Abstract
Stereotactic lesioning of the bilateral globus pallidus (GPi) was one of the first surgical treatments for medication‐refractory dystonia but has largely been abandoned in clinical practice after the introduction of deep brain stimulation (DBS). However, some patients with dystonia are not eligible for DBS. Therefore, we reviewed the efficacy, safety, and sustainability of bilateral pallidotomy by conducting a systematic review of individual patient data (IPD). Guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses and IPD were followed. In May 2020, Medline, Embase, Web of Science, and Cochrane Library were searched for studies reporting on outcome of bilateral pallidotomy for dystonia. If available, IPD were collected. In this systematic review, 100 patients from 33 articles were evaluated. Adverse events were reported in 20 patients (20%), of which 8 were permanent (8%). Pre‐and postoperative Burke‐Fahn‐Marsden Dystonia Rating Movement Scale scores were available for 53 patients. A clinically relevant improvement (>20%) of this score was found in 42 of 53 patients (79%). Twenty‐five patients with status dystonicus (SD) were described. In all but 2 the SD resolved after bilateral pallidotomy. Seven patients experienced a relapse of SD. Median‐reported follow‐up was 12 months (n = 83; range: 2–180 months). Based on the current literature, bilateral pallidotomy is an effective and relatively safe procedure for certain types of dystonia, particularly in medication‐refractory SD. Although due to publication bias the underreporting of negative outcomes is very likely, bilateral pallidotomy is a reasonable alternative to DBS in selected dystonia patients. © 2020 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: pallidotomy; dystonia; safety; efficacy; sustainability
Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions, causing abnormal, often repetitive movements, postures, or both. 1 It is a debilitating disorder with a high burden of disease. 2 If pharmacological treatment fails, stereotactic functional neurosurgery is an established next step in the treatment algorithm. It is considered the most successful strategy in life‐threatening, medication‐refractory status dystonicus (SD). 3
Nowadays, deep brain stimulation (DBS) is preferred over lesioning techniques for dystonia. 4 , 5 , 6 , 7 However, from the 1950s to the 1980s, thalamotomy and pallidotomy were the cornerstones of stereotactic neurosurgical treatment for dystonia. 8 , 9 Although DBS of the pallidum has important advantages over ablative procedures, including its reversibility and the ability to adjust stimulation parameters, it also has its limitations. Adverse events such as infection, skin erosion, and hardware malfunction, but also lifelong follow‐up and neurological side effects, for example, long‐term akinesia and gait disorder, are well known. In fact, hardware‐related issues are more common in patients with dystonia than in patients with other movement disorders. 10 Furthermore, DBS is expensive, and reimbursement and availability vary among countries. 11
Cognitive impairment, young age, cachectic state, and inability to comply with follow‐up visits are recognized as valid reasons to refrain from DBS. 12 Severe medical‐refractory dystonia and SD often coincide with impaired cognition and cachectic state, particularly in the case of neurometabolic or neurodegenerative diseases. In these patients bilateral pallidotomy is a valid treatment, but this step is not easily taken. The literature on this topic is limited to case reports and case series. Therefore, we performed this systematic review and collected the individual patient data (IPD) to assess the efficacy, safety, and sustainability of bilateral pallidotomy in patients with dystonia.
Patients and Methods
A systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA, Fig. 1). 13 A systematic search for relevant studies was performed up to May 2020, based on electronic databases Cochrane Library, Medline, Embase, and Web of Science. The search strategy was designed using the Medical Subject Headings terms “pallidotomy” and “dystonia.” The references of the included articles were scrutinized for other relevant studies.
FIG. 1.
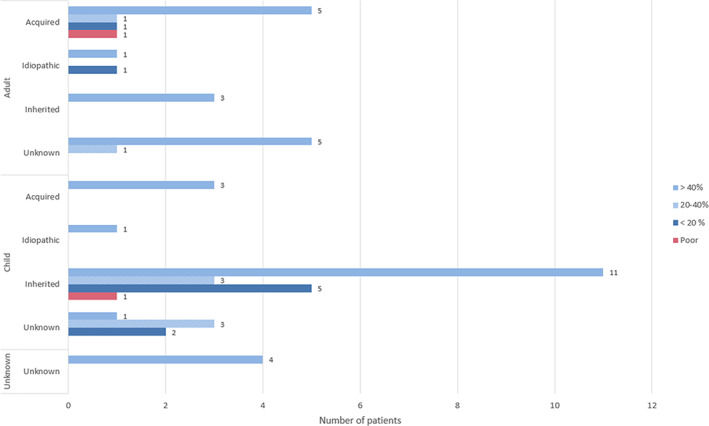
Improvement Burke‐Fahn‐Marsden Dystonia Rating Scale movement score at the end of reported follow‐up (n = 53). [Color figure can be viewed at wileyonlinelibrary.com]
Study Selection
Three authors independently made the study selection. Disagreements were solved in a consensus meeting. Peer‐reviewed full‐text papers that reported on motor outcome were selected. The initial selection was based on screening of titles and abstracts. Further selection was made after reading and cross‐reading of the full text. Patients with dystonia in the context of Parkinson's disease were excluded. Studies with overlapping patients were also excluded, except when additional IPD could be obtained by including both articles. In case of missing data, an attempt to contact the corresponding author was made twice by e‐mail.
Data Extraction
The following data were extracted: (1) Patient characteristics: age, gender, diagnosis, and dystonia type; (2) efficacy of pallidotomy, as expressed by an improvement in motor performance (Burke‐Fahn‐Marsden Dystonia Rating Scale [BFMDRS], 14 Unified Dystonia Rating Scale [UDRS], or any other qualitative or quantitative description); (3) safety in terms of reported adverse events, which were categorized as transient or permanent; and (4) sustainability of the lesion effect and duration of follow‐up. Ten corresponding authors were contacted to retrieve supplementary IPD. Four authors responded, which led to additional IPD of one patient. 15
Methodological Quality Assessment
Methodological quality of case series was assessed using the tool of Moga et al. 16 Case reports were assessed using an adapted version of the same tool (Table APPENDIX S1). To assess the methodological quality of the included case series and case reports, the overall percentage of agreement was calculated. Subsequently, two separate Cohen's kappas were calculated for both the case reports and the case series assessment to assess interrater agreement.
Data Analysis
Data were analyzed using SPSS version 22. Descriptive statistics were used for analysis of IPD. To describe age and duration of follow‐up, means and standard deviations were used. For categorical variables (gender, type of dystonia, diagnosis, UDRS, and side effects), frequencies with percentages were used. The BFMDRS movement score was assessed separately, if available. For this score, the relative change percentage was subdivided into four categories: >40%, 20–40%, <20%, and worsening (relative to baseline).
Results
Study Selection
The electronic search yielded 1149 papers (Medline: 296, Embase: 452, Web of Science: 401, and Cochrane Library: 0). No randomized clinical trials were found. After duplicates were removed, 764 papers remained. Titles and abstracts were screened for eligibility. Subsequently, 166 full‐text papers were read and assessed for eligibility. Initially non‐English articles were excluded. However, due to scarcity of literature, non‐English papers were also assessed. Finally, 33 papers met our inclusion criteria and were included in this review. 15 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 The reasons to exclude the other 133 articles were the following: congress abstracts (n = 37), described no patients (n = 25), full text not available (n = 18), unilateral pallidotomy (n = 15), Parkinson's disease (n = 12), no (motor) outcomes (n = 9), patients described before (n = 7), thalamotomy or other lesions (n = 6), no dystonia patients (n = 2), and DBS only (n = 2) ( FIGURE S1).
Patient and Study Characteristics
The 33 included studies were published between 1996 and 2020, describing 100 patients (Table 1). If sufficient data were available, IPD were collected (Table 2). The included studies were case series (n = 14), case reports (n = 18), and a letter to the editor (n = 1). The number of enrolled patients per study varied from 1 to 18. The majority of patients suffered from generalized dystonia before surgery (65%); 25 patients (25%) had SD. Seven patients (7%) had focal or other forms of dystonia. Thirty‐one patients (31%) had a confirmed genetic diagnosis, 18 of whom had DYT1 dystonia, caused by a mutation in the TOR1A gene. Table 1 presents additional information on etiology and clinical presentation. The median age at surgery was 17 years (n = 85).
TABLE 1.
Overview of studies on bilateral pallidotomy for dystonia
| Author | Gender (M/F) | Age at start of disease | Age at surgery | Symptoms pre‐op | Diagnosis | Dystonia type | BFMDRS movement | BFMDRS disability | Staged/simultaneous | Other scores | Score | Follow‐up | Child/adult | Complications | Part of case report/case series | Aftereffects | Relapse/no improvement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Levi et al (2019) Italy |
F | 2 y | 10 y | Status dystonicus | PKAN classic | Inherited | – | – | Simultaneous | BAD | SD: 32, postop: 32, last: NA | – | Child | – | Series | Ineffective, death due to SD after 35 days | Death |
| F | 4 y | 7 y | Status dystonicus | Post‐infective SD | Acquired | – | – | Simultaneous | BAD | SD: 32, post‐op: 29, last: 31 | 12 mo | Child | – | Series | 1 day | SD resolution | |
| M | 1 y | 9 y | Status dystonicus | – | – | – | – | Staged | BAD | SD: 30, post‐op: 14, last: 15 | 12 mo | Child | – | Series | 35 days | SD resolution | |
| M | 2 y | 8 y | Status dystonicus | PKAN atypical | Inherited | – | – | Simultaneous | BAD | SD: 31, post‐op: 30, last: 30 | 12 mo | Child | – | Series | 15 days | SD resolution | |
| F | 1 y | 19 y | Status dystonicus | Cerebral palsy | Acquired | – | – | Simultaneous | BAD | SD: 31, post‐op: 24, last: 28 | 38 mo | Adult | – | Series | 50 days | Death due to complications unrelated to baseline dystonia | |
| F | 1 y | 10 y | Status dystonicus | GNAO1 | Inherited | – | – | Simultaneous, after failed DBS | BAD | SD: 32, post‐op: 28, last: 28 | 180 mo | Child | – | Series | 60 days | SD resolution | |
| M | 2 y | 16 y | Status dystonicus | Cerebral palsy | Acquired | – | – | Simultaneous | BAD | SD: 30, post‐op: 28, last: 28 | 30 mo | Child | – | Series | 8 days | Death due to complications unrelated to baseline dystonia | |
|
Garone et al (2019) Italy |
M = 7, F = 1, unknown = 1 | Status dystonicus | – | Both acquired and inherited | – | – | – | DSAP | DSAP4 = 1, DSAP5 = 6, unknown = 2 | – | Child | Series | – | SD resolution in all but 1 patient; dystonia relapse in 7 of 9 patients | |||
|
Franzini et al (2018) a Italy |
F | 4 y | 6 y | Status dystonicus | Tuberculous meningoencephalitis | Acquired | Pre‐op: 110, 3 mo: 50 | Pre‐op: 30, 3 mo: 30 | Simultaneous | – | – | 3 mo | Child | – | Report | Second day after surgery: dramatic reduction of dystonic movements | Unknown |
|
Horisawa et al (2018) a Japan |
M | 36 y | 38 y | Camptocormia | Tardive dystonic camptocormia | Acquired | Pre‐op: 3, post‐op: 0 | Pre‐op: 3, post‐op: 0 | Staged | – | – | 18 mo | Adult | – | Report | Complete resolution of symptoms after unknown time parameter | Unknown |
|
Horisawa et al (2018) a Japan |
M | 43 y | 47 y | Embouchure dystonia | Embouchure dystonia | Acquired | – | – | Staged: 6‐mo interval | – | – | 12 mo | Adult | – | Report | Complete resolution of symptoms after unknown time parameter | Unknown |
|
Kohara et al (2017) Japan |
M | – | 32 y | Trunk and upper extremity dystonia | Tardive dystonia | Acquired | Pre‐op: 28.5, post‐op: 1.5, 9 mo: 0 | Simultaneous | – | – | 9 mo | Adult | – | Report | Immediately post‐surgery | Unknown | |
|
Franzini et al (2017) a Italy |
M | – | 9 y | Status dystonicus | – | Idiopathic | – | – | Staged, 2‐week interval | UDRS | Pre‐op: 110, post‐op: 41 | 6 mo | Child | – | Report | Gradual improvement over 2 mo | Unknown |
|
Minkin et al (2017) a Bulgaria |
M | 45 y | 68 y | Focal dystonia | Meige syndrome | Idiopathic | Pre‐op: 26, 6 mo: 3, 24 mo: 3 | – | Staged: 6‐mo interval | – | – | 24 mo | Adult | – | Report | Immediately | Unknown |
|
Horisawa et al (2016) a Japan |
M | 27 y | 36 y | Cervical dystonia | – | Idiopathic | – | – | Simultaneous | TWSTRS and Tsui score | Pre‐op: 12 and 6, 1 week: 1 (both) | 12 mo | Adult | Transient aggressive behavior | Report | Day after surgery | Unknown |
|
Franzini et al (2015) a Italy |
F | Birth | 15 y | Generalized dystonia | Hypoxic event | Idiopathic | Pre‐op: 56, 12 mo: 28 | – | Staged interval unspecified | – | – | 12 mo | Child | – | Report | Few weeks after lesions | Unknown |
|
Marras et al (2014) a Italy |
M | 3 y | 15 y | Status dystonicus | Chromosomopathy | Inherited | Pre‐op: 101, 22 mo: 16 | Pre‐op: 30, post‐op: 30 | Simultaneous | – | – | 22 mo | Child | – | Series | 40 days | Unknown |
| M | 1 mo | 19 y | Status dystonicus | Epileptic encephalopathy | Pre‐op: 84, 21 mo: 4 | Pre‐op: 30, post‐op: 30 | Simultaneous | – | – | 21 mo | Adult | – | Series | 30 days | Unknown | ||
| M | 4 mo | 6.5 y | Status dystonicus | Bilateral striatal necrosis | Inherited | Pre‐op: 60, 15 mo: 57 | Pre‐op: 30, post‐op: 30 | Simultaneous | – | – | 15 mo | Child | – | Series | 21 days | Unknown | |
| M | 8 mo | 12 y | Status dystonicus | Hypoxic event | Acquired | Pre‐op: 77, 15 mo: 44 | Pre‐op: 26, latest follow‐up: 28 | Simultaneous | – | – | 15 mo | Child | – | Series | 60 days | Unknown | |
|
Fonoff et al (2012)a Brazil |
F | 2 y | 23 y | Generalized dystonia | – | Idiopathic | – | – | – | – | – | 24 mo | Unknown | Severe hypophonia | Series | – | Relapse after 2 y |
| M | 11 y | 41 y | Generalized dystonia | – | Idiopathic | – | – | – | – | – | 24 mo | Unknown | – | Series | – | Relapse | |
| F | 40 y | 58 y | Generalized dystonia | – | Acquired | – | – | – | – | – | 24 mo | Unknown | Speech impairment | Series | – | Relapse after 2.5 y | |
| M | 10 y | 20 y | Generalized dystonia | – | Idiopathic | – | – | – | – | – | 24 mo | Unknown | – | Series | – | Relapse after 4.5 y | |
|
Zirn et al (2011) a Germany |
F | 7 y | 15 y | – | TOR1A gene mutation | Inherited | – | – | – | – | – | – | Child | Mutism, dysarthria, dysphagia, hyperhidrosis | Letter to the editor | Immediately, cessation of hyperkinesia | Needs assistance in all aspects of life, however, may be due to disease progression |
|
Hashimoto et al (2010) a Japan |
M | – | 56 y | Tardive jaw‐opening dystonia | Tardive dystonia | Acquired | Motor speech and eating pre‐op: 8, post‐op: 1 | Pre‐op: 8, post‐op: 2 | Simultaneous | – | 24 mo | Adult | – | Report | Immediately | Unknown | |
|
Elkay et al (2009) a United States |
F | – | 21 y | Status dystonicus | Batten disease | Inherited | Pre‐op: 120, 5 mo: 65 | – | Simultaneous | – | – | 6 y | Adult | – | Report | 10 days | Slight opisthotonus but never to prepallidotomy level |
| F | – | 19 y | Generalized dystonia | Batten disease | Inherited | – | – | – | – | – | 6 y | Child | – | Report | Immediately | Relapse after 3 weeks | |
|
Cersosimo et al (2007) a Argentina |
F | 6 y | 19 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 108, 3 mo: 70, 1 y: 42, 8 y: 21 | Pre‐op: 26, 3 mo: 15, 1 y: 9, 8 y: 8 | Simultaneous | – | – | 96 mo | Adult | – | Series | – | Unknown |
| M | 10 y | 14 y | Focal dystonia | TOR1A gene mutation | Inherited | Pre‐op: 42, 3 mo: 10, 5 y: 11, 6 y: 32, 8 y: 50 | Pre‐op: 12, 3 mo: 8, 5 y: 8, 6 y 12, 8 y: 15 | Simultaneous | – | – | 96 mo | Child | Anarthria | Series | – | Relapse after 60 mo | |
|
Teive et al (2005)a Brazil |
M | – | 8 y | Status dystonicus | Cerebral palsy | Acquired | – | – | – | – | – | Child | – | Series | – | Unknown | |
|
Hwang et al (2005) a United States |
M | 6 mo | 6 y | Generalized dystonia | Glutaric aciduria type 1 | Inherited | Pre‐op: 115, 6 mo 98 | – | Staged, 3‐mo interval | Global Dystonia Rating Score | Pre‐op: 98, post op: 78 | 6 mo | Child | – | Report | Immediately | Unknown |
|
Rakocevic et al (2004) a United States |
M | 10 mo | 18 mo | Generalized dystonia | Glutaric aciduria type 1 | Inherited | – | – | Staged | – | – | 24 mo | Child | Left horizontal gaze preference | Report | – | Unknown |
|
Kyriagis et al (2004) a United States |
M | 16 mo | 9 y | Status dystonicus | Hallervorden‐Spatz disease | Inherited | – | – | Simultaneous | – | – | 12 mo | Child | – | Report | 6 mo, with intrathecal baclofen | Alleviation of spasms with bilateral pallidotomy and baclofen |
|
Hutchison et al (2003) a Canada |
– | 4 y | 13 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 70.5, post‐op: 48 | – | – | – | – | – | Child | – | Series | – | Unknown |
| – | 7 y | 14 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 53.5, post‐op: 13.5 | – | – | – | – | – | Child | – | Series | – | Unknown | |
| – | 7 y | 9 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 75, post‐op: 52 | – | – | – | – | – | Child | – | Series | – | Unknown | |
| – | 9 y | 9 y | Generalized dystonia | – | – | Pre–op: 113.5, post–op: not available | – | – | – | – | – | Child | – | Series | – | Unknown | |
| – | 5 y | 16 y | Generalized dystonia | – | Idiopathic | Pre‐op: 49, post‐op: 45, 5 mo: 56 | – | – | – | – | – | Child | – | Series | – | Relapse at 5 mo | |
| Eltahawy et al (2004) a | M | 7 y | 14 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 75, 6 mo: 25 | – | Simultaneous | Global Outcome Score | 6 mo: 4 | 6 mo | Child | Hypophonia | Series | – | Unknown |
| M | 8 y | 15 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 71, 6 mo: 38 | – | Simultaneous | Global Outcome Score | 6 mo: 3 | 6 mo | Child | Dysphonia | Series | – | Unknown | |
| F | 7 y | 17 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 53, 6 mo: 13 | – | Simultaneous | Global Outcome Score | 6 mo: 4 | 6 mo | Child | – | Series | – | Unknown | |
| M | 5 y | 19 y | Generalized dystonia | Idiopathic | Idiopathic | Pre‐op: 49, 6 mo: 40 | – | Simultaneous | Global Outcome Score | 6 mo: 1 | 6 mo | Adult | – | Series | – | Unknown | |
| M | 1 y | 12 y | Generalized dystonia | Glutaric aciduria | Inherited | Pre‐op: 113, 6 mo: 99 | – | Simultaneous | Global Outcome Score | 6 mo: 1 | 6 mo | Child | – | Series | – | Unknown | |
|
Sanghera et al (2003) a United States |
– | 7 y | 15 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 50, post‐op: 12 | – | Simultaneous | UDRS | Pre‐op: 68, post‐op: 12 | 12 mo | Child | – | Series | – | Unknown |
| – | 8 y | 10 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 26, post‐op: 19 | – | Staged | UDRS | Pre‐op: 36, post‐op: 21 | 12 mo | Child | – | Series | – | Unknown | |
| – | 9 y | 51 y | Generalized dystonia | Pre‐op: 57, post‐op: 15 | – | Staged | UDRS | Pre‐op: 83, post‐op: 26 | 12 mo | Adult | – | Series | – | Unknown | |||
| – | 8 y | 13 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 48, post‐op: 17 | – | Simultaneous | UDRS | Pre‐op: 81, post‐op: 20 | 12 mo | Child | – | Series | – | Unknown | |
| – | 12 y | 16 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 27, post‐op: 10 | – | Simultaneous | UDRS | Pre‐op: 41, post‐op: 2 | 12 mo | Child | – | Series | – | Unknown | |
| – | 7 y | 17 y | Generalized dystonia | – | – | Pre‐op: 59, post‐op: 17 | – | Simultaneous | UDRS | Pre‐op: 101, post‐op: 27 | 12 mo | Child | – | Series | – | Unknown | |
| – | 6 y | 15 y | Generalized dystonia | – | – | Pre‐op: 56, post‐op: 45 | – | Simultaneous | UDRS | Pre‐op: 82, post‐op: 74 | 12 mo | Child | – | Series | – | Unknown | |
| – | 9 y | 19 y | Generalized dystonia | – | – | Pre‐op: 56, post‐op: 43 | – | Simultaneous | UDRS | Pre‐op: 86, post‐op: 63 | 12 mo | Adult | – | Series | – | Unknown | |
| – | 5 y | 15 y | Generalized dystonia | – | – | Pre‐op: 49, post‐op: 46 | – | Simultaneous | UDRS | Pre‐op: 78, post‐op: 66 | 12 mo | Child | – | Series | – | Unknown | |
| – | 2 y | 11 y | Generalized dystonia | – | – | Pre‐op: 58, post‐op: 36 | – | Simultaneous | UDRS | Pre‐op: 88, post‐op: 54 | 12 mo | Child | – | Series | – | Unknown | |
| – | 0.4 y | 8 y | Generalized dystonia | – | – | Pre‐op: 31, post‐op: 25 | – | Staged | UDRS | Pre‐op: 51, post‐op: 34 | 12 mo | Child | – | Series | – | Unknown | |
|
Anca et al (2003) a Israel |
F | 7 y | 15 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 78, 1 y: 42, 2 y: 52 | Pre‐op: 28, 1 y: 22, 2 y: 22 | – | – | – | 24 mo | Child | 1 patient of these aphonia after 1 y | Series | – | Significant motor improvement over first 3–4 mo but progressive worsening after 1 y |
| M | 8 y | 11 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: unknown, 1 y: 14, 2 y: 14, 3 y: 23, 4 y: 38 | Pre‐op: unknown, 1 y: 4, 2 y: 4, 3 y: 6, 4 y: 9 | – | – | – | 24 mo | Child | 1 patient of these aphonia after 1 y | Series | – | Relapse after 3 y | |
| M | 9 y | 13 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 68, 1 y: 15, 2 y: 25 | Pre‐op: 26, 1 y: 3.5, 2 y: 9 | – | – | – | 48 mo | Child | 1 patient of these aphonia after 1 y | Series | – | Significant motor improvement over first 3–4 mo but progressive worsening after 1 y | |
|
Teive et al (2001)a Brazil |
M | 37 y | 40 y | Generalized dystonia | Trauma | Acquired | Pre‐op: 51, 3 days: 15, 3 m: 51, 6 mo: 51 | – | Simultaneous | – | – | 6 mo | Adult | Track hemorrhages: motor seizure, hemiparesis | Series | Immediately but relapse 3 mo later | Relapse after 3 mo |
| M | 16 y | 35 y | Generalized dystonia | – | – | Pre‐op: 60, 3 days: 52 (stage1) /46 (stage 2), 3 mo: 18, 6 mo: 18 | – | Staged | – | – | 6 mo | Adult | – | Series | Progressive improvement up to 3 mo | Unknown | |
| – | 5 y | – | – | – | – | Pre‐op: 50, 3 days: 50, 3 mo: 28, 6 mo: 28 | – | Simultaneous | – | – | 6 mo | Child | – | Series | Progressive improvement up to 3 mo | Unknown | |
| M | 23 y | 28 y | Generalized dystonia | – | – | Pre‐op: 48, 3 days: 40, 3 mo: 6, 6 mo: 4 | – | Simultaneous | – | – | 6 mo | Adult | Transient lethargy | Series | Progressive improvement over 6 mo | Unknown | |
| ‐ | 21 y | – | – | – | – | Pre‐op: 28, 3 days: 24, 3 mo: 12, 6 mo: 12 | – | Simultaneous | – | – | 6 mo | Adult | – | Series | Progressive improvement up to 3 mo | Unknown | |
|
Cubo et al (2000) a United States |
F | 4 y | 13 y | Generalized dystonia | Westphal variant of Huntington's disease | Inherited | Pre‐op: 56, 3 mo post‐op: 49 | – | Simultaneous | – | – | 3 mo | Child | Edema on the right capsule: seizures | Report | Little improvement over 3 mo | Disease progression |
|
Iacono et al (1996) a United States |
M | 8 y | 17 y | Generalized dystonia | TOR1A gene mutation | Inherited | – | – | Simultaneous | – | – | 12 mo | Child | – | Report | Immediately | Unknown |
|
Vitek et al (1998) a United States |
– | – | – | Generalized dystonia | – | – | Pre‐op: 34, 1 mo: 10 | Pre‐op: 10, 1 mo: 3 | – | – | – | 2 mo | Unknown | – | Series | – | Unknown |
| – | – | – | Generalized dystonia | – | – | Pre‐op: 31, 2 mo: 5 | Pre‐op: 8, 2 mo: 0 | – | – | – | 2 mo | Unknown | – | Series | – | Unknown | |
| – | – | – | Generalized dystonia | – | – | Pre‐op: 41, 1 week: 6 | Pre‐op: 8.5, 1 week: 2 | – | – | – | 2 mo | Unknown | – | Series | – | Unknown | |
|
Ondo et al (1998) a United states |
M | 10 y | 51 y | Generalized dystonia | – | – | Pre‐op: 57, post‐op: 9 | Pre‐op: 16, post‐op: 8 | Staged | UDRS, ADL | Pre‐op: 83, post‐op: 20, ADL = pre‐op: 16, post‐op: 8 | 6 mo | Adult | – | Series | – | Unknown |
| – | 13 y | 18 y | Generalized dystonia | Trauma | Acquired | Pre‐op: 56, post‐op: 46 | Pre‐op: 18, post‐op: 16 | Simultaneous | UDRS, ADL | Pre‐op: 86, post‐op: 58, ADL = pre‐op: 18, post‐op: 16 | 6 mo | Adult | – | Series | – | Unknown | |
| M | 10 y | 16 y | Generalized dystonia | Hypoxic event | Acquired | Pre‐op: 59, post‐op: 17 | Pre‐op: 19, post‐op: 9 | Simultaneous | UDRS, ADL | Pre‐op: 101, post‐op: 27, ADL = pre‐op: 19, post‐op: 9 | 6 mo | Child | – | Series | 2 days | Unknown | |
| F | 7 y | 14 y | Generalized dystonia | Genetic | Inherited | Pre‐op: 50, post‐op: 17 | Pre‐op: 18, post‐op: 7 | Simultaneous | UDRS, ADL | Pre‐op: 68, post‐op: 21, ADL = pre‐op: 18, post‐op: 7 | 6 mo | Child | Transient lethargy | Series | Gradual improvement over 3 mo | Partial recurrence after 6 mo | |
| F | 8 y | 13 y | Generalized dystonia | TOR1A gene mutation | Inherited | Pre‐op: 48, post‐op: 17 | Pre‐op: 17, post‐op: 6 | Simultaneous | UDRS, ADL | Pre‐op: 81, post‐op: 20, ADL = pre‐op: 17, post‐op: 6 | 6 mo | Child | – | Series | Within 3 weeks | Unknown | |
|
Weetman et al (1997) a United Kingdom |
M | – | 31 y | Generalized dystonia | Tardive dystonia | Acquired | Pre‐op: 76, 8 mo: 21 | Pre‐op: 22, 8 mo: 4 | Simultaneous | Obeso scale | Grade 2 | 8 mo | Adult | – | Report | Immediately | Slight recurrence |
|
Lin et al (1999)a,b Taiwan |
M | – | 29 y | Generalized dystonia | Perinatal asphyxia | Acquired | Pre‐op: 51, 3 mo: 37, 6 mo: 33.5, 1 y: 33.5 | – | – | – | – | 12 mo | Adult | – | Report | Improvement over 6 mo | Unknown |
|
Taiwan |
F | 30 y | 36 y | Generalized dystonia | Dystonia due to hypovolemic shock | Acquired | Pre‐op: 74, 1 mo: 47, 3 mo: 34, 6 mo: 28, 9 mo: 28 | Pre‐op: 20, 1 mo: 12, 3 mo: 10, 6 mo: 9, 9 mo: 9 | – | – | – | 9 mo | Adult | Transient right facial weakness | Report | Improvement over 9 mo | Unknown |
|
Lin et al (2001) b Taiwan |
n = 18, 8 men, 10 women | Average = 24.8 y | BFMDRS movement | Generalized dystonia | – | – | 13% decrease | 9% decrease | 14 simultaneous, 4 staged | – | – | 12 mo | – | Transient adverse effects in 7 of 18: urinary incontinence (2), visual field defects (2), hemiparesis (2), unsteady gait (1), fever (1) | Series | – | Unknown |
| Khandelwal et al (2018) | M | – | 48 y | Cervical dystonia | Cervical dystonia | – | – | – | Simultaneous | – | – | – | Adult | Transient bilateral mydriasis and visual field defects | Report | – | Unknown |
Included in meta‐analysis.
Overlapping patient.
Abbreviations: BFMDRS, Burke‐Fahn‐Marsden Dystonia Rating Scale; DBS, deep brain stimulation; ADL, activities of daily living; UDRS, Unified Dystonia Rating Scale; BAD, Barry Albright Dystonia; SD, status dystonicus; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale; PKAN, Pantothenate Kinase‐Associated Neurodegeneration; DSAP, Dystonia Severity Action Plan.
TABLE 2.
Individual patient data group characteristics
| Characteristic | Total population |
|---|---|
| Sex (n = 75) | |
| Men | 35/75 (46%) |
| Women | 18/75 (24%) |
| Unknown | 22/75 (30%) |
| Mean age at surgery ± SD (y), n = 70 | 20 ± 13.9 |
| Type of dystonia (n = 72) | |
| Generalized | 49/72 (68%) |
| Status dystonicus | 16/72 (22%) |
| Focal | 6/72 (8%) |
| Camptocormia | 1/72 (1%) |
| Mean duration of follow‐up ± SD (mo), n = 67 | 19.7 ± 27.7 |
| Diagnosis (n = 55) | |
| Idiopathic | 9/56 (16%) |
| Acquired | 17/56 (30%) |
| Inherited | 30/56 (54%) |
| BFMDRS improvement (n = 53) | |
| >40% | 34/53 (64%) |
| 20–40% | 8/53 (15%) |
| <20% | 9/53 (17%) |
| Worsening | 2/53 (4%) |
| UDRS (n = 17) | |
| Improvement ≥20% | 11/17 (65%) |
| Score decrease <20% | 4/17 (24%) |
| No change or worsening | 2/17 (12%) |
| Side effects (n = 75) | |
| Transient | 6/75 (8%) |
| Permanent | 8/75 (11%) |
Abbreviations: BFMDRS, Burke‐Fahn‐Marsden Dystonia Rating Scale; UDRS, Unified Dystonia Rating Scale.
Methodological Quality Assessment
The overall agreement between raters was 91% (190/209) for case reports and 86% (216/252) for case series. Both Cohen's kappas were substantial (case reports: 0.76, case series: 0.67). Therefore, the interrater variability is low (APPENDIX S1).
Efficacy
Of 100 patients, BFMDRS scores were assessed for 53 patients pre‐ and postoperatively. One paper presented improvement percentage for 18 patients on a group level instead of individual scores. 34 The outcome for patients lacking a BFMDRS score was quantified by the UDRS (n = 17), a qualitative description of motor results (n = 12), or another scale (n = 3). Of note, a variety of other scales were used, and some patients were scored twice on separate scoring systems: Barry Albright Dystonia scale (n = 7), Activity of Daily Living scale (n = 5), Gross Outcome Score (n = 5), Obeso scale (n = 1), Toronto Western Spasmodic Torticollis Rating Scale (n = 1), Tsui score (n = 1), and the Global Dystonia Rating Scale (n = 1).
Motor Performance
BFMDRS scores were available for 53 of 100 (53%) patients, showing improvement after pallidotomy in 51 of 53 patients (96%). Forty‐two patients (79%) showed improvement of 20% or more. In 34 patients (64%) improvement was >40% at the end of reported follow‐up (Fig. 1). Improvement typically did not occur in the early postoperative phase but became apparent after several weeks to months (Fig. 2). Lin et al reported a mean 13% improvement at 1‐year follow‐up in a group of 18 patients with generalized dystonia (no individual scores available). 34 Cersosimo et al reported on a patient with a maximum improvement of 76.2% at 3 months followed by a secondary deterioration of 19% at 96 months. 18 Another patient experienced a temporary postoperative deterioration for a few weeks but returned to preoperative level at 3 months.
FIG. 2.
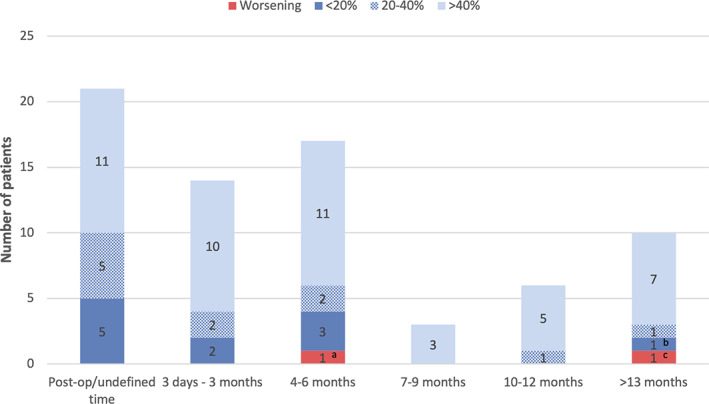
Improvement BFMDRS (Burke‐Fahn‐Marsden Dystonia Rating Scale) movement score, categorized by percentage decrease in score at latest‐reported follow‐up. Some studies report multiple follow‐up moments. Letters a–c represent relapsing patients. For exact scores of patients see Table 1. a: Hutchison et al (2003). 8.2% improvement immediately postoperatively and relapsed at 5‐month follow‐up to a level 14.3% worse than baseline. b: Anca et al (2003), maximal improvement of 46.2% at 12‐month follow‐up, but the score had dropped to 33.3% improvement at 24‐month follow‐up. c: Cersosimo et al (2008). Maximal improvement of 76.2% at 3‐month follow‐up and showed relapsing at 60‐month follow‐up. At 96‐month follow‐up, the BFMDRS score had dropped to 19.1% worse than baseline. [Color figure can be viewed at wileyonlinelibrary.com]
Disability
A disability score was available for 21 patients (21%). Fifteen patients showed an improvement (14 patients >20%). Four patients showed no improvement. The scores of 2 patients worsened, 1 of whom had an initial improvement of 33% at 60 months that dropped to –25% at 72 months. Lin et al also scored the disability scale and reported a mean improvement of 9% (no individual scores are available). 34
In 17 other patients, the UDRS score was used. Of those, the vast majority improved more than 20% (n = 15; 88.2%), 1 patient improved 15%, and 1 patient improved 10%. 40 On the remaining reported scales, all patients improved (Table 1).
Sustainability
The mean follow‐up was 18.2 months (range 2–180 months; n = 83). In 17 patients, the follow‐up duration was not (individually) reported. In most patients, the beneficial effect lasted through follow‐up. In 19 of 100 patients, a relapse of dystonic symptoms was reported (Table 1). One study reported relapsing SD. 45 Six patients were treated with DBS after bilateral pallidotomy, because their dystonic symptoms reappeared after temporary benefit. 24 , 38 , 49 The relapse time of dystonic symptoms after pallidotomy varied between 3 weeks and 4.5 years.
Safety
Adverse events were reported in 20 of 100 patients (entire data set). Of note, adverse effects were sometimes reported in case series and therefore not analyzed as adverse effects in individually reported patients. The adverse effects in individually reported patients are presented in Table 3. Twelve patients (60%) had a variety of transient side effects (lethargy, edema, subgaleal effusion, urinary incontinence, visual field defects, hemiparesis, unsteady gait, and fever). Eight patients (40%) experienced permanent deficits (mutism, hypophonia, dysphonia, anarthria, aphonia, dysarthria, dysphagia, hyperhidrosis, and limitation of left horizontal gaze). Surgical track hemorrhages were reported in 1 patient. In most cases (n = 19), adverse effects were noticed shortly after surgery. One patient reported development of aphonia at 1‐year follow‐up. 17 No deaths due to bilateral pallidotomy were registered.
TABLE 3.
Reported adverse events in all studies mentioned in this review
| Article | Patients | Permanent adverse events | Transient adverse events |
|---|---|---|---|
| Khandelwal et al (2018) | 1 | Bilateral mydriasis and visual field defects (n = 1) | |
| Horisawa et al (2016) | 1 | Transient aggressive behavior (n = 1) | |
| Fonoff et al (2012) | 2 | Severe hypophonia (n = 1), speech impairment (n = 1) | |
| Zirn et al (2011) | 1 | Mutism, dysarthria, dysphagia, and hyperhidrosis (n = 1) | |
| Cersosimo et al (2008) | 1 | Anarthria (n = 1) | |
| Rakocevic et al (2004) | 1 | Left horizontal gaze preference (n = 1) | |
| Eltahawy et al (2004) | 2 | Hypophonia (n = 1), dysphonia (n = 1) | |
| Teive et al (2001) | 2 | Transient lethargy (n = 1), track hemorrhages (n = 1) | |
| Cubo et al (2000) | 1 | Right capsule edema (n = 1) | |
| Ondo et al (1998) | 1 | Transient lethargy (n = 1) | |
| Anca et al (2003) | 1 | Aphonia (n = 1) | |
| Lin et al (1998) a | 1 | Transient right facial weakness (n = 1) | |
| Lin et al (2001) | 7 | Urinary incontinence (n = 2), visual field defects (n = 2), hemiparesis (n = 2), unsteady gait (n = 1), fever (n = 1) |
Also mentioned in Lin et al (2001).
Staged pallidotomy was reported in 12 of 48 patients and simultaneous bilateral pallidotomy in 35 of 48 patients. For the remaining 52 cases, these data were not reported. In the “staged” group, a case of unilateral horizontal gaze impairment and a case of transient lethargy were reported. The other adverse events occurred either in the “simultaneous” group or in the “unreported” group.
One paper by Khandelwal et al was not included in the overall analysis because of a lack of clinical outcome data. Nevertheless, the paper is mentioned in Table 3 because of a transient adverse effect of bilateral mydriasis and visual field defects. 50
Discussion
This systematic review evaluated the efficacy, safety, and sustainability of bilateral pallidotomy for dystonia. In 2008, Gross stated that improvement after pallidotomy was similar to DBS and that complications appeared to be rare. 51 Although this statement was based on studies of unequal levels of scientific evidence, it provides food for thought regarding whether the abandonment of bilateral pallidotomy as a treatment option in selected patients is justified. In the reviewed papers, the rationale for choosing pallidotomy over DBS is generally not mentioned. A few exceptions were as follows: patients decided against implantation of material inside their head, higher costs of DBS, and in one case DBS was ineffective for SD and it was decided to perform bilateral pallidotomy.
Efficacy and Sustainability
Bilateral pallidotomy was shown to lead to improvement in the majority of patients with a reported BFMDRS score (96%). BFMDRS score as the most relevant outcome measure revealed an improvement of 20% or more in 42 of 53 patients (79%). Of these, 34 (64%) patients had an improvement of more than 40%. Three patients experienced secondary worsening of the BFMDRS score, 2 of which returned to baseline (Fig. 2). 17 , 29 Although in some cases immediate effects were reported, the beneficial effect of bilateral pallidotomy typically took weeks or months to occur. The cause for heterogeneity in response time is unknown. After bilateral pallidotomy, the disability scale (maximal 30 points) showed overall a mean improvement of 6.5 points (22%) but was observed only in a minority of patients. Stewart and colleagues concluded that the disability score is less responsive to change in dystonic symptoms. 52 There can be a meaningful change in functioning in the absence of a disability score improvement. 53 All these aspects of pallidotomy should be mentioned in counseling patients.
No conclusions can be drawn concerning long‐term efficacy of bilateral pallidotomy in the treatment of dystonia as the median‐reported follow‐up time was only 18 months.
In medication‐refractory SD, bilateral pallidotomy initially terminated in 23 of 25 reported cases (92%). Remarkably, in a single study by Garone and colleagues after initial benefit, relapse of symptoms was reported in 7 patients. 45 Unfortunately, the (possible) explanations for these relapses are not mentioned, nor the duration of the relapse nor the type of dystonia. In combination with the fact that this is the only study that reports the recurrence of SD after bilateral pallidotomy, conclusions cannot be drawn on the risk of relapse after SD. Of note, an additional search for cases describing unilateral pallidotomy for SD was performed, but such cases were not identified.
Safety
In the literature, adverse events of bilateral pallidotomy include hemiparesis, visual field defects, and neuropsychological changes. The latter often disappear within 6 months after pallidotomy but are reported permanent if lesions encroach the anteromedial (non‐motor) portion of the pallidum. Facial paresis is also frequently reported. Limb paresis (up to 4%) and visual field deficits (up to 14%) are less common. Of note, most data on side effects of pallidotomy are from patients with Parkinson's disease. 54
In this review, transient and permanent adverse events were reported after bilateral pallidotomy. Temporary lethargy and permanent speech disorders (eg, hypophonia) were most frequently reported. Adverse events were less reported after staged bilateral pallidotomy, but in 45 patients adverse events were not specified to be related to either a staged or a simultaneous procedure, so these data should be interpreted with caution. Also, some adverse events reported in a simultaneous procedure were the result of a unilateral complication, for example, seizures by a hemorrhagic track. Therefore, the safety benefits of staging the procedure should be weighed carefully in individual cases. Based on this review, the marginal safety gain of staging, barely, if at all, outweighs the discomfort of two surgeries and the delayed relief of dystonic symptoms.
Five patients (5%) had permanent speech disorders after bilateral pallidotomy. Transient or stimulation‐dependent speech disorders are also reported after DBS. 12 On the contrary, the same authors state that DBS‐hardware problems are very common (36.1%). The adverse event profiles of both lesioning and DBS are to be kept in mind when considering surgical treatment, but as the profiles do not match, this should be done with caution.
Finally, we identified 5 patients with DBS after bilateral pallidotomy. DBS in both the internal globus pallidus and subthalamic nucleus has been reported to be successful. Although this is encouraging evidence, we cannot draw conclusions from these data. The reported cases in literature on this matter are scarce, requiring further validation in future studies.
Limitations
The main limitation of this review is that only case reports and case series on bilateral pallidotomy in dystonia were available. This comes with a high probability of underreporting of negative outcomes, leading to inevitable publication bias in this review. The second limitation of this study is that not all included patients were evaluated using standardized assessment tools (eg, BFMDRS, UDRS).
We recommend the application of standardized tools such as BFMDRS for clinical assessment in future reports on pallidotomy for dystonia. In addition, we recommend the use of personalized goals. 55 , 56 Finally, the 12‐month follow‐up sample size was considerably smaller than that at 3 and 6 months. As such, no definite conclusions can be drawn on long‐term sustainability of bilateral pallidotomy nor the risk of relapsing in the long term. Data on specifics of surgical techniques were too sparsely reported to consider in this analysis. For future reports we suggest to provide at least the following points: general anesthesia or awake surgery, use of micro‐electrode recording, target localization, probe type and diameter, lesioning duration and temperature, and number of lesions per side and preferably postoperative evaluation of lesion size and location.
The next step, a controlled trial of pallidotomy in dystonia, is worth exploring, as this review shows not only that pallidotomies in dystonia are currently being performed and reported but also that there is at least some equipoise in the field. The potential factors complicating a controlled trial include the establishment of DBS as the standard surgical treatment for dystonia in most centers and the lack of proper neurosurgical training and expertise in ablative surgeries for movement disorders.
Conclusion
Based on this systematic literature review, bilateral pallidotomy is in the short term an effective treatment for dystonia, particularly in treating medication‐refractory SD and despite a considerable number of adverse events. Given the burden of dystonia, bilateral pallidotomy should be regarded a viable tool in the armamentarium of the neurosurgeon in the treatment of dystonia, particularly for patients with contraindications for DBS or if the severity of the dystonic symptoms outweighs the risk of permanent speech disorders.
Author Roles
L.M.C.: Research project: organization, execution, Statistical analysis: design, execution, Manuscript: writing the first draft, review, and critique; D.L.M.O.: Research project: conception, organization, Statistical analysis: review and critique, Manuscript: writing, review, and critique; M.A.J.T.: Manuscript: review and critique; I.L.‐L.: Research project: organization, execution, Statistical analysis: design, execution, Manuscript: writing the first draft, review, and critique; M.E.E.: Manuscript: review and critique; J.M.C.D.: Research project: conception, organization, Statistical analysis: review and critique, Manuscript: writing, review, and critique.
Full financial disclosures for the previous 12 months
D.L.M.O. received travel grants from Medtronic and LivaNova. L.M.C., I.L.‐L. and J.M.C.D. declare no competing interests. M.E.E. received a travel grant from Medtronic. M.A.J.T. reports grants from the European Fund for Regional Development from the European Union (01492947) and the province of Friesland, ZONMW‐TOP (91218013), Dystonia Medical Research Foundation, from Stichting Wetenschapsfonds Dystonie Vereniging, from Fonds Psychische Gezondheid, and from Phelps Stichting and an unrestricted grant from Actelion and Merz.
Supporting information
APPENDIX S1 Adapted quality assessment tool for case reports
FIGURE S1 Flow diagram of study selection process, according to the PRISMA guidelines
Acknowledgments:
We are indebted to Nynke Smidt for her valuable advice on epidemiological issues of this review. We also thank the corresponding authors Dr. M. Waugh, Dr. B. Hutchison, Dr. A. Lozano, and Dr. G. Messina for their replies to our request for IPD. Finally, we thank Jojanneke Bruintjes for her efforts in editing the manuscript for publication.
Two of the authors of this publication (M.A.J.T. and M.E.E.) are members of the European Reference Network for Rare Neurological Diseases, project ID 739510.
References
- 1. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013;28(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Delnooz CC, van de Warrenburg BP. Current and future medical treatment in primary dystonia. Ther Adv Neurol Disord 2012;5(4):221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruiz‐Lopez M, Fasano A. Rethinking status dystonicus. Mov Disord 2017;32(12):1667–1676. [DOI] [PubMed] [Google Scholar]
- 4. Kupsch A, Benecke R, Muller J, et al. Pallidal deep‐brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006;355(19):1978–1990. [DOI] [PubMed] [Google Scholar]
- 5. Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep‐brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005;352(5):459–467. [DOI] [PubMed] [Google Scholar]
- 6. Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral, pallidal, deep‐brain stimulation in primary generalised dystonia: a prospective 3 year follow‐up study. Lancet Neurol 2007;6(3):223–229. [DOI] [PubMed] [Google Scholar]
- 7. Volkmann J, Wolters A, Kupsch A, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5‐year follow‐up of a randomised trial. Lancet Neurol 2012;11(12):1029–1038. [DOI] [PubMed] [Google Scholar]
- 8. Cif L, Hariz M. Seventy years with the Globus pallidus: Pallidal surgery for movement disorders between 1947 and 2017. Mov Disord 2017;32(7):972–982. [DOI] [PubMed] [Google Scholar]
- 9. Guridi J, Lozano AM. A brief history of pallidotomy. Neurosurgery 1997;41(5):1169–1180. discussion 1180‐1163. [DOI] [PubMed] [Google Scholar]
- 10. Jitkritsadakul O, Bhidayasiri R, Kalia SK, Hodaie M, Lozano AM, Fasano A. Systematic review of hardware‐related complications of deep brain stimulation: do new indications pose an increased risk? Brain Stimul 2017;10(5):967–976. [DOI] [PubMed] [Google Scholar]
- 11. Szolna A, Harat M, Gryz J. Stereotactic pallidotomy and thalamotomy in the treatment of primary dystonia. Neurol Neurochir Pol 2006;40(3):186–193. [PubMed] [Google Scholar]
- 12. Koy A, Bockhorn N, Kuhn AA, et al. Adverse events associated with deep brain stimulation in patients with childhood‐onset dystonia. Brain Stimul 2019;12(5):1111–1120. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 14. Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985;35(1):73–77. [DOI] [PubMed] [Google Scholar]
- 15. Franzini A, Levi V, Franzini A, Dones I, Messina G. Staged pallidotomy: MRI and clinical follow‐up in status dystonicus. Br J Neurosurg 2019;33(2):184–187. [DOI] [PubMed] [Google Scholar]
- 16. Moga CGB, Schopflocher D, Harstall C. Development of a Quality Appraisal Tool for Case Series using a Modified Delphi Technique. Edmonton AB: Institute of Health Economics; 2012. [Google Scholar]
- 17. Anca MH, Zaccai TF, Badarna S, Lozano AM, Lang AE, Giladi N. Natural history of Oppenheim's dystonia (DYT1) in Israel. J Child Neurol 2003;18(5):325–330. [DOI] [PubMed] [Google Scholar]
- 18. Cersosimo MG, Raina GB, Piedimonte F, Antico J, Graff P, Micheli FE. Pallidal surgery for the treatment of primary generalized dystonia: long‐term follow‐up. Clin Neurol Neurosurg 2008;110(2):145–150. [DOI] [PubMed] [Google Scholar]
- 19. Cubo E, Shannon KM, Penn RD, Kroin JS. Internal globus pallidotomy in dystonia secondary to Huntington's disease. Mov Disord 2000;15(6):1248–1251. [DOI] [PubMed] [Google Scholar]
- 20. Elkay M, Silver K, Penn RD, Dalvi A. Dystonic storm due to Batten's disease treated with pallidotomy and deep brain stimulation. Mov Disord 2009;24(7):1048–1053. [DOI] [PubMed] [Google Scholar]
- 21. Eltahawy HA, Saint‐Cyr J, Giladi N, Lang AE, Lozano AM. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery 2004;54(3):613–619. Discussion 619‐621. [DOI] [PubMed] [Google Scholar]
- 22. Franzini A, Cordella R, Penner F, et al. Posteroventrolateral pallidotomy through implanted DBS electrodes monitored by recording local field potentials. Br J Neurosurg 2015;29(6):888–890. [DOI] [PubMed] [Google Scholar]
- 23. Franzini A, Franzini A, Levi V, Cordella R, Messina G. An unusual surgical indication for cerebral tuberculosis: status dystonicus. Case report. Acta Neurochir (Wien) 2018;160(7):1355–1358. [DOI] [PubMed] [Google Scholar]
- 24. Fonoff ET, Campos WK, Mandel M, Alho EJ, Teixeira MJ. Bilateral subthalamic nucleus stimulation for generalized dystonia after bilateral pallidotomy. Mov Disord 2012;27(12):1559–1563. [DOI] [PubMed] [Google Scholar]
- 25. Hashimoto T, Naito K, Kitazawa K, Imai S, Goto T. Pallidotomy for severe tardive jaw‐opening dystonia. Stereotact Funct Neurosurg 2010;88(2):105–108. [DOI] [PubMed] [Google Scholar]
- 26. Horisawa S, Goto S, Takeda N, Terashima H, Kawamata T, Taira T. Bilateral Pallidotomy for cervical dystonia after failed selective peripheral denervation. World Neurosurg 2016;89:728 e1‐4. [DOI] [PubMed] [Google Scholar]
- 27. Horisawa S, Oka M, Kawamata T, Taira T. Bilateral pallidotomy for embouchure dystonia. Eur J Neurol 2018;25(9):e108–e109. [DOI] [PubMed] [Google Scholar]
- 28. Horisawa S, Oka M, Kohara K, Kawamata T, Taira T. Staged bilateral pallidotomy for dystonic camptocormia: case report. J Neurosurg 2018;131(3):839–842. [DOI] [PubMed] [Google Scholar]
- 29. Hutchison WD, Lang AE, Dostrovsky JO, Lozano AM. Pallidal neuronal activity: implications for models of dystonia. Ann Neurol 2003;53(4):480–488. [DOI] [PubMed] [Google Scholar]
- 30. Hwang HSDSA. Bilateral Pallidotomy for dystonia with Glutaric aciduria type 1. J Korean Neurosurg Soc 2005;38:380–383. [Google Scholar]
- 31. Iacono RP, Kuniyoshi SM, Lonser RR, Maeda G, Inae AM, Ashwal S. Simultaneous bilateral pallidoansotomy for idiopathic dystonia musculorum deformans. Pediatr Neurol 1996;14(2):145–148. [DOI] [PubMed] [Google Scholar]
- 32. Kyriagis M, Grattan‐Smith P, Scheinberg A, Teo C, Nakaji N, Waugh M. Status dystonicus and Hallervorden‐Spatz disease: treatment with intrathecal baclofen and pallidotomy. J Paediatr Child Health 2004;40(5–6):322–325. [DOI] [PubMed] [Google Scholar]
- 33. Lin JJ, Lin GY, Shih C, Lin SZ, Chang DC, Lee CC. Benefit of bilateral pallidotomy in the treatment of generalized dystonia. Case report. J Neurosurg 1999;90(5):974–976. [DOI] [PubMed] [Google Scholar]
- 34. Lin JJ, Lin SZ, Lin GY, Chang DC, Lee CC. Treatment of intractable generalized dystonia by bilateral posteroventral pallidotomy–one‐year results. Zhonghua Yi Xue Za Zhi (Taipei) 2001;64(4):231–238. [PubMed] [Google Scholar]
- 35. Lin JJ, Lin SZ, Lin GY, Chang DC, Lee CC. Application of bilateral sequential pallidotomy to treat a patient with generalized dystonia. Eur Neurol 1998;40(2):108–110. [PubMed] [Google Scholar]
- 36. Marras CE, Rizzi M, Cantonetti L, et al. Pallidotomy for medically refractory status dystonicus in childhood. Dev Med Child Neurol 2014;56(7):649–656. [DOI] [PubMed] [Google Scholar]
- 37. Minkin K, Gabrovski K, Dimova P, et al. Bilateral pallidotomy for Meige syndrome. Acta Neurochir (Wien) 2017;159(7):1359–1363. [DOI] [PubMed] [Google Scholar]
- 38. Ondo WG, Desaloms JM, Jankovic J, Grossman RG. Pallidotomy for generalized dystonia. Mov Disord 1998;13(4):693–698. [DOI] [PubMed] [Google Scholar]
- 39. Rakocevic G, Lyons KE, Wilkinson SB, Overman JW, Pahwa R. Bilateral pallidotomy for severe dystonia in an 18‐month‐old child with glutaric aciduria. Stereotact Funct Neurosurg 2004;82(2–3):80–83. [DOI] [PubMed] [Google Scholar]
- 40. Sanghera MK, Grossman RG, Kalhorn CG, Hamilton WJ, Ondo WG, Jankovic J. Basal ganglia neuronal discharge in primary and secondary dystonia in patients undergoing pallidotomy. Neurosurgery 2003;52(6):1358–1370. discussion 1370‐1353. [DOI] [PubMed] [Google Scholar]
- 41. Teive HA, Munhoz RP, Souza MM, et al. Status Dystonicus: study of five cases. Arq Neuropsiquiatr 2005;63(1):26–29. [DOI] [PubMed] [Google Scholar]
- 42. Teive HA, Sa DS, Grande CV, Antoniuk A, Werneck LC. Bilateral pallidotomy for generalized dystonia. Arq Neuropsiquiatr 2001;59(2‐B):353–357. [DOI] [PubMed] [Google Scholar]
- 43. Vitek JL, Zhang J, Evatt M, et al. GPi pallidotomy for dystonia: clinical outcome and neuronal activity. Adv Neurol 1998;78:211–219. [PubMed] [Google Scholar]
- 44. Weetman J, Anderson IM, Gregory RP, Gill SS. Bilateral posteroventral pallidotomy for severe antipsychotic induced tardive dyskinesia and dystonia. J Neurol Neurosurg Psychiatry 1997;63(4):554–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garone G, Graziola F, Nicita F, et al. Prestatus and status dystonicus in children and adolescents. Dev Med Child Neurol 2020;62(6):742–749. [DOI] [PubMed] [Google Scholar]
- 46. Levi V, Zorzi G, Messina G, et al. Deep brain stimulation versus pallidotomy for status dystonicus: a single‐center case series. J Neurosurg 2019;Dec 20:1–11. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 47. Zirn B, Korenke C, Wagner M, Rudnik‐Schoneborn S, Muller U. Concurrence of dystonia 1 and Charcot‐Marie‐tooth neuropathy, type 1 a, in a large family. Mov Disord 2011;26(2):361–362. [DOI] [PubMed] [Google Scholar]
- 48. Kohara K, Taira T, Horisawa S, Hanada T, Kawamata T. Bilateral Pallidotomy for tardive dystonia: a case report. No Shinkei Geka 2017;45(11):971–976. [DOI] [PubMed] [Google Scholar]
- 49. Waln O, Jankovic J. Bilateral globus pallidus internus deep brain stimulation after bilateral pallidotomy in a patient with generalized early‐onset primary dystonia. Mov Disord 2013;28(8):1162–1163. [DOI] [PubMed] [Google Scholar]
- 50. Khandelwal A, Pandia MP, Lamsal R. Delayed emergence from anaesthesia and bilateral mydriasis following bilateral pallidotomy. Indian J Anaesth 2018;62(6):466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gross RE. What happened to posteroventral pallidotomy for Parkinson's disease and dystonia? Neurotherapeutics 2008;5(2):281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stewart K, Harvey A, Johnston LM. A systematic review of scales to measure dystonia and choreoathetosis in children with dyskinetic cerebral palsy. Dev Med Child Neurol 2017;59(8):786–795. [DOI] [PubMed] [Google Scholar]
- 53. Gimeno H, Tustin K, Selway R, Lin JP. Beyond the Burke‐Fahn‐Marsden dystonia rating scale: deep brain stimulation in childhood secondary dystonia. Eur J Paediatr Neurol 2012;16(5):501–508. [DOI] [PubMed] [Google Scholar]
- 54. Okun MS, Vitek JL. Lesion therapy for Parkinson's disease and other movement disorders: update and controversies. Mov Disord 2004;19(4):375–389. [DOI] [PubMed] [Google Scholar]
- 55. Kubu CS, Cooper SE, Machado A, Frazier T, Vitek J, Ford PJ. Insights gleaned by measuring patients' stated goals for DBS: more than tremor. Neurology 2017;88(2):124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kubu CS, Ford PJ. Beyond mere symptom relief in deep brain stimulation: an ethical obligation for multi‐faceted assessment of outcome. AJOB Neurosci 2012;3(1):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Adapted quality assessment tool for case reports
FIGURE S1 Flow diagram of study selection process, according to the PRISMA guidelines


