 73) | 29 (1, 126) | <.001 |
| Diagnostic facility | 2 (1, 6) | 21 (3, 62) | 27 (11, 135) | 208 (33, 376) | <.001 |
| Travel time in hours (median, IQR) on the day of the visit to: | |||||
| First HCP | 0.5 (0.3, 1) | 0.7 (0.3, 1.2) | 0.8 (0.4, 2) | 1.0 (0.3, 2) | <.001 |
| Treatment facility d | 0.8 (0.4, 1) | 2.0 (0.7, 4) | 2.0 (1, 7) | 1.5 (1, 7) | <.001 |
| Self‐perceived barriers contributing to delay (yes) e | |||||
| Transport | 27 (6) | 43 (13) | 68 (18) | 54 (15) | <.001 |
| Hospital too far | 9 (2) | 16 (5) | 60 (16) | 40 (11) | <.001 |
| Other obligations/no permission from family member | 9 (2) | 4 (1) | 9 (2) | 11 (3) | .43 |
| Embarrassment | 7 (2) | 9 (3) | 8 (2) | 10 (3) | .72 |
| Pain or discomfort | 21 (5) | 23 (7) | 24 (6) | 21 (6) | .74 |
| Fear of dying/treatment | 30 (7) | 21 (6) | 22 (6) | 16 (4) | .43 |
| No trust in medicine/prefer traditional healer | 10 (2) | 15 (4) | 17 (4) | 14 (4) | .36 |
| Difficulty with making an appointment or getting a hold of doctor | 10 (2) | 17 (5) | 29 (8) | 22 (6) | .008 |
| Cost of diagnostic tests/treatment | 24 (6) | 25 (7) | 31 (8) | 28 (8) | .57 |
| Any barrier | 84 (20) | 93 (27) | 140 (36) | 102 (28) | <.001 |
Abbreviations: HCP, healthcare provider; IQR, interquartile range.
Population‐specific quartile (Quartile 1, 2, 3, 4): Namibia black: 1‐195, 196‐457, 458‐583, 584+ km; Namibia non‐black: 1, 2‐238, 239‐292, 293+ km; Nigeria: 1–2, 3–6, 7‐28, 29+ km; Uganda: 1‐15, 16‐77, 78‐196, 197+ km; Zambia: 3‐8, 9‐156, 157‐374, 375+ km.
P values were derived from ANOVA test for the continuous variables and Chi‐square test for the categorical variables.
Country‐specific tertile of socioeconomic position score (Tertile 1, 2, 3): Namibia: 0‐4, 5‐7, 8‐9; Nigeria: 0‐4, 5‐6, 7‐8; Uganda: 0‐2, 3, 4‐8; Zambia: 0‐2, 3‐5, 6‐8.
The question asked about the travel time on the day of the visit, which might not capture the entire journey from home to the treatment facility.
Participants could report more than one barrier.
3.3. Time to diagnosis of breast cancer and disease stage at diagnosis
Median time from first symptom to diagnosis of breast cancer was 7 months (Table 1). The large majority (74%, among those with nonmissing information) of the women had a delay in diagnosis. Overall, 64% of the women were diagnosed at late stage, ranging from 25% in Namibian non‐black women to 76% in Nigerian women.
In analyses adjusted for population‐group only, rural residence and longer distance to diagnostic facility were associated with delay in diagnosis (Table 3). After mutual adjustment, rural residence (OR: 1.40, 95% CI: 1.06‐1.84) and distance (OR per 50 km increment: OR = 1.04, 95% CI: 1.00‐1.09, P trend: .048; highest vs lowest quartile: OR: 1.57, 95% CI: 1.07‐2.32) were found to be independently associated with delay to diagnosis. There was some evidence that late stage at diagnosis was positively associated with longer distance to treatment facility (mutually adjusted OR per 50 km increment: 1.03, 95% CI: 1.01‐1.06, P trend: .01; highest vs lowest quartile: 1.37, 95% CI: 0.97‐1.94) and inversely with highest quartile of distance to first provider (vs lowest quartile: 0.75, 95% CI: 0.55‐1.02), but not associated with distance to first provider when treated as a continuous variable (mutually adjusted OR per 50 km increment: 1.01, 95% CI: 0.97‐1.06, P trend: .53) or rural residence (mutually adjusted OR: 1.11, 95% CI: 0.86‐1.43). Population‐specific analysis revealed, however, some heterogeneity in the direction and magnitude of the geospatial associations with late‐stage disease, particularly so in Nigeria where, for instance, rural residence was, in contrast to the other settings, inversely associated with late‐stage disease (OR: 0.49, 95% CI: 0.30‐0.79, Supplementary Table 1). Hence, in mutually adjusted analyses restricted to non‐Nigerian women, a positive association between rural residence and late stage was revealed (OR: 1.45, 95% CI: 1.08‐1.94) and the positive association between distance to treatment facility and late stage was strengthened slightly (OR for the highest vs lowest quartile: 1.64, 95% CI: 1.11‐2.44) (Table 3).
TABLE 3.
Associations of geospatial characteristics of prediagnostic journey with time to diagnosis and stage at diagnosis
| Geospatial characteristics | Time to diagnosis | Stage at diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All (N = 1417) | All (N = 1415) | Non‐Nigerian women (N = 1046) | |||||||||
| <3 months, N (%) | ≥3 months, N (%) | OR (95% CI) a | OR (95% CI) b | 0‐II N (%) | III/IV N (%) | OR (95% CI) a | OR (95% CI) b | OR (95% CI) a | OR (95% CI) b | ||
| Urbanization of area of residence | |||||||||||
| Urban (town or city) | 246 (66) | 519 (50) | 1.00 | 1.00 | 295 (58) | 467 (52) | 1.00 | 1.00 | 1.00 | 1.00 | |
| Rural (rural or village) | 127 (34) | 525 (50) | 1.34 (1.03‐1.75) f | 1.40 (1.06‐1.84) f | 218 (42) | 435 (48) | 1.11 (0.87‐1.42) | 1.11 (0.86‐1.43) | 1.45 (1.10‐1.91) g | 1.45 (1.08‐1.94) f | |
| Straight‐line distance to first HCP c | |||||||||||
| Population‐specific quartile | Quartile 1 (shortest) | 177 (49) | 388 (40) | 1.00 | 1.00 | 221 (46) | 358 (42) | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 41 (11) | 144 (15) | 1.07 (0.69‐1.66) | 1.09 (0.70‐1.70) | 53 (11) | 117 (14) | 0.86 (0.57‐1.29) | 0.88 (0.59‐1.33) | 1.28 (0.75‐2.20) | 1.33 (0.77‐2.29) | |
| Quartile 3 | 59 (16) | 203 (21) | 1.16 (0.81‐1.67) | 1.07 (0.73‐1.56) | 86 (18) | 170 (20) | 0.86 (0.62‐1.20) | 0.85 (0.60‐1.19) | 1.10 (0.75‐1.61) | 1.07 (0.73‐1.58) | |
| Quartile 4 (longest) | 83 (23) | 238 (24) | 1.11 (0.80‐1.53) | 0.98 (0.70‐1.38) | 124 (26) | 207 (24) | 0.84 (0.62‐1.12) | 0.75 (0.55‐1.02) | 0.92 (0.66‐1.28) | 0.77 (0.55‐1.09) | |
| Distance in kilometer d | Median (IQR) | 2 (1, 28) | 6 (1, 44) | 1.02 (0.97‐1.08) | 1.00 (0.94‐1.05) | 4 (1, 42) | 5 (1, 36.5) | 1.03 (0.98‐1.07) | 1.01 (0.97‐1.06) | 1.03 (0.98‐1.07) | 1.01 (0.96‐1.05) |
| Straight‐line distance to diagnostic or treatment facility e | |||||||||||
| Population‐specific quartile | Quartile 1 (shortest) | 134 (36) | 281 (27) | 1.00 | 1.00 | 157 (31) | 239 (26) | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 75 (20) | 233 (22) | 1.19 (0.84‐1.69) | 1.16 (0.81‐1.66) | 116 (23) | 196 (22) | 1.01 (0.73‐1.38) | 1.01 (0.72‐1.40) | 1.21 (0.84‐1.73) | 1.14 (0.78‐1.68) | |
| Quartile 3 | 94 (25) | 276 (26) | 1.26 (0.91‐1.75) | 1.25 (0.87‐1.81) | 136 (27) | 227 (25) | 1.05 (0.77‐1.42) | 0.99 (0.70‐1.40) | 1.27 (0.90‐1.79) | 1.08 (0.73‐1.59) | |
| Quartile 4 (longest) | 70 (19) | 254 (24) | 1.64 (1.16‐2.33) g | 1.57 (1.07‐2.32) f | 104 (20) | 240 (27) | 1.48 (1.08‐2.04) f | 1.37 (0.97‐1.94) | 1.92 (1.34‐2.75) h | 1.64 (1.11‐2.44) f | |
| Distance in kilometer d | Median (IQR) | 7 (1, 61) | 20 (3, 128) | 1.05 (1.00‐1.09) f | 1.04 (1.00‐1.09) f | 77 (5, 293) | 61 (6, 288) | 1.04 (1.01‐1.06) g | 1.03 (1.01‐1.06) f | 1.04 (1.01‐1.06) g | 1.03 (1.00‐1.06) f |
Abbreviations: CI, confidence interval; HCP, healthcare provider; N, number; OR, odds ratio.
Logistic regression models were adjusted for population group.
In addition to population group, distance to diagnostic/treatment facility, distance to first HCP and urban/rural variables were included in the same model.
Due to missing information on location of first provider, 84 women were excluded from the analysis of time to diagnosis and 79 women were excluded from the analysis of stage at diagnosis.
When the distance variables were treated as continuous variables, OR associated with 50 km increment was estimated.
In the analysis of time to diagnosis, distance to the nearest laboratory within the network of National Institute of Pathology that provides diagnostic services was used for Namibian women who did not go directly to the Windhoek Central Hospital. Distance to the Windhoek Central Hospital was used if they went directly to the hospital. In the analysis of stage at diagnosis, distance to the treatment facility (ie, place of recruitment) was used.
0.01 ≤ P value < 0.05.
0.001 ≤ P value < 0.01.
P value < 0.001.
Further adjustment for sociodemographic variables (ie, age at diagnosis, educational level, SEP [country‐specific tertiles]) and modes of transport used to reach the first healthcare provider, and the treatment facility showed that distance to the diagnostic facility was the only geospatial variable independently associated with delay in diagnosis (OR per 50 km increment: 1.05, 95% CI: 1.01‐1.09, P trend: .02; highest vs lowest quartile: 1.56, 95% CI: 1.08‐2.27, Table 4) and late stage at diagnosis (OR per 50 km increment: 1.04, 95% CI: 1.01‐1.06, P trend: .004; highest vs lowest quartile: OR: 1.47, 95% CI: 1.05‐2.06), with the late‐stage associations for the quartiles being somewhat strengthened in analysis restricted to non‐Nigerian women (OR for the highest vs lowest quartile: 1.73, 95% CI: 1.18‐2.54). For both outcomes, significant associations were not found with rural residence after adjustment for distance to diagnostic/treatment facility and the aforementioned covariates. A forest plot of the estimated ORs associated with geospatial variables is shown in Figure 2.
TABLE 4.
Associations of geospatial and other characteristics of women with delay in diagnosis and stage at diagnosis
| Time to diagnosis: all (n = 1417) | Stage at diagnosis: all (n = 1415) | Stage at diagnosis: non‐Nigerian women (n = 1046) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <3 months, N (%) | ≥3 months, N (%) | OR (95% CI) a | 0‐II, N (%) | III/IV, N (%) | OR (95% CI) a | 0‐II, N (%) | III/IV, N (%) | OR (95% CI) a | ||
| Geospatial characteristics | ||||||||||
| Urbanization of area of residence | ||||||||||
| Urban (town or city) | 246 (66) | 519 (50) | 1.00 | 295 (58) | 467 (52) | 1.00 | 249 (59) | 277 (44) | 1.00 | |
| Rural (rural or village) | 127 (34) | 525 (50) | 1.06 (0.76‐1.47) | 218 (42) | 435 (48) | 0.89 (0.66‐1.21) | 174 (41) | 346 (56) | 1.17 (0.82‐1.67) | |
| Straight‐line distance to diagnostic/treatment facility b | ||||||||||
| Population‐specific quartile | Quartile 1 (shortest) | 134 (36) | 281 (27) | 1.00 | 157 (31) | 239 (26) | 1.00 | 140 (33) | 153 (25) | 1.00 |
| Quartile 2 | 75 (20) | 233 (22) | 1.15 (0.80‐1.64) | 116 (23) | 196 (22) | 0.96 (0.69‐1.34) | 92 (22) | 135 (22) | 1.07 (0.73–1.56) | |
| Quartile 3 | 94 (25) | 276 (26) | 1.12 (0.78‐1.61) | 136 (27) | 227 (25) | 0.99 (0.70‐1.39) | 110 (26) | 161 (26) | 1.08 (0.73–1.59) | |
| Quartile 4 (longest) | 70 (19) | 254 (24) | 1.56 (1.08‐2.27) d | 104 (20) | 240 (27) | 1.47 (1.05–2.06) d | 81 (19) | 174 (28) | 1.73 (1.18–2.54) e | |
| Distance in kilometer c | Median, IQR | 7 (1, 61) | 20 (3, 128) | 1.05 (1.01–1.09) d | 77 (5, 293) | 61 (6, 288) | 1.04 (1.01‐1.06) e | 158 (8, 375) | 229 (31, 457) | 1.04 (1.01‐1.06) e |
| Other characteristics of prediagnostic journey | ||||||||||
| Mode of transport to first HCP | ||||||||||
| Public transport/foot/other | 264 (71) | 876 (84) | 1.00 | 366 (71) | 756 (84) | 1.00 | 289 (68) | 494 (79) | 1.00 | |
| Car | 109 (29) | 168 (16) | 1.07 (0.70‐1.63) | 147 (29) | 146 (16) | 1.01 (0.69‐1.50) | 134 (32) | 129 (21) | 1.00 (0.67‐1.51) | |
| Mode of transport to treatment facility | ||||||||||
| Public transport/foot/other | 274 (73) | 918 (88) | 1.00 | 388 (76) | 798 (88) | 1.00 | 312 (74) | 537 (86) | 1.00 | |
| Car | 99 (27) | 126 (12) | 0.91 (0.58‐1.44) | 125 (24) | 104 (12) | 0.94 (0.61‐1.43) | 111 (26) | 86 (14) | 1.17 (0.74‐1.85) | |
| Outcome of first visit to a HCP | ||||||||||
| Breast cancer not suspected/tests done but no results | 69 (18) | 384 (37) | 2.03 (1.48‐2.80) f | 140 (27) | 323 (36) | 1.25 (0.95‐1.65) | 128 (30) | 259 (42) | 1.22 (0.91‐1.64) | |
| Breast cancer suspected/referral | 226 (61) | 468 (45) | 1.00 | 278 (54) | 412 (46) | 1.00 | 257 (61) | 336 (54) | 1.00 | |
| Went directly to the treatment facility (ie, place of recruitment) | 78 (21) | 192 (18) | 1.04 (0.70‐1.56) | 95 (19) | 167 (19) | 0.53 (0.36‐0.78) e | 38 (9) | 28 (4) | 0.40 (0.23‐0.71) e | |
| Sociodemographic factors | ||||||||||
| Age at breast cancer diagnosis (mean, SD) | 52 (14) | 50 (14) | 0.99 (0.98‐1.00) d | 52 (14) | 50 (14) | 0.99 (0.98–1.00) e | 53 (14) | 50 (14) | 0.98 (0.97‐0.99) e | |
| Site/race | ||||||||||
| Namibia—black | 106 (28) | 267 (26) | 1.00 | 142 (28) | 255 (28) | 1.00 | 142 (34) | 255 (41) | 1.00 | |
| Namibia—non‐black | 60 (16) | 38 (4) | 0.38 (0.23‐0.64) f | 78 (15) | 26 (3) | 0.27 (0.16‐0.45) f | 78 (18) | 26 (4) | 0.26 (0.15‐0.46) f | |
| Nigeria | 113 (30) | 255 (24) | 1.01 (0.71‐1.43) | 90 (18) | 279 (31) | 1.84 (1.30‐2.60) e | — | — | — | |
| Uganda | 45 (12) | 340 (33) | 2.80 (1.84‐4.27) f | 136 (27) | 247 (27) | 0.95 (0.68‐1.33) | 136 (32) | 247 (40) | 0.90 (0.64‐1.28) | |
| Zambia | 49 (13) | 144 (14) | 1.21 (0.80‐1.82) | 67 (13) | 95 (11) | 0.81 (0.54‐1.19) | 67 (16) | 95 (15) | 0.85 (0.57‐1.26) | |
| Education | ||||||||||
| Primary school or less | 122 (33) | 514 (49) | 1.00 | 200 (39) | 431 (48) | 1.00 | 176 (42) | 354 (57) | 1.00 | |
| Secondary/high school | 127 (34) | 344 (33) | 0.72 (0.51‐1.02) | 172 (34) | 303 (34) | 0.73 (0.54‐1.00) d | 138 (33) | 200 (32) | 0.75 (0.54‐1.06) | |
| Technical/university | 124 (33) | 186 (18) | 0.46 (0.31‐0.68) f | 141 (27) | 168 (19) | 0.51 (0.35‐0.74) f | 109 (26) | 69 (11) | 0.38 (0.25‐0.59) f | |
| SEP score (country‐specific tertile) | ||||||||||
| Tertile 1 (lowest) | 124 (33) | 496 (48) | 1.00 | 188 (37) | 431 (48) | 1.00 | 149 (35) | 308 (49) | 1.00 | |
| Tertile 2 | 130 (35) | 345 (33) | 1.05 (0.75‐1.45) | 157 (31) | 320 (35) | 1.07 (0.79‐1.44) | 125 (30) | 194 (31) | 1.08 (0.75‐1.54) | |
| Tertile 3 (highest) | 119 (32) | 203 (19) | 0.81 (0.54‐1.23) | 168 (33) | 151 (17) | 0.65 (0.45‐0.94) d | 149 (35) | 121 (19) | 0.77 (0.50‐1.18) |
Abbreviations: CI, confidence interval; HCP, healthcare provider; N, number; OR, odds ratio; SEP, social economic position.
OR and CI were adjusted for all the variables in the table except for outcome of first visit to a HCP.
In the analysis of time to diagnosis, distance to the nearest laboratory within the network of the National Institute of Pathology that provides diagnostic services was used for Namibian women who did not go directly to the Windhoek Central Hospital. Distance to the Windhoek Central Hospital was used if they went directly to the hospital. In the analysis of stage at diagnosis, distance to the treatment facility (ie, place of recruitment) was used.
When the distance variable was treated as a continuous variable, OR associated with 50 km increment was estimated.
0.01 ≤ P value < 0.05.
0.001 ≤ P value < 0.01.
P value < 0.001.
FIGURE 2.
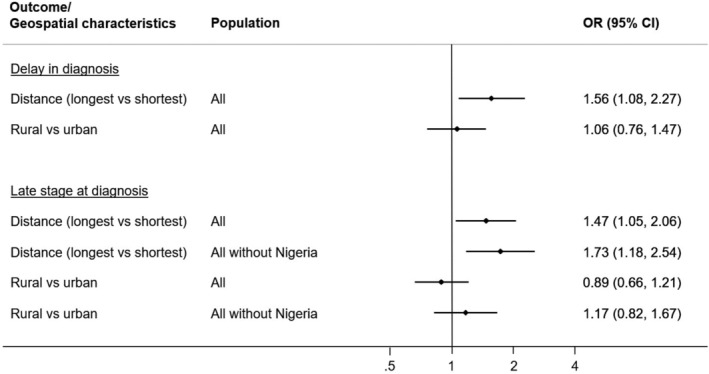
Forest plot of the odds ratios (ORs) with corresponding 95% confidence intervals (CIs) of delay in diagnosis and late stage at diagnosis on geospatial characteristics. ORs and CIs were derived from logistic regression models adjusted for urbanization of area of residence or straight‐line distance to diagnostic/treatment facility,modes of transport used to reach the first healthcare provider and the treatment facility, population, age, educational status and socioeconomic position score. In the analysis of delay in diagnosis, distance to diagnostic facility was examined, whereas in the analysis of late stage at diagnosis, distance to treatment facility was examined. The ORs for distance are based on the comparison between the highest and lowest quartiles (with the lowest quartile as reference)
Upon examination of effect modification of the effects of distance on delay to diagnosis and on late stage at diagnosis, SEP modified the distance‐late‐stage association (p for interaction = .006), but SEP did not modify the distance‐delay association (p for interaction = .37, Supplementary Table 2). The distance‐late‐stage association was observed in women with higher SEP (OR = 1.71, 95% CI: 1.19‐2.43) and not in those with lower SEP, but the latter group had later stage regardless of residential location. Similar results were observed in analysis restricted to non‐Nigerian women. No effect modification by rural residence was found (p for interaction >.65 for all).
Finally, the exploratory analysis in Namibia showed that among women living outside Windhoek, those living more than 50 km from the nearest pathological laboratory had elevated odds of delay in diagnosis than those living <50 km from the nearest pathological laboratory after adjusting for age at diagnosis, educational status and SEP (OR = 1.54, 95% CI: 0.93‐2.57, Supplementary Table 3). The corresponding OR for black women was 1.60 (95% CI: 0.90‐2.84). No substantial difference in odds of being diagnosed at late stage was observed between women living outside Windhoek and more than 50 km from the nearest pathological laboratory (OR: 1.16, 95% CI: 0.74‐1.82) and those living outside Windhoek and <50 km from the nearest pathological laboratory (reference group).
4. DISCUSSION
Our study of women diagnosed with breast cancer in four SSA countries (Namibia, Nigeria, Uganda and Zambia) showed large distances traveled and long travel time taken to reach cancer services, illustrating obstacles that women might face during their prediagnostic journeys. The study further showed that the geospatial characteristics, particularly travel distance to cancer diagnostic or treatment facilities, were associated with both delay in diagnosis and more advanced stage at diagnosis, with some heterogeneity across SSA settings and by SEP.
The observed association between distance to cancer treatment facility and late stage at diagnosis largely support the finding from a recent review of 27 studies that greater travel burden is associated with late cancer diagnosis and poorer outcomes. 13 Of the 27 studies reviewed, only one was from Africa. The results from the latter, which recruited over 1000 women newly diagnosed with breast cancer at a South African public hospital, are also in line with our finding that greater distance to the tertiary hospital was associated with a later stage at diagnosis. 12 To our knowledge, no other studies have so far examined the association between distance to cancer care service and cancer stage at diagnosis in SSA, but geospatial barriers in access to obstetrics care have been investigated more extensively. A systematic review of 57 studies conducted in SSA concluded that women living farther away from health facilities equipped to provide skilled care for childbirth are less likely to use such service. 14
Geospatial barriers to early breast cancer diagnosis are likely multifactorial and partly attributable to financial and logistic challenges (eg, needing financial support, transport for traveling and accommodation near the hospital) or others due to competing priorities (eg, work, care of children). To reduce geospatial barriers to healthcare access, measures to improve patients' transport or lower its financial burden could be implemented. 18 For instance, lowering patient's financial burden of travel may be feasible at local level by using community‐based loan funds 19 or transport provided by hospitals. In Namibia, 46% of the study participants reached the Windhoek Central Hospital, which provides a major National Oncology center, via transport provided by the hospitals. Most of these women were living far away from the Windhoek Central Hospital. In Zambia and Uganda, 21% to 24% of women reported cost or availability of transport as a barrier contributing to delay in reaching the treatment facility. In these settings, providing transport or financial support to those living in a remote area as done in Namibia may help narrow the gap in cancer care access. Further setting specific investigation should be followed to inform interventions to be implemented in each setting.
Another possible approach to reduce the barrier of distance to cancer treatment facility is to decentralize part of the initial diagnostic work‐up to lower‐level facilities, for instance, from national to district level. Such partial decentralization of cancer diagnostic services is being considered in Zambia 20 and is recommended by the Breast Health Global Initiative (BHGI) as a phased implementation step in improving access to prompt and effective breast cancer diagnosis and treatment. 21 Namibia has a network of pathological laboratories across the country that allows women to undergo biopsy and receive diagnostic test results without having to travel to the central hospital; instead, the FNA/tumor specimens are sent to the capital city and only those women with a histological/cytological‐confirmed diagnosis need to travel to the central hospital for treatment. In the present study, the median time from first symptom recognition to specimen collection was longer (3.3 vs 6.2 months) (data not shown) and the odds of having a delay to diagnosis and being diagnosed at late stage were higher in women living outside Windhoek compared to those living in Windhoek (Supplementary Table 3), but the median time from specimen collection to presentation at the Windhoek Central Hospital was similar (0.8 months and 1.1 months, respectively). Furthermore, among black women living outside Windhoek, those who lived greater than 50 km from a pathological laboratory had greater odds of delay in diagnosis compared to those who lived less than 50 km from a pathological laboratory. This is consistent with a positive effect of a partial decentralization of cancer diagnostic services, that is, decentralized tissue collection with centralized pathological review contributing to improvement of access to early diagnosis of breast cancer.
Alongside a full or partial decentralization of diagnostic services, an effective system of referral to the diagnostic services, transport of samples and result feedback, followed by referral for treatment should also be in place. On a positive note, distance to first provider did not contribute to delays or late stage, and many first providers were contacted early in the prediagnostic journey. 22 This first provider thus represents a critical first interaction of the women with the health system, a point at which interventions (financial assistance, guidance and support) can be made to accelerate the subsequent journey to treatment. The present study revealed that 32% of women were not suspected of having breast cancer or were not referred for further evaluation by the first healthcare provider visited and were more likely to have a delay in diagnosis. This points to the need to improve awareness and training about breast cancer symptoms and available cancer services among healthcare providers throughout the referral system 10 , 23 while balancing against the burden of false positives, that is, referring women with benign diseases for diagnostics and treatment. As a nonnegligible proportion of women, particularly in Nigeria (7%) and Uganda (13%), sought care from an informal care provider, involving these providers in the pyramid of referrals should also be considered to facilitate timely diagnosis and treatment.Additional approaches to the improvement of breast cancer diagnosis and pathology have been proposed by BHGI, the International Society of Clinical Pathologists (ISCP) and others for resource‐limited settings, such as training pathologists and organizing international pathology services. 24 , 25
Among the settings examined in the ABC‐DO study, we also observed some heterogeneity with Nigeria having a particularly distinct profile. In this setting, women were recruited at the public hospitals (the Federal Medical Centre in Owerri and university teaching hospital in Aba) and at a private clinic in Aba, with 73% of the women living within a radius of 25 km. Although the time from first symptom recognition to diagnosis was shorter than that of other settings, the proportion of women diagnosed at late stage was higher. The geospatial association with late stage at diagnosis in Nigeria was inconsistent to that seen in the other settings. The associations of late stage at diagnosis with rural residence (Supplementary Table 1) and higher quartiles of distance to treatment facility (data not shown) were reversed. Although the peculiarity of distance‐stage association in Nigerian women appears to be driven by certain sites (data not shown), the reasons for the discrepancy are unclear. The inverse associations could be in part due to the small variations of distance and stage, residual confounding from determinants of advanced stage at diagnosis, such as educational and socioeconomic statuses, 11 or distance bias effect that was suspected in previous studies, 26 that is, those with an advanced disease are less likely to reach the hospital if they are living far away from the hospital. The latter raises an important question about the extent of undiagnosed cases in the SSA settings. Furthermore, we observed an effect modification of the distance‐stage association by SEP, with the proportion of late stage at diagnosis being high regardless of distance in women with lower SEP, suggesting that they might have additional obstacles that need to be addressed in order to reduce delays in diagnosis. These heterogeneities underscore the importance of evaluating the specificities of each population when designing measures for down‐staging in SSA.
Unlike many of the previous studies of cancer diagnosis in SSA settings, the present study featured geospatial dimension to prediagnostic journey, which has been underinvestigated in cancer care in SSA. The results of our study, together with the other key determinants of delay in breast cancer diagnosis 22 and advanced stage at diagnosis 11 (eg, lack of breast cancer awareness), can guide future implementation research and governments and their partners to make informed decisions about the planning and development of strategies to mitigate breast cancer deaths in SSA settings.
Nevertheless, in interpreting the results of the present study, potential limitations of the study must be considered. One of the limitations of the study is that the geocodes were based on the names of places that may cover a large area, making the locations less precise. Some of the locations of diagnostic centers might also not be precise if the samples were collected at another laboratory/clinic. Also, travel distance was calculated based on straight‐line distance and not on road network, which can also introduce information bias. However, a high correlation was observed between the straight‐line distance and road distance in a random sample of women from Uganda (r = 0.91, data not shown). Therefore, the ranking of the distance is likely to reflect that of the road distance. Furthermore, there is potential misclassification of the outcomes under study. We determined delay based on date of first symptom recognition, which might not reflect the date of symptom onset due to a lack of awareness about breast cancer symptoms or a difficulty with remembering when. However, as breast cancer knowledge score did not substantially differ by distance, we would expect the resultant bias would be minimal. Another potential limitation is that the present study included only women who attended the selected treatment facilities. Therefore, it is possible that the stage distribution differs from what would be observed in the totality of breast cancer cases in the respective countries. Also, underestimation of cancer stage was suspected in this cohort. 4 Since all women were staged at the treatment facility, the misclassification is unlikely to be differential, and thus the results would be biased towards the null. Nevertheless, given the large variety of distance in SSA except in Nigeria, we were still able to capture the gradient of distances and associated odds of delay in diagnosis and late stage at diagnosis.
Although the focus of the present study was on geospatial barriers to early diagnosis of breast cancer, such barriers can also delay treatment or hinder adherence to treatment and receipt of other cancer services, such as follow‐up examinations and counseling. 13 The efforts to tackle the geospatial barrier need to be extended to also ensure access to care after a diagnosis and accompanied by ensuring the availability of appropriate equipment, drugs and healthcare providers in order to achieve the aims to reduce the morbidity and mortality of cancer and to improve the quality of life of the women affected by breast cancer. 27
5. CONCLUSIONS
The present study provides evidence that geographical distance to cancer care services is a barrier to early diagnosis of breast cancer in SSA, indicating potential avenues for downstaging this disease. Potential approaches to reducing this barrier include providing transport or travel allowance to women, particularly those living far away from the hospitals, or considering decentralizing diagnostic services in conjunction with accelerated referral and follow‐up. Population‐specific interventions will be needed to develop targeted approaches to tackle these barriers to early diagnosis of breast cancer.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DISCLAIMER
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
ETHICS STATEMENT
The ABC‐DO study was approved by the ethics committees of all involved institutions. All participants provided written informed consent or, if illiterate, a fingerprint.
Supporting information
Appendix S1: Supplementary Information
ACKNOWLEDGEMENTS
The authors thank the women who participated in our study. They also thank the dedicated team of study research assistants, notably J. Pontac, A. Naamala, A. Kaggwa, A. Nteziryayo, T. Nakazibwe, C. Sule Oyamienlen, K. Iwuoha, E. Ezeigbo, M.Lusaka, M. Mudolo and E. Bakengesa. They would also like to acknowledge C. Ekwunife, R. Enendu, O. Duru, D. Oparocha, N. Eleweke and Ezeonwumelu for their help and assistance with the study.
The study was funded by Susan G Komen (IIR 13264158, GSP18IARC001 and GSP19IARC001, “Implementing breast cancer care efficiency in Zambia through specialized health provider training and m‐health evaluation of patient outcomes”) and by IARC through an internal process to support etiological research in Sub‐Saharan Africa by the IARC Section of Environment and Radiation.
Togawa K, Anderson BO, Foerster M, et al. Geospatial barriers to healthcare access for breast cancer diagnosis in sub‐Saharan African settings: The African Breast Cancer—Disparities in Outcomes Cohort Study. Int. J. Cancer. 2021;148:2212–2226. 10.1002/ijc.33400
Funding information Centre International de Recherche sur le Cancer; Susan G. Komen, Grant/Award Numbers: GSP18IARC001, GSP19IARC001, IIR 13264158
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. International Agency for Research on Cancer . In: Parkin DM, Jemal JFA, Borok M, et al., eds. Cancer in Sub‐Saharan Africa (IARC Scientific Publications No. 167). Lyon, France: International Agency for Research on Cancer; 2018. [Google Scholar]
- 2. Ferlay JEM, Lam F, Colombet M, et al. Global Cancer Observatory: Cancer Tomorrow. Lyon, France: International Agency for Research on Cancer; 2018. [Google Scholar]
- 3. Joko‐Fru WY, Miranda‐Filho A, Soerjomataram I, et al. Breast cancer survival in sub‐Saharan Africa by age, stage at diagnosis and human development index: A population‐based registry study. Int J Cancer. 2020;146:1208‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCormack V, McKenzie F, Foerster M, et al. Breast cancer survival and survival gap apportionment in sub‐Saharan Africa (ABC‐DO): a prospective cohort study. Lancet Glob Health. 2020;8:e1203‐e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995‐2009: analysis of individual data for 25,676,887 patients from 279 population‐based registries in 67 countries (CONCORD‐2). Lancet. 2015;385:977‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pruitt L, Mumuni T, Raikhel E, et al. Social barriers to diagnosis and treatment of breast cancer in patients presenting at a teaching hospital in Ibadan, Nigeria. Glob Public Health. 2015;10:331‐344. [DOI] [PubMed] [Google Scholar]
- 7. Tetteh DA, Faulkner SL. Sociocultural factors and breast cancer in sub‐Saharan Africa: implications for diagnosis and management. Womens Health (Lond Engl). 2016;12:147‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akuoko CP, Armah E, Sarpong T, Quansah DY, Amankwaa I, Boateng D. Barriers to early presentation and diagnosis of breast cancer among African women living in sub‐Saharan Africa. PLoS One. 2017;12:e0171024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Espina C, McKenzie F, Dos‐Santos‐Silva I. Delayed presentation and diagnosis of breast cancer in African women: a systematic review. Ann Epidemiol. 2017;27:659.e7‐71.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scheel JR, Anderson S, Foerster M, Galukande M, McCormack V. Factors contributing to late‐stage breast cancer presentation in sub‐Saharan Africa. Curr Breast Cancer Res. 2018;10:142‐147. [Google Scholar]
- 11. McKenzie F, Zietsman A, Galukande M, et al. Drivers of advanced stage at breast cancer diagnosis in the multicountry African breast cancer—disparities in outcomes (ABC‐DO) study. Int J Cancer. 2018;142:1568‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a peri‐urban setting: a South African public hospital case series of over 1000 women. International journal of cancer. J Int Cancer. 2014;135:2173‐2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a Barrier to Cancer Diagnosis and Treatment: Review of the Literature. Oncologist. 2015;20:1378‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong KLM, Benova L, OMR C. A look back on how far to walk: systematic review and meta‐analysis of physical access to skilled care for childbirth in sub‐Saharan Africa. PLoS One. 2017;12:e0184432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farber SH, Vissoci JR, Tran TM, et al. Geospatial analysis of unmet surgical need in Uganda: an analysis of SOSAS survey data. World J Surg. 2017;41:353‐363. [DOI] [PubMed] [Google Scholar]
- 16. McKenzie F, Zietsman A, Galukande M, et al. African Breast Cancer‐Disparities in Outcomes (ABC‐DO): protocol of a multicountry mobile health prospective study of breast cancer survival in sub‐Saharan Africa. BMJ Open. 2016;6:e011390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Network of Cancer Registries , Recommendations for Coding Incidence Date, 1995.
- 18. O'Donnell O. Access to health care in developing countries: breaking down demand side barriers. Cad Saude Publica. 2007;23:2820‐2834. [DOI] [PubMed] [Google Scholar]
- 19. Nwolise CH, Hussein J, Kanguru L, Bell J, Patel P. The effectiveness of community‐based loan funds for transport during obstetric emergencies in developing countries: a systematic review. Health Policy Plan. 2015;30:946‐955. [DOI] [PubMed] [Google Scholar]
- 20. Cabanes A, Kapambwe S, Citonje‐Msadabwe S, et al. Challenges, opportunities, and priorities for advancing breast cancer control in Zambia: a consultative meeting on breast cancer control. J Global Oncol. 2019;5:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mutebi M, Anderson BO, Duggan C, et al. Breast cancer treatment: a phased approach to implementation. Cancer. 2020;126:2365‐2378. [DOI] [PubMed] [Google Scholar]
- 22. Foerster M, McKenzie F, Zietsman A, et al. Dissecting the journey to breast cancer diagnosis in sub‐Saharan Africa: Findings from the multicountry ABC‐DO cohort study. Int J Cancer. 2020. 10.1002/ijc.33209. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clegg‐Lamptey J, Dakubo J, Attobra YN. Why do breast cancer patients report late or abscond during treatment in Ghana? A pilot study. Ghana Med J. 2009;43:127‐131. [PMC free article] [PubMed] [Google Scholar]
- 24. Shyyan R, Masood S, Badwe RA, et al. Breast cancer in limited‐resource countries: diagnosis and pathology. Breast J. 2006;12(Suppl 1):S27‐S37. [DOI] [PubMed] [Google Scholar]
- 25. Anglade F, Milner DA, Brock JE. Can pathology diagnostic services for cancer be stratified and serve global health? Cancer. 2020;126:2431‐2438. [DOI] [PubMed] [Google Scholar]
- 26. Kelly C, Hulme C, Farragher T, Clarke G. Are differences in travel time or distance to healthcare for adults in global north countries associated with an impact on health outcomes? A systematic review. BMJ Open. 2016;6:e013059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization . Module 3: Early Detection Cancer Control: Knowledge into Action. Geneva, Switzerland: WHO Guide for Effective Programmes; 2007. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


