Abstract
The genus Lolium comprises many species, of which L. perenne ssp. multiflorum, L. perenne ssp. perenne, and L. rigidum are of worldwide agricultural importance as both pasture crops and as weeds. These three species are inter‐fertile, obligate out‐crossers with a self‐incompatible reproduction system. This combination contributes to high genetic diversity that supplies new variants during expansion to new natural areas and agricultural environments. Human dispersal, de‐domestication and crop‐weed hybridization events between Lolium spp., or with others such as Festuca spp., are likely associated with their distinct weediness abilities. Furthermore, new introductions followed by introgression may hasten adaptation to new environments. Most Lolium‐related weed science studies have focused on adaptation leading to herbicide resistance, but other forms of adaptation may also occur. In this review, we explore how the wide genetic variation among Lolium species and hybridization with other species may contribute to range expansion, and adaptation to both new agricultural practices and future predicted climate change scenarios. © 2020 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: adaptation, evolution, genetic variation, herbicide resistance, hybridization, self‐incompatibility
The Lolium genus comprises of many species, of which L. perenne spp. multiflorum, L. perenne spp. perenne, and L. rigidum are of worldwide agricultural importance. Here, we explore questions related to the implication of the mating system of Lolium spp. and how it contributed to the wide genetic variation among populations. We also discuss questions related to intra‐specific and inter‐specific hybridization of Lolium spp., and the effect of admixture among Lolium populations and its contribution to rapid evolution of complex traits, adaptation to both new agricultural practices and predicted climate change scenarios.
1. INTRODUCTION
1.1. Taxonomy and distribution Lolium spp.
The Lolium genus (Poaceae) is comprised of several perennial and annual species. 1 Lolium spp. are native to Europe, temperate Asia, and North Africa but have spread over the last 200 years to southern parts of Africa, Australia, South America, New Zealand, and North America. 2 Lolium spp. were introduced into new regions mainly as pasture plants, turf and cover crops, in contaminated commercial crop seed, and livestock feed. Plant morphology is very similar among members of the genus Lolium. Species distinction can be detected in the flowering stage, where the number of flowers in each spike, as well as the inflorescence shape and structure, are distinguishable features. 3 L. perenne L. ssp. perenne (synonym Lolium perenne, common name; perennial ryegrass), L. perenne L. ssp. multiflorum (synonym Lolium multiflorum, common name; Italian ryegrass), and L. rigidum (common name; rigid ryegrass) are the most distinctive members of the Lolium genus (hereinafter referred to collectively as Lolium spp.). As crops, Lolium spp. are adapted to different climates and, consequently, their use patterns are divergent depending on the region grown and end use. L. perenne is adapted primarily to temperate climates, possesses greater cold tolerance compared to the other two species, 4 and is widely adopted around the globe for either turf or pasture due to its high feed value and perenniality. Conversely, L. multiflorum and L. rigidum exhibit mainly annual behavior, and the majority of their use is for pasture in warmer regions (e.g. southern United States and Australia). However, in the process of domestication, a natural and deliberate adaptation has occurred for Lolium spp. to establish as pastures. Thus, as part of the domestication process, different cultivars have been breed for greater heat or cold tolerance. However, and most importantly, they are considered major weeds of worldwide importance in cropping systems that infest numerous agricultural and non‐agricultural areas.
1.2. Yield reduction caused by Lolium spp.
Under severe infestation, L. rigidum can cause yield reductions of more than 30% in wheat. 5 L. rigidum is present in more than 800 000 ha of Spanish cereal fields where barley yield reductions of up to 85% were documented in field studies. 6 The evolution of herbicide resistance in Lolium populations makes control more difficult, thus increasing the likelihood of yield loss. Herbicide resistance to 14 herbicide mechanisms of action (MOA) has been reported in Lolium spp. 7 No other weed species has evolved herbicide resistance at this pace. A L. rigidum population originating from Western Australia evolved resistance to herbicides from seven different MOA, the most severe case of multiple herbicide resistance recorded to date. Lolium spp. have evolved both target site and non‐target site mechanisms of resistance. Furthermore, different herbicide resistance mechanisms to the same MOA have been reported within a single population. 8
It is clear that Lolium spp. possess a distinctive ability to rapidly adapt to new environments. However, in some parts of the world, Lolium spp. have colonized new areas where their presence had been previously prevented by environmental constraints. 9 We argue that the context in which Lolium spp. are dispersed, their mating system, and the genetic diversity inherent to this species complex play important roles in the current and future global spread. In this review, we (i) discuss the implication of the mating system of Lolium spp. and how it contributed to the wide genetic variation among populations, (ii) explore questions related to intra‐specific and inter‐specific hybridization of Lolium spp., (iii) discuss the effect of admixture among Lolium populations and its contribution to rapid evolution of complex traits, (iv) adaptation to both new agricultural practices and predicted climate change scenarios, and (v) future research directions.
2. SELF‐INCOMPATIBILITY AND GENETIC VARIATION
Pollination and fertilization in flowering plants involves a series of interactive events between the male (pollen grain) and the female (stigma) structures. Pollen may be rejected or accepted by the stigma depending on underlying genetic factors inherent to the organisms. Self‐incompatibility can depend entirely on the recognition of the genetic composition of the haploid pollen by the stigma (gametophytic), or can involve secretions from the stigmatic or the transmitting tissue that prevent the germination or growth of incompatible pollen. Gametophytic self‐incompatibility is the mechanism present in Lolium spp. that reduces the frequency of successful fertilization of pollen with unmatched loci. Gametophytic self‐incompatibility in flowering plants is controlled by several independent multi‐allelic loci. 10
2.1. Self‐incompatibility
The gametophytic self‐incompatibility system in Lolium spp. is governed by two multi‐allelic independent loci, S and Z. 11 In contrast to gametophytic self‐incompatibility mating systems in other grasses, not all alleles have to be matched in order to produce a self‐incompatibility reaction in Lolium spp. Thus, differences in reproduction success rate may be dependent on the direction of reciprocal crosses and the degree of compatibility between individuals. Additional loci may be involved in the gametophytic self‐incompatibility system in Lolium spp., 12 thus the complexity of the gametophytic self‐incompatibility mating system in Lolium spp. remains unclear. While the gametophytic self‐incompatibility system is efficient under normal environmental conditions, under adverse conditions (e.g. high temperature) the self‐pollination rate may be as high as 30%, a phenomenon known as pseudo‐self‐compatibility (Fig. 1). 13 , 14 Although these species are grown under mild temperatures during the vegetative stage, 4 late season seed production may occur under high temperatures where pseudo‐self‐compatibility is more likely to happen, considering these are winter annual species that flower as temperatures warm in the early summer. This fact may have been overlooked by weed scientists and may have been the cause for inconsistencies in some population genetics‐related studies where the assumption is made that Lolium spp. are obligate outcrossers regardless of the environmental conditions. One possible outcome of that assumption is that crossing of a herbicide‐susceptible and a herbicide‐resistant biotype for inheritance studies at high temperatures may result in seeds originating from the susceptible or resistant individual that have undergone selfing and thus may shift the ratios of susceptible to resistant individuals in the progeny. This shift may lead to herbicide resistance inheritance ratios in the population not following Mendelian segregation, resulting in inaccurate herbicide evolution prediction models. Using multiple families, pollinating plants at cool temperatures, and emasculation may aid in avoiding these issues.
Figure 1.
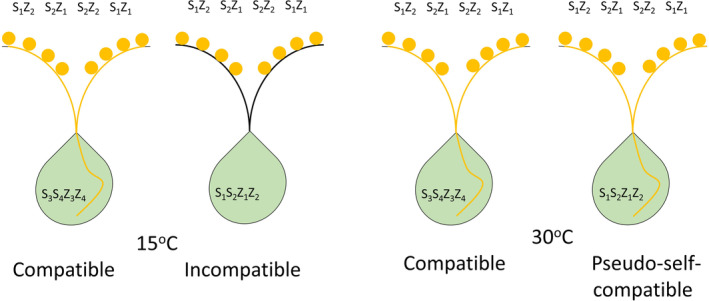
Effect of temperature on the reproduction system of Lolium spp. While under normal conditions Lolium spp. possess a gametophytic self‐incompatibility system, under high temperatures self‐pollination is enabled.
2.2. Effective population size
Due to their obligate outcrossing breeding systems, Lolium spp. are likely to exhibit a large effective population size (indexed size of a population that accounts for genetic events), with high heterozygosity and recombination rates resulting in large standing genetic variation. The effective population size of Lolium spp. has been inferred indirectly by means of linkage disequilibrium, because this estimate is dependent on the effective population size and the map distance between loci. 15 The gametophytic self‐incompatibility breeding system reduces the accumulation of deleterious alleles that may cause inbreeding depression, 16 and is a key trait in the recovery of populations (i.e. evolutionary rescue) following population bottlenecks.
Alleles that confer adaptation may arise in a population via different evolutionary modes: (i) de novo mutations; (ii) selection on standing genetic variation; and (iii) immigration. 17 Standing genetic variation and initial allele frequency may explain the variation in life history traits within a population attributing to the fast adaptability of Lolium spp. Thus, although initial allele frequency and population size may contribute to the rate of adaptive evolution, allelic exchange through the gametophytic self‐incompatibility mating system may have a more crucial role for adaptation processes. As obligate outcrossing species with wind‐mediated pollen movement, Lolium spp. contain high genetic variation within populations but low genetic differentiation between populations. 18 , 19 Successive selection by the same stress agent will have less effectiveness in reducing the census population size. This will potentially lead to rapid restoration of populations to their original size, but with decreased genetic diversity and greater tolerance to the initial stress among individuals. A widespread example of this scenario is the selection of Lolium populations to herbicides. Although this adaptability may be specific to the stress agent, this is not always the case. For instance, recurrent selection with a herbicide may result in metabolic cross resistance to several other herbicides from different MOA. 20 Other examples are greater seed dormancy levels 21 evolved under intensive cultivation, where late‐germinating individuals may avoid early season weed control, and selection for germination from greater soil depths to avoid shallow cultivation, and seedling avoidance of low‐mobility pre‐emergence herbicides. Differences in inter‐species competitive abilities, 22 such as higher seed retention and seed production were also recorded in some populations of L. rigidum, in response to intensive cultivation using the harvest weed seed control. Thus, reducing the overall effectiveness of this new weed control tactic.
2.3. Genetic variation in Lolium spp.
As a pasture species, the genetic variability in populations of L. perenne was mainly studied for its effect on vegetative persistence 23 and morphology. 24 L. multiflorum and L. rigidum are also economically significant weeds of cropping systems, where genetic variation has been studied mainly in the context of genetic inheritance and variability of herbicide resistance mechanisms. 18 , 25 In addition to the evolution of herbicide resistance, high genetic variation may also lead to biotic and abiotic stress adaptation, which are highly valuable characteristics in a breeding program. 26 , 27 However, these traits may also increase the weediness of the species and thus result in crop yield losses. High adaptability resulting in reduced sensitivity to various chemical and non‐chemical weed management approaches may be attributed to the large genetic variation observed among populations of Lolium spp. Evidence for this is in L. multiflorum and L. rigidum being reported among the ten weed species to evolve resistance to the greatest number of herbicides in a single field population. 7 These resistant populations exhibit both target site and non‐target site mechanisms of resistance.
Genetic variation as a result of intentional crop improvement efforts likely plays an important role in the introgression of new traits into local populations. Several regions around the world exhibit appropriate conditions for the establishment of cool‐season grass breeding programs. In Oregon, grass seed production dates back to the 1930s, when commercial production of L. multiflorum and L. perenne seed became popular, and by the 1960s, 11 varieties of both species were adopted by growers, where the majority were public varieties. In the 1980s, over 38 varieties were available and, currently, there are 76 L. multiflorum and 348 L. perenne certified varieties, produced and commercialized by many local grass seed farmers and breeding companies (https://www.oecd.org). In Australia, the early introductions of Lolium spp. were a combination of deliberate introductions and accidental introductions as contaminants. Starting in the early 20th century there was a more organized spread of Lolium spp. cultivars originally selected from sites where Lolium spp. had persisted and adapted to local conditions. Following this, there were deliberate introductions of new cultivars from South Africa, Tunisia and New Zealand. 28 Prior to 1990, the cultivars available for sowing were mainly L. perenne cultivars, but also included a small number of L. multiflorum, L. perenne × L. multiflorum hybrids and L. rigidum cultivars. There has been a marked increase in new cultivars since then. In 1993, there were six recommended cultivars of L. perenne including hybrids and three of L. multiflorum. Since 1993 there has been a large increase in new cultivars available in Australia, mainly from New Zealand, but also some from the United States and other countries and by 2013 there were 65 L. perenne and hybrid cultivars and 73 L. multiflorum and hybrid cultivars available. Around the world, there are over 2200 L. perenne and L. multiflorum varieties registered with the Organization for Economic Co‐operation and Development (OECD, https://www.oecd.org). Overall, the main breeding objectives for Lolium spp. have included: improved persistence of Lolium spp. cultivars; increased dry matter production; shorter flowering time to suit dry environments; the presence of endophytes that do not induce mammalian toxicities; tolerance to abiotic stresses; rapid regrowth after grazing; high persistence, and pest and disease tolerance. 28 These traits are likely to confer improved competitive abilities to weedy populations, particularly to new, challenging environments.
2.4. Symbiosis with endophytes
Epichloe spp. are fungal organisms that form symbiotic associations with Lolium spp. They inhabit the intercellular space of the host organism, and are often associated with increases in production of secondary metabolites (e.g. molecules involved in defence against herbivores), protection against a range of fungal pathogens, and enhanced protection against abiotic stresses, such as drought. Epichloe spp. provide drought tolerance to Lolium spp. by enhancing antioxidant production that buffers the damaging effects of reactive oxygen species. 29 Endophytes were reported in symbiosis within 77% of L. perenne populations from Denmark 30 and 100% of the populations from Australia. 31 Likewise endophytes were identified in 100% of 299 populations of L. rigidum from Australia. 32
There is limited research addressing the effects of fungal endophytes in Lolium spp. adaptation. Endophyte‐host associations are generally specific, and deviations from this may result in negative effects for the host and/or endophyte. Furthermore, host genetic background, in particular in situations with genetic structure in isolated populations or populations that experienced genetic bottleneck, may affect the compatibility with endophyte organisms. 33 Endophytes common to Lolium spp. are transmitted asexually, and inherently the genetic variability of these organisms is low, 32 whereas the breeding system of Lolium spp. results in high genetic variability of host populations.
A model has been proposed to explain the correlation between host genetic diversity and symbiosis with endophytes. 33 When the endophyte‐infected host population that possesses low levels of genetic variability, reduced fitness is expected. Despite the high host‐endophyte compatibility in this scenario, reduced fitness is likely associated with the high energy costs of maintaining the fungal organisms and host fitness compromised due to inbreeding depression. At high levels of genetic diversity in the host population, symbiotic interactions are expected to reduce fitness, because of incompatibilities between host and endophyte. Finally, at intermediate levels of host genetic diversity, host fitness is expected to be at the highest level, likely associated with heterosis and reduction in the energy expenses with maintaining the endophyte.
It is still unclear why L. rigidum and L. multiflorum exhibit high levels of endophyte infections, while still maintaining high levels of genetic variability. A possible explanation is that endophytes common to these species are not as specific as observed in other hosts species. If that is the case, then even small, founder populations after bottlenecks may take advantage of the symbiotic associations with endophytes to increase their fitness under new environmental conditions, or under conditions of stress (e.g. drought, flooding, herbicide, herbivory, etc.). Small increments in plant fitness may not avert extinction under challenging new environments, but could delay extinction until evolution takes place (by introduction of new genotypes, new mutations, or epigenetics).
3. INTRA‐SPECIFIC AND INTER‐SPECIFIC HYBRIDIZATION AND EPIGENETIC EFFECTS IN LOLIUM SPP.
Human‐mediated movement of Lolium spp. seed is an important component of the Lolium spp. allele dispersal network (Fig. 2). Although the center of origin of L. multiflorum and L. perenne is the Mediterranean basin, 2 the main seed production areas for commercial seed crops are in temperate regions of the globe, notably Oregon in the United States, Canada, Denmark, and New Zealand. These seed production areas typically host breeding programs to improve desirable traits for end users in Lolium spp., such as nitrogen content, digestibility, and pest resistance. From those countries, seeds have been shipped to the rest of the world, which is the main mechanism of long distance dispersal and source of new introduced genes to the gene pools of local Lolium populations. Dispersal of Lolium spp. seeds can also be via contaminated seed shipments. Lolium spp. seeds were found in wheat, barley and oilseed rape grain shipments imported from Western Australia to Japan, leading to plant dispersal around grain‐importation ports. 34 Once new gene pools are introduced locally, short‐distance dispersal by wind (via pollen) and commodity transport (via seed) are the predominant forms of the local allele dispersal network. With the free movement of alleles to new locations, de‐domestication processes start to take place, as newly introduced genotypes may exchange genetic material with locally adapted genotypes.
Figure 2.
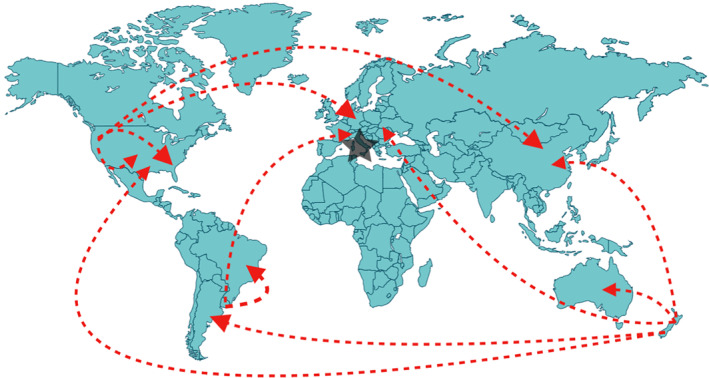
Current dispersion routes of alleles in Lolium spp. The star represents the center of origin of Lolium spp. Lolium spp. were initially grown for commercial reasons (mainly for pasture). At present, Lolium spp. seed production takes place in many countries around the world and are commercialized for pasture, turf, and as a cover crop and sold to many different markets (red dashed arrows). This human‐mediated long‐distance transport facilitates the introduction of new alleles to local populations around the world. 2
Species in the genus Lolium may also hybridize with members of the genus Festuca, where F. arundinacea and F. pratensis are the two most important cultivated forage species. Festuca spp. exhibit a wide range of ploidy levels, ranging from diploids to octoploid species. 35 Similar, to Lolium spp., Festuca spp. are also native to the Mediterranean basin, and spontaneous hybridization may occur. Lolium spp. and Festuca spp. hybrids may form, and typically these crosses result in male sterility (indehiscent anthers), and a small percentage of female fertility. 36 Festuca spp. are generally regarded as better adapted to abiotic and biotic stresses compared to Lolium spp., such as tolerance to harsh winter and summer drought, and leaf fungal diseases. 37 Therefore, crosses between members of these two genus may result in hybrids with superior performance as agricultural crops. However, given the high levels of sterility of intergeneric hybrids with Festuca spp. these are likely to contribute less to adaptation of Lolium populations as weeds of agricultural regions than hybrids among the Lolium spp. Further research is needed to fully understand this phenomenon.
3.1. Adaptation to new environments
Once a Lolium population experiences a new challenging environment (e.g. a new management practice or biotic/abiotic stress), elimination of most local individuals occurs, and it is expected that the genetic diversity (and effective population size) of the population will decrease. Unless new mutations arise in a timely manner, adaptation from standing genetic variation is the most common way that populations evolve. It is important to note, however, that because of the reduction in genetic diversity in the surviving individuals, the likelihood of adaptation to any additional environmental stress becomes low, simply because the required adaptive alleles might have become extinct from the population during the prior event. Therefore, additional immigration events may be the best way to supply new adaptive alleles in a timely manner. In this context, Lolium spp. are in a particularly advantageous position to recurrently adapt to new environments, as not only intra‐specific (Fig. 3(A)), but also inter‐specific (Fig. 3(B)) gene flow occurs from a number of close‐related species. Gene flow via pollen is known to occur among Lolium spp., as these species are inter‐fertile. For instance, when L. multiflorum is grown in close proximity to L. perenne, the hybridization rate may be as high as 47.5%. 38 The ability to intermate among Lolium spp. is a tremendous advantage to populations that are colonizing new environments or are exposed to challenging selection pressures. There are many examples of inter‐specific hybridization in the Lolium genus. Glyphosate‐susceptible L. multiflorum and glyphosate‐resistant L. perenne were crossed to study the inheritance pattern of glyphosate resistance. 39 Offspring of this cross showed moderate glyphosate‐resistance and 75% of the plants survived the treatment with recommended field rate, suggesting glyphosate resistance was inherited through a single locus with incomplete dominance. Gene flow via pollen transfer between different Lolium spp. may facilitate adaptation under field conditions. Inter‐specific hybrids may also be used in breeding systems to isolate genes of interest. Using crosses between the self‐incompatible L. multiflorum and the self‐fertile L. temulentum, a genetic linkage map was produced in order to study important agronomic traits in Lolium spp. 40 In regions where Lolium spp. are grown for seeds, as in Oregon, USA, and in regions of Canada and New Zealand, this may be an important source of contamination of feral traits to the seed crop, especially if highly undesirable traits, such as high dormancy levels, are present in the feral populations. The opposite is also likely to be true: gene flow from the crop may be an important source of new alleles in weedy populations. Alleles related to increased vigor and competition abilities at different developmental stages may be harnessed by weedy populations. This would allow weedy populations to ‘exploit’ the crop diversity and evolve enhanced growth characteristics, increasing their fitness and competitive abilities. Although both crop and weedy Lolium spp. are grown in close proximity in several countries, studies exploring the movement of crop productivity characteristics to weed populations are scarce.
Figure 3.
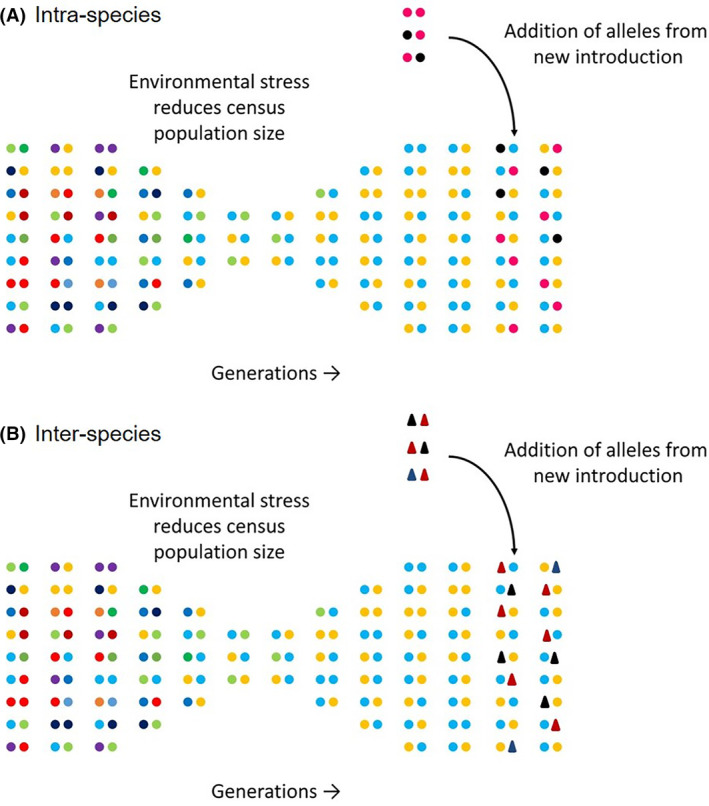
Following population bottlenecks, alleles that confer adaptation may arise in a population via immigration. This may be related to introduction of new alleles from new introduction of the same species (A) or via hybridization with other close‐related species (B). These attributes may explain the variation in life history traits within a population and facilitate evolution to new agricultural environments.
3.2. Stress‐induced adaptation
Weed management practices affect the overall plant performance, not only those traits involved in the survival in the new environment, but also to other unrelated traits. To illustrate this idea, ecological studies conducted to test the fitness cost of herbicide‐sensitive and ‐resistant Lolium populations can show differences in life history traits between the populations. 41 , 42 However, since many of these studies compare populations with different genetic backgrounds, the differences observed may be attributed to the high variability within Lolium spp., associated with a genetic bottleneck after recurrent herbicide applications, and may not be directly linked to the herbicide resistance alleles.
Given the rapid evolution observed in Lolium populations, the relative contribution between genetic and epigenetic modifications in the adaptability to challenging environments remains unclear. The rate of epigenetic alterations have been reported to be far greater than that of genetic mutations. 43 More epigenetics research is needed to assess the importance of this mechanism in the evolution of Lolium populations as major weed species, as limited research has addressed this topic in weeds and other non‐model plants in general. Epigenetic mechanisms might be particularly important in Lolium populations that do not carry the adapted standing alleles in environments that change continually. Furthermore, inter‐specific hybridization not only recruits new alleles to plant populations, but may also alter the epigenome of the offspring. 44 , 45 DNA methylation has been shown in other plant species to be the main epigenetic mechanism that allows plant populations to respond to sudden environment changes, 46 , 47 as frequently as it happens in agricultural systems. Environmental clues, particularly non‐lethal stresses, elicit epigenetic variations in plants that are inheritable and may be stable for as long as the stress continues. This mechanism may provide the phenotypic plasticity necessary to survive while waiting for additional introductions to occur or new mutations to arise. A common example of non‐lethal stress to weeds is the use of low herbicide rates, or herbicide applications on older weeds. For example, a L. rigidum population from Australia was exposed to sub‐lethal rates of pyroxasulfone, a very‐long chain fatty acid inhibitor, for three generations, resulting in an offspring that exhibited increased levels of resistance to this herbicide. 48 In another L. rigidum population from Australia, recurrent selection for three subsequent generations with reduced rates of diclofop‐methyl, resulted in resistance to the commercial field rate of diclofop‐methyl. Increased resistance to other acetyl‐coenzyme A carboxylase inhibitors (fluazifop‐P‐butyl, haloxyfop‐R‐methyl and clethodim) and to acetolactate synthase inhibiting herbicides (imazethapyr) was also observed. 49 Three subsequent selections have led to reduced sensitivity to glufosinate in a L. multiflorum population from California, USA, however, in this case, no cross resistance to other herbicides from different modes of action was recorded. 50 Although the presence of epigenetic modifications have not been tested in low‐dose selection studies, the observed increase in survival frequency may be associated with modifications in the epigenome.
4. ADAPTATION TO NEW AGRICULTURAL PRACTICES AND PREDICTED CLIMATE CHANGE SCENARIOS
4.1. Phenological adaptation to weed management practices
Resistance to herbicides is not the only adaptation by Lolium spp. to agricultural practices. In southern Australia, Lolium spp. was widely planted as a pasture species in provide valuable fodder for sheep production that dominated agricultural activity until the late 1980s. Following a decline in wool prices, more land was devoted to growing grain crops. Early studies with Lolium spp. seed germination in the 1970s and early 1980s showed low persistence of seed in the soil seed bank with less than 10% persisting between years. 51 By the 2000s, 20% of the seed remained dormant between years. 52 This change in seed dormancy is likely driven by continuous crop production systems where early germinating weeds were controlled by herbicides. In Western Australia, a correlation was observed between the extent of herbicide resistance in populations and increased seed dormancy. 22 Populations of Lolium spp. from continuously cropped fields have also changed with more of the population emerging later to avoid early season controls. 53 Similar patterns of phenotypic shifts have been identified in the germination rate, dormancy, and seed aging, as well as response to herbicide treatment, after L. multiflorum was allowed to grow in isolation or in mixture with the close‐related species F. rubra over a 5‐year period. 53 The shifts in germination response were likely due to the survival of late emerging individuals that carry alleles for increased dormancy. Because sublethal doses of herbicides were used, it is possible that epigenetic alterations may be involved in the shifts observed.
Early emerging weed populations are less likely to be exposed to post‐emergence wheat herbicides, therefore, selection pressure from herbicides is more pronounced on weeds that germinate after the wheat sowing operations are concluded. The presence of sub‐populations within Lolium populations is a clear example of the genetic diversity in these species, and the likelihood of adaptation to new management practices. Late germinating sub‐populations of L. multiflorum evolved diclofop resistance, whereas the early germinating remained susceptible. 54 The differences in germination timing could be attributed to different temperature requirements of the sub‐populations, an example of within population variability that allows Lolium spp. to thrive not only in the presence of herbicide (because there are resistant and susceptible sub‐populations), but also to tillage (because of the non‐uniform germination pattern). Early germinating seeds are exposed to tillage operations as part of the soil preparation in the wheat crop, whereas late germinating seeds will develop plants that are exposed solely to the herbicide.
4.2. Adaptation to altered environmental conditions
There has been adaptation to changing environments in Lolium spp. For example, time to flowering in Lolium spp. collected from Western Australian fields was correlated with the extent of growing season. 55 Flowering occurred earlier in collections from lower latitudes, where the growing season is shorter. Flowering occurred later in collections from higher latitudes (southern locations in the wheatbelt of Western Australia) with a 25 day difference in time to 50% spike emergence over 6° of latitude. There has also been range expansion of Lolium spp. in Australia. Despite being a weed of Mediterranean climate systems, Lolium spp. is now present in the summer rainfall dominant northern grain growing region of Australia where it was traditionally absent. 8 There has also been seasonal expansion where recently Lolium spp. has become a serious weed of cotton production systems in Australia, even though Lolium spp. is usually a winter weed and cotton is a summer crop. 56 Lolium populations expanding to new environments may be negatively affected by inbreeding depression and low genetic variation (Fig. 4). These effects may be reduced by an inter‐specific hybridization event, serving as an additional source of variation, allowing them to restore the genetic diversity and counter inbreeding depression. Hybridization may also produce new phenotypes that were absent in the parental populations as reviewed elsewhere. 57 The role of heterosis in weed populations and its importance in adaptation in Lolium populations remain unclear.
Figure 4.
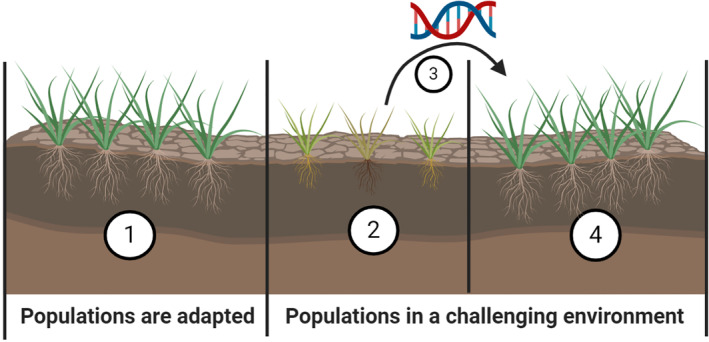
Constraints for range expansion of Lolium populations. A population adapted to local conditions will not expand to new environments unless beneficial alleles are introduced (1). If individuals manage to establish, plant development will be compromised because of inbreeding depression and lack of adapted alleles (2). Once new alleles are introduced to the population (3), colonization of new environments is now possible and genetic diversity is partially restored (4).
4.3. Genetic diversity hastens the adaptation of Lolium spp. as an invasive species
The preceding examples show how Lolium spp. can adapt to changing agricultural practices due to the high genetic diversity present within populations, 18 , 19 potential involvement in epigenetics in the rapid adaptation, and migration. The movement of Lolium populations to agricultural landscapes provides an opportunity for exchange of genetic material to create new combination of traits that enable adaptation. Australia is a good example where there have been multiple introductions of Lolium spp. overlaid by the widespread distribution of ‘Wimmera’ cultivar (L. rigidum) as a pasture species. 58 The introduction of new genotypes may be sufficient to drive populations that are currently of little concern to expand their range and become more troublesome. Lolium spp., as a result of its forage value, have multiple avenues for distribution: through deliberately sown seed; as a contaminant in hay or grain; inside animals shipped between countries; and on vehicles. Because of the recruitment of new allele sources associated with the movement of Lolium spp., the expansion of these species to new agricultural and non‐agricultural environments, as well as adaptation to new management practices, are inevitable.
An obvious example of how Lolium spp. adapt to management practices is the evolution of herbicide resistance. Initially, resistant individuals are at extremely low frequencies in a population. However, repeated use of herbicides will increase the frequency of resistant individuals, shifting the mean response to a herbicide that is repeatedly utilized. Although other examples of agricultural adaptation in Lolium spp. are scarce, one could hypothesize a number of ways populations could adapt to management. For example, no‐till farming systems result in earlier sowing of crops. Where post‐emergent herbicides are unavailable, due to resistance, evolution of increased seed dormancy could be an advantage in these systems allowing individuals to avoid pre‐emergent herbicide controls. There is some limited evidence of this occurring in L. rigidum in Australia. 21 Likewise, Lolium spp. could adapt to the use of crop residue used as mulch by delaying its germination. This is because seed cohorts that germinate early in the season cannot push through the crop residue, but late‐germination cohorts may be successful after partial degradation of the crop residue.
There is no doubt that Lolium spp. will continue to evolve as agricultural practices change. The extent of herbicide resistance in Lolium spp. in Australia has resulted in the adoption of a range of harvest weed seed control practices to reduce the amount of weed seed returning to the weed seed bank. 59 Differences in the timing of seed shedding in Lolium populations exist 60 , 61 and could be easily selected. Early data suggests no significant increase in early seed shattering, 22 but the introduction of new genotypes with higher shattering may provide the trigger for change. Harvest weed seed control techniques challenge for the adoption of these techniques in wheat production regions in the Pacific Northwest in the United States.
The climate of much of the world's agricultural production areas is changing. The climate is predicted to become warmer in most locations, wetter in some, drier in others, and rainfall is predicted to become more erratic. Lolium spp. has the ability to become a more problematic weed with climate change, due to its ability to rapidly adapt to new environments. The examples earlier from around the world show the ability of Lolium populations to adapt to different climatic conditions.
5. PERSPECTIVES AND CHALLENGES FOR FUTURE RESEARCH
Although Lolium spp. have been studied for decades, many issues regarding weed biology, ecology, and evolution remain unanswered. Specifically, topics on (i) rapid distinction among Lolium spp., (ii) better understanding of trait dominance in hybrids, (iii) drivers of evolution to different management practices, and (iv) the use of basic knowledge to drive management decisions, require further attention from the scientific community.
5.1. Distinction among Lolium spp.
It is inarguable that it is difficult to tell apart Lolium spp., particularly in geographic locations where their distributions overlap. However, it is clear that, although these species are very close phylogenetically, their responses to the environment and management practices are distinct. Therefore, their correct identification is crucial before future studies on their biology are conducted. For instance, although L. perenne and L. multiflorum co‐occur in the Pacific Northwest in the United States, they have distinct biology and responses to the environment. L. multiflorum is more adapted to flooding conditions, whereas L. perenne is not. L. perenne requires a lengthy vernalization period in order to flower, whereas L. multiflorum does not exhibit such a requirement. More strikingly is the fact that herbicide resistance is more common in L. multiflorum than in L. perenne. These distinctions demand a more accurate identification of the Lolium spp. Even more problematic is that the inter‐fertility of the three species leads to introgression between these species, resulting in numerous hybrids. 62 However, pure populations still exist and even in a hybrid population the dominance of one species over the other in the genetic admixture may be pronounced. The correct identification of the genetic material being investigated is important, particularly in studies comparing the biology and ecology of Lolium populations. Molecular markers have been used in the past for this purpose and are relatively easy to perform. 34 , 63 Several of these approaches have been used to distinguish between Lolium spp., aimed at improving the effectiveness of breeding. Although hybrid cultivar plants of L. multiflorum × L. perenne were successfully detected using simple sequence repeat (SSR) markers, 34 more data and development of new molecular techniques are needed for faster identification of hybrids between Lolium spp. and of weed field populations. Here, we have suggested that hybridization between Lolium spp. has aided the adaptation and spread of weedy Lolium spp. types. However, there is currently little genetic evidence available that addresses this issue. It will be important to unravel the role of hybridization in the invasiveness of these weed species to better understand the risks for future range expansion.
5.2. Drivers of evolution to new management practices and environmental conditions
Throughout this article, we argued that the high levels of population heterozygosity maintained by the mating system in Lolium spp., along with the long‐distance gene flow, and potential involvement of epigenetics, are keys to the rapid adaptability to challenging environments. An example of range expansion may be the colonization of temperate and humid areas that are prone to flooding. However, limited research has focused on the phenotypic traits of hybrids and how trait dominance is expressed. The admixture proportions from each species should be examined, as it may affect the overall genetic diversity within the studied samples and likely affect the results.
Lolium spp. have exhibited an astonishing ability to adapt to new environments. However, much of the underlying adaptability processes remain unknown, especially because of the difficulties associated with correlating the genotypes with the phenotypes and the limited genomic resources available for this species. Some tools that could be used to investigate the genetic basis of evolution in Lolium spp. are, therefore, the various ‐omics. A chromosome level genome assembly could facilitate the identification of candidate genes involved in the various phenotypes observed in Lolium spp. There are currently several research groups interested in providing this resource to the scientific community.
5.3. The use of basic knowledge to drive management decisions
Given that Lolium spp. range expansion to new environments is unavoidable, predictive tools must be developed to diminish the impact of these new introductions. In particular, climate change predictions could be paired with other predictive models to anticipate where Lolium spp. have the potential to colonize and establish. 64 As previously noted, shifts in germination patterns and seed longevity have direct impact on the infestation potential of weeds, and knowing when seeds are more prone to germinate may assist land managers in the decision‐making process. An important approach that has recently emerged as a useful tool in weed management is harvest weed seed control. 22 This Australian innovation has been developed to target weed seed retained at crop maturity and thereby reduce the soil seed bank for next year. This tool was developed mainly for the harvest of grass weed seeds such as L. rigidum, a major weed in commercial wheat growing in several places around the world. Although no evidence for adaptation has yet been observed, 22 increased use of this tool could lead to the evolution of early flowering and seed shattering L. rigidum populations. As we have seen for many weed management practices (e.g. herbicides, tillage, etc.) the evolutionary response will not take long, as early flowering and seed shattering alleles probably exist in the genetic pool of these populations. Lolium spp. have colonized all types of agricultural and non‐agricultural environments, and have shown a tremendous potential to adapt to new environments. It is crucial that more basic knowledge is obtained to reduce the impact of Lolium spp. on agricultural areas, reduce their interference, and maintain the economic sustainability of production systems.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Jonathan Gressel for the opportunity to write this review and for his valuable comments and suggestions. The authors would also like to thank Dr Carol Mallory‐Smith for providing feedback and valuable discussion on an early version of this manuscript. CAB was partially supported by grants from the Oregon Department of Agriculture, Oregon Seed Council, Oregon Mint Commission, Oregon Wheat Commission, and OSU Agricultural Research Foundation. Figures were created with Biorender (Biorender.com).
Contributor Information
Maor Matzrafi, Email: maorm@volcani.agri.gov.il.
Caio Augusto Brunharo, Email: caio.brunharo@oregonstate.edu.
REFERENCES
- 1. Catalán P, Kellogg EA and Olmstead RG, Phylogeny of Poaceae subfamily Pooideae based on chloroplast ndhF gene sequences. Mol Phylogenet Evol 8:150–166 (1997). [DOI] [PubMed] [Google Scholar]
- 2. Humphreys M, Feuerstein U, Vandewalle M and Baert J, Ryegrasses, in Fodder Crops and Amenity Grasses, ed. by Boller B, Posselt UK and Veronesi F. Springer, New York, pp. 211–260 (2010). [Google Scholar]
- 3. Terrell EE, A taxonomic revision of the genus Lolium, Technical Bulletin 1392. US Department of Agriculture, Washington, DC, p. 2 (1968). [Google Scholar]
- 4. Zhang C, Zhang FS, Warnke S, Li L and Hannapel D, Identification of genes associated with cold acclimation in perennial ryegrass. J Plant Physiol 166:1436–1445 (2009). [DOI] [PubMed] [Google Scholar]
- 5. Lemerle D, Verbeek B and Coombes N, Losses in grain yield of winter crops from Lolium rigidum competition depend on crop species, cultivar and season. Weed Res 35:503–509 (1995). [Google Scholar]
- 6. Izquierdo J, Recasens J, Fernández‐Quintanilla C and Gill G, Effects of crop and weed densities on the interactions between barley and Lolium rigidum in several Mediterranean locations. Agronomie 23:529–536 (2003). [Google Scholar]
- 7. Heap I, The international survey of herbicide resistant weeds. Available: http://www.weedscience.com [Novenmber 2020].
- 8. Han H, Yu Q, Owen MJ, Cawthray GR and Powles SB, Widespread occurrence of both metabolic and target‐site herbicide resistance mechanisms in Lolium rigidum populations. Pest Manag Sci 72:255–263 (2016). [DOI] [PubMed] [Google Scholar]
- 9. Narwal S, Sindel BM and Jessop RS, Dormancy and longevity of annual ryegrass (Lolium rigidum) as affected by soil type, depth, rainfall, and duration of burial. Plant Soil 310:225–234 (2008). [Google Scholar]
- 10. Takayama S and Isogai A, Self‐incompatibility in plants. Annu Rev Plant Biol 56:467–489 (2005). [DOI] [PubMed] [Google Scholar]
- 11. Klaas M, Yang B, Bosch M, Thorogood D, Manzanares C, Armstead IP et al., Progress towards elucidating the mechanisms of self‐incompatibility in the grasses: further insights from studies in Lolium . Ann Bot 108:677–685 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slatter LM, Barth S, Manzanares C, Velmurugan J, Place I and Thorogood D, A new genetic locus for self‐compatibility in the outcrossing grass species perennial ryegrass (Lolium perenne). Ann Bot (2020). 10.1093/aob/mcaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilkins PW and Thorogood D, Breakdown of self‐incompatibility in perennial ryegrass at high temperature and its uses in breeding. Euphytica 64:65–69 (1992). [Google Scholar]
- 14. Bullitta S, Floris R, Hayward MD and Veronesi F, The reproductive system of a Lolium rigidum gaud. Population from Sardinia and its implications for breeding. Plant Breed 111:312–317 (1993). [Google Scholar]
- 15. Hayes BJ, Cogan NOI, Pembleton LW, Goddard ME, Wang J, Spangenberg GC et al., Prospects for genomic selection in forage plant species. Plant Breed 132:133–143 (2013). [Google Scholar]
- 16. Brown KE and Kelly JK, Severe inbreeding depression is predicted by the “rare allele load” in Mimulus guttatus . Evolution 74:587–596 (2020). [DOI] [PubMed] [Google Scholar]
- 17. Lee KM and Coop G, Population genomics perspectives on convergent adaptation. Philos Trans R Soc B Biol Sci 374:20180236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karn E and Jasieniuk M, Genetic diversity and structure of Lolium perenne ssp. multiflorum in California vineyards and orchards indicate potential for spread of herbicide resistance via gene flow. Evol Appl 10:616–629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang J, Dobrowolski MP, Cogan NOI, Forster JW and Smith KF, Assignment of individual genotypes to specific forage cultivars of perennial ryegrass based on SSR markers. Crop Sci 49:49–58 (2009). [Google Scholar]
- 20. Neve P and Powles S, High survival frequencies at low herbicide use rates in populations of Lolium rigidum result in rapid evolution of herbicide resistance. Heredity 95:485–492 (2005). [DOI] [PubMed] [Google Scholar]
- 21. Owen MJ, Goggin DE and Powles SB, Intensive cropping systems select for greater seed dormancy and increased herbicide resistance levels in Lolium rigidum (annual ryegrass). Pest Manag Sci 71:966–971 (2015). [DOI] [PubMed] [Google Scholar]
- 22. Walsh MJ, Broster JC, Aves C and Powles SB, Influence of crop competition and harvest weed seed control on rigid ryegrass (Lolium rigidum) seed retention height in wheat crop canopies. Weed Sci 66:627–633 (2018). [Google Scholar]
- 23. O'Connor JR, Jahufer MZZ and Lyons T, Examining perennial ryegrass (Lolium perenne L.) persistence through comparative genetic analyses of two cultivars after nine years in the field. Euphytica 216:36 (2020). [Google Scholar]
- 24. Katova A and Vulchinkov J, Variability of morphological characters of collection accessions of perennial ryegrass (Lolium perenne L.). Bulg J Agric Sci 25:1015–1023 (2019). [Google Scholar]
- 25. Yu Q, Han H, Nguyen L, Forster JW and Powles SB, Paraquat resistance in a Lolium rigidum population is governed by one major nuclear gene. Theor Appl Genet 118:1601–1608 (2009). [DOI] [PubMed] [Google Scholar]
- 26. Hulke BS, Watkins E, Wyse DL and Ehlke NJ, Freezing tolerance of selected perennial ryegrass (Lolium perenne L.) accessions and its association with field winterhardiness and turf traits. Euphytica 163:131–141 (2008). [Google Scholar]
- 27. Pearson A, Cogan NOI, Baillie RC, Hand ML, Bandaranayake CK, Erb S et al., Identification of QTLs for morphological traits influencing waterlogging tolerance in perennial ryegrass (Lolium perenne L.). Theor Appl Genet 122:609–622 (2011). [DOI] [PubMed] [Google Scholar]
- 28. Reed KFM, Perennial pasture grasses – an historical review of their introduction, use and development for southern Australia. Crop Pasture Sci 65:691–712 (2014). [Google Scholar]
- 29. Hamilton CE and Bauerle TL, A new currency for mutualism? Fungal endophytes alter antioxidant activity in hosts responding to drought. Fungal Divers 54:39–49 (2012). [Google Scholar]
- 30. Mette Dahl Jensen A and Roulund N, Occurrence of Neotyphodium endophytes in permanent grassland with perennial ryegrass (Lolium perenne) in Denmark. Agric Ecosyst Environ 104:419–427 (2004). [Google Scholar]
- 31. Reed KFM, Leonforte A, Cunningham PJ, Walsh JR, Allen DI, Johnstone GR et al., Incidence of ryegrass endophyte (Neotyphodium lolii) and diversity of associated alkaloid concentrations among naturalised populations of perennial ryegrass (Lolium perenne L.). Aust J Agr Res 51:569–578 (2000). [Google Scholar]
- 32. Kirkby KA, Pratley JE, Hume DE, Faville MJ, An M and Wu H, Incidence of endophyte Neotyphodium occultans in Lolium rigidum from Australia. Weed Res 51:261–272 (2011). [Google Scholar]
- 33. Gundel PE, Omacini M, Sadras VO and Ghersa CM, The interplay between the effectiveness of the grass‐endophyte mutualism and the genetic variability of the host plant. Evol Appl 3:538–546 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kurokawa S, Kobayashi H and Ikeda K, Genetic background of an invasive Lolium population in central Japan using chloroplast DNA and SSR markers. Weed Res 50:245–252 (2010). [Google Scholar]
- 35. Cheng Y, Zhou K, Humphreys MW, Harper JA, Ma X, Zhang X et al., Phylogenetic relationships in the Festuca–Lolium complex (Loliinae; Poaceae): new insights from chloroplast sequences. Front Ecol Evol 4:89 (2016). [Google Scholar]
- 36. Thomas HM, Morgan WG and Humphreys MW, Designing grasses with a future – combining the attributes of Lolium and Festuca . Euphytica 133:19–26 (2003). [Google Scholar]
- 37. Oertel C and Matzk F, Introgression of crown rust resistance from Festuca spp. into Lolium multiflorum . Plant Breed 118:491–496 (1999). [Google Scholar]
- 38. Arcioni S and Mariotti D, Selfing and interspecific hybridization in Lolium perenne L. and Lolium multiflorum Lam. evaluated by phosphoglucoisomerase as isozyme marker. Euphytica 32:33–40 (1983). [Google Scholar]
- 39. Yanniccari M, Istilart C, Giménez DO and Castro AM, Inheritance of glyphosate resistance in Lolium perenne and hybrids with Lolium multiflorum . Crop Prot 71:72–78 (2015). [Google Scholar]
- 40. Guan X, Hirata M, Ding C, Xu N, Yuyama N, Tan L et al., Genetic linkage map of Lolium multiflorum Lam. constructed from a BC1 population derived from an interspecific hybridization, L. multiflorum × Lolium temulentum L. × L. temulentum . Grassl Sci 60:142–149 (2014). [Google Scholar]
- 41. Matzrafi M, Gerson O, Rubin B and Peleg Z, Different mutations endowing resistance to acetyl‐coA carboxylase inhibitors results in changes in ecological fitness of Lolium rigidum populations. Front Plant Sci 8:1078 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vila‐Aiub MM, Neve P, Steadman KJ and Powles SB, Ecological fitness of a multiple herbicide‐resistant Lolium rigidum population: dynamics of seed germination and seedling emergence of resistant and susceptible phenotypes. J Appl Ecol 42:288–298 (2005). [Google Scholar]
- 43. Miryeganeh M and Saze H, Epigenetic inheritance and plant evolution. Popul Ecol 62:17–27 (2020). [Google Scholar]
- 44. Chodavarapu RK, Feng S, Ding B, Simon SA, Lopez D, Jia Y et al., Transcriptome and methylome interactions in rice hybrids. Proc Natl Acad Sci 109:12040–12045 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He G, Zhu X, Elling AA, Chen L, Wang X, Guo L et al., Global epigenetic and transcriptional trends among two rice subspecies and their reciprocal hybrids. Plant Cell 22:17–33 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nicotra AB, Segal DL, Hoyle GL, Schrey AW, Verhoeven KJF and Richards CL, Adaptive plasticity and epigenetic variation in response to warming in an alpine plant. Ecol Evol 5:634–647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang YY, Fischer M, Colot V and Bossdorf O, Epigenetic variation creates potential for evolution of plant phenotypic plasticity. New Phytol 197:314–322 (2013). [DOI] [PubMed] [Google Scholar]
- 48. Busi R, Gaines TA, Walsh MJ and Powles SB, Understanding the potential for resistance evolution to the new herbicide pyroxasulfone: field selection at high doses versus recurrent selection at low doses. Weed Res 52:489–499 (2012). [Google Scholar]
- 49. Neve P and Powles S, Recurrent selection with reduced herbicide rates results in the rapid evolution of herbicide resistance in Lolium rigidum . Theor Appl Genet 110:1154–1166 (2005). [DOI] [PubMed] [Google Scholar]
- 50. Matzrafi M, Morran S and Jasieniuk M, Recurrent selection with glufosinate at low rates reduces the susceptibility of a Lolium perenne ssp. multiflorum population to glufosinate. Agronomy 10:1288 (2020). [Google Scholar]
- 51. Gramshaw D, Germination of annual ryegrass seeds (Lolium rigidum Gaud.) as influenced by temperature, light, storage environment, and age. Aust J Agr Res 23:779–787 (1972). [Google Scholar]
- 52. Chauhan BS, Gill G and Preston C, Influence of tillage systems on vertical distribution, seedling recruitment and persistence of rigid ryegrass (Lolium rigidum) seed bank. Weed Sci 54:669–676 (2006). [Google Scholar]
- 53. Gundel PE, Martínez‐Ghersa MA and Ghersa CM, Dormancy, germination and ageing of Lolium multiflorum seeds following contrasting herbicide selection regimes. Eur J Agron 28:606–613 (2008). [Google Scholar]
- 54. Ghersa CM, Martinez‐Ghersa MA, Brewer TG and Roush ML, Selection pressures for diclofop‐methyl resistance and germination time of Italian ryegrass. Agron J 86:823–828 (1994). [Google Scholar]
- 55. Gill GS, Cousens RD and Allan MR, Germination, growth, and development of herbicide resistant and susceptible populations of rigid ryegrass (Lolium rigidum). Weed Sci 44:252–256 (1996). [Google Scholar]
- 56. Manalil S, Werth J, Jackson R, Chauhan BS and Preston C, An assessment of weed flora 14 years after the introduction of glyphosate‐tolerant cotton in Australia. Crop Pasture Sci 68:773–780 (2017). [Google Scholar]
- 57. Pfennig KS, Kelly AL and Pierce AA, Hybridization as a facilitator of species range expansion. Proceedings Biol Sci 283:20161329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kloot PM, The genus Lolium in Australia. Aust J Bot 31:421–435 (1983). [Google Scholar]
- 59. Walsh M, Ouzman J, Newman P, Powles S and Llewellyn R, High levels of adoption indicate that harvest weed seed control is now an established weed control practice in Australian cropping. Weed Technol 31:341–347 (2017). [Google Scholar]
- 60. Harun RMR and Bean EW, Seed development and seed shedding in north Italian ecotypes of Lolium multiflorum . Grass Forage Sci 34:215–220 (1979). [Google Scholar]
- 61. Elgersma A, Leeuwangh JE and Wilms HJ, Abscission and seed shattering in perennial ryegrass (Lolium perenne L.). Euphytica 39:51–57 (1988). [Google Scholar]
- 62. Dinelli G, Bonetti A, Lucchese C, Catizone P, Bravin F and Zanin G, Taxonomic evaluation of Italian populations of Lolium spp. resistant and susceptible to diclofop‐methyl. Weed Res 42:156–165 (2002). [Google Scholar]
- 63. Wu YG, Dong R, Luo D, Liu WX, Wang YR and Liu ZP, A rapid one‐step PCR protocol to distinguish between perennial (Lolium perenne) and annual (L. multiflorum) ryegrass seeds. Seed Sci Technol 45:444–454 (2017). [Google Scholar]
- 64. Castellanos‐Frías E, Garcia De León D, Bastida F and Gonzalez‐Andujar JL, Predicting global geographical distribution of Lolium rigidum (rigid ryegrass) under climate change. J Agric Sci 154:755–764 (2016). [Google Scholar]


