Abstract
Aim
To investigate the effectiveness of premixed insulin for achieving glycaemic outcomes in clinical practice in the UK.
Materials and Methods
Electronic medical record data from The Health Improvement Network database were captured for adults with type 2 diabetes (T2D) uncontrolled (HbA1c ≥9%) on two or more oral antihyperglycaemic drugs (OADs) initiating premixed insulin. Effectiveness of premixed insulin was assessed by the probability and incidence of achieving glycaemic outcomes (target HbA1c <7.5% [<58 mmol/mol] and a ≥1% or ≥2% HbA1c reduction) over 24 months.
Results
Data from 974 participants (mean age 62 years; 56% male; 52% obese or extremely obese; mean HbA1c 11.3% [100 mmol/mol]; hypertension 64%, dyslipidaemia 23% and nephropathy 21%) were analysed. The probability of achieving HbA1c <7.5% was highest during months 3‐6 (18.2%), while the cumulative probability of achieving this target plateaued between months 15‐24 (15.7%‐16.0%). Incidence of achieving all glycaemic outcomes plateaued after 12 months and differed by baseline HbA1c, but not OAD use. Factors affecting some glycaemic outcomes included a body mass index >40 kg/m2 and co‐morbidities including nephropathy and stroke.
Conclusions
In people with uncontrolled T2D (HbA1c ≥9%), glycaemic outcome achievement on premixed insulin was low at 6 months with little additional clinical benefit beyond 12 months, suggesting a high unmet need for early, timely treatment changes with more effective, simpler therapies.
Keywords: database research, glycaemic control, insulin therapy, type 2 diabetes
1. INTRODUCTION
Diabetes is a global epidemic affecting ~463 million adults, of whom 90% have type 2 diabetes (T2D). 1 It is anticipated that the prevalence of diabetes will increase by 51% by 2045, affecting 700 million people worldwide. 1 In the UK, an estimated 4.7 million people have diagnosed or undiagnosed diabetes, which is expected to rise to more than 5.5 million by 2030. 2
Poor glycaemic control in people with T2D can lead to an increased risk of microvascular and macrovascular complications, such as cardiovascular disease, chronic kidney disease and diabetic foot syndrome. 3 , 4 Many guidelines consider a target HbA1c of ≤7.0% (≤53 mmol/mol) as appropriate for managing most adults with T2D. 5 , 6 , 7 , 8 However, individualized HbA1c targets based on life expectancy, frailty, patient preferences, co‐morbidities and risk of adverse effects, including hypoglycaemia and hypoglycaemia awareness, are also recommended. 5 , 6 , 7 , 8
Due to the progressive decline in β‐cell function in people with T2D, drug therapy will need to be intensified over time to maintain glycaemic control. 9 According to the UK National Institute for Health and Care Excellence (NICE) guidelines, people with T2D require drug intensification if they are inadequately controlled (HbA1c ≥7.5% [≥58 mmol/mol]) by either a single oral antihyperglycaemic drug (OAD) or by two OADs. 8 Furthermore, the UK NICE guidelines recommend insulin‐based treatment as an option for people with T2D inadequately controlled (HbA1c ≥7.5%) with two OADs, and premixed insulin (a combination of neutral protamine Hagedorn insulin and a short‐acting insulin) as a treatment option for those with HbA1c ≥9% (≥75 mmol/mol). 8 Previous studies have shown that in the UK, 22.8% of people with T2D are receiving insulin therapy, 10 of whom ~40% are using premixed insulin. 11 , 12
Treatment with premixed insulin is commonly used as both initial insulin therapy and when intensification is required, although initial insulin treatment comprising the addition of basal insulin to OADs is currently recommended for the majority of people with T2D, 5 , 6 , 7 , 8 as most individuals usually maintain sufficient β‐cell function to respond to OADs. Twice‐daily doses of premixed insulin are a more convenient means of spreading insulin across the day compared with administering basal insulin and prandial insulin separately, 5 with an improved postprandial glucose level compared with basal‐only insulin regimens. 13 , 14 However, a high proportion of people with T2D still fail to achieve HbA1c ≤7% with premixed insulin, 15 , 16 and evidence suggests that premixed insulin may be associated with a higher risk of hypoglycaemia compared with basal insulin analogues. 13 , 14 , 15 , 16 It should be noted that the NICE guidelines suggest relaxing the HbA1c goal for people for whom reaching HbA1c ≤7% may not be feasible, including people with co‐morbidities for T2D such as established cardiovascular disease. 8 Furthermore, premixed insulin regimens are strict, requiring either two or three daily injections based on carbohydrate intake estimation with each meal, 17 and people with diabetes are more likely to skip insulin injections if their treatment regimen includes multiple daily injections, interferes with their daily activities or increases the risk of hypoglycaemia. 18 , 19
In this retrospective cohort study, we investigated the real‐world effectiveness of premixed insulin for achieving glycaemic control in routine clinical practice in the UK.
2. METHODS
2.1. Study design and data sources
This was a retrospective, population‐based, observational, cohort study in adults with T2D who had switched from OADs to premixed insulin. Adults (aged ≥18 years) with T2D who received their first prescription for premixed insulin from 1 January 2010 to 31 December 2016 (date of first prescription = index date) and who had used at least two OADs in the previous 12 months were identified from The Health Improvement Network (THIN) database. A diagnosis of T2D was identified using the ICD‐10‐CM code E11 or the corresponding THIN medical codes (INUK.C109.13, INUK.C109.12, INUK.C109.11, INUK.C109.00, INUK.C10F.11 or INUK.C10F.00). Individuals were eligible for inclusion in the study if they also had at least 6 months of follow‐up after the initiation of premixed insulin or at least two prescriptions for premixed insulin; at least three HbA1c measurements (baseline HbA1c ≥9% recorded between 90 days prior to and 14 days after premixed insulin initiation, and at least two HbA1c measurements in the 15‐720 days after premixed insulin initiation); and no insulin treatment in the 12 months prior to premixed insulin initiation. Individuals who received glucagon‐like peptide‐1 receptor agonists prior to initiating premixed insulin were not excluded. THIN, set up in 2003, is a database of anonymized primary care records from ~850 general practices in the UK and has been used in more than 1000 publications to date.
Follow‐up for participants ranged from their index date until whichever of the following occurred first: they achieved the glycaemic outcome under analysis; they required additional antihyperglycaemic therapy (intensification); they discontinued premixed insulin; their last record in the database; they died; or the end of the study period. The study period was from 1 January 2009 (to include a 12‐month baseline period) to 31 December 2018. Information on the treatment algorithms followed by healthcare professionals while using premixed insulin is not reliably recorded in THIN database.
The study was conducted in accordance with ethical principles that are consistent with the Declaration of Helsinki, International Conference on Harmonisation, Good Clinical Practice, Good Pharmacoepidemiology Practice, and the applicable legislation on non‐interventional studies and real‐world evidence and epidemiology studies.
2.2. Outcomes and assessments
The primary objective of the study was to investigate the real‐world effectiveness of premixed insulin, assessed as the probability of reaching glycaemic control (defined as HbA1c <7.5% [<58 mmol/mol]), the incidence of reaching glycaemic control (HbA1c <7.5%), and the mean change in HbA1c, all over 24 months post‐premixed insulin initiation. Sensitivity analyses evaluated improvement in glycaemic control as the incidence of achieving a ≥1% or ≥2% reduction from baseline in HbA1c over 24 months post‐premixed insulin initiation. Secondary outcomes included determining if there were any associations between baseline characteristics (sex, age, HbA1c, previous OADs and the presence of common co‐morbidities during the baseline period) and the achievement of glycaemic outcomes. Age and baseline HbA1c were assessed as continuous and categorical variables. The categories used for age were 18‐44, 45‐54, 55‐64, 65‐75 and >75 years and the categories used for HbA1c were ≥9% and ≤10% (≥75 and ≤86 mmol/mol); >10% and ≤11% (>86 and ≤97 mmol/mol); and >11% (>97 mmol/mol). Previous OAD use at baseline was assessed using binary variables for each of the following OAD categories: biguanides, sulphonylureas, meglitinides, thiazolidinediones, dipeptidyl peptidase‐4 (DPP‐4) inhibitors and α‐glucosidase inhibitors; and as an ordinal variable for the number of OAD categories used (2 and ≥3). The co‐morbidities assessed during the baseline period were overweight/obesity (not overweight or obese: body mass index [BMI] ≤25 kg/m2; overweight: BMI >25 and ≤30 kg/m2; obese: BMI >30 and ≤40 kg/m2; extremely obese: BMI >40 kg/m2; or classified based on medical codes if BMI unavailable), nephropathy (binary variable based on medical diagnosis codes or at least two glomerular filtration rate measurements more than 90 days apart <60 mL/min/1.73 m2), hypertension (binary variable based on medical diagnosis codes or at least two abnormal blood pressure measures [systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg]), and peripheral vascular disease, valvular heart disease, neuropathy, retinopathy, congestive heart failure, stroke and dyslipidaemia (all binary variables based on medical diagnosis codes). All the medical diagnosis codes used are listed in Table S1 (Appendix S1).
2.3. Statistical analysis
Sample size was calculated based on assessing the percentage change in mean HbA1c from baseline to 6 months or the first available measurement of HbA1c with sufficient precision, powered based on two‐sided significance (α = 0.05). Based on preliminary sample counts, it was calculated that the study should include ~1000 participants.
Baseline characteristics were analysed using descriptive statistics, with continuous variables reported as mean (standard deviation [SD]) and categorical variables reported as percentages. The primary outcome was analysed as the conditional probability of first achieving glycaemic control (HbA1c <7.5%) in every 3‐month period up to 24 months post‐premixed insulin initiation, the cumulative incidence of achieving glycaemic control (HbA1c <7.5%) over 24 months post‐premixed insulin initiation as a Kaplan–Meier estimation, and the mean (standard error [SE]) change in HbA1c levels over 24 months after premixed insulin initiation. If participants had multiple HbA1c measurements during the baseline HbA1c period (−90 to 14 days postpremixed insulin initiation), the measurement closest to the index date was used as the baseline level. If a participant had multiple HbA1c measurements during any 3‐month period, the lowest value closest to the 90‐day point was used.
Sensitivity analyses evaluated the cumulative incidence of improving glycaemic control (defined as a reduction in HbA1c of ≥1% or ≥2% from baseline) over 24 months postpremixed insulin initiation as a Kaplan–Meier estimation.
Kaplan–Meier curves were generated for participants stratified by HbA1c category at baseline (≥9% and ≤10%, >10% and ≤11%, and >11%), age group (18‐44, 45‐54, 55‐64, 65‐75 and >75 years), and number of OADs at baseline (2 and ≥3); and prespecified pairwise log‐rank regression analyses were performed stratified by age group, baseline HbA1c and the number of OADs used at baseline.
Cox proportional‐hazards regression models were used to assess any associations between baseline characteristics and glycaemic control outcomes postpremixed insulin initiation following confirmation of the proportionality of hazards (e.g. Schoenfeld residual test). Alternative semi‐parametric methods were used if the proportionality assumption was violated. Baseline characteristics were included as covariates following a stepwise procedure (backwards until P < .2). Data are presented as hazard ratios (HR) and 95% confidence intervals (CIs) for each outcome.
For Kaplan–Meier estimates and Cox regression analyses, the event of interest was achieving glycaemic control. Participants were censored at treatment intensification, premixed insulin discontinuation, death, or end of follow‐up. Adjustment for multiplicity was unnecessary.
The influence of missing values was limited by the requirement for participants to have complete data at baseline and in the follow‐up period, and at least one valid HbA1c measurement both at baseline and during the follow‐up period.
3. RESULTS
Of 30,103 people who initiated premixed insulin from 1 January 2010 to 31 December 2016, 974 fulfilled the eligibility criteria (Table S2, Appendix S1). The majority of participants were male (n/N = 550/974: 56.5%), at least 55 years of age (720/974: 73.9%), obese or extremely obese (BMI >30 kg/m2; 507/974: 52.1%) and had a baseline HbA1c >11% (490/974: 50.3%; Table 1). Mean (SD) participant age was 62 (11.0) years. The most common co‐morbidities were hypertension (622/974: 63.9%), dyslipidaemia (220/974: 22.6%) and nephropathy (201/974: 20.6%). At least three OADs were used in the 12‐month preindex period in 440 (45.2%) participants, the most common being metformin (960/974: 98.6%), DPP‐4 inhibitors (493/974: 50.6%) and thiazolidinediones (413/974: 42.4%). The mean (SD) number of general practitioner (GP) visits in the year prior to index date was 17.1 (10.5) for the overall population.
TABLE 1.
Baseline demographics and disease characteristics
| Baseline characteristics | All participants (N = 974) |
|---|---|
| Age, years | 61.6 ± 10.96 |
| Age group, years | |
| 18‐44 | 62 (6.4) |
| 45‐54 | 192 (19.7) |
| 55‐64 | 284 (29.2) |
| 65‐75 | 326 (33.5) |
| >75 | 110 (11.3) |
| Sex, male | 550 (56.5) |
| Baseline HbA1c, % | 11.3 ± 1.66 |
| Baseline HbA1c categories, % | |
| ≥9‐10 | 247 (25.4) |
| >10‐11 | 237 (24.3) |
| >11 | 490 (50.3) |
| BMI, kg/m2 | 30.9 ± 6.20 |
| BMI category, kg/m2 | |
| ≤25 | 152 (15.6) |
| >25‐30 (overweight) | 266 (27.3) |
| >30‐40 (obese) | 432 (44.4) |
| >40 (extremely obese) | 75 (7.7) |
| Missing and unknown | 49 (5.0) |
| Co‐morbidities | |
| Hypertension | 622 (63.9) |
| Dyslipidaemia | 220 (22.6) |
| Nephropathy (including CKD) | 201 (20.6) |
| Peripheral vascular disease | 64 (6.6) |
| Congestive heart failure | 61 (6.3) |
| Stroke | 41 (4.2) |
| Valvular heart disease | 25 (2.6) |
| Neuropathy | 16 (1.6) |
| Retinopathy | 11 (1.1) |
| OAD use in 12‐month preindex period | |
| Metformin | 960 (98.6) |
| DPP‐4 inhibitors | 493 (50.6) |
| Thiazolidinediones | 413 (42.4) |
| Sulphonylureas | 182 (18.7) |
| SGLT‐2 inhibitors | 5 (0.5) |
| Number of OADs used in 12‐month preindex period | |
| 2 | 534 (54.8) |
| ≥3 | 440 (45.2) |
| GP visits in 1 year preindex | 17.1 ± 10.5 |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; DDP‐4, dipeptidyl peptidase‐4; GP, general practitioner; OAD, oral antihyperglycaemic drug; SD, standard deviation; SGLT‐2, sodium‐glucose linked co‐transporter‐2.
Data are n (%) or mean ± SD.
3.1. Achievement of glycaemic control (HbA1c <7.5%)
Over the 24‐month follow‐up period, the estimated probability of achieving glycaemic control defined as HbA1c <7.5% was highest during months 3‐6, with 18.2% of participants who had not achieved HbA1c <7.5% by month 3 having achieved this target by month 6 (Table 2). The probability of achieving glycaemic control was between 10% and 20% from month 3 to month 18. The cumulative probability of achieving glycaemic control plateaued between months 15‐24 (15.7%‐16.0%; Table 2).
TABLE 2.
Conditional probability of achieving glycaemic control by time after premixed insulin initiation
| Time after premixed insulin initiation, months | Participants who had not achieved glycaemic control, a n | Participants who first achieved glycaemic control within period, n | Estimated probability, b % | Cumulative probability, % |
|---|---|---|---|---|
| Glycaemic control: HbA1c <7.5 % | ||||
| 0‐3 | 459 | 26 | 5.7 | 2.7 |
| 3‐6 | 346 | 63 | 18.2 | 9.1 |
| 6‐9 | 204 | 28 | 13.7 | 12.0 |
| 9‐12 | 125 | 19 | 15.2 | 14.0 |
| 12‐15 | 76 | 8 | 10.5 | 14.8 |
| 15‐18 | 71 | 9 | 12.7 | 15.7 |
| 18‐21 | 40 | 3 | 7.5 | 16.0 |
| 21‐24 | 40 | 0 | 0 | 16.0 |
Abbreviation: n, number of participants.
Participants were required to be still receiving premixed insulin and have at least one valid HbA1c measurement recorded during the 3‐month period.
Estimated probability was calculated as the percentage of participants who first achieved glycaemic control during each 3‐month period among those who had not achieved glycaemic control previously and who were still receiving premixed insulin and had at least one valid HbA1c measurement recorded during that 3‐month period.
The cumulative incidence of achieving glycaemic control plateaued after month 12 and increased faster in participants with a baseline HbA1c of 9%‐10% or >10%‐11% than in those with a baseline HbA1c >11% (Figure 1A). The cumulative incidence of achieving glycaemic control was also significantly higher in those with baseline HbA1c 9%‐10% than in those with baseline HbA1c >11% (P = .0060). It was also significantly higher in participants aged 65‐75 years compared with those aged 45‐54 years (P = .017). There were no other notable differences in the cumulative incidence of achieving glycaemic control according to age or the number of OADs used at baseline. According to the Cox proportional hazards models, participants with baseline HbA1c 9%‐10% were significantly more likely to achieve glycaemic control compared with those with HbA1c >11% (HR 1.54, 95% CI 1.11‐2.14; P < .05; Figure 2). No other available baseline characteristics had a clinically significant effect on achieving glycaemic control (Figure 2). The mean (SD) number of GP visits in the year prior to index date was similar for those who did and did not achieve HbA1c <7.5% (17.2 [10.79] vs. 17.0 [10.36], respectively).
FIGURE 1.
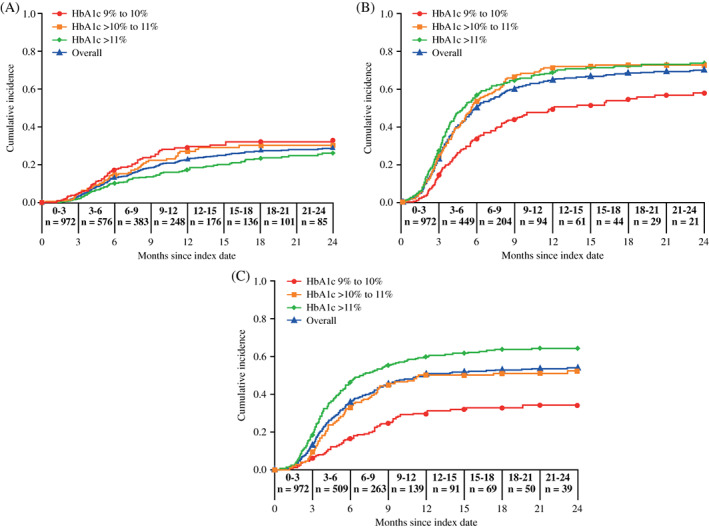
Cumulative incidence of glycaemic control achievement (A, HbA1c <7.5%) and glycaemic control improvement (B, ≥1% HbA1c reduction from baseline and C, ≥2% HbA1c reduction from baseline) in the overall population and by baseline HbA1c. n, number of participants
FIGURE 2.
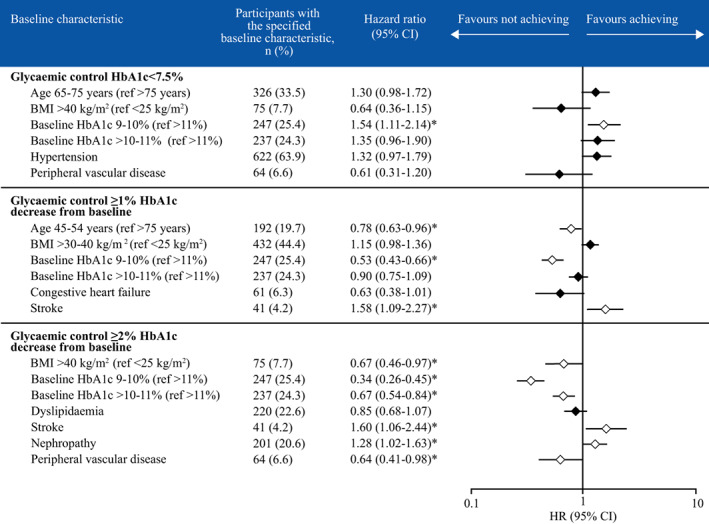
Associations between baseline characteristics and glycaemic control achievement or improvement. *P <.05. Cox proportional hazards model with stepwise variable selection (backwards selection until P <.2). BMI, body mass index; CI, confidence interval; HR, hazard ratio
Mean (SE) change in HbA1c from baseline was −1.43% (0.08%) at months 0‐3, before remaining between −2.14% and −2.41% from 3‐6 months to 21‐24 months (Figure 3).
FIGURE 3.
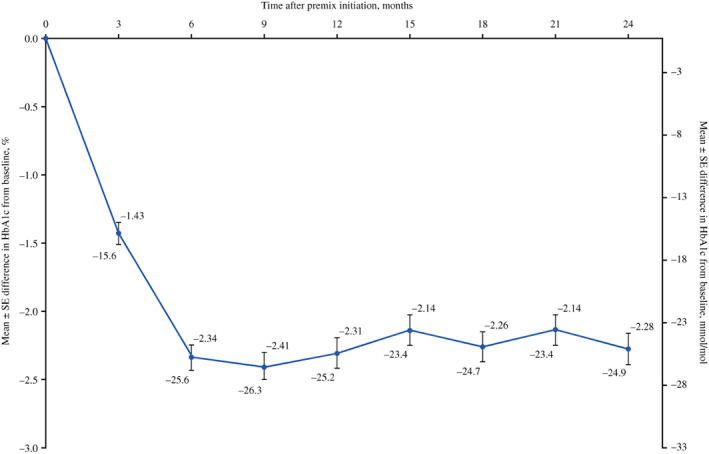
Mean (SE) HbA1c difference from baseline over time after premixed insulin initiation. SE, standard error
3.2. Improvement of glycaemic control
3.2.1. ≥1% HbA1c reduction from baseline
In the sensitivity analysis, the cumulative incidence of improving glycaemic control (defined as a ≥1% reduction in HbA1c from baseline) plateaued after 12 months (Figure 1B). The cumulative incidence of achieving a ≥1% reduction in HbA1c was significantly lower in participants with baseline HbA1c 9%‐10% compared with participants with baseline HbA1c >10%‐11% (P <.0005) or >11% (P < .0005). The cumulative incidence of improving glycaemic control was significantly higher in participants aged 65‐75 years compared with those aged 45‐54 years (P = .011). There were no other notable differences in the cumulative incidence of improving glycaemic control according to age or the number of OADs used at baseline. Based on the Cox proportional hazards models, participants were significantly less likely to achieve a ≥1% reduction in HbA1c if they were 45‐54 years of age compared with those aged >75 years (HR 0.78, 95% CI 0.63‐0.96; P <.05) or had baseline HbA1c 9%‐10% compared with an HbA1c >11% (HR 0.53, 95% CI 0.43‐0.66; P <.05; Figure 2). Conversely, participants were significantly more likely to achieve a ≥1% HbA1c decrease if they had experienced stroke (HR 1.58, 95% CI 1.09‐2.27; P < .05). No other available baseline characteristics had a significant effect on improving glycaemic control (Figure 2).
3.2.2. ≥2% HbA1c reduction from baseline
In the sensitivity analysis, the cumulative incidence of improving glycaemic control (defined as a ≥2% HbA1c reduction from baseline) plateaued after 12 months (Figure 1C). The cumulative incidence of achieving a ≥2% reduction in HbA1c was significantly lower in participants with baseline HbA1c 9%‐10% compared with participants with baseline HbA1c >10%‐11% (P < .0005) and HbA1c >11% (P < .0005), and in participants with baseline HbA1c >10%‐11% compared with baseline HbA1c >11% (P = .0008). By comparison, there was no significant difference in improvement in glycaemic control by age or the number of OADs used at baseline. Based on the Cox proportional hazards models, participants were significantly less likely to achieve a ≥2% reduction in HbA1c if they had a BMI >40 kg/m2 compared with a BMI <25 kg/m2 (HR 0.67, 95% CI 0.46‐0.97; P < .05), had a baseline HbA1c of 9%‐10% (HR 0.34, 95% CI 0.26‐0.45; P < .05) or >10%‐11% (HR 0.67, 95% CI 0.54‐0.84; P < .05) compared with >11%, or had peripheral vascular disease (HR 0.64, 95% CI 0.41‐0.98; P < .05; Figure 2). Conversely, participants were significantly more likely to achieve a ≥2% HbA1c decrease if they had a history of stroke (HR 1.60, 95% CI 1.06‐2.44; P < .05) or nephropathy (HR 1.28, 95% CI 1.02‐1.63; P < .05). No other available baseline characteristics had a significant effect on improving glycaemic control (Figure 2).
4. DISCUSSION
In this cohort of people with inadequately controlled (HbA1c ≥9%) T2D in the UK who switched from OADs to premixed insulin, the cumulative probability of achieving glycaemic control defined as an HbA1c <7.5% was generally low and was highest during months 3‐6 (18.2%). Likewise, the cumulative incidence of achieving glycaemic control was low, and little additional benefit in terms of achieving glycaemic control was achieved beyond month 12 after initiating premixed insulin. Notably, HbA1c did not decrease further beyond month 6, although the reduction in HbA1c was sustained until month 24. The cumulative incidence of achieving glycaemic control (HbA1c <7.5%) was significantly higher in those with lower baseline HbA1c levels, as might be expected. However, interestingly, in the pairwise log‐rank regression analyses, those aged 65‐75 years had a significantly higher cumulative incidence of glycaemic control than those aged 45‐54 years. This may be because older individuals have more time available to adhere to a strict insulin regimen because of reduced work commitments, a greater awareness in the healthcare profession that older people are a high‐risk group, or involvement from other healthcare providers in the treatment and management of T2D in older individuals.
The cumulative incidence of improving glycaemic control (defined as a ≥1% or ≥2% reduction in HbA1c from baseline) also plateaued after 12 months and as expected was lower in those with the lowest baseline HbA1c.
Our results show that despite not reaching glycaemic target (HbA1c <7.5%), many participants remained on premixed insulin at 6 months (n = 204) and 12 months (n = 76). These data provide evidence for the therapeutic inertia and unmet medical need that exists regarding treatment intensification in people with T2D. 5 , 6 , 7 , 8 While therapeutic inertia, a recognized and significant barrier to optimal glycaemic control in people with T2D, occurs at all stages of diabetes treatment, the longest delays are reported for the initiation and intensification of insulin therapy. 20 Delaying insulin therapy is often caused by fear of hypoglycaemia, multiple daily injections and weight gain, leading to patients staying on the same therapy despite not achieving or maintaining glycaemic control. 20
In patients with a high baseline HbA1c, an improvement of ≥1% or ≥2% in HbA1c may be more achievable than reaching HbA1c <7.5%. The NICE guidelines suggest relaxing the HbA1c goal for people for whom reaching HbA1c ≤7% may not be feasible, including people with co‐morbidities for T2D such as established cardiovascular disease. 8 This might explain why participants with peripheral vascular disease were less likely to see greater improvements in glycaemic control (≥2% decrease in HbA1c). However, participants were more likely to achieve HbA1c reduction (≥1% and ≥2% from baseline) if they had previously experienced a stroke. There is no clear explanation for these contrasting results, although participants with a history of stroke could be more rigorously supervised, particularly if a loss of function results in a third party administering insulin therapy.
In a similar manner, participants were more likely to improve their glycaemic control (≥2% decrease in HbA1c) if they had nephropathy. Renal impairment reduces the renal clearance of insulin, 21 which may result in higher levels of premixed insulin being retained in this subgroup of participants, possibly resulting in the observed improvement in glycaemic control. However, impaired kidney function is a well‐known risk factor for hypoglycaemia, 22 , 23 and therefore other insulin therapy regimens with a lower hypoglycaemia risk may be more suitable in some cases. A history of chronic kidney disease has been shown to be one of the factors that increases the probability of treatment intensification with insulin therapy in people with T2D uncontrolled on non‐insulin antidiabetic drugs. 24 Oral medications, such as metformin or sodium‐glucose linked co‐transporter‐2 inhibitors, are not suitable for individuals with more advanced chronic kidney disease as they are not licensed for use if the estimated glomerular filtration rate is below 30 mL/min/1.73 m2.
Participants were less likely to improve glycaemic control if they had a BMI of >40 kg/m2, which aligns with previous results showing that obesity is associated with inadequate response to insulin therapy initiation in people with T2D and secondary failure. 25
A strength of this study is the use of data from THIN database, which is generalizable to the UK national population in terms of demographics, the prevalence of various chronic conditions and mortality rates. 26 However, certain data are not available in THIN database, such as socioeconomic status, some lifestyle factors, detailed and complete information on insulin dose, duration of diabetes, and weight and hypoglycaemia data. This represents a limitation of the current analysis as these factors may have affected glycaemic control but could not be included as covariates. Other limitations include that HbA1c was not measured according to a set schedule, which may have introduced surveillance bias if participants had their HbA1c levels measured more often because of a poorer state of health or major lifestyle changes. Stratification according to the type of premixed insulin used was not performed; as such it is not possible to determine HbA1c target achievement according to use of human or analogue premixed insulin. In addition, the approach used to handle missing data may have mitigated, but not entirely excluded, the bias introduced by missing data.
In conclusion, the incidence of achieving glycaemic targets on premixed insulin was low at 6 months with little additional clinical benefit beyond 12 months in people with uncontrolled T2D (HbA1c ≥9%) treated with premixed insulin as per the NICE recommendations. This suggests a high unmet need for early and timely therapy change with newer therapies that enable a higher proportion of patients to reach HbA1c targets with a lower risk of hypoglycaemia and a simpler treatment regimen.
CONFLICT OF INTEREST
EJ has received advisory board honoraria and grant/research support from Sanofi, and has received speaker honoraria from Bayer AG, Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Takeda. CT has been an investigator in clinical trials for Eli Lilly and Sanofi, and has acted as a speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi. RE has acted as an advisor and speaker for AstraZeneca, Boehringer Ingelheim, Lilly, MSD, Novo Nordisk and Sanofi. AA has received research support and honoraria from Amgen, Eli Lilly, Gelesis, NAPP, Novartis, Novo Nordisk and Sanofi. RL and KP are employees of Sanofi. AS was an employee of Sanofi at the time the analyses were performed and is currently an employee of CSL Behring. NN has no disclosures to declare. PR has received research support from Gan & Lee Pharmaceuticals, Mylan Pharma and Novo Nordisk. FGP has acted as an advisor for Abbott Diabetes, AstraZeneca, Novartis, Novo Nordisk and Sanofi; has been an investigator in clinical trials for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi; and has acted as a speaker for Abbott Diabetes, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Novartis, Novo Nordisk and Sanofi.
AUTHOR CONTRIBUTIONS
All the named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. RL, KP and AS designed and conceptualized the study. RL contributed to the acquisition of data. RL contributed to the analysis of data. All the authors participated in the interpretation of the data, the writing, reviewing and editing of the manuscript, and had final responsibility for approving the published version.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14298.
Supporting information
Appendix S1 Supporting Information
Table S1 Comorbidity medical diagnosis codes.
Table S2. Participant attrition at screening.
ACKNOWLEDGEMENTS
The authors would like to thank Ana Merino‐Trigo, PhD (Sanofi) for co‐ordinating the development, facilitating author discussions and review of this manuscript. Editorial assistance was provided by Tamsin Brown, MSc, and Jo Bentley, PhD, of Fishawack Communications Ltd, and was funded by Sanofi. All the authors take complete responsibility for the interpretation of the data in this paper. These data were previously partially presented at the 80th American Diabetes Association Scientific Sessions virtual meeting, 12‐16 June 2020, and at the 56th annual meeting of the European Association for the Study of Diabetes (EASD) virtual meeting, 21‐25 September 2020. This study was funded by Sanofi, Paris, France. The funder of the study was involved in the study design; data collection, analysis and interpretation; and writing of the report.
Jude EB, Trescoli C, Emral R, et al. Effectiveness of premixed insulin to achieve glycaemic control in type 2 diabetes: A retrospective UK cohort study. Diabetes Obes Metab. 2021;23:929–937. 10.1111/dom.14298
Funding information Sanofi
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from CEGEDIM Health data. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors at https://www.cegedim-health-data.com/cegedim-health-data/thin-the-health-improvement-network/ with the permission of CEGEDIM Health data.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas, 9th edn. Brussels, Belgium; 2019. Available at: http://www.diabetesatlas.org. [Google Scholar]
- 2. Diabetes UK . Us, diabetes and a lot of facts and stats. https://www.diabetes.org.uk/resources-s3/2019-02/1362B_Facts%20and%20stats%20Update%20Jan%202019_LOW%20RES_EXTERNAL.pdf. Accessed February 2020.
- 3. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta‐analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(Suppl 2):S22‐S28.e21‐e22. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association . Standards of medical care in diabetes ‐ 2020. Diabetes Care. 2020;43(Suppl 1):S1‐S212. [DOI] [PubMed] [Google Scholar]
- 6. Davies M, D'Alessio D, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461‐2498. [DOI] [PubMed] [Google Scholar]
- 7. The Society for Endocrinology Metabolism and Diabetes of South Africa Type 2 Diabetes Guidelines Expert Committee . SEMDSA 2017 guidelines for the management of type 2 diabetes. JEMDSA. 2017;22(1(Supplement 1)):S1‐S196. [Google Scholar]
- 8. National Institute for Health and Care Excellence . NICE guideline [NG28]. Type 2 diabetes in adults: management. August 2019. https://www.nice.org.uk/guidance/ng28. Accessed January 2020.
- 9. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281(21):2005‐2012. [DOI] [PubMed] [Google Scholar]
- 10. Nuhoho S, Vietri J, Worbes‐Cerezo M. Increased cost of illness among European patients with type 2 diabetes treated with insulin. Curr Med Res Opin. 2017;33(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 11. Anyanwagu U, Owen K, Mamza J, et al. Demographics, insulin use and clinical targets in type 2 diabetes insulin users: comparison of a local integrated diabetes services vs a UK‐wide cohort. Pract Diabetes. 2017;34(4):123‐128. [Google Scholar]
- 12. Blak B, Smith H, Hards M, Maguire A, Gimeno V. A retrospective database study of insulin initiation in patients with type 2 diabetes in UK primary care. Diabet Med. 2012;29(8):e191‐e198. [DOI] [PubMed] [Google Scholar]
- 13. Bellido V, Suarez L, Galiana Rodriguez M, et al. Comparison of basal‐bolus and premixed insulin regimens in hospitalized patients with type 2 diabetes. Diabetes Care. 2015;38:2211‐2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qayyum R, Bolen S, Maruthur N, et al. Systematic review: comparative effectiveness and safety of premixed insulin analogues in type 2 diabetes. Ann Intern Med. 2008;149(8):549‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holman RR, Farmer AJ, Davies MJ, et al. Three‐year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736‐1747. [DOI] [PubMed] [Google Scholar]
- 16. Janka HU, Plewe G, Riddle MC, Kliebe‐Frisch C, Schweitzer MA, Yki‐Jarvinen H. Comparison of basal insulin added to oral agents versus twice‐daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care. 2005;28(2):254‐259. [DOI] [PubMed] [Google Scholar]
- 17. Wu T, Betty B, Downie M, et al. Practical guidance on the use of premix insulin analogs in initiating, intensifying, or switching insulin regimens in type 2 diabetes. Diabetes Ther. 2015;6:273‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peyrot M, Rubin R, Kruger D, Travis L. Correlates of insulin injection omission. Diabetes Care. 2010;33:240‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giugliano D, Maiorino MI, Bellastella G, Esposito K. Clinical inertia, reverse clinical inertia, and medication non‐adherence in type 2 diabetes. J Endocrinol Invest. 2019;42(5):495‐503. [DOI] [PubMed] [Google Scholar]
- 21. Cavanaugh K. Diabetes management issues for patients with chronic kidney disease. Clin Diabetes. 2007;25(3):90‐97. [Google Scholar]
- 22. Mezquita‐Raya P, Reyes‐García R, Moreno‐Pérez Ó, et al. Position statement: hypoglycemia management in patients with diabetes mellitus. Diabetes Mellitus Working Group of the Spanish Society of Endocrinology and Nutrition. Endocrinol Nutr. 2013;60(9):517.e1‐517.e18. [DOI] [PubMed] [Google Scholar]
- 23. Chow LS, Zmora R, Ma S, Seaquist ER, Schreiner PJ. Development of a model to predict 5‐year risk of severe hypoglycemia in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2018;6(1):e000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mata‐Cases M, Franch‐Nadal J, Real J, et al. Therapeutic inertia in patients treated with two or more antidiabetics in primary care: factors predicting intensification of treatment. Diabetes Obes Metab. 2017;20:103‐112. [DOI] [PubMed] [Google Scholar]
- 25. Lee Y‐H, Lee B‐W, Chun S, Cha B, Lee H. Predictive characteristics of patients achieving glycaemic control with insulin after sulfonylurea failure. Int J Clin Pract. 2011;65(10):1076‐1084. [DOI] [PubMed] [Google Scholar]
- 26. Blak B, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19:251‐255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Table S1 Comorbidity medical diagnosis codes.
Table S2. Participant attrition at screening.
Data Availability Statement
The data that support the findings of this study are available from CEGEDIM Health data. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors at https://www.cegedim-health-data.com/cegedim-health-data/thin-the-health-improvement-network/ with the permission of CEGEDIM Health data.


