Abstract
Background
A prolonged prothrombin time (PT) is a common feature in sepsis indicating consumptive coagulopathy.
Objectives
To determine the association between a prolonged PT and aberrations in other host response mechanisms in sepsis.
Methods
Patients admitted to the intensive care unit with sepsis were divided in quartiles according to the highest PT value measured within 24 h after admission. The host response was evaluated by measuring 19 plasma biomarkers reflecting pathways implicated in sepsis pathogenesis and by blood leukocyte gene expression profiling.
Measurements and Main Results
Of 1524 admissions for sepsis, 386 (25.3%) involved patients with a normal PT (≤12.7 s); the remaining quartiles entailed 379 (24.9%) patients with a slightly prolonged PT (12.8 ≤ PT ≤ 15.0 s), 383 (25.1%) with an intermediately prolonged PT (15.1 ≤ PT ≤ 17.2 s), and 376 (24.7%) with an extremely prolonged PT (≥17.3 s). While patients with an extremely prolonged PT showed an increased crude mortality up to 1 year after admission, none of the prolonged PT groups was independently associated with 30‐day adjusted mortality. Comparison of the host response between patients with a normal PT or an extremely prolonged PT matched for baseline characteristics including severity of disease showed that an extremely prolonged PT was associated with impaired anticoagulant mechanisms, a more disturbed endothelial barrier integrity and increased systemic inflammation, and blood leukocyte transcriptomes indicating more prominent metabolic reprogramming and protein catabolism.
Conclusion
A prolonged PT is associated with stronger anomalies in pathways implicated in the pathogenesis of sepsis, suggesting that activation of coagulation impacts other host response mechanisms.
Keywords: endothelium inflammation, host response, intensive care unit, prothrombin time, sepsis
Essentials.
A prolonged prothrombin time (PT) is a feature in sepsis indicating consumptive coagulopathy.
We report an observational study in sepsis patients admitted to the intensive care unit of two Dutch hospitals.
A prolonged PT was associated with a more disturbed endothelial barrier integrity and increased inflammation.
This association occurred independent of differences in disease severity.
1. INTRODUCTION
Prothrombin time (PT) is a widely used test to detect coagulation abnormalities in critically ill patients. 1 , 2 The reported incidence of a prolonged PT, indicative of consumptive coagulopathy, ranges from 30% to 66% in the overall intensive care unit (ICU) population, 3 , 4 with an even higher incidence in critically ill patients with sepsis (up to 93%). 5 A prolonged PT on ICU admission was reported to be an independent risk factor for mortality in both critically ill patients 4 and patients with severe sepsis. 5 , 6 , 7
Besides PT, platelet counts are commonly used to determine the extent of coagulation activation. 1 , 2 The recently introduced term “sepsis‐associated coagulopathy” is defined by the presence of low platelet counts and a prolonged PT. 6 We previously reported that low platelet counts in ICU patients with sepsis are independently associated with increased mortality and a more disturbed host response. 8 Sepsis patients with platelet counts below 50 × 109/L showed enhanced activation of the cytokine network and the vascular endothelium; a more profound loss of vascular integrity; and a distinct whole‐blood leukocyte transcriptome pattern revealing decreased leukocyte adhesion, diapedesis, and extravasation signaling. 8 This observational study provided validity to a series of mouse studies documenting a protective and immune regulatory role of platelets in experimental sepsis. 9 , 10 , 11 Considering the pleiotropic functions of platelets beyond their role in hemostasis, 9 , 10 , 11 our earlier investigation left unanswered whether coagulation activation per se impacts the host response in patients with sepsis. This question is relevant in view of laboratory and animal studies pointing at an extensive cross‐talk between coagulation factors and endothelial cell function and inflammation, as well as investigations reporting protection against the lethal consequences of fulminant sepsis by inhibition of components of the coagulation system. 12 , 13
Our objective was to assess the association between consumptive coagulopathy, as reflected by a prolonged PT, and host response aberrations in patients admitted to the ICU with sepsis. To this end we measured 19 biomarkers indicative of coagulation activation, endothelial cell activation, and systemic inflammation in sepsis patients stratified according to admission PT. Additionally, in an unbiased approach we compared genome‐wide messenger (m)RNA profiles in blood leukocytes obtained from patients with a normal or extremely prolonged PT.
2. METHODS
2.1. Study design, patients, and definitions
This study was conducted as part of the Molecular Diagnosis and Risk Stratification of Sepsis (MARS) project, a prospective observational study in the mixed ICUs of two tertiary teaching hospitals (Academic Medical Center in Amsterdam and University Medical Center in Utrecht). 8 , 14 , 15 , 16 All consecutive patients above 18 years of age who presented with an expected length of stay longer than 24 h admitted between January 2011 and January 2014 were included via an opt‐out method approved by the medical ethical committees of the participating hospitals. The likelihood of infection for which the clinical team initiated antibiotics was classified for each infectious source as none, possible, probable, or definite by research physicians using Centers for Disease Control and Prevention 17 and International Sepsis Forum consensus definitions, 18 as described in Klein Klouwenberg et al. 14 For the current study sepsis was defined in accordance with the Sepsis‐3 definition 19 using data that were prospectively collected during the study period as the presence of infection diagnosed within 24 h after ICU admission with a likelihood of at least possible, accompanied by organ failure as indicated by a Sequential Organ Failure Assessment (SOFA) score of two or more (central nervous system was excluded). When the SOFA score was not available (n = 68) the following variables were used to define organ failure: mechanical ventilation, acute kidney injury (AKI), acute respiratory distress syndrome (ARDS), or shock, all on the first day of ICU admission. Hence, for the present investigation data contained within the MARS database were used to identify patients from the entire MARS cohort that fulfilled the Sepsis‐3 definition. AKI and ARDS were defined using strict pre‐set criteria. 20 , 21 Shock was defined by the use of noradrenaline for hypotension in a dose of >0.1 µg/kg/min during at least 50% of the ICU day. Complications that started 2 days or more after ICU admission were defined as ICU‐acquired. Patients were divided into quartiles using the highest PT value measured within 24 h after ICU admission. The first quartile was defined as normal. Patients with a PT in quartiles 2–4 were defined as admissions with a prolonged PT: slightly prolonged PT, intermediately prolonged PT, and extremely prolonged PT. Exclusion criteria were: the absence of a PT measurement or intravenous administration of heparin in the first 24 h after ICU admission, chronic use of a vitamin K antagonist prior to ICU admission, liver cirrhosis, readmissions for an infectious diagnosis within the same hospital admission or within 30 days after the first ICU admission, transferals from other ICUs, and patients with major bleeding on admission defined based on the consensus definition 22 , 23 as a fall in hemoglobin level of ≥2 g/dl within the first 24 h combined with transfusion of at least two red blood cell units during the first 24 h of ICU stay. For patients who were readmitted to the ICU after 30 days demographic and long‐term mortality data (≥30 days and more) are given for the first ICU admission.
2.2. Assays
PT and activated partial thromboplastin time (APTT) were measured daily using photometric methods with Dade Innovin Reagent and Dade Actin FS Activated PTT Reagent, respectively (Siemens Healthcare Diagnostics). Host response biomarkers were measured within 16 h after admission. For immune assays please see supporting information.
2.3. Blood gene expression microarrays
Whole blood was collected in PAXgene™ tubes (Becton‐Dickinson) within 24 h after ICU admission. Messenger RNA profiles were analyzed using Human Genome U219 arrays (Affymetrix) as described. 8 , 15 For details see supporting information.
2.4. Statistical analysis
All data distribution was analyzed for parametric distribution by Shapiro‐Wilk tests and using histogram plots. A Mann‐Whitney U or a Kruskal‐Wallis test was used to analyze continuous nonparametric data, presented as median and interquartile ranges (IQR, 25th and 75th percentiles). Continuous parametric data, presented as means ±standard deviation (SD), were analyzed using a Student's t‐test or analysis of variance when appropriate. Post hoc testing was performed using Dunn's test of multiple comparisons using rank sums for nonparametric continuous data, a Tukey post hoc testing for parametric continuous data, and a Bonferroni correction for categorical variables. Categorical data, presented as numbers (percentages), were analyzed using a chi‐square test. A multivariable Cox proportional hazard model was used to determine the association between PT categories (normal, slightly prolonged, intermediately prolonged, or extremely prolonged) and survival by day 30. Based on literature review and expert opinion, the model adjusted for age and APACHE IV score (model 2), and a more extensive model adjusted for the following confounders: age, sex, race, Acute Physiology and Chronic Health Evaluation (APACHE) IV acute physiology score (APS), immunocompromised state, renal compromise, malignancy, pulmonary or abdominal site of infection, infection by a Gram‐negative pathogen, lowest platelet count in the first 24 h, and number of plasma units transfused over the first 24 h (model 3).
We also investigated the association between PT as a continuous variable and 30‐day mortality by logistic regression. The same confounders as those used in the Cox proportional hazard model were considered for inclusion. Confounders were individually selected using a “change‐in‐estimate” approach, 24 with a cutoff of 10%. The absence of collinearity between variables was ascertained by a variance inflation factor lower than 4 and interactions between these variables and PT were investigated on a multiplicative scale.
To determine the association between PT and host response biomarkers in clinically similar patients, propensity score matching was used to select comparable patients from those with an extremely prolonged PT (cases) and those with normal PT levels (controls). For details see the supporting information.
All data were analyzed using R studio built under R version 3.0.2 (R Core Team 2013). A p value <.05 was considered to be of statistical significance.
3. RESULTS
3.1. Patients
The 3‐year study period included 2880 consecutive admissions for sepsis (Figure 1). After exclusion of patients with cirrhosis (n = 50, 1.7%), transfers from other ICUs (n = 294, 10.2%), readmissions within the same hospital stay or within 30 days after ICU discharge (n = 351, 12.2%), bleeding (n = 98, 3.4%), intravenous heparin administration (n = 288, 10.0%), chronic use of vitamin K antagonist prior to admission (n = 205, 7.1%), 1594 sepsis admissions remained. Of these, PT was not measured in 70 (4.4%) in the first 24 h after ICU admission, resulting in a final cohort of 1524 ICU admissions for sepsis. These admissions were divided into quartiles: 386 (25.3%) admissions involved patients with normal PT levels (≤12.7 s), 379 (24.9%) involved admissions with a slightly prolonged PT (12.8 ≤ PT ≤ 15.0 s), 383 (25.1%) involved admissions with an intermediately prolonged PT (15.1 ≤ PT ≤ 17.2 s), and 376 (24.7%) involved admissions with an extremely prolonged PT (≥17.3 s).
FIGURE 1.
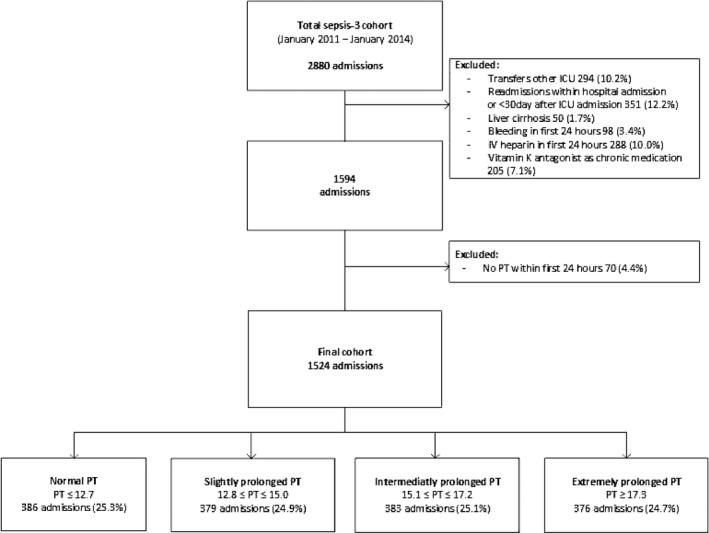
Flowchart of patient inclusion. ICU, intensive care unit; IV, intravenous; PT, prothrombin time
Patients with an extremely prolonged PT more often had comorbidities, particularly malignancy (31.3% vs. 13.1% in normal PT and 24.9% in slightly prolonged PT, overall p < .001), renal insufficiency (18.0% vs. 8.5% in normal PT, 8.8% in slightly prolonged PT, and 9.5% in intermediately prolonged PT, overall p < .001) and immunocompromise (26.4% vs. 17.1% in normal PT, overall p = .02; Table 1). Patients with an extremely prolonged PT were most severely ill on ICU admission relative to patients in the other PT groups, as reflected by the highest APACHE IV (acute physiology; 76 [59–97] vs. 53 [38–70] in normal PT, 60 [45–76] in slightly prolonged PT, and 64 [50–79] in intermediately prolonged PT, overall p < .0001) and SOFA scores (8 [6–10] vs. 6 [4–8] in normal PT, 5 [3–8] in slightly prolonged PT, and 6 [4–8] in intermediately prolonged PT, overall p < .0001), more often presenting with shock (154 [41.0%] vs. 55 [14.2%] in normal PT, 64 [16.9%] in slightly prolonged PT, and 85 [22.2%] in intermediately prolonged PT, overall p < .001) and AKI (191 [50.8%] vs. 114 [29.5%] in normal PT, 101 [26.6%] in slightly prolonged PT, and 111 [29.0%] in intermediately prolonged PT, overall p < .001), and more frequently in need of mechanical ventilation (298 [79.3%] vs. 270 [69.9%] in normal PT, 287 [75.7%] in slightly prolonged PT, overall p = .02). Abdominal sepsis was over‐represented in patients with an extremely prolonged PT (104 [27.7%] vs. 37 [9.6%] in normal PT, 50 [13.2%] in slightly prolonged PT, and 74 [19.3%] in intermediately prolonged PT, overall p < .001), while pneumonia and central nervous system infection were less frequent in this group (140 [37.2%] versus 221 [57.3%] in normal PT, 195 [51.5%] in slightly prolonged PT, and 195 [50.9%] in intermediately prolonged PT, overall p < .001 and 8 [2.1%] vs. 33 [8.5%] in normal PT, 33 [8.7%] in slightly prolonged PT, overall p = .02, respectively). Gram‐positive bacterial infection was more often documented in admissions with intermediately and extremely prolonged PT (170 [46.3%] and 177 [48.2%], respectively) compared to admissions with normal or slightly prolonged PT (128 [33.2%] and 132 [36.5%], respectively, overall p < .001). Gram‐negative bacterial infection was more often documented in patients with extremely prolonged PT (196 [52.1%]) compared to admissions with normal or slightly prolonged PT (149 [38.6%] and 156 [41.2%], respectively, overall p = .001). Patients with extremely prolonged PT were more often treated with fresh frozen plasma, vitamin K, and prothrombin complex concentrate on ICU admission and during ICU stay (Table S1 in supporting information).
TABLE 1.
Baseline characteristics and outcome of patient admissions for sepsis stratified by prothrombin time on admission
| Normal PT | Slightly prolonged PT | Intermediately prolonged PT | Extremely prolonged PT | p value | |
|---|---|---|---|---|---|
| PT ≤ 12.7 s | 12.8 ≤ PT ≤ 15.0 s | 15.1 ≤ PT ≤ 17.2 s | PT ≥ 17.3 s | ||
| Patients | 375 | 362 | 367 | 367 | |
| Admissions | 386 | 379 | 383 | 376 | |
| Age, mean (SD) | 59.2 (16.0) | 56.4 (16.7) | 60.0 (15.4) b | 61.2 (14.9) b | <.001 |
| Race, white, n (%) | 308 (82.1%) | 318 (87.8%) | 342 (93.2%) a | 340 (92.6%) a | <.001 |
| Gender, male, n (%) | 237 (63.2%) | 215 (59.4%) | 237 (64.6%) | 224 (61.0%) | .48 |
| Medical admission, n (%) | 292 (75.6%) | 280 (73.9%) | 276 (72.1%) | 281 (74.7%) | .06 |
| Chronic comorbidities, n (%) | |||||
| None | 143 (38.1%) | 113 (31.2%) | 90 (24.5%) a | 87 (23.7%) a | <.001 |
| Cardiovascular compromise | 81 (21.6%) | 58 (16.0%) | 77 (21.0%) | 93 (25.3%) b | .02 |
| COPD | 62 (16.5%) | 45 (12.4%) | 56 (15.3%) | 43 (11.7%) | .19 |
| Diabetes | 65 (17.3%) | 63 (17.4%) | 76 (20.7%) | 78 (21.3%) | .39 |
| Hypertension | 96 (25.6%) | 92 (25.4%) | 128 (34.9%) a , b | 108 (29.4%) | .02 |
| Immunocompromise | 64 (17.1%) | 84 (23.2%) | 80 (21.8%) | 97 (26.4%) a | .02 |
| Malignancy | 49 (13.1%) | 90 (24.9%) a | 109 (29.7%) a | 115 (31.3%) a , b | <.001 |
| Renal insufficiency | 32 (8.5%) | 32 (8.8%) | 35 (9.5%) | 66 (18.0%) a , b , c | <.001 |
| Respiratory insufficiency | 73 (19.5%) | 65 (18.0%) | 63 (17.2%) | 56 (15.3%) | .49 |
| Charlson comorbidity index | 4 [2–5] | 4 [2–5] | 4 [3–6] b | 4 [2–6] a , b | <.001 |
| Severity of disease on ICU admission | |||||
| APACHE IV Score, median [IQR] | 64 [51–82] | 71 [55–90] a | 77 [62–94] a , b | 91 [71–114] a , b , c | <.0001 |
| APACHE APS median [IQR] | 53 [38–70] | 60 [45–76] a | 64 [50–79] a , b | 76 [59–97] a , b , c | <.0001 |
| SOFA score, median [IQR] | 6 [4–8] | 5 [3–8] | 6 [4–8] | 8 [6–10] a , b , c | <.0001 |
| Mechanical ventilation, n (%) | 270 (69.9%) | 287 (75.7%) | 294 (76.8%) | 298 (79.3%) a , b | .02 |
| Shock, n (%) | 55 (14.2%) | 64 (16.9%) | 85 (22.2%) a | 154 (41.0%) a , b , c | <.001 |
| Acute kidney injury, n (%) | 114 (29.5%) | 101 (26.6%) | 111 (29.0%) | 191 (50.8%) a , b , c | <.001 |
| Acute respiratory distress syndrome, n (%) | 93 (24.1%) | 73 (19.3%) | 91 (23.8%) | 98 (26.1%) | .15 |
| Source of infection, n (%) | |||||
| Pulmonary | 221 (57.3%) | 195 (51.5%) | 195 (50.9%) | 140 (37.2%) a , b , c | <.001 |
| Abdominal | 37 (9.6%) | 50 (13.2%) | 74 (19.3%) a | 104 (27.7%) a , b , c | <.001 |
| Urinary tract | 31 (8.0%) | 21 (5.5%) | 24 (6.3%) | 35 (9.3%) | .19 |
| Cardiovascular infection | 7 (1.8%) | 10 (2.6%) | 9 (2.3%) | 20 (5.3%) a | .02 |
| CNS | 33 (8.5%) | 33 (8.7%) | 22 (5.7%) | 8 (2.1%) a , b | <.001 |
| Skin | 6 (1.6%) | 8 (2.1%) | 4 (1.0%) | 16 (4.3%) c | .02 |
| Other | 51 (13.2%) | 62 (16.4%) | 55 (14.4%) | 53 (14.1%) | .63 |
| Causative pathogens, n (%) | |||||
| Gram‐positive bacteria | 128 (33.2%) | 132 (36.5%) | 170 (46.3%) a , b | 177 (48.2%) a , b | <.001 |
| Gram‐negative bacteria | 149 (38.6%) | 156 (41.2%) | 172 (44.9%) | 196 (52.1%) a , b | .001 |
| Fungi | 30 (7.7%) | 35 (9.2%) | 36 (10.4%) | 29 (7.7%) | .75 |
| Viruses | 14 (3.6%) | 30 (7.9%) a | 15 (3.9%) | 17 (4.5%) | .02 |
| Other | 17 (4.4%) | 19 (5.0%) | 32 (8.4%) | 21 (5.6%) | .24 |
| Unknown | 178 (46.7%) | 165 (43.5%) | 175 (45.7%) | 147 (39.1%) | .18 |
| Outcome | |||||
| Length of stay, median days [IQR] | 3 [2–7] | 3 [2–7] | 4 [2–8] | 5 [2–10] a , b , c | <.001 |
| ICU‐acquired complications, n (%) | |||||
| None | 338 (87.6%) | 345 (91.0%) | 335 (87.5%) | 308 (81.9%) b | <.01 |
| Acute kidney injury | 31 (8.0%) | 18 (4.7%) | 22 (5.7%) | 26 (6.9%) | .26 |
| Acute respiratory distress syndrome | 10 (2.6%) | 7 (1.8%) | 21 (5.5%) b | 14 (3.7%) | .03 |
| ICU‐acquired infection | 9 (6.0%) | 28 (5.7%) | 34 (7.0%) | 49 (10.3%) b | .04 |
| Mortality, n (%) | |||||
| ICU mortality | 32 (8.3%) | 51 (13.5%) | 47 (12.3%) | 96 (25.5%) a , b , c | <.001 |
| Hospital mortality | 67 (17.9%) | 88 (24.3%) | 73 (19.9%) | 132 (36.0%) a , b , c | <.001 |
| 30‐day mortality | 62 (16.5%) | 78 (21.5%) | 62 (16.9%) | 121 (33.0%) a , b , c | <.001 |
| 90‐day mortality | 82 (21.9%) | 102 (28.2%) | 95 (25.9%) | 154 (42.0%) a , b , c | <.001 |
| 1‐year mortality | 113 (30.1%) | 132 (36.5%) | 133 (36.2%) | 186 (50.7%) a , b , c | <.001 |
Age, race, gender, and chronic comorbidities are provided for unique patients, all other variables are provided for each admission.
In 57 (14.8%) infectious events in normal PT, 61 (16.1%) in slightly prolonged PT, 82 (21.4%) in intermediately prolonged PT, and 75 (19.9%) extremely prolonged PT multiple pathogens were assigned as causative (p = .05).
Mortality, except for ICU mortality, is given for unique patients, all other variables are given for every admission.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; APS, Acute Physiology Score; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile ratio; PT, prothrombin time; SD, standard deviation; SOFA, Sequential Organ Failure Assessment.
Significant versus normal PT using a Dunn's test of multiple comparisons using rank sums for nonparametric continues variables, a Tuckey test for parametric continues variables and a Bonferroni correction for categorical variables.
Significant versus slightly prolonged PT using a Dunn's test of multiple comparisons using rank sums for nonparametric continues variables, a Tuckey test for parametric continues variables and a Bonferroni correction for categorical variables.
Significant versus intermediately prolonged PT using a Dunn's test of multiple comparisons using rank sums for nonparametric continues variables, a Tukey test for parametric continuos variables, and a Bonferroni correction for categorical variables.
3.2. Outcome
Patients with an extremely prolonged PT had an increased ICU length of stay (5 [2–10] versus 3 [2–7] in normal PT, 3 [2–7] in slightly prolonged PT and 4 [2–8] in intermediately prolonged PT, overall p < .001). Both short‐ and long‐term mortality were higher in patients with an extremely prolonged PT compared to the other PT levels. Thirty‐day mortality was 33.0% in patients with an extremely prolonged PT, versus 16.5%, 21.5%, and 16.9% in patients with a normal, slightly prolonged, and intermediately prolonged PT, respectively (overall p < .001); 1‐year mortality was 50.7% versus 30.1%, 36.5%, and 36.2% respectively (overall p < .001; Table 1 and Figure S1 in supporting information).
We previously reported on the association between platelet counts and mortality in the same cohort. 8 In this earlier publication the older Sepsis‐2 definition was used to identify patients with sepsis. 8 , 25 Because in the current article the Sepsis‐3 definition was used, we determined whether the findings of our previous article could be confirmed in the present sepsis group and assessed the crude association between platelet counts and mortality. To do so, patients were stratified into clinically relevant subgroups of platelet counts on admission to the ICU as described in our previously published paper 8 : very low <50 × 109/L, intermediate‐low 50–99 × 109/L, low 100–149 × 109/L, or normal 150–399 × 109/L. Patients with thrombocytosis (≥400 × 109/L; n = 145) or unknown platelet counts in the first 24 h after ICU admission (n = 4) were excluded. Consistent with our previous publication, 8 mortality was highest in patients with very low platelet counts (n = 133); 34.6% for ICU mortality compared to 24.4% in patients with intermediate‐low platelet counts (n = 156), 12.6% in patients with low platelet counts (n = 230), and 11.3% in patients with normal platelet counts (n = 856; overall p < .001). The same was true for 30‐day mortality, which was highest in patients with very low platelet counts, 44.9% versus 30.0% in patients with intermediate‐low platelet counts; 18.6% in patients with low platelet counts, and 19.1% in patients with normal platelet counts (overall p < .001).
In a Cox proportional hazard model an extremely prolonged PT was associated with an increased risk for 30‐day mortality compared to a normal PT (unadjusted hazard ratio [HR] 2.21, 95% confidence interval [CI] 1.63–2.98; Table 2). However, when corrected for confounders (see Methods), none of the prolonged PT levels independently associated with increased risk for 30‐day mortality. Likewise, in a logistic regression analysis PT assessed as a continuous variable was associated with an increased crude risk of mortality by day 30 (unadjusted OR 1.27, 95% CI 1.13–1.42 per 10% increase), but this association also did not remain after adjustment for confounding factors (Table S2 in supporting information). In this adjusted analysis platelet counts were inversely associated with 30‐day mortality (OR 0.97, 95% CI 0.96–0.99 per 10% increase).
TABLE 2.
Cox proportional hazard analyses for 30‐day mortality risk
| Model 1 ‐ unadjusted | Model 2 ‐ adjusted | Model 3 ‐ adjusted | |||
|---|---|---|---|---|---|
| Covariables | HR [95% CI] | Covariables | HR [95% CI] | Covariables | HR [95% CI] |
| Slightly prolonged PT | 1.28 [0.92–1.77] | Slightly prolonged PT | 1.13 [0.81–1.57] | Slightly prolonged PT | 1.11 [0.80–1.55] |
| Intermediately prolonged PT | 1.10 [0.78–1.54] | Intermediately prolonged PT | 0.83 [0.59–1.17] | Intermediately prolonged PT | 0.80 [0.56–1.13] |
| Extremely prolonged PT | 2.21 [1.63–2.98] | Extremely prolonged PT | 1.15 [0.83–1.59] | Extremely prolonged PT | 1.13 [0.81–1.60] |
| Age | 1.02 [1.01–1.02] | Age | 1.02 [1.01–1.02] | ||
| APACHE IV APS | 1.02 [1.02–1.03] a | Gender, male | 0.91 [0.73–1.14] | ||
| White race | 1.21 [0.82–1.79] | ||||
| APACHE IV APS | 1.02 [1.02–1.03] a | ||||
| Malignancy | 1.32 [1.03–1.69] | ||||
| Immunocompromise | 1.21 [0.93–1.57] | ||||
| Renal compromise | 0.92 [0.67–1.27] | ||||
| Lung infection | 1.38 [1.06–1.78] | ||||
| Abdominal infection | 1.34 [0.97–1.85] | ||||
| Gram negative infection | 0.92 [0.74–1.67] | ||||
| Lowest platelet count first 24 h | 0.999 [0.998–0.999] | ||||
| Fresh frozen plasma units transfused in first 24 h | 0.87 [0.74–1.01] | ||||
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; APS, Acute Physiology Score; CI, confidence interval; HR, hazard ratio; PT, prothrombin time.
Proportionality was not met for APACHE IV APS.
3.3. Activation of the coagulation system
In patients with an extremely prolonged admission PT, PT decreased during the first 7 days after admission, while in the other three PT groups PT remained relatively stable (Figure 2). Patients with an extremely prolonged PT had the lowest platelet counts (Figure 2, Figure S2 in supporting information).
FIGURE 2.
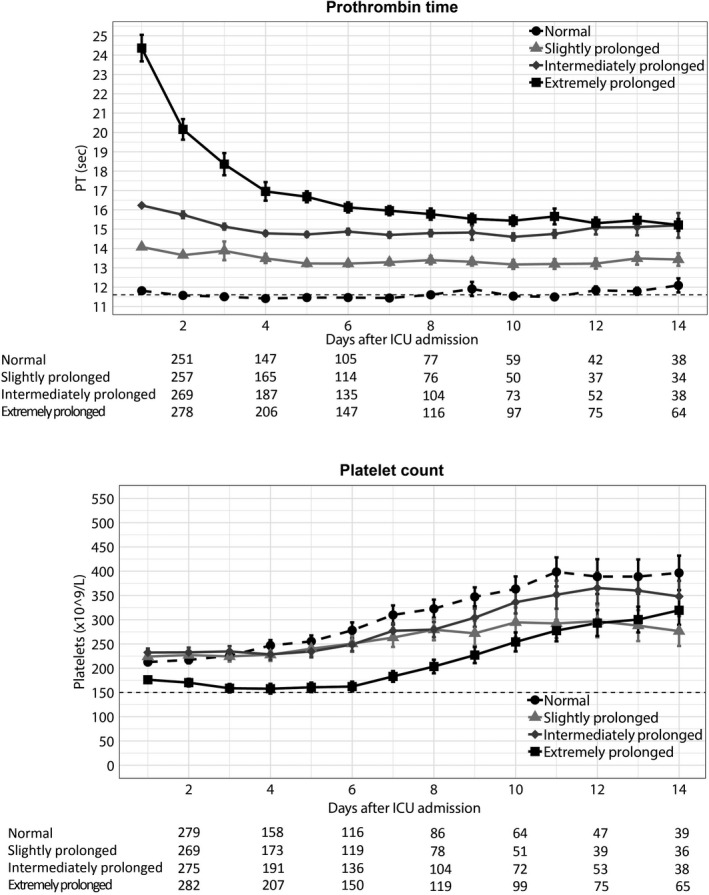
Prothrombin time and platelet counts over time in patients with sepsis stratified according to prothrombin time on admission to the intensive care unit (ICU). Data are mean and standard error of the mean. Numbers below x‐axis indicate number of patients still present in the intensive care unit for each group
In the subgroup of patients with sepsis and an infection likelihood of definite or probable (785 admissions) a comprehensive set of host response biomarkers, including additional markers for coagulation activation, was measured on admission to the ICU. This subgroup was comparable to the total cohort with regard to baseline characteristics (Table S3 in supporting information) and outcome (Table S4 in supporting information). Patients with an extremely prolonged PT had higher plasma D‐dimer concentrations, lower antithrombin and protein C levels, and longer APTT values (Figure S2). Hence, patients with an extremely prolonged PT overall showed signs of more severe coagulation disorders.
3.4. Endothelial cell activation
The coagulation system is tightly connected to endothelial cell function. 26 , 27 We measured biomarkers for endothelial cell activation (plasma levels of soluble E‐selectin, soluble intercellular adhesion molecule [ICAM]‐1, and fractalkine) and vascular integrity (plasma angiopoetin‐1 and ‐2) on admission in patients with sepsis with an infection likelihood of definite or probable stratified according to PT. Patients with extremely prolonged PT values had the highest plasma soluble E‐selectin, ICAM‐1, and fractalkine levels, indicative of more pronounced endothelial cell activation (Figure S3 in supporting information). In addition, patients with extremely prolonged PT values had the highest plasma angiopoietin‐2 levels and angiopoietin‐2/angiopoietin‐1 ratios, indicative of a more disturbed endothelial barrier function (Figure S3).
3.5. Systemic inflammatory responses
Patients with an extremely prolonged PT displayed signs of enhanced systemic inflammation, as reflected by the highest plasma levels of interleukin (IL)‐6, IL‐8, IL‐10, and matrix metalloproteinase (MMP)8 (Figure S4 in supporting information). Tumor necrosis factor‐α, IL‐1β, IL‐13, and interferon‐γ remained undetectable in most patients (data not shown).
3.6. Evaluation of the host response in patients stratified according to PT and matched for severity of disease
To exclude the influence of baseline differences between patients with different PT values on host response biomarkers, we used propensity score matching to create groups with normal or extremely prolonged PT values and comparable baseline characteristics and severity of disease on ICU admission. This resulted in a cohort of 114 admissions with a normal PT and 114 admissions with an extremely prolonged PT in whom the severity of disease (APACHE IV, APACHE IV Acute Physiology, and SOFA scores, proportion of shock) did not differ (Table S5 in supporting information). In this matched cohort, patients with an extremely prolonged PT had similar platelet counts compared to patients with normal PT values (Figure 3). Plasma D‐dimer concentrations were not different between groups. However, patients with an extremely prolonged PT still had lower antithrombin and protein C levels and more prolonged APTT values. In addition, patients with an extremely prolonged PT showed stronger endothelial cell activation reflected by higher fractalkine and angiopoietin‐2 levels (Figure 4). Patients with an extremely prolonged PT had higher plasma levels of IL‐6, IL‐8, IL‐10, and MMP8, compared to matched patients with normal PT values (Figure 5).
FIGURE 3.
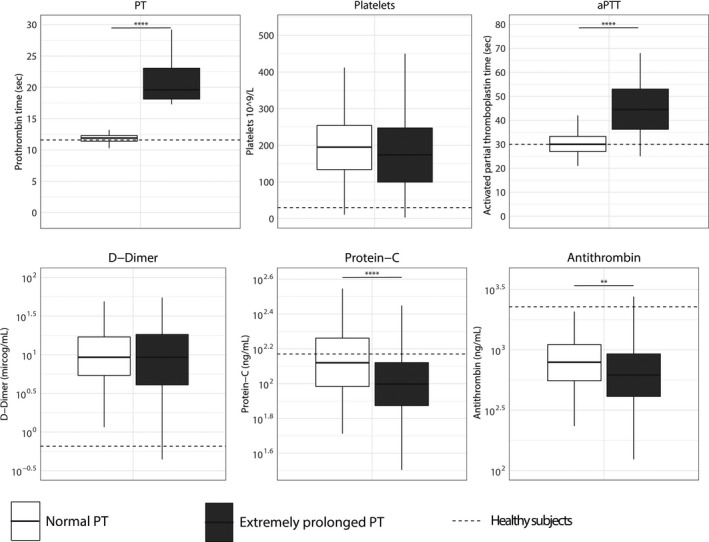
Biomarkers reflecting coagulation activation in patients with sepsis stratified according to prothrombin time on admission and matched for discordant baseline parameters. Data are expressed as box and whisker diagrams depicting the median and lower quartile, upper quartile, and their respective 1.5 interquartile range as whiskers (as specified by Tukey). Dotted lines indicate median values obtained in 27 healthy age‐matched subjects. Biomarker distribution on ICU admission was compared using a nonparametric Mann‐Whitney U test. **p < .01, ***p < .001, ****p < .0001. APTT, activated partial thromboplastin time; PT, prothrombin time
FIGURE 4.
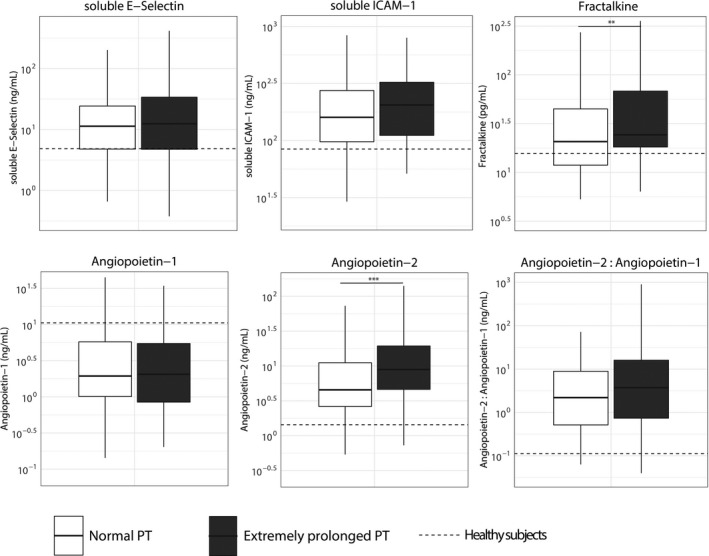
Biomarkers reflecting endothelial cell activation and barrier function in patients with sepsis stratified according to prothrombin time on admission and matched for discordant baseline parameters. Data are expressed as box and whisker diagrams depicting the median and lower quartile, upper quartile, and their respective 1.5 interquartile range as whiskers (as specified by Tukey). Dotted lines indicate median values obtained in 27 healthy age‐matched subjects. Biomarker distribution on intensive care unit admission was compared using a nonparametric Mann‐Whitney U test. **p < .01. ***p < .001. ANG, angiopoietin; sE‐Selectin, soluble E‐selectin; sICAM, soluble intercellular adhesion molecule
FIGURE 5.
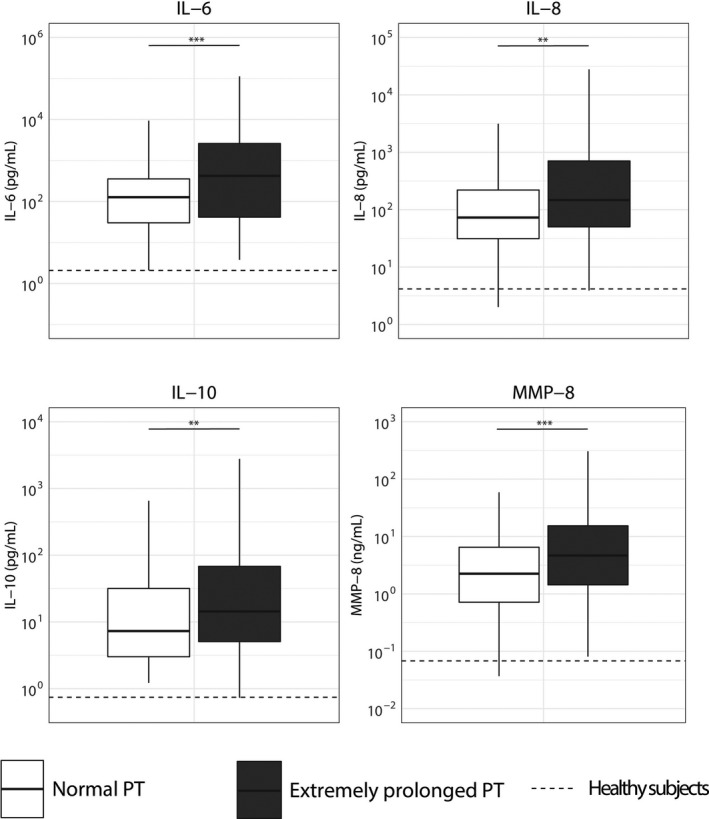
Biomarkers reflecting systemic inflammation in patients with sepsis stratified according to prothrombin time on admission and matched for discordant baseline parameters. Data are expressed as box and whisker diagrams depicting the median and lower quartile, upper quartile, and their respective 1.5 interquartile range as whiskers (as specified by Tukey). Dotted lines indicate median values obtained in 27 healthy age‐matched subjects. Biomarker distribution on intensive care unit admission was compared using a nonparametric Mann‐Whitney U test. **p < .01, ***p < .001. IL, interleukin; MMP, matrix metalloproteinase
3.7. Blood leukocyte transcriptome analysis
We compared the blood leukocyte transcriptome of patients with a normal PT (n = 43) to those with an extremely prolonged PT (n = 43); this analysis comprised the propensity matched cohort, using the subgroup of consecutive patients enrolled during the first 1.5 years of this study. Genome‐wide blood gene expression profiles of these sepsis patients were initially compared to 42 healthy controls. Both patients with a normal PT and an extremely prolonged PT demonstrated profound transcriptional alterations (Figure 6A). Eighty‐two percent of the altered transcriptome was common to both groups (Figure 6B), and this common response showed a strong correlation (Figure 6C). Pathway analysis revealed common overexpressed genes associated with innate pro‐inflammatory (IL‐6, IL‐8, TREM‐1 pathways) and anti‐inflammatory (IL‐10) pathways as well as metabolic pathways (mitochondrial dysfunction, oxidative phosphorylation, HIF‐1α signaling; Figure S5 in supporting information). Common underexpressed genes associated with lymphocyte and antigen‐presentation pathways (Figure S5). Differential gene expression analysis revealed moderate differences between PT groups, encompassing 277 significantly altered genes in patients with an extremely prolonged relative to normal PT (multiple‐comparison adjusted p < .05; Figure 6D). Pathway analysis showed that overexpressed genes in patients with an extremely prolonged PT were significantly associated to a more prominent metabolic reprogramming (oxidative phosphorylation, mitochondrial dysfunction), transcriptional regulation (sirtuin signaling pathway), and protein catabolism (ubiquitination pathway), whereas underexpressed genes were associated with a relative impairment of post‐translational protein modifications (sumoylation) and specific signaling pathways (glucocorticoid and retinoic acid receptor pathways; Figure 6E).
FIGURE 6.
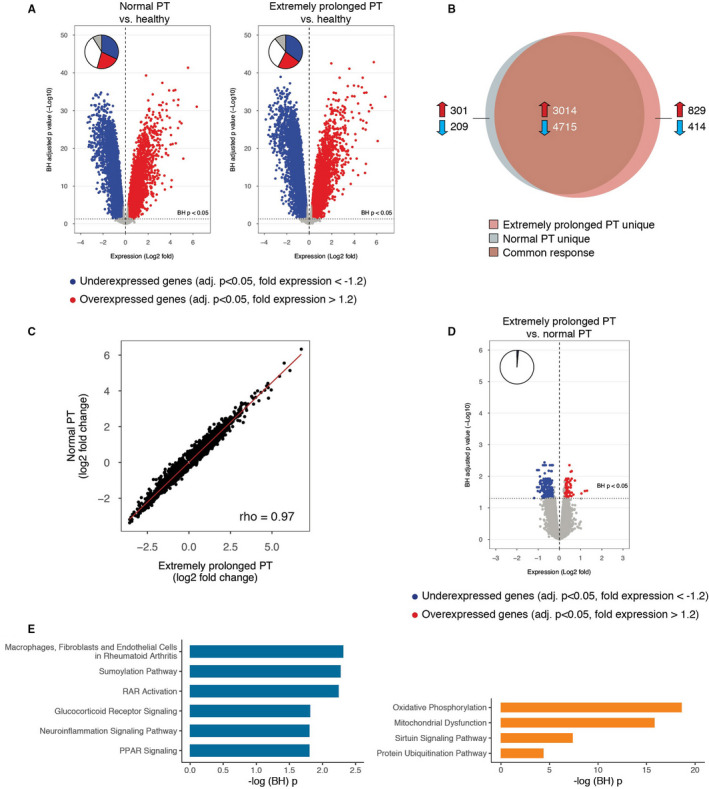
Leukocyte genomic responses and associated biological pathways in patients with sepsis stratified according to prothrombin time on admission and matched for discordant baseline parameters. A, Volcano plot illustrating the differences in leukocyte genomic responses (integrating log2 foldchanges and multiple‐test adjusted probabilities) between sepsis patients with normal PT (PT ≤ 12.7 s) and healthy subjects (left) and patients with extremely prolonged PT (PT ≥ 17.3 s) and healthy subjects (right). Considering adjusted p < .05, 8239 and 8972 genes were identified as differentially expressed in patients with normal PT versus healthy subjects, and patients with extremely prolonged PT versus healthy subjects, respectively. Blue dots represent significantly underexpressed genes (adjusted p < .05, fold expression <−1.2) whereas red dots represent significantly overexpressed genes (adjusted p < .05, fold expression >1.2) in patients relative to healthy controls. Horizontal dotted line indicates multiple‐test adjusted Benjamini‐Hochberg (BH) p < .05 threshold. B, Venn‐Euler representation of differentially expressed genes in sepsis patients with extremely prolonged PT and normal PT versus healthy subjects (adjusted p < .05). Red arrows denote overexpressed genes; blue arrows denote underexpressed genes. C, Dot plot depicting the common response (log2 foldchanges) of patients with extremely prolonged PT and normal PT compared to healthy subjects. r, Spearman's correlation coefficient. D, Volcano plot illustrating the differences in leukocyte genomic responses between sepsis patients with extremely prolonged PT and patients with normal PT upon admission. Considering adjusted p < .05, 277 genes were identified as differentially expressed in patients with extremely prolonged PT versus patients with normal PT. E, Ingenuity pathway analysis of commonly underexpressed genes in patients with extremely prolonged PT versus patients with normal PT. –log (Benjamini‐Hochberg (BH)) p value, negative log10‐transformed p value corrected for multiple comparisons; PPAR, peroxisome proliferator‐activated receptor; PT, prothrombin time; RAR, retinoid acid receptor
4. DISCUSSION
Consumptive coagulopathy is a common complication in patients with sepsis caused by strong activation of the coagulation system and manifested by a prolonged PT. 1 , 2 , 5 , 6 While multilevel interactions between mediators of coagulation and other host response mechanisms have been documented in laboratory and animal investigations, 12 , 13 knowledge of associations between plasmatic coagulation and the dysregulated host response in patients with sepsis is limited. We here utilized a large prospectively enrolled cohort of sepsis patients to show that a prolonged PT is associated with stronger aberrations in distinct host response mechanisms implicated in the pathogenesis of sepsis, including endothelial cell function and systemic inflammation, even after adjusting for confounding factors such as severity of disease.
In contrast to previous studies we did not find an independent association between admission PT and mortality. 5 , 6 , 7 Notably, two of these earlier investigations did not correct for severity of disease in their analyses, 5 , 7 while the third used the total SOFA score for this. 6 In our stringent analysis, low platelet counts remained independently associated with 30‐day mortality, confirming our previous report. 8 This earlier publication also includes crude analyses on the association between platelet counts and mortality. 8 These results suggest that in patients with a prolonged PT, severity of disease rather than the extent of consumptive coagulopathy is the main (or sole) driver of mortality.
Patients with an extremely prolonged PT had lower plasma levels of the anticoagulant proteins protein C and antithrombin compared to patients with a normal PT matched for disease severity and several other factors that could influence plasma biomarker levels. Low antithrombin and protein C levels are common features in sepsis patients, likely caused by a combination of impaired synthesis, ongoing consumption, extravascular loss due to increased vascular permeability, increased clearance, and proteolytic degradation. 12 , 13 Our data suggest that activation of the coagulation cascade may play a role in one or more of these processes, either directly (e.g., through increased complex formation between thrombin and antithrombin, resulting in enhanced clearance) or indirectly (e.g., through enhanced systemic inflammation and a more disturbed endothelial barrier function). Platelet counts did not differ between patients with an extremely prolonged PT versus those with a normal PT matched for disease severity, suggesting that activation of the coagulation cascade does not impact platelet activation and consumption. Conversely, we previously reported that thrombocytopenia in patients with sepsis is not associated with consumptive coagulopathy, as reflected by similar PTs in those with low (<50 × 109/L) and normal platelet counts. 8
Plasma biomarkers indicative of endothelial cell activation and function were different between patients with an extremely prolonged and normal PT to a modest extent. While plasma fractalkine levels were higher in patients with an extremely prolonged PT, the concentrations of soluble ICAM‐1 and soluble E‐selectin were not different between groups. The higher plasma angiopoietin‐2 levels in patients with an extremely prolonged PT are suggestive of a more disturbed endothelial barrier integrity in this group. Activation of coagulation can impact endothelial cell function in several ways. 26 , 27 The tissue factor‐‐factor VIIa‐‐factor Xa complex, factor Xa, and thrombin can selectively activate cell surface protease activated receptors (PARs), which are considered to be the main link between coagulation and inflammation, on the vasculature. 26 , 28 High thrombin concentrations induce proinflammatory responses in endothelial cells through activation of PAR1, resulting in activation of nuclear factor‐κB, production of cytokines such as IL‐6 and cytoskeletal derangements, and cell contraction and rounding, thereby weakening cell‐cell contacts and decreasing barrier function. 26 , 28 The net effect of coagulation proteases on endothelial function likely depends on the balance between procoagulant factors and the anticoagulant protein C system, wherein PAR1 activation by activated protein C evokes anti‐inflammatory and cytoprotective responses. 28 , 29 In this respect it is important to note that the function of the protein C system is strongly impaired in patients with sepsis 12 , 13 , 30 and that in the present study patients with an extremely prolonged PT showed lower plasma protein C levels than those with a normal PT. Thus, our observational results in patients provide some in vivo validity to preclinical data on the effects of coagulation factors on the endothelium, but the effect size seems relatively low.
An extremely prolonged PT was associated with increased systemic inflammation (higher IL‐6, IL‐8, and MMP8 concentrations). In agreement, preclinical studies have provided ample evidence of activation of proinflammatory mechanisms in parenchymal and immune cells by coagulation factors, predominantly through cleavage of PARs. 12 , 13 , 26 , 27 , 28 , 29 Blood leukocytes of patients with an extremely prolonged PT did not show increased proinflammatory signaling compared to leukocytes of patients with a normal PT, as determined by genome‐wide transcriptional profiling, suggesting that elevated circulating IL‐6, IL‐8, and MMP8 levels originated from extravascular cellular sources. Pathways that were overexpressed in blood leukocytes of patients with an extremely prolonged PT included metabolic reprogramming and protein catabolism. Eighty‐two percent of the leukocyte genomic response was common in patients with an extremely prolonged and those with a normal PT. This common response included increased expression of pro‐inflammatory, anti‐inflammatory, and metabolic pathways, and reduced expression of genes associated with lymphocyte and antigen‐presentation pathways, findings that are in agreement with previous reports on the blood transcriptome in patients with sepsis. 31
Our study has strengths and limitations. We studied a large prospectively enrolled cohort in which patients were punctiliously characterized according to strict criteria. We implemented propensity matching to adjust for differences in disease severity and other potential confounders between patients with different PTs; nevertheless, a bias may have remained after matching due to unmeasured confounders. Data were collected in two ICUs in the Netherlands, which may limit the generalizability.
In conclusion, we here show that a prolonged PT in patients with sepsis upon admission to the ICU is associated with alterations in plasma biomarkers reflecting stronger anomalies in pathways implicated in the pathogenesis of sepsis. Although causality cannot be inferred from our observational study, these results taken together with preclinical data 12 , 13 suggest that activation of the coagulation system impacts other host response mechanisms in sepsis.
CONFLICTS OF INTEREST
All authors have read and approved the manuscript. None of the authors declare any conflicts of interest.
AUTHOR CONTRIBUTIONS
L. A. van Vught, F. Uhel, C. Ding, and T. van der Poll contributed to the design of the study. L. A. van Vught, F. Uhel, B. P. Scicluna, A. J. Hoogendijk, P. M. C. Klein Klouwenberg, O. L. Cremer, M. J. Schultz, and T. van der Poll acquired the data. F. Uhel, B. P. Scicluna, and P. Nürnberg did the array analyses. L. A. van Vught had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. L. A. van Vught and T. van der Poll were involved in the interpretation of the data. L. A. van Vught and T. van der Poll drafted the manuscript, and all authors revised it critically for important intellectual content.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
Members of the MARS consortium: Amsterdam University Medical Centers, Academic Medical Center, University of Amsterdam, the Netherlands: Friso M. de Beer, Lieuwe D. J. Bos, Gerie J. Glas, Arie J. Hoogendijk, Roosmarijn T. M. van Hooijdonk, Janneke Horn, Mischa A. Huson, Laura R. A. Schouten, Marcus J. Schultz, Brendon P. Scicluna, Marleen Straat, Lonneke A. van Vught, Luuk Wieske, Maryse A. Wiewel, Esther Witteveen. University Medical Center Utrecht, Utrecht, the Netherlands: Marc J.M. Bonten, Olaf M. Cremer, David S.Y. Ong, Jos F. Frencken, Peter M.C. Klein Klouwenberg, Maria E. Koster‐Brouwer, Kirsten van de Groep, Diana M. Verboom.
A full list of members of the MARS consortium can be found in the Acknowledgments.
Manuscript handled by: Patricia Liaw
Funding information
The MARS project was supported by the Center for Translational Molecular Medicine (http://www.ctmm.nl), project MARS (grant 04I‐201). Chao Ding was supported by the Chinese Scholarship Council (CSC).
Contributor Information
Tom van der Poll, Email: t.vanderpoll@amsterdamumc.nl.
the MARS consortium:
Friso M. de Beer, Lieuwe D. J. Bos, Gerie J. Glas, Arie J. Hoogendijk, Roosmarijn T. M. van Hooijdonk, Janneke Horn, Mischa A. Huson, Laura R. A. Schouten, Marcus J. Schultz, Brendon P. Scicluna, Marleen Straat, Lonneke A. van Vught, Luuk Wieske, Maryse A. Wiewel, Esther Witteveen, Marc J.M. Bonten, Olaf M. Cremer, David S.Y. Ong, Jos F. Frencken, Peter M.C. Klein Klouwenberg, Maria E. Koster‐Brouwer, Kirsten van de Groep, and Diana M. Verboom
REFERENCES
- 1. Yamakawa K, Yoshimura J, Ito T, Hayakawa M, Hamasaki T, Fujimi S External validation of the two newly proposed criteria for assessing coagulopathy in sepsis. Thromb Haemost. 2019;119:203‐212. 10.1055/s-0038-1676610. [DOI] [PubMed] [Google Scholar]
- 2. Iba T, Levy JH, Wada H, Thachil J, Warkentin TE, Levi M Differential diagnoses for sepsis‐induced disseminated intravascular coagulation: communication from the SSC of the ISTH. J Thromb Haemost. 2019;17:415‐419. 10.1111/jth.14354. [DOI] [PubMed] [Google Scholar]
- 3. Chakraverty R, Davidson S, Peggs K, Stross P, Garrard C, Littlewood TJ The incidence and cause of coagulopathies in an intensive care population. Br J Haematol. 1996;93:460‐463. [DOI] [PubMed] [Google Scholar]
- 4. Walsh TS, Stanworth SJ, Prescott RJ, Lee RJ, Watson DM, Wyncoll D Prevalence, management, and outcomes of critically ill patients with prothrombin time prolongation in United Kingdom intensive care units. Crit Care Med. 2010;38:1939‐1946. 10.1097/CCM.0b013e3181eb9d2b. [DOI] [PubMed] [Google Scholar]
- 5. Dhainaut JF, Shorr AF, Macias WL, et al. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med. 2005;33:341‐348. [DOI] [PubMed] [Google Scholar]
- 6. Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J New criteria for sepsis‐induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7:e017046. 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iba T, Di Nisio M, Thachil J, et al. A proposal of the modification of japanese society on thrombosis and hemostasis (JSTH) disseminated intravascular coagulation (DIC) diagnostic criteria for sepsis‐associated DIC. Clin Appl Thromb Hemost. 2018;24:439‐445. 10.1177/1076029617720069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Claushuis TA, van Vught LA, Scicluna BP, et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127:3062‐3072. 10.1182/blood-2015-11-680744. [DOI] [PubMed] [Google Scholar]
- 9. Semple JW, Italiano JE Jr, Freedman J Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264‐274. 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 10. de Stoppelaar S, van’t Veer C, Poll TVD The role of platelets in sepsis. Thromb Haemost. 2014;112:666‐677. [DOI] [PubMed] [Google Scholar]
- 11. Li JL, Zarbock A, Hidalgo A Platelets as autonomous drones for hemostatic and immune surveillance. J Exp Med. 2017;214:2193‐2204. 10.1084/jem.20170879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gando S, Levi M, Toh CH Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:16037. 10.1038/nrdp.2016.37. [DOI] [PubMed] [Google Scholar]
- 13. Levi M, van der Poll T Coagulation and sepsis. Thromb Res. 2017;149:38‐44. 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 14. Klein Klouwenberg PM, Ong DS, Bos LD, et al. Interobserver agreement of Centers for Disease Control and Prevention criteria for classifying infections in critically ill patients. Crit Care Med. 2013;41:2373‐2378. 10.1097/CCM.0b013e3182923712. [DOI] [PubMed] [Google Scholar]
- 15. van Vught LA, Klein Klouwenberg PMC, Spitoni C, et al. Risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016;315:1469‐1479. 10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 16. van Vught LA, Wiewel MA, Hoogendijk AJ, et al. The host response in patients with sepsis developing intensive care unit‐acquired secondary infections. Am J Respir Crit Care Med. 2017;196:458‐470. 10.1164/rccm.201606-1225OC. [DOI] [PubMed] [Google Scholar]
- 17. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128‐140. [DOI] [PubMed] [Google Scholar]
- 18. Calandra T, Cohen J The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538‐1548. [DOI] [PubMed] [Google Scholar]
- 19. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315:801‐810. 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute renal failure ‐ definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204‐R212. 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernard GR, Artigas A, Brigham KL, et al. The American‐European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respirat Crit Care Med. 1994;149:818‐824. 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 22. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S , Standardization Committee of the International Society on T , Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thrombosis Haemostasis: JTH. 2005;3:692‐694. 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 23. Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thrombosis Haemostasis: JTH. 2010;8:202‐204. 10.1111/j.1538-7836.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 24. Budtz‐Jorgensen E, Keiding N, Grandjean P, Weihe P Confounder selection in environmental epidemiology: assessment of health effects of prenatal mercury exposure. Ann Epidemiol. 2007;17:27‐35. 10.1016/j.annepidem.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 25. Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2001;2003(31):1250‐1256. 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 26. Opal SM, van der Poll T Endothelial barrier dysfunction in septic shock. J Intern Med. 2015;277:277‐293. 10.1111/joim.12331. [DOI] [PubMed] [Google Scholar]
- 27. Jackson SP, Darbousset R, Schoenwaelder SM Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906‐918. 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 28. Rezaie AR Protease‐activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost. 2014;112:876‐882. 10.1160/TH14-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mosnier LO, Zlokovic BV, Griffin JH The cytoprotective protein C pathway. Blood. 2007;109:3161‐3172. [DOI] [PubMed] [Google Scholar]
- 30. Danese S, Vetrano S, Zhang L, Poplis VA, Castellino FJ The protein C pathway in tissue inflammation and injury: pathogenic role and therapeutic implications. Blood. 2010;115:1121‐1130. 10.1182/blood-2009-09-201616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407‐420. 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


